SUMMARY
Intestinal microbes are recognized for their role in human disease. Enterotoxigenic Bacteroides fragilis (ETBF) has been implicated in inflammatory bowel disease and colorectal cancer; however, colonization alone is insufficient to cause these illnesses. We hypothesized that homeostasis in healthy carriers is maintained by colonic mucus, the major constituent of which is the glycoprotein Muc2. We found that Muc2-deficient mice succumb to lethal disease from ETBF colonization in a B. fragilis toxin (BFT)-dependent manner. We identify a toxin regulator, the two-component system RprXY, which suppresses BFT expression in vitro and in vivo. Overexpression of either component was sufficient to prevent lethal disease in Muc2-deficient mice. Our studies demonstrate that homeostasis in the context of ETBF colonization is dependent on a dynamic interaction between intestinal mucus, a bacterial toxin, and a toxin regulatory system. Regulation of virulence may offer a therapeutic target to maintain intestinal homeostasis in susceptible patients.
In Brief
Enterotoxigenic B. fragilis is associated with inflammatory disease of the colon. Hecht, Casterline, and colleagues report that mucus-deficient mice are susceptible to lethal colitis. Suppressing toxin expression by manipulating a bacterial two-component system restores homeostasis, linking the host environment to pathogen virulence and providing a strategy to modify disease outcome.
Bacteroides fragilis is a common commensal anaerobe (Newton et al., 2015), identified as an important species to enteric microbial ecology and health (Fisher and Mehta, 2014; Trosvik and de Muinck, 2015). Molecules elaborated by B. fragilis shape and limit inflammation to the mutual benefit of host and bacterium (Mazmanian et al., 2005, 2008; Round et al., 2011). However, the effects on host health are highly strain dependent. Enterotoxigenic B. fragilis (ETBF) strains express a metalloprotease, termed B. fragilis toxin (BFT), which is activated upon cleavage by the cysteine protease Fragipain (Choi et al., 2016), inducing colonocyte E-cadherin cleavage and inflammatory cytokine secretion (Wu et al., 2004, 2006). ETBF is a causative agent of acute diarrhea among humans and livestock (San Joaquin et al., 1995; Zhang et al., 1999), and is correlated with active inflammatory episodes in patients with inflammatory bowel disease (IBD) (Prindiville et al., 2000). The overrepresentation of ETBF strains in the microbiome of colorectal cancer (CRC) patients (Toprak et al., 2006), and their physical association with neoplastic tissue further implicates these organisms in human disease (Boleij et al., 2015). ETBF virulence has been attributed to the activity of BFT, which enhances colon tumorigenesis and exacerbates IBD-like symptoms in mouse models (Housseau et al., 2016; Rhee et al., 2009; Wu et al., 2009). B. fragilis is also the leading cause of anaerobic sepsis (Redondo et al., 1995), in which BFT contributes to pathogenesis (Choi et al., 2016). Colonization with nontoxigenic B. fragilis (NTBF) is sufficient to pre-empt colonization with ETBF and prevent disease, further supporting the strain dependence of this phenotype (Hecht et al., 2016). Nonetheless, up to 20% of humans are asymptomatically colonized by ETBF (San Joaquin et al., 1995; Zhang et al., 1999). These data are consistent with the conceptual model that clinically significant disease depends on both canonical microbial virulence factors and host susceptibility factors (Casadevall and Pirofski, 2003). Understanding the factors that contribute to ETBF disease susceptibility may thus provide considerable insight into the pathogenesis of IBD and colorectal cancer.
Mouse modeling to date has not replicated host-ETBF interaction without disease. Thus, we sought to develop a model of homeostatic ETBF colonization. Consistent with previous studies (Choi et al., 2016; Rhee et al., 2009), we found that orogastric gavage of 109 colony-forming units (CFU) of wild-type (WT) ETBF into specific pathogen-free (SPF) C57Bl/6 mice treated with continuous antibiotics induces significant epithelial damage (Figure 1A), dependent on BFT expression (Figure 1B). In contrast, mice pretreated with 24 hr of antibiotics showed no evidence of colonic injury when colonized with ETBF WT or ETBF deleted for the bft gene (ETBF Δbft) (Figures 1C and 1D). There was no significant difference in colonization between the continuous and 24-hr antibiotic groups (Figure 1 legend). Thus, 24-hr antibiotic pretreatment models a homeostatic interaction between ETBF and the WT host, permitting investigation of the factors required for maintenance of disease-free colonization.
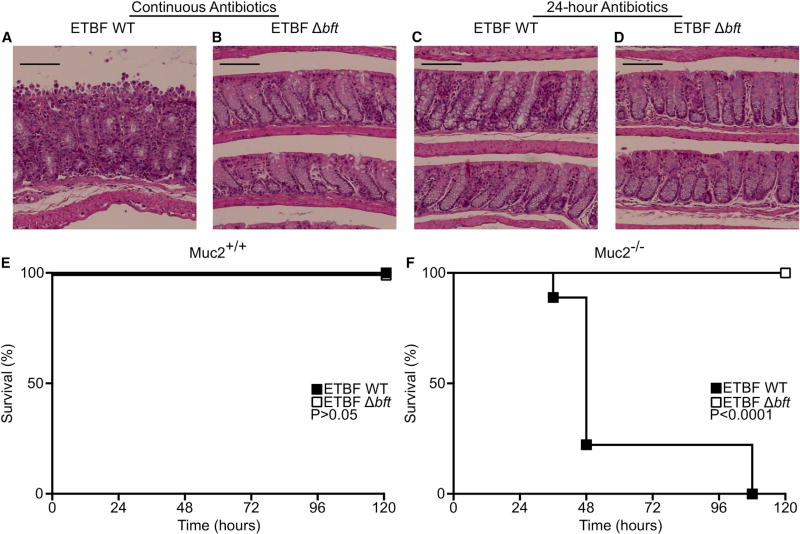
Figure 1. Colonization of Muc2-Deficient Mice with ETBF Results in Lethal BFT-Dependent Disease.
(A and B) Mice were pretreated with antibiotics for 7 days prior to orogastric gavage with ETBF ATCC 43859 WT (A) or Δbft (B) and continued on antibiotics for the duration of the experiment. At 21 days post inoculation the mice were euthanized, the colons fixed, and the tissue stained with H&E. Feces were collected and plated for CFU, demonstrating no significant difference in colonization (WT: 1.36 × 1010 ± 8.51 × 109 CFU/g feces; Δbft: 1.40 × 1010 ± 8.26 × 109). Data are representative of three independent experiments with five mice in each group. Scale bars, 100 µm.
(C and D) Mice were pretreated with antibiotics 24 hr prior to orogastric gavage with ETBF ATCC 43859 WT (A) or Δbft (B) and the antibiotics held thereafter. At 21 days post inoculation the mice were euthanized, the colons fixed, and the tissue stained with H&E. Feces were collected and plated for CFU, demonstrating no significant difference in colonization (WT: 8.00 × 109 ± 3.38 × 109 CFU/g feces; Δbft: 6.56 × 109 ± 4.17 × 109). Data are representative of three independent experiments with five mice in each group. Scale bars, 100 µm. p Values were calculated by one-way ANOVA comparing fecal CFU corresponding to panels (A) to (D).
(E and F) Survival of WT (E) or Muc2−/− mice (F) after orogastric gavage with ETBF ATCC 43859 WT (closed squares) or ETBF Δbft (open squares) clones at 120 hr post colonization.
Results are representative of three independent experiments (E) or are a pooling of three independent experiments (F). Group sizes were as follows: WT mice, ETBF WT (E, closed squares, n = 5); WT mice, ETBF Δbft (E, open squares, n = 5); Muc2−/− mice, ETBF WT (F, closed squares, n = 9); Muc2−/− mice, ETBF Δbft (F, open squares, n = 10). p Values were calculated by log-rank Mantel-Cox test comparing ETBF WT and Δbft groups.
Colonic mucus is one host factor intimately engaged with bacterial interaction (Sun et al., 2016). Muc2, a secreted intestinal glycoprotein, forms the mucus barrier that protects the epithelium from enteric microbes (Bergström et al., 2016; Bergstrom et al., 2010; Johansson et al., 2008). This barrier serves as a reservoir for host-secreted antimicrobial peptides (Antoni et al., 2013), and is mobilized to expel bacteria during host stress (McLoughlin et al., 2016). Mucus niche ablation in mice through deletion of the Muc2 gene (Muc2−/−) dysregulates host-microbe interactions (Johansson et al., 2008; Bergstrom et al., 2010). Conversely, the mucus layer is rich in glycans that provide carbon substrates for bacterial growth (Sonnenburg et al., 2005), specifying B. fragilis niche occupancy and stable colonization (Lee et al., 2013; Roberton and Stanley, 1982; Round et al., 2011). Furthermore, exploitation of host-derived mucopolysaccharides promotes colonization and pathogenesis of Escherichia coli (Alteri et al., 2009; Chang et al., 2004), Campylobacter jejuni (Hofreuter et al., 2008), and Salmonella species (Thiennimitr et al., 2011). This tension between the roles of colonic mucus as microbial barrier and carbon source positions it as a driver of host-microbe homeostasis.
We hypothesized that loss of intestinal mucus would disrupt the ETBF-host relationship. To test this, we colonized Muc2−/− mice and WT littermates with ETBF utilizing the 24-hr antibiotic model. WT mice were grossly and microscopically unaffected by ETBF inoculation through 5 and 21 days post inoculation, respectively (Figures 1C and 1E, closed squares). However, ETBF colonization caused rapid lethality in Muc2−/− mice (Figure 1F, closed squares). Consistent with the centrality of BFT to ETBF virulence, Muc2−/− animals colonized with ETBF Δbft were protected from lethal disease (Figures 1E and 1F, open squares). These results demonstrate that stable equilibrium between the host and ETBF is perturbed by mucus deficiency, with bft deletion sufficient to rescue homeostasis.
The potentially deleterious consequences of host colonization by ETBF led us to examine whether B. fragilis regulates BFT to maintain stability. BFT production is dependent upon the region upstream of the translational initiation site (Franco et al., 2002), which includes five putative promoters, designated P1–P5 (Figure S1A) (Bayley et al., 2000). Serial truncation of this upstream region revealed an effect on toxin production by truncation of P4, with no detectable full-length toxin (FLBFT) or active toxin (BFT*) remaining (Figure S1B). Mutation of only P4 (−7 mutant) produced a similar phenotype (Figure S1B). For confirmation of P4 as the bft promoter, we utilized 5′-RACE (5′ rapid amplification of cDNA ends). This revealed a single full-length bft mRNA with a transcriptional start site downstream of the predicted P4 site (Figures S1C and S1D), matching expectations of transcriptional start site location in B. fragilis (Bayley et al., 2000; Smith et al., 1992). These data confirm P4 as the functional promoter of bft expression.
To identify putative transcriptional regulators, we pursued a pull-down approach whereby DNA from the P4 promoter region was used as bait in ETBF cell lysate. Several proteins specifically bound the P4 promoter region relative to irrelevant DNA (Irr) from within the coding sequence of bft or no DNA (beads). After elution, proteins enriched in the promoter sample were analyzed by mass spectrometry sequencing, leading to the identification of RNA polymerases α (RpoA), β (RpoB), β′ (RpoC), and RprY, a two-component system response regulator (Figure S1E and Table S1).
Bacterial two-component systems (TCSs) transduce external stimuli into a transcriptional response via sensor-regulator pairs: an inner membrane-embedded histidine kinase (HK), and a cytoplasmic DNA-binding response regulator (RR; Stock et al., 2000). Changes in environmental conditions are detected by the HK, inducing autophosphorylation, and are relayed to the cognate RR via phosphotransfer. Bacteroides species require particular TCSs for dietary saccharide sensing and colonization fitness (Sonnenburg et al., 2006). Moreover, TCSs regulate virulence factors of enteric pathogens during intestinal colonization (Pacheco et al., 2012).
We find that rprX and rprY are broadly conserved in the Bacteroidetes. A homolog of RprY was previously identified in Porphyromonas gingivalis (Duran-Pinedo et al., 2007; Krishnan and Duncan, 2013). B. fragilis RprY and its cognate HK, RprX, have active transcriptional effects when heterologously expressed in E. coli (Rasmussen and Kovacs, 1993); however, this TCS has not been explored in B. fragilis. To confirm RprY binding of the P4 promoter region, we performed an anti-RprY immunoblot following DNA pull-down of ETBF lysate, wherein RprY bound selectively to the P4 promoter region (Figure S1F). Electrophoretic mobility shift assays (EMSA) performed with recombinant RprY (rRprY) protein showed that increasing quantities of rRprY caused a shift of the promoter DNA (Figure 2A), at concentrations similar to that of previously tested TCS RRs (Wen et al., 2006). Addition of a high-energy phosphodonor increased rRprY binding to the P4 promoter region (Figure 2A). rRprY in its native state or when combined with phosphodonor had negligible binding affinity to Irr (Figure S2A). Competition with the cold bft promoter precluded DNA shift while cold Irr had no effect (Figure S2B). Specific binding of RprY to the P4 promoter region suggests that BFT transcription is modulated by this interaction. We were unable to generate an rprY mutant through allelic exchange, consistent with studies suggesting that rprY is an essential gene in B. fragilis (Veeranagouda et al., 2014).
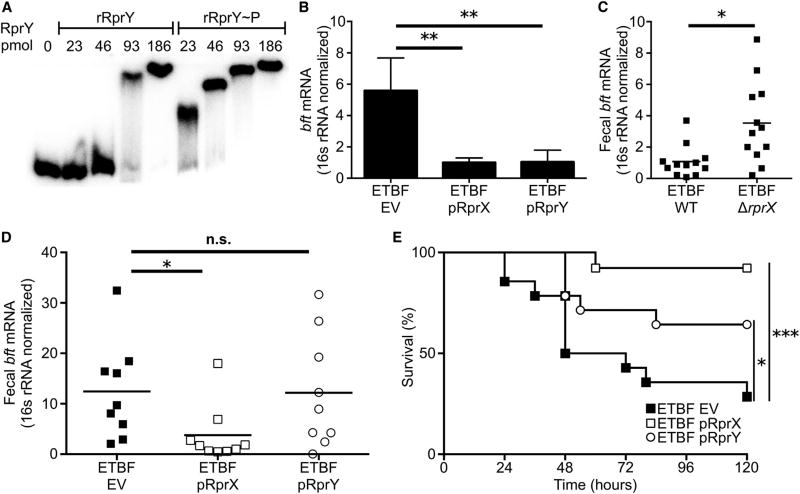
Figure 2. The Two-Component System RprXY Is a BFT Suppressor and Protects Susceptible Mice from Lethal ETBF Colonization.
(A) EMSA was performed with labeled bft promoter and increasing concentrations of rRprY with (rRprY~P) or without acetyl-phosphate treatment. Results are representative of three independent experiments.
(B) RprX or RprY were overexpressed downstream of the GAPDH promoter on a plasmid in ETBF ATCC 43858 (ETBF pRprX and ETBF RprY, respectively) and compared with ETBF encoding empty vector (ETBF EV). RNA was extracted from these ETBF clones and bft transcript quantified using qRT-PCR, normalized to 16S RNA. Results are pooled from four biological replicates of independent trials. Data are presented as mean ± SD; p value was calculated using one-way ANOVA with Dunnett’s multiple comparisons test, **p < 0.01.
(C) WT mice were orally gavaged with ETBF ATCC 43858 WT (n = 12) or ETBF ΔrprX (n = 12). Two days after colonization, fecal RNA was extracted and quantified for bft mRNA using qRT-PCR, normalized to B. fragilis 16S rRNA. The results are pooled from three independent experiments. All data points are shown. p Value was calculated using the Mann-Whitney U test, *p < 0.05.
(D) WT mice were orally gavaged with ETBF EV (n = 9), ETBF pRprX (n = 9), or ETBF pRprY (n = 9). Two days after colonization, fecal RNA was extracted and quantified for bft mRNA using qRT-PCR, normalized to B. fragilis 16S rRNA. Results are pooled from two independent experiments. Data are presented as the mean. p Value was calculated using one-way ANOVA with Dunnett’s multiple comparisons test: *p < 0.05; n.s., not significant.
(E) Survival of Muc2−/− mice after oral gavage with ETBF EV (n = 14), ETBF pRprX (n = 13) or ETBF pRprY (n = 14) clones at 120 hr post colonization. Results depict pooled data of three independent experiments. p Values were calculated by log-rank Mantel-Cox test comparing ETBF EV and ETBF RprX or ETBF EV and ETBF RprY groups: *p < 0.05, ***p < 0.001.
To determine the effect of RprX and RprY on BFT production, we conjugated plasmids for overexpression of RprY (pRprY) or RprX (pRprX) into ETBF (ETBF pRprY and ETBF pRprX, respectively). Overexpression of RprY or RprX suppressed both FLBFT production and bft transcript in vitro (Figures S2D and 2B). These findings suggested that the RprXY TCS may modulate host-microbe commensalism. While a deletion mutant of rprX (ETBF ΔrprX) did not produce detectable changes in FLBFT or BFT* expression in vitro (Figure S2C), loss of RprX resulted in overexpression of bft in vivo (Figure 2C). WT mice inoculated with ETBF pRprX, ETBF pRprY, or ETBF encoding empty vector (ETBF EV) demonstrated that overexpression of RprX reduced fecal bft transcript compared with ETBF EV (Figure 2D), despite similar capacity for colonization (Figure S2E). The RprXY TCS is therefore an in vivo toxin suppressor.
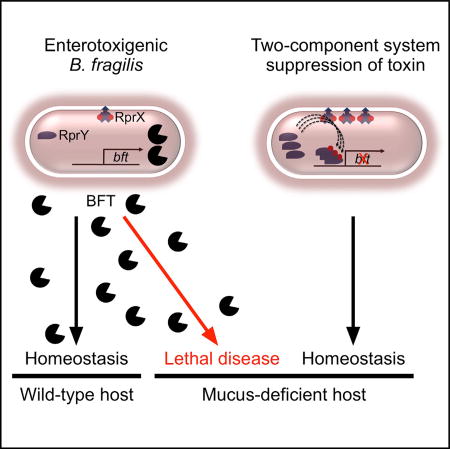
We hypothesized that overexpression of this regulatory system would rescue homeostasis in animals susceptible to BFT-dependent disease. Indeed, Muc2−/− mice colonized with either ETBF pRprX or ETBF pRprY were protected from lethality relative to those inoculated with ETBF EV (Figure 2E), demonstrating that overexpression of the RprXY TCS regulates toxin expression in vivo sufficiently to ameliorate disease in susceptible hosts. Genetic recalibration of a toxin-regulatory sense/response system thus restores balance to a host-microbe interaction that may favor development of an injurious state. These findings lend experimental support to the “damage-response framework of microbial pathogenesis,” in which the state of either health or disease is a product of the exquisite interaction between a microorganism and its host (Casadevall and Pirofski, 2003).
Host injury has been described as a competitive strategy for several pathogens including Salmonella enterica serovar Typhimurium and Citrobacter rodentium (Faber et al., 2016; Lopez et al., 2016). B. fragilis differs from these organisms in several important respects, including its ubiquitous presence and stability within healthy human microbiota (Newton et al., 2015). As up to 20% of human-associated strains of B. fragilis encode bft (Toprak et al., 2006; Zhang et al., 1999), significant disease pathogenesis is a rare outcome of colonization. Thus, the selective advantage of BFT expression and potentially lethal disruption of host homeostasis is unclear (Casterline et al., 2017; Wagner et al., 2016).
Both host and bacterial factors are required to maintain stable, quiescent ETBF colonization. Mucus may physically entrap BFT and facilitate its expulsion (McLoughlin et al., 2016) or prevent ETBF colonization within sufficient distance of the epithelium to cause injury. Alternatively, secreted mucopolysaccharides may protect the host by modulating BFT expression. A number of carbohydrates are known to suppress bft transcription, including those found in colonic glycans (Casterline et al., 2017; Van Tassell et al., 1992). The mechanism by which carbohydrates suppress BFT, and whether the RprXY TCS is involved, remain unclear.
We theorize that bacterial sensing of enteric signals elicits regulatory control that calibrates virulence to the host environment. Subacute or self-resolving colitis may be advantageous for B. fragilis colonization, while severe disease is a stochastic consequence of ETBF exposure in the context of a heterogeneous host population (Casterline et al., 2017; Wagner et al., 2016; Figure S2F). Our findings suggest that the native RprXY TCS is encoded to ensure stability within WT hosts but is insufficiently regulated to maintain host-ETBF homeostasis in susceptible animals. Baseline expression of RprX is lower than that of RprY (Figure S2D); overexpression of RprX, but not of RprY, suppressed fecal bft transcript and enhanced host survival (Figures 2D and 2E). We thus speculate that RprX, serving as a genetic bottleneck in BFT regulation, is critical to both ETBF colonization and host-ETBF homeostasis (Figure S2F).
The high rate of ETBF colonization in the asymptomatic adult population suggests that a long-lasting homeostasis is commonly reached. We propose that the association between ETBF and CRC, a result of elevated epithelial cell turnover from sustained toxin exposure, represents a cost of maintaining homeostasis. Acute diarrhea may represent an episode of brief disequilibrium induced by temporary ETBF expansion or bft upregulation with disruptions of the enteric ecosystem. In contrast, IBD is a chronic failure of host-microbe homeostasis, associated with both host susceptibility and dysbiosis. We assert that ETBF is a paradigmatic “pathobiont” on the basis of the above observations: long-term health risks associated with maintaining homeostasis (CRC), episodes of short-term disease precipitated by unknown triggers (diarrhea), and chronic disease in susceptible hosts (IBD). We add insight into these disease processes, demonstrating that alterations in the balance of host environment and microbial sense/response systems can be deleterious to health. Our results suggest that targeted upregulation of the RprXY TCS or its activating signals may ameliorate BFT-dependent disease. As with the majority of TCSs, the RprX activating signal is unknown. Future studies into these environmental cues would add considerable mechanistic insight into enteric homeostasis and potentially allow for therapeutic repression of bft transcription to restore health in susceptible hosts.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
CONTACT FOR REAGENT AND RESOURCE SHARING
- EXPERIMENTAL MODEL AND SUBJECT DETAILS
-
◦Bacteria
-
◦Mice
-
◦
- METHOD DETAILS
-
◦Plasmids
-
◦Conjugations
-
◦Bacterial Mutants
-
◦Promoter Truncation
-
◦Protein Overexpression
-
◦Recombinant Protein and PURIFICATION
-
◦Antibodies
-
◦B. fragilis Cell Pellet and Supernatant Immunoblot
-
◦5’RACE
-
◦qRT-PCR
-
◦DNA Pulldown
-
◦EMSA
-
◦Bioinformatics
-
◦Fecal CFU and bft Transcript Quantification
-
◦Experimental Design
-
◦
QUANTIFICATION AND STATISTICAL ANALYSIS
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| BFT antiserum | Choi et al., 2016 | N/A |
| RprX antiserum | This paper | N/A |
| RprY antiserum | This paper | N/A |
| E. coli RpoA antibody | Biolegend | Cat# 663102; RRID: AB_2564409 |
| Goat anti-rabbit IgG 680 | Life Technologies | Cat# A-21109; RRID: AB_2535758 |
| Goat anti-mouse IgG 800 | Life Technologies | Cat# SA5-10176; RRID: AB_2556756 |
| Bacterial and Virus Strains | ||
| Bacteroides fragilis | ATCC | ATCC 43858 |
| Bacteroides fragilis | ATCC | ATCC 43859 |
| Escherichia coli | ATCC | ATCC BAA-2428 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| rRprY | This paper | N/A |
| rRprX | This paper | N/A |
| cDNA synthesis kit | Bio-Rad | Cat# 1708891 |
| IQ Sybr Green Super Mix | Bio-Rad | Cat# 1708882 |
| ATP-γ32P | Perkin Elmer | Cat# NEG002A100UC |
| Critical Commercial Assays | ||
| FirstChoice RLM-RACE kit | Invitrogen (Fisher) | Cat# AM1700 |
| ZR soil/fecal RNA microprep kit | Zymo Research | Cat# R2040 |
| Experimental Models: Organisms/Strains | ||
| Jackson C57Bl/6J mice | Jackson Labs | 000664 |
| Muc2−/− C57Bl/6J mice | Velcich et al., 2002 | N/A |
| Oligonucleotides | ||
| See Table S2 for oligonucleotides used in these studies | This paper | N/A |
| Recombinant DNA | ||
| pRprY | This paper | N/A |
| pRprX | This paper | N/a |
| pAH1 | Choi et al., 2016 | N/A |
| pET28b RprY | This paper | N/A |
| pET28b RprX | This paper | N/A |
| pFD340-BFTFL | This paper | N/A |
| pFD340-BFTP5 | This paper | N/A |
| pFD340-BFTP4 | This paper | N/A |
| pFD340-BFTP3 | This paper | N/A |
| pFD340-BFTP2 | This paper | N/A |
| Software and Algorithms | ||
| Geneious version 6.0.5 | www.geneious.com | N/A |
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Juliane Bubeck Wardenburg, jbubeck@wustl.edu.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Bacteria
Human isolated ETBF strains ATCC 43858 (ATCC strain designation 2-078382-3) and ATCC 43859 (ATCC strain designation 20793-3) were utilized in these studies. Both of these strains encode the bft-2 isotype (Franco et al., 1999; Scotto d’Abusco et al., 2000) B. fragilis strains were grown in Brain Heart Infusion (BHI) broth anaerobically at 37°C with a gas mix of 5% H2, 10% CO2 and 85% N2. BHI was supplemented with 0.0005% hemin and 0.5 µg/mL vitamin K1 for optimal growth (BHIS). E. coli S17-1 was used for cloning of shuttle and suicide plasmids and conjugation into B. fragilis. Escherichia coli strains were grown in LB aerobically at 37°C. Antibiotics used were as follows: ampicillin (100 µg/mL), kanamycin (50 µg/mL), gentamicin (200 µg/mL), and clindamycin (5 µg/mL).
Mice
All animal studies were conducted in accord with ethical regulations under protocols approved by the University of Chicago or Washington University Institutional Animal Care and Use and Biosafety Committees. SPF C57BL/6 mice were bred in-house from mice originally purchased from Jackson Laboratory or purchased from Jackson Laboratory and maintained under SPF conditions for use in experimentation at 4 weeks of age. Muc2+/− mice were bred in-house as Muc2+/− × Muc2+/− pairs (Velcich et al., 2002). All pups were genotyped (Table S2). Littermate Muc2−/− and Muc2+/+ mice of both sexes were used at 7 weeks of age. At the time of weaning, animals were randomly distributed for use in experimentation. There was no investigator blinding in animal experimentation, and no animals were excluded from analysis.
Microscopic disease was achieved by treatment of SPF C57BL/6 mice with 100mg/L clindamycin in drinking water for one week prior to orogastric gavage of 109 CFU ATCC 43859. Clindamycin treatment was continued until sacrifice and harvest of colonic tissue in 10% formalin on 21 post-inoculation. Disease-free colonization was achieved by overnight treatment of SPF C57BL/6 mice with 100mg/L clindamycin in drinking water, which was subsequently replaced by regular drinking water at the time of orogastric gavage of 109 CFU ATCC 43859. Colonic tissue was harvested in 10% formalin on day 21 post-inoculation. Fixed tissues were paraffin embedded, sectioned at 5 µm, and stained with H&E.
For survival modeling, Muc2−/− and Muc2+/+ mice were pre-treated with 100mg/L clindamycin in drinking water for 24 hours, which was subsequently replaced by regular drinking water. Mice were then orally gavaged with 109 CFU of various ETBF clones. The mice were monitored for 120 hours and euthanized upon meeting IACUC criteria. For the testing of ETBF WT and ETBF Δbft, the strain ATCC 43859 was utilized with the vector pAH1 integrated into the genome of those clones. For testing of ETBF EV, ETBF pRprX and ETBF pRprY, the strain ATCC 43858 was used.
METHOD DETAILS
Plasmids
pRK231 is a conjugation helper vector used to increase transfer from E. coli to B. fragilis. Allelic exchange mutagenesis was completed with the suicide vector pKNOCK. The shuttle vectors pFD340 and pAH2 were used to determine the bft promoter site in B. fragilis and for mutant complementation, respectively (Hecht et al., 2016). For stable insertion of clindamycin resistance into the genome, the vector pAH1 was utilized (Choi et al., 2016). The vector pET28b was used for recombinant protein production and the vector pKNOCK was used for allelic exchange mutation.
Conjugations
For conjugation of plasmids from E. coli into B. fragilis strains, a previous published protocol was used (Hecht et al., 2016). In brief, appropriate B. fragilis and E. coli strains were grown to mid-log phase in BHIS anaerobically and LB aerobically, respectively. Equal volumes of these cultures were sedimented into a single tube, resuspended in BHI, pooled onto a BHIS plate and grown aerobically at 37°C overnight. This mix was spread onto a selective BHIS plate with gentamicin and clindamycin and grown anaerobically at 37°C.
Bacterial Mutants
In-frame deletion of rprX was generated through allelic exchange using a previously published protocol (Hecht et al., 2016). In brief, regions 1kb upstream and downstream of the rprX gene were amplified and fused via PCR (Table S2). This construct was cloned into pKNOCK and conjugated into strain ATCC 43859. Single clones resistant to clindamycin, indicating genomic integration, were passaged (1:100) daily without antibiotics. After 5-10 passages, single clones were patched onto selective (clindamycin) and nonselective plates. Sensitive colonies were PCR screened for loss of rprX.
Promoter Truncation
To delineate the bft promoter, the toxin and its upstream region were cloned into the pFD340 vector in the KpnI and BamHI sites with a 6xHis tag encoded at the 3’ end of the gene to produce pFD340-BFTFL. The upstream region was serially truncated with primers around the putative promoters P2-5 (diagramed in Figure S1; primers in Table S2). Mutation of the P4 -7 site was performed through amplification of the region upstream of the -7 site and downstream of the site, incorporating mutation of key nucleotides (Table S2, bold). These products were PCR fused and cloned into the pFD340 vector.
Protein Overexpression
To overexpress RprX and RprY in ETBF strain ATCC 43858, the constructs pRprX and pRprY were cloned downstream of a B. fragilis constitutive promoter, similar to a previously published method (Hecht et al., 2016). To accomplish this, the rprX and rprY genes were amplified separately and each fused to the GAPDH promoter and ribosomal binding site via PCR (Table S2). These products were ligated into the pFD340 vector at the BamHI and KpnI sites and conjugated into ETBF strain ATCC 43858.
Recombinant Protein and PURIFICATION
The entire rprY gene was amplified from genomic DNA of ETBF strain ATCC 43858 with a nucleotide sequence appended to the 3’ terminus encoding a 6xHis tag. The DNA sequence encoding the C-terminal region of RprX, downstream of the predicted transmembrane domain, was similarly amplified with a 3’ 6xHis tag appended. Both were cloned into the pET28b vector into the NcoI and XhoI restriction enzyme sites. These were then sequence verified and transformed into BL21 cells for protein expression. Overnight cultures of these clones were diluted 1:50 into fresh LB media, grown to OD600= 0.5 and induced for 4 hours with 1mM IPTG. The cells were pelleted and resuspended in buffer composed of 50mM Tris-base, 500mM NaCl, 20mM imidazole, pH 7.4. The resuspended cells were French pressed 3 times. After centrifugation for 30 minutes at 12,000g, the supernatant was incubated with nickel-NTA beads, rocking at 4°C for one hour. The column was washed with the resuspension buffer and eluted with the same buffer, supplemented with 250mM imidazole. Elution fractions above a concentration of 1mg/mL were dialyzed against PBS overnight at 4°C.
Antibodies
For immunoblots, primary antibody generation and usage were as follows: rabbit anti-BFT antibody was generated, as previously reported (Choi et al., 2016). The rabbit anti-RprX and anti-RprY antibodies were created with recombinant RprX and RprY as a service from Pocono Rabbit Farm and Laboratory, under IACUC approved protocol PRF2A. Mouse monoclonal anti-E. coli RNA-polymerase α (RpoA) antibody was obtained from Biolegend, which we found reactive against B. fragilis RpoA. Primary antibodies were used at the following dilutions in TBST: BFT-1:2000, RprY-1:2000, RprX-1:1000, RpoA-1:2000. Secondary antibodies were as follows: goat anti-rabbit IgG 680 and goat anti-mouse IgG 800 (Life Technologies) were used at a 1:10,000 concentration in TBST.
B. fragilis Cell Pellet and Supernatant Immunoblot
For detection of BFT, RprX, RprY or RpoA in the cell pellet or supernatant fraction, the samples were prepared as follows: 1mL of culture at the indicated time point was pelleted at 5,000g for 5 minutes at room temperature, directly after removal from the anaerobic environment. The cell pellet was resuspended in 2× Laemmli sample buffer and heated to 95°C for 10 minutes. The supernatant was removed and precipitated in a final concentration of 10% TCA. This was incubated for 1 hour on ice, spun at a maximum speed for 30 minutes on a benchtop centrifuge, washed with 100% acetone, and spun at maximum speed for 10 minutes. The wash was repeated once, air-dried for 30 minutes and resuspended in 2× Laemmli sample buffer.
Samples were run on SDS-PAGE gels (10% for pro-toxin detection, 15% for active toxin, RprX, RprY and RpoA) and transferred onto PVDF membrane. Membranes were blocked with 5% skim milk in TBS buffer supplemented with 0.1% Tween-20 (TBST). Membranes were subsequently incubated with primary antibody for one hour, followed by three washes in TBST for 5 minutes each. Secondary antibody was incubated with the membrane for one hour, followed by three TBST washes. This was subsequently imaged on a Li-Cor Odyssey system.
5’RACE
To determine the transcriptional start site of bft, 5’ Rapid Amplification of DNA Ends (5’RACE) was performed. To accomplish this, early stationary phase ETBF strain ATCC 43858 was pelleted and RNA extracted as previously described. 5’RACE was performed with the FirstChoice RLM-RACE kit (Invitrogen) according to manufacturer’s instructions with two alterations. In brief, Tobacco Acid Pyrophosphatase (TAP) either was or was not added to ETBF RNA to distinguish full-length non-degraded mRNA. Adaptor DNA was then ligated to the 5’ end of the mRNA. cDNA was generated as previously described, but a bft-specific primer was used instead of random priming, in order to increase the signal (Table S2). PCR was performed with this same BFT-specific primer and the adaptor-specific primer. This was run on a 2%agarose gel and imaged. One band was found specific to the +TAP lane and was sequenced through Sanger sequencing. Published bft sequence with associated upstream region was compared to sequence from 5’RACE reaction via Map to Reference function of Geneious 6.0.5.
qRT-PCR
To test the transcription levels of bft, quantitative reverse transcription PCR (qRT-PCR) was used. RNA was collected from cell culture using the RNeasy kit and RNA protect (Qiagen), according to manufacturer’s instructions. For fecal pellets, RNA was collected with the ZR soil/fecal RNA microprep kit (Zymo Research). RNase-free DNase (Fisher) was used to digest contaminating genomic DNA. First strand cDNA synthesis was performed with iScript cDNA synthesis kit (Bio-Rad) and qPCR was performed with SYBR Green (Bio-Rad) on Bio-Rad CFX96 or Applied Biosystems 7500 machines. Transcript was quantified with bft-specific primers and normalized to B. fragilis 16s rRNA (Hecht et al., 2016). Efficiency of each primer set was determined to calculate accurate fold-differences and melt curves were examined to confirm the specificity of each reaction.
DNA Pulldown
To determine the binding proteins of the bft promoter from ETBF lysate, a modified protocol from a previously published method was utilized (Jutras et al., 2012).
A 5’ biotin-tagged primer was used with an untagged primer to amplify a ~300bp sequence flanking the P4 toxin promoter from a plasmid template encoding the bft gene and its upstream region (Table S2). This product was column purified to a total of 50µg of DNA per reaction. For irrelevant DNA control, a sequence of the same length was amplified from within the bft coding region with a 5’biotin tagged-untagged primer pair (Table S2). Strepavidin-agarose beads (Thermo-Fisher) were washed two times with 2× B/W buffer (10mM Tris pH 7.5, 1mM EDTA, 2M NaCl). The amplified products were bound to 200µL of beads for 1 hour, rotating at room temperature and washed thrice with TE buffer. The beads were then washed with BS/THES (5× BS buffer: 50mM HEPES, 25mM CaCl2, 250mM KCl, 60% glycerol; 2.25× THES: 50mM Tris pH 7.5, 10mM EDTA, 20% sucrose, 140mM NaCl) twice, followed by one wash with BS/THES supplemented with 10µg/mL salmon sperm DNA (Fisher).
ETBF ATCC 43858 was grown overnight, diluted 1:50 into fresh BHIS and grown to early stationary phase, the point of maximal toxin induction. 500mL of the culture was pelleted and resuspended in 1×BS/1×THES buffer supplemented with complete, EDTA-free protease inhibitor (Roche). These cells were French pressed four times and spun at 20,000rpm for 30 minute to clear cell debris. 100µg of salmon sperm DNA was added to the supernatant.
The prepared beads (promoter, irrelevant, no DNA) were combined with 1mL of cell lysate and incubated by rotating for one hour at room temperature. The beads were then washed five times with BS/THES supplemented with 10µg/mL of salmon sperm DNA. Finally, the beads were washed twice more with BS/THES and the supernatant discarded.
Elution was performed with elution buffer (25mM Tris pH 7.5, varying NaCl), serially increasing NaCl during each elution to remove more tightly bound proteins. Each elution was performed through rolling incubation for 5 minutes, followed by spin and storage of the supernatant. This was performed with 100, 200, 300, 500, 750mM and 1M NaCl concentrations. Each fraction was run on an SDS-PAGE gel (15%), comparing promoter DNA to irrelevant DNA and no DNA controls. Silver stain (Pierce) was performed, bands of interest excised and sent for mass spectrometry peptide sequencing (Taplin Biological Mass Spectrometry Facility, Harvard University).
EMSA
To determine the specificity of RprY binding to the toxin promoter, we performed an electrophoretic mobility shift assay (EMSA). A protocol was adapted from a previously published method (Hellman and Fried, 2007). Briefly, promoter or irrelevant DNA was amplified as in section “Methods: DNA pulldown” and radiolabeled with 32P using ATP-γ32P (Perkin Elmer) and T4 polynucleotide kinase (NEB) according to manufacturer instructions. The DNA was column purified and diluted to a concentration of 5fmol/µL. Recombinant RprY was prepared via incubation with or without acetyl phophate in 1× buffer (2× buffer: 50mM Tris pH 7.5, 20mM MgCl2, 0.1mM DTT, ± 20mM acetyl phosphate, frozen in aliquots at −20°C immediately after preparation) to generate RprY or RprY~P, respectively. Incubation of RprY or RprY~P with 5fmol of DNA was performed for 1 hour in 1× binding buffer (5× binding buffer: 100mM HEPES pH 7.9, 300mM KCl, 25mM MgCl, 5mM EDTA, 5mM DTT, 1.5mg/mL BSA, 1mg/mL salmon sperm DNA, 50% glycerol). After 1 hour of binding, the samples were run immediately on a 5%TAE acrylamide gel. The gels were dried, exposed overnight and imaged on a phosphorimager.
For cold-competitor experiments, varying concentrations of cold promoter or irrelevant DNA were added to the incubation mix as noted in Figure S2. The EMSA protocol was otherwise performed as stated above.
Bioinformatics
BLASTn search was conducted with nucleotide sequences of B. fragilis rprX and rprY. Using an E-value cut off of 0.0001 65 cultured organisms (67 total) with homologs of the rprX coding sequence were uncovered. All 65 were in Bacteroidetes phylum, including the major classes Bacteroidia (39), Cytophagia (16), Chitinophagaceae (5), and Flavobacteriaceae (3). The same search parameters found 77 (78 total) cultured organisms with homologs for rprY. 69 of these are in Bacteroidetes, including the major classes Bacteroidia (49), Cytophagia (9), and Flavobacteriaceae (16). RprY was also found in Firmicutes (7 hits), including the human pathogen Clostridium botulinum.
Fecal CFU and bft Transcript Quantification
SPF C57BL/6 mice were pre-treated with 100mg/L clindamycin in drinking water for 24 hours, which was subsequently replaced by regular drinking water. Mice were then orally gavaged with 109 CFU of ETBF EV, ETBF pRprX, ETBF pRprY, or ETBF ΔrprX KO. After 48 hours, fecal pellets were collected from individual mice, weighed, and vortexed in 1 mL PBS to achieve homogenization. Serial 10-fold dilutions were plated on BHIS agar containing gentamicin and clindamycin. CFU/g feces for each clone was calculated, log10 transformed and plotted. For transcript quantification, fecal pellets were collected at 48 hours post-gavage and RNA was extracted with the ZR soil/fecal RNA microprep kit (Zymo Research). RNase-free DNase (Fisher) was used to digest genomic DNA in the samples. First-strand cDNA synthesis was accomplished with iScript cDNA synthesis kit (Bio-Rad), and qPCR was performed with SYBR Green (Bio-Rad) on a Bio-Rad CFX96 machine.
Experimental Design
For all experimentation, at least two independent replicates were performed and the pooled data or a representative replicate is shown, delineated in the figure legends. For all mouse experiments, mice were randomized to equivalent groups. No investigator blinding was used in these studies. Sample size was estimated using α=0.05, β=0.20, and means specific to each experiment. No data points were excluded in these studies.
QUANTIFICATION AND STATISTICAL ANALYSIS
The details of statistical testing, sample size (n), mean, and standard deviation are reported in the figures and corresponding legends. Statistical analysis was performed using GraphPad Prism software. One-way ANOVA, with Dunnett’s multiple comparisons test was used to compare CFU and qRT-PCR for three or more experimental groups while a Mann-Whitney t test was used to compare two groups. Comparison of variances was performed in GraphPad utilizing the F test, providing documentation of similar variance between groups. Log-Rank Mantel-Cox test was used to compare survival curves.
Supplementary Material
Highlights.
Enterotoxigenic B. fragilis causes lethal disease in mice lacking colonic mucus
A two-component system regulates B. fragilis toxin in vitro and in vivo
Suppressing toxin expression restores homeostasis and prevents lethality
Colonic disease is a manifestation of both host susceptibility and bacterial virulence
Acknowledgments
This work was supported by a Pilot and Feasibility Award from the Digestive Diseases Research Core Center at the University of Chicago (NIDDK P30DK42086). J.B.W. is a recipient of a Burroughs Wellcome Foundation Investigators in the Pathogenesis of Infectious Disease Fellowship. B.W.C. is supported by the National Institute of Allergy and Infectious Diseases of the NIH (F30AI126791). A.L.H., B.W.C., and V.M.C. are trainees of the NIH Medical Scientist Training Program at the University of Chicago (GM007281). We thank Drs. Lucia Rothman-Denes and Sean Crosson for their insight into DNA-binding proteins and TCSs.
Footnotes
Supplemental Information includes two figures and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.chom.2017.08.007.
AUTHOR CONTRIBUTIONS
A.L.H., B.W.C., and J.B.W. conceived, designed, and analyzed the experiments. A.L.H., B.W.C., and V.M.C. performed the experiments. A.L.H., B.W.C., and J.B.W. wrote the manuscript.
References
- Alteri CJ, Smith SN, Mobley HLT. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 2009;5:e1000448. doi: 10.1371/journal.ppat.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni L, Nuding S, Weller D, Gersemann M, Ott G, Wehkamp J, Stange EF. Human colonic mucus is a reservoir for antimicrobial peptides. J. Crohns Colitis. 2013;7:e652–664. doi: 10.1016/j.crohns.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Bayley DP, Rocha ER, Smith CJ. Analysis of cepA and other Bacteroides fragilis genes reveals a unique promoter structure. FEMS Microbiol. Lett. 2000;193:149–154. doi: 10.1111/j.1574-6968.2000.tb09417.x. [DOI] [PubMed] [Google Scholar]
- Bergström JH, Birchenough GMH, Katona G, Schroeder BO, Schütte A, Ermund A, Johansson MEV, Hansson GC. Gram-positive bacteria are held at a distance in the colon mucus by the lectin-like protein ZG16. Proc. Natl. Acad. Sci. USA. 2016;113:13833–13838. doi: 10.1073/pnas.1611400113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom KSB, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015;60:208–215. doi: 10.1093/cid/ciu787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Pirofski L. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casterline BW, Hecht AL, Choi VM, Wardenburg JB. The Bacteroides fragilis pathogenicity island links virulence and strain competition. Gut Microbes. 2017;23:1–10. doi: 10.1080/19490976.2017.1290758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D-E, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA. 2004;101:7427–7432. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi VM, Herrou J, Hecht AL, Teoh WP, Turner JR, Crosson S, Bubeck Wardenburg J. Activation of Bacteroides fragilis toxin by a novel bacterial protease contributes to anaerobic sepsis in mice. Nat. Med. 2016;22:563–567. doi: 10.1038/nm.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Pinedo AE, Nishikawa K, Duncan MJ. The RprY response regulator of Porphyromonas gingivalis. Mol. Microbiol. 2007;64:1061–1074. doi: 10.1111/j.1365-2958.2007.05717.x. [DOI] [PubMed] [Google Scholar]
- Faber F, Tran L, Byndloss MX, Lopez CA, Velazquez EM, Kerrinnes T, Nuccio S-P, Wangdi T, Fiehn O, Tsolis RM, et al. Host-mediated sugar oxidation promotes post-antibiotic pathogen expansion. Nature. 2016;534:697–699. doi: 10.1038/nature18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CK, Mehta P. Identifying keystone species in the human gut microbiome from metagenomic timeseries using sparse linear regression. PLoS One. 2014;9:e102451. doi: 10.1371/journal.pone.0102451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AA, Cheng RK, Chung GT, Wu S, Oh HB, Sears CL. Molecular evolution of the pathogenicity island of enterotoxigenic Bacteroides fragilis strains. J. Bacteriol. 1999;181:6623–6633. doi: 10.1128/jb.181.21.6623-6633.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AA, Cheng RK, Goodman A, Sears CL. Modulation of bft expression by the Bacteroides fragilis pathogenicity island and its flanking region. Mol. Microbiol. 2002;45:1067–1077. doi: 10.1046/j.1365-2958.2002.03077.x. [DOI] [PubMed] [Google Scholar]
- Hecht AL, Casterline BW, Earley ZM, Goo YA, Goodlett DR, Bubeck Wardenburg J. Strain competition restricts colonization of an enteric pathogen and prevents colitis. EMBO Rep. 2016;17:1281–1291. doi: 10.15252/embr.201642282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman LM, Fried MG. Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nat. Protoc. 2007;2:1849–1861. doi: 10.1038/nprot.2007.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofreuter D, Novik V, Galán JE. Metabolic diversity in Campylobacter jejuni enhances specific tissue colonization. Cell Host Microbe. 2008;4:425–433. doi: 10.1016/j.chom.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Housseau F, Wu S, Wick EC, Fan H, Wu X, Llosa NJ, Smith KN, Tam A, Ganguly S, Wanyiri JW, et al. Redundant innate and adaptive sources of IL17 production drive colon tumorigenesis. Cancer Res. 2016;76:2115–2124. doi: 10.1158/0008-5472.CAN-15-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras BL, Verma A, Stevenson B. Identification of novel DNA-binding proteins using DNA-affinity chromatography/pull down. Curr. Protoc. Microbiol. 2012 doi: 10.1002/9780471729259.mc01f01s24. Chapter 1 http://dx.doi.org/10.1002/9780471729259.mc01f01s24, Unit1F.1. [DOI] [PMC free article] [PubMed]
- Krishnan K, Duncan MJ. Role of sodium in the RprY-dependent stress response in Porphyromonas gingivalis. PLoS One. 2013;8:e63180. doi: 10.1371/journal.pone.0063180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Donaldson GP, Mikulski Z, Boyajian S, Ley K, Mazmanian SK. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013;501:426–429. doi: 10.1038/nature12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, Miller BM, Rivera-Chávez F, Velazquez EM, Byndloss MX, Chávez-Arroyo A, Lokken KL, Tsolis RM, Winter SE, Bäumler AJ. Virulence factors enhance Citrobacter rodentium expansion through aerobic respiration. Science. 2016;353:1249–1253. doi: 10.1126/science.aag3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McLoughlin K, Schluter J, Rakoff-Nahoum S, Smith AL, Foster KR. Host selection of microbiota via differential adhesion. Cell Host Microbe. 2016;19:550–559. doi: 10.1016/j.chom.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Newton RJ, McLellan SL, Dila DK, Vineis JH, Morrison HG, Eren AM, Sogin ML. Sewage reflects the microbiomes of human populations. MBio. 2015;6:e02574. doi: 10.1128/mBio.02574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. Fucose sensing regulates bacterial intestinal colonization. Nature. 2012;492:113–117. doi: 10.1038/nature11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg. Infect. Dis. 2000;6:171–174. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BA, Kovacs E. Cloning and identification of a two-component signal-transducing regulatory system from Bacteroides fragilis. Mol. Microbiol. 1993;7:765–776. doi: 10.1111/j.1365-2958.1993.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Redondo MC, Arbo MD, Grindlinger J, Snydman DR. Attributable mortality of bacteremia associated with the Bacteroides fragilis group. Clin. Infect. Dis. 1995;20:1492–1496. doi: 10.1093/clinids/20.6.1492. [DOI] [PubMed] [Google Scholar]
- Rhee K-J, Wu S, Wu X, Huso DL, Karim B, Franco AA, Rabizadeh S, Golub JE, Mathews LE, Shin J, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 2009;77:1708–1718. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton AM, Stanley RA. In vitro utilization of mucin by Bacteroides fragilis. Appl. Environ. Microbiol. 1982;43:325–330. doi: 10.1128/aem.43.2.325-330.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Joaquin VH, Griffis JC, Lee C, Sears CL. Association of Bacteroides fragilis with childhood diarrhea. Scand. J. Infect. Dis. 1995;27:211–215. doi: 10.3109/00365549509019011. [DOI] [PubMed] [Google Scholar]
- Scotto d’Abusco AS, Del Grosso M, Censini S, Covacci A, Pantosti A. The alleles of the bft gene are distributed differently among enterotoxigenic Bacteroides fragilis strains from human sources and can be present in double copies. J. Clin. Microbiol. 2000;38:607–612. doi: 10.1128/jcm.38.2.607-612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Rogers MB, McKee ML. Heterologous gene expression in Bacteroides fragilis. Plasmid. 1992;27:141–154. doi: 10.1016/0147-619x(92)90014-2. [DOI] [PubMed] [Google Scholar]
- Sonnenburg ED, Sonnenburg JL, Manchester JK, Hansen EE, Chiang HC, Gordon JI. A hybrid two-component system protein of a prominent human gut symbiont couples glycan sensing in vivo to carbohydrate metabolism. Proc. Natl. Acad. Sci. USA. 2006;103:8834–8839. doi: 10.1073/pnas.0603249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL, Xu J, Leip DD, Chen C-H, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu. Rev. Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Sun J, Shen X, Li Y, Guo Z, Zhu W, Zuo L, Zhao J, Gu L, Gong J, Li J. Therapeutic potential to modify the mucus barrier in inflammatory bowel disease. Nutrients. 2016;8:E44. doi: 10.3390/nu8010044. http://dx.doi.org/10.3390/nu8010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiennimitr P, Winter SE, Winter MG, Xavier MN, Tolstikov V, Huseby DL, Sterzenbach T, Tsolis RM, Roth JR, Bäumler AJ. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl. Acad. Sci. USA. 2011;108:17480–17485. doi: 10.1073/pnas.1107857108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 2006;12:782–786. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Trosvik P, de Muinck EJ. Ecology of bacteria in the human gastrointestinal tract–identification of keystone and foundation taxa. Microbiome. 2015;3:44. doi: 10.1186/s40168-015-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tassell RL, Lyerly DM, Wilkins TD. Purification and characterization of an enterotoxin from Bacteroides fragilis. Infect. Immun. 1992;60:1343–1350. doi: 10.1128/iai.60.4.1343-1350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranagouda Y, Husain F, Tenorio EL, Wexler HM. Identification of genes required for the survival of B. fragilis using massive parallel sequencing of a saturated transposon mutant library. BMC Genomics. 2014;15:429. doi: 10.1186/1471-2164-15-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Wagner VE, Dey N, Guruge J, Hsiao A, Ahern PP, Semenkovich NP, Blanton LV, Cheng J, Griffin N, Stappenbeck TS, et al. Effects of a gut pathobiont in a gnotobiotic mouse model of childhood undernutrition. Sci. Transl. Med. 2016;8:366ra164. doi: 10.1126/scitranslmed.aah4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Feng J, Scott DR, Marcus EA, Sachs G. Involvement of the HP0165-HP0166 two-component system in expression of some acidic-pH-upregulated genes of Helicobacter pylori. J. Bacteriol. 2006;188:1750–1761. doi: 10.1128/JB.188.5.1750-1761.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway. Infect. Immun. 2004;72:5832–5839. doi: 10.1128/IAI.72.10.5832-5839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Shin J, Zhang G, Cohen M, Franco A, Sears CL. The Bacteroides fragilis toxin binds to a specific intestinal epithelial cell receptor. Infect. Immun. 2006;74:5382–5390. doi: 10.1128/IAI.00060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Rhee K-J, Albesiano E, Rabizadeh S, Wu X, Yen H-R, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Svenungsson B, Kärnell A, Weintraub A. Prevalence of enterotoxigenic Bacteroides fragilis in adult patients with diarrhea and healthy controls. Clin. Infect. Dis. 1999;29:590–594. doi: 10.1086/598639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.