Abstract
Background
Detection of below-threshold first-phase insulin release or FPIR (1 + 3 minute insulin concentrations during an intravenous glucose tolerance test [IVGTT]) is important in type 1 diabetes prediction and prevention studies including the TrialNet Oral Insulin Prevention Trial. We assessed whether an insulin immunoenzymometric assay (IEMA) could replace the less practical but current standard of a radioimmunoassay (RIA) for FPIR.
Methods
One hundred thirty-three islet autoantibody positive relatives of persons with type 1 diabetes underwent 161 IVGTTs. Insulin concentrations were measured by both assays in 1056 paired samples. A rule classifying FPIR (below-threshold, above-threshold, uncertain) by the IEMA was derived and validated against FPIR by the RIA.
Results
The insulin IEMA-based rule accurately classified below- and above-threshold FPIRs by the RIA in 110/161 (68%) IVGTTs, but was uncertain in 51/161 (32%) tests for which FPIR by RIA is needed. An uncertain FPIR by the IEMA was more likely among below-threshold vs above-threshold FPIRs by the RIA (64% [30/47] vs. 18% [21/114], respectively; p < 0.05).
Conclusions
An insulin IEMA for FPIR in subjects at risk for type 1 diabetes accurately determined below- and above-threshold FPIRs in 2/3 of tests relative to the current standard of the insulin RIA, but could not reliably classify the remaining FPIRs. TrialNet is limiting the insulin RIA for FPIR to the latter given the practical advantages of the more specific IEMA.
Keywords: first-phase insulin release, insulin radioimmunoassay, insulin immunoenzymometric assay, prediction and prevention of type 1 diabetes
INTRODUCTION
Low first-phase insulin release (FPIR) – as measured by the sum of the 1 and 3 min insulin concentrations during a 10-min intravenous glucose tolerance test (IVGTT) - is strongly predictive of future type 1 diabetes in persons with islet autoantibodies and a family history of the disease [1 – 3]. This has made FPIR important to type 1 diabetes prediction and prevention studies [3 – 6]. For example, in the Diabetes-Prevention Trial – Type 1 (DPT-1), a below-threshold FPIR indicated high risk for diabetes (> 50%/5 yrs) and justified entry to the more intensive DPT-1 Parenteral Insulin Trial whereas an above-threshold FPIR indicated lower risk (25 – 50%/5 yrs) and was an entry criterion for the DPT-1 Oral Insulin Trial [4,6].
The method to determine FPIR was standardized in 1992 [2]. The 1 + 3 minute insulin concentrations defining below- and above-threshold FPIRs were subsequently determined in healthy controls using insulin concentrations measured by a radioimmunoassay (RIA) by the DPT-1 Study Group [7,8]. Other insulin assays have since become available that offer major advantages over the RIA including greater specificity for insulin, less within-assay variation, better sensitivity for lower insulin concentrations, smaller volumes, and faster throughput [9,10]. Another limitation of the DPT-1 insulin RIA is its dependence on a finite supply of polyclonal antibodies to insulin. Despite these strengths, measurement of FPIR by the original insulin RIA remains the reference standard for type 1 diabetes prediction and prevention studies because FPIR by newer insulin assays have not been validated against the RIA-based FPIR. This is relevant to all type 1 diabetes prediction and prevention studies that require FPIR but is especially pertinent to the TrialNet Oral Insulin Type 1 Diabetes Prevention Trial that is now testing a DPT-1 subgroup finding that suggested that oral insulin prevented diabetes in subjects with strongly positive insulin autoantibodies and above-threshold FPIRs by the RIA [6,11].
MATERIALS AND METHODS
Subjects
All subjects were enrolled between 2004 and 2009 in the TrialNet Natural History Study (NHS) of type 1 diabetes [12] and provided informed consent prior to study. The NHS entry criteria include non-diabetic relatives (1st, 2nd or 3rd degree) of persons with type 1 diabetes and age between 1 and 45 y. Subjects were screened for autoantibodies (Insulin Autoantibodies [mIAA], Glutamic Acid Decarboxylase Autoantibodies [GADA], and IA-2 Autoantibodies [IA-2A]); if positive, they were also tested for Islet Cell Antibodies (ICA) and offered follow-up for development of diabetes including oral glucose tolerance tests (OGTTs) [12]. Autoantibody positive subjects were potentially eligible for the TrialNet Oral Insulin Prevention Trial and underwent a 10-min IVGTT for FPIR [11]. We identified 133 consecutive NHS subjects between 2004 and 2009 undergoing IVGTTs who had 1 and 3 minute insulin concentrations determined by both RIA and IEMA. Twenty-eight subjects had 2 FPIR tests yielding 161 IVGTTs and, because the IVGTT included samples at up to 5 other times, a total of 1056 pairs of insulin concentrations by RIA and IEMA for analysis.
FPIR determinations
The IVGTT used the standardized protocol [2]. Insulin concentrations were measured by RIA and IEMA in the TrialNet Beta-Cell Function Core Laboratory (Seattle, Washington) which was also the laboratory that determined FPIR by the RIA in the DPT-1 [4,6]. Samples for analysis by the two insulin assays were stored at −80°C under strict monitoring and without freeze thaw cycles. Under these conditions insulin concentrations have been found to remain stable.
Total immunoreactive insulin analysis was performed by a double-antibody in-house developed RIA [13]. The procedure uses a 48-hour PEG-accelerated assay involving a guinea pig anti-human insulin primary antibody, and a goat anti-guinea pig second antibody. The pig anti-human insulin antibody was produced in the laboratory and is available in a very large quantity, therefore ensuring assay consistency over many years. The standard curve range is 360 µU/ml and has a sensitivity of 3 µU/ml. The assay is not insulin specific. A set of high and low insulin quality control samples are analyzed in each batch of samples to monitor assay performance. The CVs for the high (mean 66.7 µU/ml) and low (mean 22.7 µU/ml) insulin quality control are 4.5% and 6.9% respectively. As part of a quality control program, the laboratory received masked split-duplicate samples. The CV on a large number of blind split-duplicates was consistently <8.5%.
The insulin IEMA was performed using a AIA-1800 auto-analyzer (Tosoh Bioscience, South San Francisco, CA) [9]. Insulin present in the test sample is bound with a monoclonal antibody immobilized on a magnetic solid phase and an enzyme labeled monoclonal antibody. The magnetic beads are washed to remove unbound enzyme labeled monoclonal antibody and then incubated with a fluorogenic substrate, 4-methylumbelliferyl phosphate (4MUP). The amount of enzyme-labeled monoclonal antibody that binds to the beads was directly proportional to the immuno-reactive insulin concentration in the test sample. A standard curve (range up to 330 µU/ml) constructed with calibrators of known concentration is used for calculating insulin concentrations in unknown samples. The assay calibrator is standardized against WHO Ist IRP 66/304 (1974) and has a sensitivity concentration of 0.5 µU/ml. The assay has high specificity with low cross-reactivity with human C-peptide (0%), intact proinsulin (2.3%) and split 32,33 proinsulin (2.6%) [9]. A set of high and low insulin concentration quality control samples are analyzed in each batch of samples to monitor assay performance. The assay variability is low as the CVs for the high (mean 66.3 µU/ml) and low (mean 20.1 µU/ml) insulin concentration quality control are 2.5% and 3.0% respectively. The CV on a large number of blind split-duplicates was 2.8%.
Sums of the 1 and 3 minute insulin RIA concentrations defined below-threshold and above-threshold FPIRs according to established cutpoints based on subject age and relationship to the type 1 diabetes proband [7,8]. Below-threshold FPIRs required: a) 1 + 3 min insulin concentrations < 60 µU/ml for parents or for siblings and offspring between ages 3 and 7 years; b) 1 + 3 min insulin concentrations < 100 µU/ml for siblings and offspring ≥ 8 years old; or c) 1 + 3 min insulin concentrations < 100 µU/ml for 2nd and 3rd degree relatives between age 8 and 20 y.
Analysis
We used paired RIA and IEMA insulin concentrations to develop regression models that predicted the insulin RIA concentration from the insulin IEMA concentration. We considered 4 models: a) simple linear; b) quadratic; c) linear-on-log (natural) transformed insulin RIA and IEMA concentrations; and d) quadratic-on-log transformed insulin RIA and IEMA concentrations. Each model was assessed for statistical significance and R2 value. Prior to analysis the best model was defined as the one that was statistically significant, used the fewest terms, and had an acceptable R2 value. We did not specify a hierarchy among these criteria in advance of choosing a model, and found the simple linear model to be the best option.
Using insulin RIA concentrations of 60 and 100 µU/ml that define below-threshold FPIRs according to subject age and relationship to the proband, and inverting the linear model (insulin RIA concentration – 3.8290]/1.52813 = IEMA insulin concentration), yielded respective below-threshold FPIRs by the insulin IEMA of 36.76 µU/ml (95% CLs: 17.17, 56.35) and 62.93 µU/ml (95% CLs: 43.34, 82.53). We used Fieller’s theorem to estimate the 95% confidence limits about the interpolated IEMA-based FPIR thresholds [14]. We then created a rule to classify each subject’s FPIR by the insulin IEMA based on the confidence limits. Thus, the IVGTT was classified as “below-threshold FPIR” if the IEMA FPIR value was below the lower 95% confidence limits (< 17.17 or < 43.34 µU/ml depending on subject age and relationship to proband), as “above-threshold FPIR” if the IEMA FPIR value exceeded the upper 95% confidence limit (> 56.35 or > 82.53 µU/ml), and as “uncertain FPIR” if the IEMA FPIR value fell within the 95% confidence limits. As part of the rule, we decided that subjects with an uncertain FPIR would require that their FPIR be determined by insulin RIA concentrations. We chose 95% confidence limits on the interpolated IEMA thresholds to define below- and above-threshold FPIRs in advance of the analysis but also tested the effect of narrower confidence limits (eg., 90%) on misclassification error rates.
Rates of misclassification errors using the insulin IEMA-based rule were computed by the method of “Leave-One-Out-Cross-Validation (LOOCV)” [15]. In LOOCV, each subject is withheld one-at-a-time and a linear model is fit to the data from the remaining subjects. Inversely interpolated thresholds and confidence regions are recalculated, the held-out subject’s FPIR is reclassified, and the error for classifying that subject is recorded. This is repeated for all subjects. The error rates are then averaged to derive an estimate of error that is free from the bias that arises from validating the model on the same subjects used to create the model.
The fitted regression models used all available paired insulin RIA and IEMA concentrations at all time points during subjects’ IVGTTs, including paired concentrations in subjects who underwent more than one IVGTT. The presence of within-subject correlation does not affect least-squares regression estimates, and the use of all available insulin data increased the precision of our estimates. Model selection was partially based on p-values and could potentially be affected by within-subject correlation. However, because model validation by the LOOCV required removal of FPIR results from the withheld subject including subjects who underwent two IVGTTs, model validation was based on statistically independent data and was therefore not affected by within-subject correlation.
RESULTS
Subjects’ mean age was 9.6 y (range 3 to 45 y), 51/133 (38%) were female, and 123/133 (92%) were first-degree relatives (parent, sibling, or child) of persons with type 1 diabetes. All subjects were positive for at least one islet autoantibody (mIAA, 121/133 [91%]; GADA, 114/133 [86%]; IA-2A, 72/133 [54%]; ICA, 63/133 [47%]); 126/133 (95%) subjects were positive for at least 2 autoantibodies; and 7/133 (5%) were positive for mIAA only.
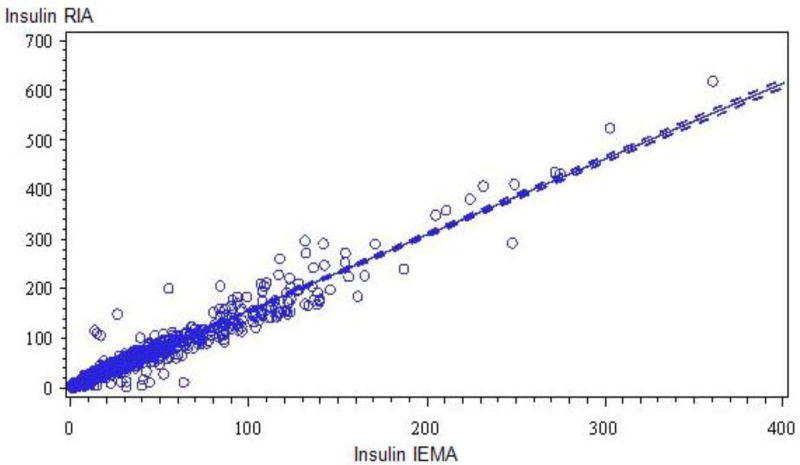
The relationship between paired insulin RIA and IEMA determinations is shown in the Figure. Results from fitting the four regression models are given in Table 1. While each model was statistically significant (p < 0.05), we chose Model 1 because of its comparatively high R2 (0.94) value and fewest (2) terms. A similar, statistically significant R2 (0.92) value was seen using Model 1 that was limited to the 1 and 3 minute insulin RIA and IEMA paired results (n = 322 pairs).
Figure.
Scatter plot and Least Square regression line for insulin concentrations (µU/ml) by the insulin RIA and insulin IEMA in 1056 paired samples in 133 subjects (R2 = 0.94, p < 0.05).
Table 1.
Model Results
| Model | R2 |
|---|---|
| Model 1 (Linear): 3.82980+1.52813(IEMA) | 94.0% |
| Model 2 (Quadratic): 6.01070+1.41031(IEMA)-0.00066(IEMA)2 | 94.2% |
| Model 3 (Log-Linear: 1.03312+.84773Ln(IEMA) | 90.7% |
| Model 4 (Log-Quadratic): 1.31336+.60022Log(IEMA)+.04485*Log(IEMA)2 | 91.3% |
“IEMA”= Insulin level by the Immunoenzymometric Assay. All models and all terms in each model were statistically significant (p < 0.05).
Table 2 shows the results of the cross-validation procedure by LOOCV. Of 161 IVGTTs, 47 (29%) were classified by the insulin RIA as having below-threshold FPIR and 114 (71%) as above-threshold FPIR. Using the proposed rule to determine FPIR according to insulin IEMA concentrations, no IVGTTs were incorrectly classified as below- or above-threshold relative to FPIRs using the insulin RIA. Among 47 IVGTTs yielding below-threshold FPIRs by the insulin RIA, 17 (36%) were correctly classified by the insulin IEMA to have a below-threshold FPIR, none were misclassified as being above-threshold FPIR, and 30 (64%) were classified as uncertain and therefore required the insulin RIA to determine FPIR. Similarly, among 114 IVGTTs that yielded an above-threshold FPIR by the insulin RIA, 93 (82%) were correctly classified by the IEMA-based rule as having an above-threshold FPIR, none were misclassified as having a below-threshold FPIR, and 21 (18%) were classified as uncertain. The proportion of uncertain FPIRs by the insulin IEMA was significantly higher among subjects with a below-threshold vs. above-threshold FPIR by the insulin RIA (30/47 [64%] vs. 21/114 [18%], respectively, p < 0.05).
Table 2.
Cross-validation Results
| FPIR by Insulin IEMA | FPIR by Insulin RIA Below-threshold |
FPIR by Insulin RIA Above-threshold |
Total |
|---|---|---|---|
| Below-threshold | 17 | 0 | 17 |
| Uncertain | 30 | 21 | 51 |
| Above-threshold | 0 | 93 | 93 |
| Total | 47 | 114 | 161 |
Each cell shows the number of IVGTTs yielding simultaneous FPIR results by the insulin RIA (the “reference standard”) and the insulin IEMA. Below- and above-threshold FPIRs by the insulin RIA used the established cutpoints of 60 and 100 µU/ml depending on subject’s age and relationship to the proband with type 1 diabetes (see text), which corresponded to the respective cutpoints (95% CI) of 36.76 µU/ml (17.17, 56.35) and 62.93 µU/ml (43.34, 82.53) by the insulin IEMA as interpolated using Model 1 (Table 1). A Below-threshold FPIR by the insulin IEMA meant that the subject’s value was below the lower 95% confidence limits (<17.17 or < 43.34 µU/mL), an Above-threshold FPIR meant that the subject’s value exceeded the upper 95% CI (> 56.35 or > 82.53 µU/ml), and an Uncertain FPIR meant that the subject’s value was within the 95% CI.
Using 90% confidence limits on the interpolated insulin IEMA values for the insulin RIA thresholds of 60 and 100 µU/ml reduced the total number of FPIRs categorized as uncertain (N = 40 vs. 51) and did not misclassify any FPIRs by the IEMA as below-threshold relative to the insulin RIA. However, 2/47 (4%) below-threshold FPIRs by the insulin RIA were misclassified as above-threshold by the IEMA.
DISCUSSION
In assessing subjects for a type 1 diabetes prevention trial we derived a rule to determine FPIR using an automated, highly specific insulin IEMA. The rule was validated against the existing reference standard for FPIR that uses the DPT-1 Study Group insulin RIA. The insulin IEMA-based rule resulted in no subjects’ FPIRs being misclassified as below- or above-threshold relative to the insulin RIA, and identified a group for whom the insulin RIA is needed to determine FPIR. Our study’s strengths include the large number of subjects undergoing simultaneous FPIR determinations by the insulin RIA and IEMA, use of the standardized procedure for the 10-min IVGTT to measure FPIR in all subjects, and comparison of the insulin IEMA against the insulin RIA that was used to determine below- and above-threshold FPIRs in previous type 1 diabetes prediction and prevention studies [4,6,7].
The practical advantages of the insulin IEMA, including smaller volumes (IEMA, 25 µl vs. RIA, 100 µl) and faster throughput (IEMA ~ 500 samples/d vs. RIA ~ 500 samples/wk), make it more attractive than the RIA for type 1 diabetes prediction and prevention studies. These studies usually include smaller children for whom there are limits on the blood volumes that can be drawn for research. An advantage of faster throughput occurs because type 1 diabetes prevention trials often require baseline tests that must be done on separate days (e.g., OGTT, IVGTT) but within a short time window. Faster FPIR determinations can avoid the need to repeat the IVGTT and other baseline tests because the window has been exceeded.
While our rule correctly classified 68% (110/161) of IVGTTs into below- and above-threshold FPIRs using the insulin IEMA, it did not eliminate the need for the insulin RIA in the remaining 32% (51/161) because of the IEMA-based FPIR fell into the uncertain category. We found a higher proportion of uncertain FPIRs by the IEMA among IVGTTs that were below the FPIR threshold by the insulin RIA (64%) compared to IVGTTs above the FPIR threshold by the insulin RIA (18%). This difference could be explained by reduced precision of the assay at very low insulin concentrations or because the windows defining below-threshold FPIRs by the lower 95% CLs for the insulin IEMA (< 17.2 and < 43.3 µU/ml) are narrow compared to those defining above-threshold FPIRs (> 56.3 and > 82.5 µU/ml).
More generally, our rule is conservative where this considers the trade-off of more subjects having their FPIR misclassified by the insulin IEMA relative to the insulin RIA, versus fewer subjects needing the insulin RIA because their FPIR is uncertain by the IEMA. The effect of choosing 90% confidence limits on the interpolated insulin IEMA thresholds shows this. It reduced the number of subjects with uncertain FPIRs from 51 to 40 but also resulted in some misclassification (specifically, 2/47 [4%] below-threshold FPIRs by the insulin RIA were above-threshold by the IEMA). This error, though small, has potential implications to type 1 diabetes prediction and prevention studies that use FPIR to assess participants. For example, the TrialNet Oral Insulin Prevention Trial that is now underway has the primary aim of testing the DPT-1 subgroup finding that oral insulin prevented type 1 diabetes in subjects with above-threshold FPIRs by the insulin RIA [6]. To be confident that the TrialNet study will reliably confirm or refute the DPT-1 subgroup finding, it is essential to reconstitute subjects’ characteristics as closely as possible to the original DPT-1 subgroup including accurate characterization of FPIR status. Our choice of 95% confidence limits on the interpolated insulin IEMA FPIR thresholds becomes attractive in this context because it resulted in no misclassification yet still offered the practical advantage of eliminating the insulin RIA in two-thirds of subjects.
Our study has limitations. First, while we validated our rule by the Leave-One-Out Cross-Validation (LOOCV) method which helps limit the bias inherent to testing a rule in the same subjects from which the rule was derived, this is less rigorous compared to prospectively testing the rule in a second, independent sample of subjects. However, we are continuing to measure FPIR in potential subjects for the TrialNet Oral Insulin Prevention Trial and can prospectively re-validate the insulin IEMA-based rule against the insulin RIA using stored samples. Second, we cannot generalize our findings to other insulin immunoassays given reported differences in their operating properties [7,8]. Third, we did not assess the impact of intra-subject variability on FPIR determinations during serial IVGTTs which is an important source of FPIR misclassification [16]. However, this should not affect comparisons of FPIRs that are simultaneously measured by the insulin IEMA and insulin RIA in subjects during any single IVGTT.
In conclusion, we have shown that use of a more specific, automated insulin IEMA to measure FPIR in subjects at risk for future type 1 diabetes provides an accurate determination of below- and above-threshold FPIRs in 2/3 of tests relative to the current standard of the DPT-1 insulin RIA. The remaining subjects’ FPIRs could not be reliably classified and require that the insulin RIA be used. Given the practical advantages of the insulin IEMA, TrialNet is now limiting use of the insulin RIA for FPIR to the latter group.
Supplementary Material
Highlights.
Low first-phase insulin release (FPIR) is important to type 1 diabetes prediction and prevention studies.
The reference standard for FPIR uses an insulin radioimmunoassay (RIA).
We compared FPIRs by the insulin RIA to a more specific, faster insulin immunoenzymometric assay (IEMA).
The insulin IEMA accurately classified FPIRs compared to RIA-based FPIRs in 68% of subjects.
FPIR by the less practical insulin RIA can be limited to subjects at risk for type 1 diabetes with an uncertain FPIR by an insulin IEMA.
Acknowledgments
TrialNet is funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Center for Research Resources; the Juvenile Diabetes Research Foundation International; and the American Diabetes Diabetes Association.
LIST OF ABBREVIATIONS
- DPT-1
Diabetes Prevention Trial – Type 1
- FPIR
First-Phase Insulin Release
- GADA
Glutamic Acid Decarboxylase Autoantibodies
- OGTT
Oral Glucose Tolerance Test
- IA-2A
IA-2Autoantibodies
- ICA
Islet Cell Autoantibodies
- IEMA
Immunoenzymometric Assay
- IVGTT
Intravenous Glucose Tolerance Test
- mIAA
Insulin Autoantibodies
- NHS
TrialNet Natural History Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS’ CONTRIBUTIONS
J.M. contributed to discussion and wrote the manuscript. C.B. performed the statistical analyses, contributed to discussion and reviewed/edited the manuscript. S.M. performed the insulin assays, contributed to discussion, and reviewed/edited the manuscript. D.B. performed the statistical analyses and reviewed/edited the manuscript. W.W. contributed to discussion and reviewed the manuscript. J.P. contributed to discussion and reviewed/edited the manuscript. J.S. contributed to discussion and reviewed/edited the manuscript. J.K. contributed to discussion and reviewed/edited the manuscript.
References
- 1.Srikanta S, Ganda OP, Gleason RE, Jackson RA, Soeldner JS, Eisenbarth GS. Pre-type 1 diabetes. Linear loss of beta cell response to intravenous glucose. Diabetes. 1984;33:717–20. doi: 10.2337/diab.33.8.717. [DOI] [PubMed] [Google Scholar]
- 2.Bingley PJ, Colman P, Eisenbarth GS, Jackson RA, McCulloch DK, Riley WJ, Gale EA. Standardization of IVGTT to predict IDDM. Diabetes Care. 1992;15:1313–16. doi: 10.2337/diacare.15.10.1313. [DOI] [PubMed] [Google Scholar]
- 3.Bingley PJ, Gale EA. For the European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia. 2006;49:881–90. doi: 10.1007/s00125-006-0160-4. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Prevention Trial – Type 1 Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 5.European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. ENDIT: A randomized controlled trial of intervention before onset of type 1diabetes. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Trial – Type 1 Study Group. Effects of oral insulin in relatives of patients with type 1 diabetes. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 7.Chase HP, Cuthbertson DD, Dolan LM, Kaufman F, Krischer JP, Schatz DA, White NH, Wilson DM, Wolfsdorf J for the Diabetes Prevention Trial – Type 1 Study Group. First-phase insulin release during the intravenous glucose tolerance test as a risk factor for type 1 diabetes. J Pediatr. 2001;138:244–9. doi: 10.1067/mpd.2001.111274. [DOI] [PubMed] [Google Scholar]
- 8.Schatz D, Krischer J, Horne G, Riley W, Spillar R, Silverstein J, et al. Islet cell antibodies predict insulin dependent diabetes in U.S. school age children as powerfully as unaffected relatives. J Clin Invest. 1994;20:1183–97. doi: 10.1172/JCI117247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcovina S, Bowsher RR, Miller WG, Staten M, Myers G, Caudill SP, Campbell SE, Steffes MW for the Insulin Standardization Workshop. Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clinical Chemistry. 2007;53:711–716. doi: 10.1373/clinchem.2006.082214. [DOI] [PubMed] [Google Scholar]
- 10.Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clinical Chemistry. 2007;53:922–932. doi: 10.1373/clinchem.2006.077784. [DOI] [PubMed] [Google Scholar]
- 11.Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, Spain L Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet--an international collaborative clinical trials network. Ann N Y Acad Sci. 2008;1150:14–24. doi: 10.1196/annals.1447.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, et al. for theTrialNet Natural History Committee and the Type 1 Diabetes TrialNet Study Group. The TrialNet Natural History Study of the development of type 1 diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenbaum CJ, Sears KL, Kahn SE, Palmer JP. Relationship of beta-cell function and autoantibodies to progression and nonprogression of subclinical Type–1 Diabetes. Diabetes. 1999;48:170–175. doi: 10.2337/diabetes.48.1.170. [DOI] [PubMed] [Google Scholar]
- 14.Fleiss JL. The Design and Analysis of Clinical Experiments. John Wiley & Sons, Inc; New York: 1999. pp. 41–43. [Google Scholar]
- 15.Efron B, Gong G. A leisurely look at the Bootstrap, the Jackknife, and Cross-Validation. The American Statistician. 1983;37(1):36–48. [Google Scholar]
- 16.Allen HF, Jeffers BW, Klingensmith GJ, Chase HP. First-phase insulin release in normal children. J Pediatr. 1993;123:733–8. doi: 10.1016/s0022-3476(05)80847-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.