Abstract
Infantile hemangiomas (IHs) are the most common vascular tumors in infants, appearing in early infancy and ultimately regressing with time. Clinical presentation may vary, with a minority of lesions causing impairment of vital function (e.g., respiratory or visual obstruction), permanent scarring, and/or disfigurement. The pathogenesis of IH is complex and poorly understood. Risk factors implicated in their development include preterm birth and placental anomalies. IH presents a myriad of clinical challenges, including correct diagnosis and whether or not to pursue treatment. This article is a review of the current literature regarding pathogenesis, clinical presentation, treatment, and prognosis of IH.
Keywords: beta blockade, glucose transporter-1 (GLUT-1), hemangioma, hypoxia, pregnancy, vascular endothelial growth factor (VEGF)
Introduction
Hemangioma: Derived from the Ancient Greek αίμα (blood), ἀγγεῖον (vessel), and ὄγκοϛ (mass or tumor) (Diab, 1999)
Infantile hemangiomas (IHs) are the most common vascular tumors of infants. First distinguished as a separate entity from other congenital vascular malformations by Mulliken and Glowacki (1982) based on unique characteristics, IH are typically absent at birth, undergo a phase of increased cellular proliferation followed by an involution phase, and ultimately regress with time. Morbidity may include disfigurement and scarring, and, in more serious cases, vision loss, airway compromise, congestive heart failure, and death (Chang et al., 2008). Clinical presentation of IH may vary, and early clinical evaluation of potentially problematic lesions by a specialist may help prevent further morbidity. Although the preferred treatment agents at this time are nonselective beta blockers, there is still a fair amount unknown regarding the pathogenesis of IH, and further research is needed to identify alternative treatment agents.
EPIDEMIOLOGY AND RISK FACTORS
Data on the prevalence of IH vary. In one prospective study in the United States of 594 infants through 9 months of age, the reported prevalence was 4.5% (Munden et al., 2014). This study may have underrepresented the number of IH, however, as the results relied on parents responding to telephone call queries and returning for follow-up examinations. A recent retrospective study of patients in Minnesota demonstrated an increasing population-based incidence of IH over the past 3 decades, which strongly correlated to a decline in birth weight, as well as an increase in preterm birth and pregnancy complications (Anderson et al., 2016). The risk associated with the white race has also been reported to significantly increase over the past few decades. However, this seems to be mirroring the increased incidence of preterm deliveries in the populations studied, and may relate to changes in reporting over time (Amrock and Weitzman, 2013).
Female gender, preterm birth, low birth weight, and products of multiple gestations have all been linked to the development of IH. A case-controlled study by Drolet et al. (2008) identified an increased risk of IH of 40% for every 500 gm decrease in birth weight. Various antenatal associations have also been identified as IH risk factors, including maternal vaginal bleeding during the first trimester, progesterone use, as well as preeclampsia, older maternal age, placenta previa, and use of in vitro fertilization (Hemangioma Investigator Group et al., 2007; Chen et al., 2013). Family history was also identified as having an association with IH risk (Drolet et al., 2008). Conversely, when looking at the incidence of IH in monozygotic and dizygotic twins in a study by Cheung et al. (1997), there was no significant difference between the two groups, perhaps suggesting that environmental factors play a more important role than underlying genetics.
CLINICAL CHARACTERISTICS AND ASSOCIATED CONGENITAL SYNDROMES
IHs are classified as superficial or deep, or a combination. They may be present as a solitary lesion or one of many, and can range in size from a few millimeters to many centimeters in diameter (Drolet et al., 1999). The superficial lesions grow to become bright red or “strawberry” in color, raised, firm or slightly compressible papules, nodules, or plaques. Deeper lesions tend to have more of a bluish hue or may appear as a skin-colored nodule. Mixed lesions, or those that involve both the epidermis and deeper subcutaneous tissues, can display features of both (Chiller et al., 2002).
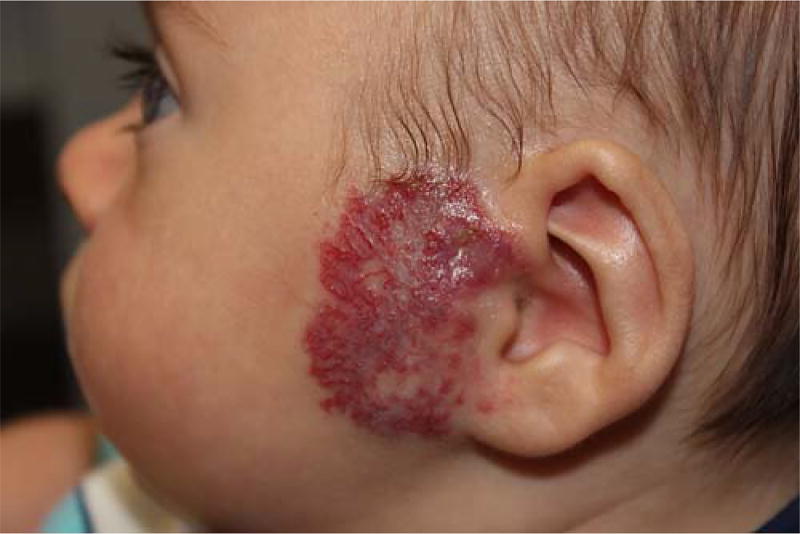
IH follows a characteristic pattern of timing; most are absent at birth and appear during the first few weeks to months of life. A premonitory sign may be present, such as a patch of pallor, signifying a vasoconstricted, bruise-like area, or group of telangiectasias at the site of future growth (Frieden et al., 2009). Proliferation occurs until approximately 1 year of age with mean growth achieved by 3 months of age, and with growth after the first year being uncommon. Of note, deep hemangiomas have been found to proliferate at a later stage than superficial hemangiomas, and their growth has been noted to last for a longer period of time, sometimes through 12 or 14 months, even up to 2 years (Chang et al., 2008; Drolet et al., 1999). The IH then enters an involution stage and in time subsides completely, in contrast with other congenital vascular malformations, which do not regress spontaneously and continue to grow proportionally with the child’s growth (Wójcicki and Wójcicka, 2014). Figure 1 demonstrates an example of an IH with both superficial and deep components that has started to enter the involution phase.
FIGURE 1.
Large preauricular mixed (with superficial and deep components) infantile hemangioma. Portions of the central section show evidence of regression (involution) with segments of normal epithelium evident, as well as super-imposed telangiectasias.
IHs may be further classified as localized or segmental, and rarely have visceral involvement. Localized lesions can be defined as nodules or plaques contained entirely within a focal anatomic area, as opposed to segmental lesions, which tend to demonstrate a linear or geometric pattern associated with an area of developmental growth. Segmental lesions often are larger and more frequently associated with developmental abnormalities than localized lesions (Chiller et al., 2002). If lesions do not fit either classification, they may be labeled as indeterminate.
Anatomic locations of an IH may have prognostic implications; for example, those infants with segmental or large focal IHs overlying the lumbosacral spine may be at increased risk for tethering of the spinal cord or genitourinary abnormalities (Chiller et al., 2002). Other IHs involving the head and neck may put infants at risk for ocular axis occlusion, astigmatism, amblyopia, tear-duct occlusion, airway hemangiomas, and overall risk for ulceration and disfigurement (Chang et al., 2008).
The presence of multiple lesions (typically more than five) is associated with an increased risk for visceral involvement, most notably in the liver and gastrointestinal tract (Chang et al., 2008). Visceral lesions are frequently asymptomatic, benign, and can be managed with observation alone, although rarely can cause a high-output cardiac failure by a shunt mechanism (Christison–Lagay et al., 2007). In the case of suspected visceral hemangiomas, evaluation utilizing ultrasound, computed tomographic imaging, or magnetic resonance imaging can help to delineate an IH lesion from another more virulent malignant process (Dubois et al., 1998).
Certain congenital syndromes have been linked to the presence of IHs, often specific to anatomic location. Large (>5 cm) segmental hemangiomas, particularly those on the face, may be associated with PHACE(S), a congenital syndrome characterized by Posterior fossa brain malformations, Hemangiomas, Arterial anomalies, Coarctation of the aorta and cardiac defects, Eye abnormalities, and occasionally ventral Sternal defects (Frieden et al., 1996). PHACE(S) is relatively uncommon but not rare; one prospective cohort study by Metry et al. (2006) identified 25 of 1096 children with IH (2.3%) who met criteria for PHACE(S) and an incidence of 20% among those with segmental facial hemangiomas. In this study, 90% of the subjects were female, a comparable finding to prior reports. Very rarely were all features of the disease present. Cerebrovascular and cardiovascular manifestations were the most common, and ranged from structural brain defects (posterior fossa malformations, hypoplasia or agenesis of brain tissue, subependymal and arachnoid cysts, polymicrogyria, and microcephaly), to various cerebrovascular and cardiovascular anomalies (carotid arterial defects, persistent embryonic arteries, aortic coarctation, aortic or subclavian aneurysms, anomalous coronary arteries, ventral and atrial septal defects, patent ductus arteriosus, pulmonary stenosis, and anomalous pulmonary veins), among others. Another more recent prospective study by Haggstrom et al. (2010) identified 33 of 108 infants (31%) with large facial hemangiomas that met criteria for PHACE(S). This study also saw a prevalence of cerebrovascular (91%) and cardiovascular (67%) anomalies over other manifestations of the congenital syndrome.
Other associations have been made between hemangiomas of the lumbosacral region and underlying congenital abnormalities, and include syndromes, such as LUMBAR, named for Lower body hemangioma and other cutaneous defects, Urogenital anomalies, Ulceration, Myelopathy, Bony deformities, Anorectal malformations, Arterial anomalies, and Renal anomalies (Iacobas et al., 2010); PELVIS, or Perineal hemangioma, External genital malformations, Lipomyelomeningocele, Vesicorenal abnormalities, Imperforate anus and Skin tag) (Girard et al., 2006); and SACRAL, Spinal dysraphism, Anogenital, Cutaneous, Renal and urologic anomalies, Associated with an Angioma of Lumbosacral localization (Stockman et al., 2007). LUMBAR syndrome carries many similar features to PHACE(S) syndrome, including segmental IH, female predominance, regional occurrence of IH with underlying anomalies, and potential for vascular anomalies, and may be considered analogous to PHACE(S) but for the lower half of the body (Iacobas et al., 2010).
PATHOGENESIS
The pathogenesis of IH is poorly understood, but several hypotheses have been advanced. Glucose transporter-1 (GLUT-1) is an erythrocyte-type glucose transporter protein that has been identified as a highly selective marker of IH at all stages as compared with other vascular malformations (North et al., 2000; North et al., 2001; Leon–Villapalos et al., 2005). In a study by North et al. (2001), microvascular endothelial GLUT-1 immunoreactivity was identified in all 66 IHs tested, whereas the other vascular malformations, pyogenic granulomas, and normal granulation tissue did not express GLUT-1. The protein has been identified in the normal human brain and placental tissue, but not in normal skin or subcutaneous tissue. The robust and unique expression of GLUT-1 in IH tissue, therefore, suggests a possible pathogenic association.
Certain clinical observations have recently led to the suggestion that IH is triggered and maintained by hypoxia, both as a consequence of maternal events creating hypoxic stress as well as by the infant’s own hypoxia-induced factors. This further supports the association of IH with GLUT-1, as GLUT-1 is a downstream target of hypoxiainducible factor-1-alpha (HIF-1α), along with vascular endothelial growth factor A (VEGF-A) and insulin-like growth factor 2 (IGF-2). The idea that hypoxia may trigger the development of IH has been recently explored by de Jong et al. (2016), who propose that factors induced by hypoxia are crucial for the vasculogenesis seen with IHs. Indeed, many of the conditions that predispose an infant to development of an IH (i.e., preterm birth, low birth weight, and preeclampsia) are associated with hypoxia. For example, preterm infants are more at risk for neonatal respiratory morbidity than full-term infants. Similarly, very low birth weight infants may show more underdevelopment of the lungs and breathing system, therefore, undergoing a significant risk for apnea and lack of oxygenation (Brown et al., 2014; Mohr et al., 2015).
IHs have also been shown at all stages to express vascular antigens that are otherwise only observed in fetal microvessels of human placental tissue (Fc-gamma-receptor II, merosin, and Lewis Y antigen). The significance of this coexpression of vascular antigens remains unclear. Some proposed mechanisms to explain the origin of these cells include possible embolization of placental chorionic mesenchymal core cells, or differentiation of placental microvascular phenotype in skin and subcutaneous tissue during fetal mesenchymal growth (North et al., 2001; Itinteang et al., 2014).
Certain key molecular markers have been identified in the proliferating IH endothelial cell. The coexpression of CD34 (an endothelial cell marker) and CD133 (an endothelial progenitor cell marker) were detected by flow cytometry in 11 of 12 infantile hemangioma specimens analyzed during the proliferative phase in one study by Yu et al. (2004). Another study found that lymphatic vessel endothelial hyaluronan receptor 1, a marker for lymphatic vessels, was strongly expressed in IH cells, also preferentially during the proliferative phase rather than during the involution stage (Dadras et al., 2004). This study highlights that proliferating IH endothelial cells share a similar immunophenotype with embryonic veins during normal development, suggesting that IH endothelial cells are arrested in an early stage of normal vascular differentiation. This idea is further corroborated by Ritter et al. (2002), who identified that IGF-2, a growth factor involved in angiogenesis and a downstream target of HIF-1α, was highly expressed during the proliferative phase of IH development, and significantly decreased during the involution phase.
It has been suggested more recently that the renin-angiotensin system (RAS) may play a role in the endothelial cell proliferation of IHs. Serum renin levels are markedly elevated during the first 3 months of life, and taper down in a pattern that may in fact parallel the natural growth pattern of IH (Fiselier et al., 1983). Furthermore, higher renin levels have been detected in females, whites, and premature births; all of which are risk factors for developing IHs (Broughton Pipkin et al., 1981; Stephenson et al., 1991). Itinteang et al. (2011) reported that IH endothelial cells in the proliferative phase express ACE and angiotensin receptor II, both integral components of the RAS. They hypothesized that the high serum levels of renin, together with the local expression of ACE, leads to a high concentration of angiotensin II (ATII), which, along with VEGF, drives cell proliferation. This theory may explain the mechanism behind the use of beta blockers, which, among other functions, ultimately lead to decreased levels of ATII by blocking renin production in the kidney (Tan et al., 2012). The spontaneous involution that occurs in IH may be related to plummeting serum renin levels with age and/or depletion of the endothelial progenitor cells with time.
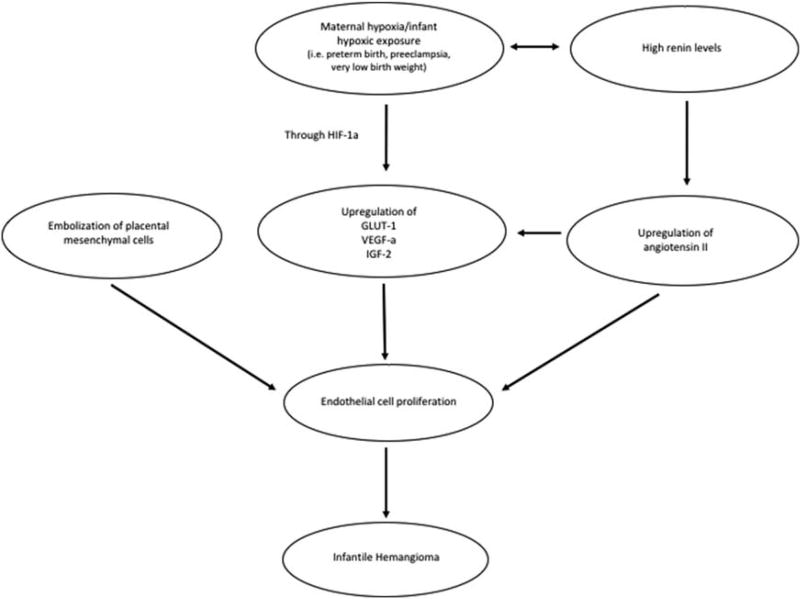
Many of the aforementioned proposed theories for IH pathogenesis can ultimately be tied back to hypoxia and the HIF pathway, the RAS being no exception. Indeed, ATII has been shown to upregulate GLUT-1 mRNA in a time-dependent and dose-dependent manner in mesangial cells (Nose et al., 2003). Furthermore, a recent study analyzing placental tissue demonstrated an increase in RAS activity in the setting of hypoxia (i.e., at high altitudes) as well as in the setting of oxidative stress (Kurlak et al., 2016). Figure 2 illustrates possible connections involved in IH pathogenesis, linking hypoxia and the HIF pathway to the RAS pathway and endothelial cell proliferation.
FIGURE 2.
Interplay between proposed theories of infantile hemangioma pathogenesis. GLUT-1, glucose transporter-1; HIF-1α, hypoxia-inducible factor-1-alpha; IGF-2, insulin-like growth factor 2; VEGF-A, vascular endothelial growth factor A.
DIAGNOSIS AND PROGNOSIS
The diagnosis of IH is made clinically, and large or potentially problematic lesions should typically be confirmed by a specialist. IHs are often misdiagnosed, as they can mimic a myriad of other vascular abnormalities, including capillary malformations (or port-wine stains), arteriovenous malformations, pyogenic granulomas, lymphatic malformations, dermoid cysts, spitz nevi, as well as noninvoluting congenital hemangiomas that are present at birth, among many others. The rapidly proliferating nature of IH can lead some to misdiagnose a patient with malignancy, as they may mimic dermatofibrosarcoma, infantile fibrosarcoma, and, in some cases, lymphoblastic lymphoma or rhabdomyosarcoma (Frieden et al., 2009).
In a prospective cohort study by Chang et al. (2008) of 433 patients with IH, almost all lesions were noted before 1 month of age, but referral to a pediatric dermatology specialist was not made until an average of 5 months. Delay in assessment by a specialist may in turn lead to a delay in diagnosis, or an incorrect diagnosis, and thus a delay in early treatment, if deemed appropriate. This emphasizes the importance of early referral to a specialist, when an appropriate diagnosis can be made, and follow-up intervals can be tailored to the particular individual.
Most IHs completely resolve with time, but many leave a small parchment-like scar. A small subset, however, are more prone to significant permanent scarring, ulceration, bleeding, or disfigurement. High-risk lesions include those that have the potential to interfere with a vital structure or function, such as lesions around the eye, nasal tip, mouth, in the airway, gastrointestinal tract, or liver (Chang et al., 2008). Hemangiomas of the segmental type as a whole have been demonstrated to require more intensive and prolonged therapy, and have been associated with more complications. Interestingly, Hispanic race may also play a prognostic role, as IHs in this population have been shown to be more complex and associated with more morbidity (Chiller et al., 2002).
TREATMENT AND MANAGEMENT
Most cutaneous IHs are uncomplicated and do not require any treatment. Lesions that are higher risk for complications, however, are managed with more aggressive intervention. Emergent treatment for potentially life-threatening complications or functional impairments should be prioritized. Additionally, a thorough evaluation should be performed of possible structural anomalies that may be associated with IHs. Ultimately, one may choose to intervene with elective treatment to prevent permanent disfigurement or scarring (Darrow et al., 2015).
Depending on the anatomic location, various IHs require additional help of a specialist. Pediatric ophthalmologists and early ophthalmologic examination, for example, are integral to the management of periocular IHs, with or without PHACE(S) syndrome, as there is potential for visual compromise, as well as astigmatism, ptosis, proptosis, and strabismus (Ceisler et al., 2004). Visceral hemangiomas with the potential to cause congestive heart failure can often be managed with systemic treatments, but refractory cases may need percutaneous embolization or surgery (Tal et al., 2016).
Various systemic and topical therapies have been studied in the treatment of IHs. Propranolol has been demonstrated to be a well-tolerated medication, with effectiveness in all stages, both halting growth during the proliferative phase as well as hastening the involution phase. In a randomized controlled trial (RCT) by Hogeling et al. (2011), propranolol administered orally at 2 mg/kg led to improvement in size, color, and elevation of both localized and segmental hemangiomas in newborns and children up to age 5 years. More recently, an RCT of propranolol at 3 mg/kg dosing successfully led to complete or near resolution of IH as compared with placebo, without a significant difference in adverse events (Léauté-Labrèze et al., 2015). Topical timolol has also been found to be efficacious, particularly if utilized early in the development of the IH, or on small thin lesions. Although systemic glucocorticoids have been demonstrated to halt tumor growth in the early proliferative phase, they are less useful at later stages, and come with a significant amount of comorbidity (Bennett et al., 2001). Vincristine and interferon alpha have been studied in aggressive hemangiomas, but carry a significant risk of adverse drug reactions, such as peripheral neuropathy and spastic diplegia (Perez et al., 2002; Michaud et al., 2004).
Evidence for the efficacy and safety of oral and topical nonselective beta blockers continues to accumulate, and propranolol is now being recommended as a first-line treatment for complicated IHs (Schiestl et al., 2011). Timolol is now widely used for small, thin, and early lesions. Although adverse events are infrequently reported in the RCTs studying propranolol, it is important to note that potential side effects may include hypotension, bradycardia, and hypoglycemia. In those patients who are unable to tolerate beta blockers, such as those with PHACE(S) syndrome who may have concomitant vascular abnormalities, alternative agents, such as glucocorticoids, interferon alpha, or vincristine, may be useful. Furthermore, the use of angiotensin converting enzyme (ACE) inhibitors is a consideration, given the increasing amount of evidence that the RAS is implicated in IH disease pathogenesis (Itinteang et al., 2011). Thus far, however, the data have favored propranolol over ACE inhibitors in terms of efficacy (Zaher et al., 2015).
Follow-up for IH lesions after treatment varies depending on the presence of any complications. Typically, patients with IH are followed closely by a pediatrician, a pediatric dermatologist, and/or another subspecialist until the lesions resolve or their complications are stably managed.
CONCLUSION
In summary, IHs are a common and generally benign entity. Various factors have been identified that increase the risk of IH, including female gender, preterm birth, and other prenatal complications, and their correlation with IH may continue to rise with time. More studies are warranted to evaluate the impact of various environmental factors on IH prevalence. The exact mechanism for the pathogenesis of IH is still under debate, but their strong association with the molecular marker GLUT-1, and their potential relationship with the RAS have contributed to a number of recent theories. IHs have a unique and characteristic timing, but vary in how they present clinically, as lesions may be superficial or deep, localized or segmental, singular, or one of multiple, making them challenging to include as an outcome in observational studies as well as diagnose in the clinic. IHs present a number of diagnostic and therapeutic challenges; thus, early identification and referral to a specialist are key in preventing associated morbidity with these vascular lesions.
Acknowledgments
We would like to acknowledge the help and assistance of Kenneth Lyons Jones, MD, Robert Terkeltaub, MD, Gretchen Bandoli, PhD, and Elizabeth Eddy in helping to create this review article.
References
- Amrock SM, Weitzman M. Diverging racial trends in neonatal infantile hemangioma diagnoses, 1979–2006. Pediatr Dermatol. 2013;30:493–494. doi: 10.1111/pde.12075. [DOI] [PubMed] [Google Scholar]
- Anderson KR, Schoch JJ, Lohse CM, et al. Increasing incidence of infantile hemangiomas (IH) over the past 35 years: correlation with decreasing gestational age at birth and birth weight. J Am Acad Dermatol. 2016;74:120–126. doi: 10.1016/j.jaad.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Fleischer AB, Jr, Chamlin SL, Frieden IJ. Oral corticosteroid use is effective for cutaneous hemangiomas: an evidence-based evaluation. Arch Dermatol. 2001;137:1208–1213. doi: 10.1001/archderm.137.9.1208. [DOI] [PubMed] [Google Scholar]
- Broughton Pipkin F, Smales OR, O’Callaghan M. Renin and angiotensin levels in children. Arch Dis Child. 1981;56:298–302. doi: 10.1136/adc.56.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HK, Speechley KN, Macnab J, et al. Neonatal morbidity associated with late preterm and early term birth: the roles of gestational age and biological determinants of preterm birth. Int J Epidemiol. 2014;43:802–814. doi: 10.1093/ije/dyt251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceisler EJ, Santos L, Blei F. Periocular hemangiomas: what every physician should know. Pediatr Dermatol. 2004;21:1–9. doi: 10.1111/j.0736-8046.2004.21101.x. [DOI] [PubMed] [Google Scholar]
- Chang LC, Haggstrom AN, Drolet BA, et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360–367. doi: 10.1542/peds.2007-2767. [DOI] [PubMed] [Google Scholar]
- Chen XD, Ma G, Chen H, et al. Maternal and perinatal risk factors for infantile hemangioma: a case-control study. Pediatr Dermatol. 2013;30:457–461. doi: 10.1111/pde.12042. [DOI] [PubMed] [Google Scholar]
- Cheung DS, Warman ML, Mulliken JB. Hemangioma in twins. Ann Plast Surg. 1997;38:269–274. doi: 10.1097/00000637-199703000-00014. [DOI] [PubMed] [Google Scholar]
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567–1576. doi: 10.1001/archderm.138.12.1567. [DOI] [PubMed] [Google Scholar]
- Christison–Lagay ER, Burrows PE, Alomari A, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62–67. doi: 10.1016/j.jpedsurg.2006.09.041. discussion 67–68. [DOI] [PubMed] [Google Scholar]
- Dadras SS, North PE, Bertoncini J, et al. Infantile hemangiomas are arrested in an early developmental vascular differentiation state. Mod Pathol. 2004;17:1068–1079. doi: 10.1038/modpathol.3800153. [DOI] [PubMed] [Google Scholar]
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma: executive summary. Pediatrics. 2015;136:786–791. doi: 10.1542/peds.2015-2482. [DOI] [PubMed] [Google Scholar]
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219–227. doi: 10.1007/s00403-016-1635-x. [DOI] [PubMed] [Google Scholar]
- Diab M. Lexicon of Orthopaedic Etymology. Seattle, WA: Harwood Academic Publishers; 1999. [Google Scholar]
- Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med. 1999;341:173–181. doi: 10.1056/NEJM199907153410307. [DOI] [PubMed] [Google Scholar]
- Drolet BA, Swanson EA, Frieden IJ Hemangioma Investigator Group. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153:712–715. doi: 10.1016/j.jpeds.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Dubois J, Garel L, Grignon A, et al. Imaging of hemangiomas and vascular malformations in children. Acad Radiol. 1998;5:390–400. doi: 10.1016/s1076-6332(98)80158-x. [DOI] [PubMed] [Google Scholar]
- Fiselier TJ, Lijnen P, Monnens L, et al. Levels of renin, angiotensin I and II, angiotensin-converting enzyme and aldosterone in infancy and childhood. Eur J Pediatr. 1983;141:3–7. doi: 10.1007/BF00445660. [DOI] [PubMed] [Google Scholar]
- Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol. 1996;132:307–311. doi: 10.1001/archderm.132.3.307. [DOI] [PubMed] [Google Scholar]
- Frieden IJ, Rogers M, Garzon MC. Conditions masquerading as infantile haemangioma: part 1. Australas J Dermatol. 2009;50:77–97. doi: 10.1111/j.1440-0960.2009.00514_1.x. quiz 98. [DOI] [PubMed] [Google Scholar]
- Girard C, Bigorre M, Guillot B, Bessis D. PELVIS syndrome. Arch Dermatol. 2006;142:884–888. doi: 10.1001/archderm.142.7.884. [DOI] [PubMed] [Google Scholar]
- Haggstrom AN, Garzon MC, Baselga E, et al. Risk for PHACE syndrome in infants with large facial hemangiomas. Pediatrics. 2010;126:e418–e426. doi: 10.1542/peds.2009-3166. [DOI] [PubMed] [Google Scholar]
- Hemangioma Investigator Group. Haggstrom AN, Drolet BA, et al. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150:291–294. doi: 10.1016/j.jpeds.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:e259–e266. doi: 10.1542/peds.2010-0029. [DOI] [PubMed] [Google Scholar]
- Iacobas I, Burrows PE, Frieden IJ, et al. LUMBAR: association between cutaneous infantile hemangiomas of the lower body and regional congenital anomalies. J Pediatr. 2010;157:795–801. doi: 10.1016/j.jpeds.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Itinteang T, Brasch HD, Tan ST, Day DJ. Expression of components of the renin-angiotensin system in proliferating infantile haemangioma may account for the propranolol-induced accelerated involution. J Plast Reconstr Aesthet Surg. 2011;64:759–765. doi: 10.1016/j.bjps.2010.08.039. [DOI] [PubMed] [Google Scholar]
- Itinteang T, Withers AH, Davis PF, Tan ST. Biology of infantile hemangioma. Front Surg. 2014;1:38. doi: 10.3389/fsurg.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlak LO, Mistry HD, Cindrova–Davies T, et al. Human placental renin-angiotensin system in normotensive and preeclamptic pregnancies at high altitude and after acute hypoxiareoxygenation insult. J Physiol. 2016;594:1327–1340. doi: 10.1113/JP271045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735–746. doi: 10.1056/NEJMoa1404710. [DOI] [PubMed] [Google Scholar]
- Leon–Villapalos J, Wolfe K, Kangesu L. GLUT-1: an extra diagnostic tool to differentiate between haemangiomas and vascular malformations. Br J Plast Surg. 2005;58:348–352. doi: 10.1016/j.bjps.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Metry DW, Haggstrom AN, Drolet BA, et al. A prospective study of PHACE syndrome in infantile hemangiomas: demographic features, clinical findings, and complications. Am J Med Genet A. 2006;140:975–986. doi: 10.1002/ajmg.a.31189. [DOI] [PubMed] [Google Scholar]
- Michaud AP, Bauman NM, Burke DK, et al. Spastic diplegia and other motor disturbances in infants receiving interferon-alpha. Laryngoscope. 2004;114:1231–1236. doi: 10.1097/00005537-200407000-00017. [DOI] [PubMed] [Google Scholar]
- Mohr MA, Vergales BD, Lee H, et al. Very long apnea events in preterm infants. J Appl Physiol. 2015;118:558–568. doi: 10.1152/japplphysiol.00144.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907–913. doi: 10.1111/bjd.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North PE, Waner M, Mizeracki A, Mihm MC., Jr GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11–22. doi: 10.1016/s0046-8177(00)80192-6. [DOI] [PubMed] [Google Scholar]
- North PE, Waner M, Mizeracki A, et al. A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol. 2001;137:559–570. [PubMed] [Google Scholar]
- Nose A, Mori Y, Uchiyama–Tanaka Y, et al. Regulation of glucose transporter (GLUT1) gene expression by angiotensin II in mesangial cells: involvement of HB-EGF and EGF receptor transactivation. Hypertens Res. 2003;26:67–73. doi: 10.1291/hypres.26.67. [DOI] [PubMed] [Google Scholar]
- Perez J, Pardo J, Gomez C. Vincristine--an effective treatment of corticoid-resistant life-threatening infantile hemangiomas. Acta Oncol. 2002;41:197–199. doi: 10.1080/028418602753669607. [DOI] [PubMed] [Google Scholar]
- Ritter MR, Dorrell MI, Edmonds J, et al. Insulin-like growth factor 2 and potential regulators of hemangioma growth and involution identified by large-scale expression analysis. Proc Natl Acad Sci U S A. 2002;99:7455–7460. doi: 10.1073/pnas.102185799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl C, Neuhaus K, Zoller S, et al. Efficacy and safety of propranolol as first-line treatment for infantile hemangiomas. Eur J Pediatr. 2011;170:493–501. doi: 10.1007/s00431-010-1324-2. [DOI] [PubMed] [Google Scholar]
- Stephenson TJ, Broughton Pipkin F, Elias–Jones AC. Factors influencing plasma renin and renin substrate in premature infants. Arch Dis Child. 1991;66(10 Spec No):1150–1154. doi: 10.1136/adc.66.10_spec_no.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman A, Boralevi F, Taïeb A, Léauté–Labrèze C. SACRAL syndrome: spinal dysraphism, anogenital, cutaneous, renal and urologic anomalies, associated with an angioma of lumbosacral localization. Dermatology. 2007;214:40–45. doi: 10.1159/000096911. [DOI] [PubMed] [Google Scholar]
- Tal R, Dotan M, Lorber A. Approach to haemangiomatosis causing congestive heart failure. Acta Paediatr. 2016;105:600–604. doi: 10.1111/apa.13359. [DOI] [PubMed] [Google Scholar]
- Tan ST, Itinteang T, Day DJ, et al. Treatment of infantile haemangioma with captopril. Br J Dermatol. 2012;167:619–624. doi: 10.1111/j.1365-2133.2012.11016.x. [DOI] [PubMed] [Google Scholar]
- Wójcicki P, Wójcicka K. Epidemiology, diagnostics and treatment of vascular tumours and malformations. Adv Clin Exp Med. 2014;23:475–484. doi: 10.17219/acem/37149. [DOI] [PubMed] [Google Scholar]
- Yu Y, Flint AF, Mulliken JB, et al. Endothelial progenitor cells in infantile hemangioma. Blood. 2004;103:1373–1375. doi: 10.1182/blood-2003-08-2859. [DOI] [PubMed] [Google Scholar]
- Zaher H, Rasheed H, El-Komy MM, et al. Propranolol versus captopril in the treatment of infantile hemangioma (IH): a randomized controlled trial. J Am Acad Dermatol. 2015;74:499–505. doi: 10.1016/j.jaad.2015.09.061. [DOI] [PubMed] [Google Scholar]