Abstract
Background
Exercise-mediated cognitive improvements can be at least partly attributed to neuroplastic changes in the nervous system, and may be influenced by the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene. Transcranial magnetic stimulation (TMS) can be used to assess mechanisms of plasticity in humans noninvasively.
Objectives
To assess the feasibility of evaluating the effects of short-term regular exercise on cognitive performance, and to evaluate the relationship between these effects, TMS measures of plasticity, and BDNF Met carrier status in young healthy sedentary adults.
Methods
Of the 19 participants who enrolled in the study, 14 sedentary adults (12 females, age mean ± SD, 27 ± 12.3 yr), with less than two sessions of physical exercise in the preceding 2 months, completed an aerobic exercise regimen including four 30-min daily sessions per week for 4 weeks (for a total of 16 sessions) delivered at 55–64% of age-predicted maximal heart rate. Prior to and following the exercise regimen, participants performed a neuropsychological test battery and an intermittent theta-burst TMS plasticity protocol.
Results
All participants completed the various measures and adhered to the exercise regimen. There were no complications and the results obtained were reliable. The feasibility of the approach is thus well established. Between-group comparisons of pre-post change revealed trends toward increased performance on the Stroop and faster reaction times in the CPT detectability in the Val66Val subgroup (p = 0.07 and p = 0.08), and a reduction in TBS-induced modulation of TMS responses in Met carriers (p = 0.07).
Conclusion
Acute exercise interventions in sedentary adults can be meaningfully conducted along with cognitive and neurophysiologic measures to assess behavioral and neurobiological effects and assessment of BDNF polymorphism. TMS measures of plasticity can be used to evaluate the effects of exercise on brain plasticity, and relate them to neuropsychological measures of cognition.
Keywords: aerobic exercise, transcranial magnetic stimulation, cognition, iTBS, plasticity, BDNF
1. Introduction
There is ample evidence to support the idea that regular exercise contributes to cognitive function. Much of this evidence comes from experimental studies in animal models demonstrating that rodents with access to running wheels (aerobic exercise) show increased performance in cognitive tests of episodic memory and visuospatial processes, which rely heavily on hippocampal function (van Praag, 2008; van Praag, 2009). The mechanisms responsible for cognitive improvements associated with aerobic exercise are not fully understood, but the current evidence indicates the important role of neuroplasticity. Long-term potentiation is enhanced after regular exercise, and is correlated with cognitive performance (van Praag, Christie, Sejnowski, & Gage, 1999). At the molecular level, aerobic exercise has been found to create a propitious cellular environment for hippocampal neuronal activity, growth and survival, which among others includes: increased synthesis and release of brain-derived neurotrophic factor (BDNF), a neurotransmitter known to support cognitive processes; and increases in P75NTR and N-methyl-D-aspartate (NMDA) receptors, which support neuronal survival (Aguiar et al., 2011; Neeper, Gomez-Pinilla, Choi, & Cotman,1995).
The evidence for behavioral impact of exercise on cognition in humans is less consistent. Several meta-analyses of long-term exercise interventions (typically delivered over 9–12 months) collectively suggest a small, but significant, impact on cognitive performance (Angevaren, Aufdemkampe, Verhaar, Aleman, & Vanhees, 2008; Colcombe & Kramer, 2003; Etnier, Nowell, Landers, & Sibley, 2006; Scherder et al., 2014; Smith et al., 2010). However, there is little agreement on the specific cognitive domains influenced by exercise and the potential effects associated with shorter interventions. A greater understanding of the exercise-mediated effects on cognitive function is relevant to maintenance of cognitive function during aging.
Furthermore, recent evidence suggests that genetic factors may influence the responsiveness to the exercise-mediated improvements in cognitive function. In one study in young adults (Hopkins, Davis, Vantieghem, Whalen, & Bucci, 2012), after 4 weeks of regular aerobic exercise, improvements in memory were seen only in BDNF Val/Val individuals but not present in those with the common Val66Met polymorphism that alters the activity-dependent secretion of BDNF. This polymorphism has been reported to be present in approximately 30% of individuals (Cargill et al., 1999), so a characterization of BDNF allelic status may help explain the interindividual variability in exercise-mediated effects.
While improvements in cognition following regular exercise are attributed to increased brain plasticity (van Praag et al., 1999), this hypothesis has not been really tested in humans. Single-pulse transcranial magnetic stimulation interleaved with theta-burst stimulation (TMS-TBS) can be used to probe the cortical circuits involved in cortical plasticity in humans (Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005). Furthermore, paired-pulse TMS offers insights into the balance of excitatory and inhibitory influences in intracortical circuitry (Kujirai et al., 1993). To our knowledge, no study to date has measured the effects of regular exercise on TMS indices of the mechanisms of cortical plasticity.
The purpose of the present study was to establish the feasibility of evaluating the effects of a month-long aerobic exercise on TMS-TBS measures of cortical plasticity, and a neuropsychological test battery designed to measure cognitive performance in young healthy sedentary adults. In addition, we wanted to be able to consider the effect of BDNF polymorphism on the potential exercise-mediated effects. We hypothesized that after a regular aerobic exercise protocol, participants would exhibit increased TMS-TBS plasticity and improved cognitive performance. Furthermore, we hypothesized that BDNF Val/Val individuals would show larger improvements in neuroplasticity and cognitive function compared to individuals who carried the Val66Met polymorphism. This feasibility pilot study aimed to establish the foundation for a future larger trial informed by lessons learned.
2. Methods
This study was approved by the institutional review board at Beth Israel Deaconess Medical Center (BIDMC). Participants were recruited from the BIDMC and the larger Boston community via flyers, and those who expressed interest were screened for eligibility. Upon fulfilling the criteria, individuals who chose to give informed consent were enrolled in the study. Inclusion criteria were: adult males and females aged 18–60 years old whose primary language was English, and were sedentary, defined as not engaging in purposeful physical activity more than two times over the last two months. Exclusion criteria were: presence of any cognitive, neurologic or orthopedic condition that could affect performance in testing or training procedures, or any contraindication to TMS (Rossi, Hallett, Rossini, Pascual-Leone, Safety of TMS Consensus Group, 2009; Rossini et al., 2015).
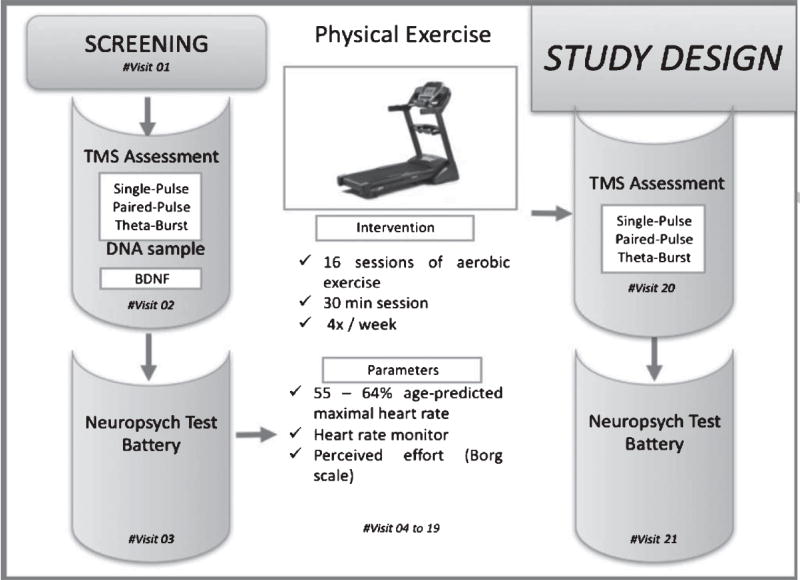
This was an open-label feasibility pilot study. Each participant underwent a total of 21 visits including a screening visit, a pretest (baseline) assessment, 16 aerobic exercise sessions delivered in 30 minute sessions, 3 times per week for 4 weeks, and a posttest assessment. Figure 1 illustrates the experimental design. During the screening session, the following data were collected: demographic data, medical history, medications in use, vital signs, physical activity readiness questionnaire (PAR-Q)(Thomas, Reading, & Shephard, 1992), physical activity level using the Physical Ability Questionnaire (George Stone, & Burkett, 1997), and a TMS screening questionnaire. Verbally reported height and weight were used to calculate body mass index (BMI). A physician within the Berenson-Allen Center provided exercise clearance upon a review of the information listed above. The pre-test assessment consisted of collection of saliva sample for BDNF analysis, TMS testing, and neuropsychological testing. At posttest, the TMS and neuropsychological testing were repeated.
Fig. 1.
Study design flowchart
2.1. BDNF testing
Saliva samples were collected utilizing the Oragene Discover OGR-250 kit (DNA Genotek Inc., Ottawa, ON, Canada) to assess the BDNF Val66Met polymorphism. Aliquot (700 ml) extraction of genomic DNA was performed on saliva samples collected using the Oragene Discover OGR-250 Kit (DNA Genotek Inc., Ottawa, ON, Canada). DNA was extracted from samples using standard methodology and the prepIT•L2P reagent (DNA Genotek Inc., 2015). The following quality control metrics were performed on each sample: PicoGreen fluorometry for double stranded DNA quantification, Nanodrop spectrophotometry as an estimate of sample purity using A260/A280 ratios and agarose gel electrophoresis for visualization of DNA integrity. TaqMan single-nucleotide polymorphism (SNP) genotyping was performed on the rs6265 SNP of the BDNF gene.
2.2. TMS testing
Participants were seated in a comfortable chair with the right arm and hand in a pronated position rested on a pillow. They were instructed to keep their right hand as still and relaxed as possible throughout the experiment. Participants were also monitored for drowsiness and asked to keep their eyes open through-out the experiment. All study parameters followed the current guidelines for the safe application of TMS recommended by the International Federation of Clinical Neurophysiology (Rossi, Hallett, Rossini, Pascual-Leone, 2009; Rossini et al., 2015).
To collect electromyogram (EMG) signal, two surface electrodes were placed on the belly and on the tendon of the right first dorsal interosseous (FDI) muscle and connected to the Nexstim data acquisition device (Nexstim Ltd., Helsinki, Finland). The TMS system delivered triggered pulses that synchronized the TMS and EMG systems. Peak-to-peak MEP amplitude of the non-rectified signal was calculated on individual waveforms by the Nexstim system. Live EMG was monitored in order to maintain hand relaxation throughout the experiment.
Single TMS pulses used to measure resting motor threshold (RMT) and cortico-motor reactivity, and paired TMS pulses were delivered by the Nexstim system. Single TMS pulses used to measure active motor threshold (AMT) and intermittent TBS (iTBS) pulses were delivered by a MagPro X100 stimulator (MagVenture A/S, Farum, Denmark). The Nexstim TMS neuronavigation system was used with a brain MRI template to ensure consistent targeting throughout the experiment. Single TMS pulses were delivered by a figure-of-eight coil utilizing biphasic pulses with an inter-pulse interval automatically randomized (5–6 seconds) to avoid train effects. The coil was held tangentially to the participant’s head surface, with the handle pointing occipitally and positioned at 45° relative to the mid-sagittal axis of the participant’s head. The optimal spot for the maximal responses of the right FDI was localized.
Upon determination of the optimal spot for activation of the FDI, we measured the RMT according to the guidelines established by the International Federation for Clinical Neurophysiology (Rossini et al., 2015). RMT was defined as the lowest stimulation intensity that would elicit MEPs of at least 50 mV (peak to peak) in at least 5 of 10 consecutive trials with the muscles at rest. After this period, 3 sets of 30 pulses were delivered at 120% of RMT to estimate baseline cortico-motor reactivity. These number of pulses were higher than the minimum number of pulses required to obtain reliable estimates of MEP amplitude at a given time point (Chang et al., 2016; Goldsworthy, Hordacre, & Ridding, 2016).
Paired-pulse TMS was delivered with a figure-of-eight coil utilizing monophasic pulses with an automatically randomized inter-pulse interval (5–6 seconds). First, RMT was estimated with monophasic single pulses, and cortico-motor reactivity was measured by delivering 50 single pulses at 120% of RMT. Following this procedure, three paired-pulse paradigms were utilized: short-interval intracortical inhibition (SICI), intracortical facilitation (ICF) and long-interval intracortical inhibition (LICI) (Kujirai et al., 1993). For SICI and ICF, the conditioning pulse was set at 80% of RMT and the test pulse was set at 120% of RMT. The inter-pulse interval was 3 ms for SICI and 12 ms for ICF. For LICI, both conditioning and test stimuli were delivered at 120% of RMT and the inter-pulse interval was 100 ms. For each paradigm, 50 conditioned TMS pulses were applied and individual MEPs >2.5 standard deviations (SD) from the mean were excluded. The percent change in amplitude from baseline to conditioned MEPs for SICI, ICF and LICI were calculated.
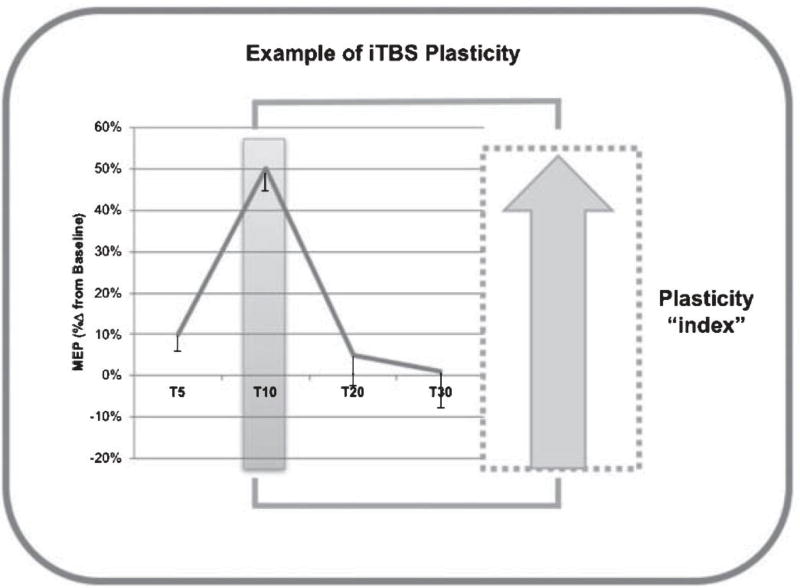
Prior to iTBS, active motor threshold (AMT) was measured. AMT was defined as the lowest stimulation intensity that would elicit MEPs ≥200 mV in at least 5 of 10 consecutive pulses with the FDI slightly contracted. Monitoring of live EMG during the AMT measurement ensured consistent effort. To control the effects of voluntary hand movements on iTBS aftereffects (Iezzi et al., 2008), there was a 2–5 min break between the AMT measurement and the onset of iTBS, during which participants were instructed to maintain hand relaxation. The iTBS protocol was delivered at 80% of individual AMT in 2-second trains interleaved with 8-second intervals for 192 seconds. Within each train, bursts of 3 pulses at 50 Hz were repeated at 200 ms inter-burst intervals (for a total of 600 pulses). This protocol has been shown to increase MEP amplitude (i.e., facilitate cortico-motor reactivity) for up to 60 minutes in healthy individuals (Wischnewski & Schutter, 2016). Once MEP amplitudes have been altered by iTBS, the time it takes for MEP amplitudes to return to their base-line levels is considered a neurophysiologic index of cortical plasticity (Oberman et al., 2010; Pascual-Leone et al., 2011; Suppa et al., 2016; Wischnewski & Schutter, 2016) (Fig. 2). Thirty single TMS pulses were delivered at 5, 10, 20 and 30 minutes post-iTBS (T5, T10, etc.). For each time point, MEP amplitudes (peak-to-peak) were calculated separately for each condition and individual MEPs >2.5 SD from the mean were excluded. Plasticity was calculated as the percent change in MEP amplitude from baseline at each post-iTBS time point.
Fig. 2.
Schematic figure depicting the interpretation of the results from the TMS-TBS plasticity assessment.
2.3. Neuropsychological test battery
A neuropsychological test battery designed to mea-sure a broad range of cognitive domains was used. Memory was assessed using the Brief Visual Mem-ory Test (BVMT-R) (Benedict, 2005) and the Rey Auditory-Verbal Learning Test (RAVLT) (Callahan Johnstone, 1994). Distinct equivalent forms (i.e., versions) were used at pre-test and post-test for the BVMT-R and RAVLT tests. Testing involved learning and delayed recall phases. During the learning phase for both verbal and visual tests, stimuli were presented to the participants over three consecutive trials. After a 20-minute delay, participants were asked to recall items from the test list and then complete a yes/no recognition test. Attention and executive functions were assessed with the Conners’ Continuous Performance Test (CPT-3) (Homack & Riccio, 2006), a computerized test of attention that requires participants to respond to target letters presented briefly on the screen. Reaction times, changes in reaction time, speed, and consistency, signal detection, omission and commission errors were recorded. Visuomotor processing speed and divided attention/task switching were assessed using Trail-making Test Parts A and B (Giovagnoli et al., 1996). In Part A of the test, participants were instructed to connect a set of 25 dots in sequential order as fast as possible while maintaining accuracy. In Part B, participants were asked to connect dots while alternating between numbers and letters (1-A-2-B, etc.).
Response inhibition, mental flexibility, and attentional control were assessed using the Stroop Color Word Test (Morrow, 2013). The test consists of three components, with each component consisting of 100 items. On the first portion, participants were asked to read words printed in black ink (RED/GREEN/BLUE). On the second portion, participants were asked to identify the color of ink in which a series of Xs were printed. In the final section, participants were asked to identify the color of ink the color words were printed in. Participants were instructed to attempt to complete each component as quickly and as accurately as possible. Each section was scored based on the number of items completed accurately within a 45-second period.
2.4. Physical exercise intervention
The physical exercise intervention was administered at Tanger Be Well (BIDMC) and was carried out on a treadmill. Each participant engaged in 30-minute daily sessions, 4 times/week for 4 consecutive weeks (for a total of 16 sessions). Participants were encouraged to keep a regular exercise timeslot for the duration of the study, but rescheduling of study visits was allowed if needed. Physical exercise was supervised by a study investigator at all times.
At the beginning of each session, participants were fitted with a heart rate monitor and proceeded to the treadmill, where they were instructed to walk (or trot) to maintain 55–64% of their age-predicted maxi-mal heart rate (220–age) (Tanaka, Monahan, & Seals, 2001) throughout the exercise session. Participants were given the opportunity to rest if they indicated feeling uncomfortable, and a physician was available by page in case a participant reported any adverse event. Participant’s perceived effort was measured with the 10-point Borg scale (Borg, 1982; Callahan & Johnstone, 1994) and heart rate was monitored prior to the session, every 10 minutes during the 30-minute session, and 5 minutes after the end of each session. The Borg scale is a widely used clinical numerical scale for the assessment of perceived effort, where zero represents rest and 10 represents maximal effort.
2.5. Data analysis
Statistical analyses were performed with SAS University Edition software version 9.4 (SAS Institute, Cary, NC, USA). Data were tested for normality of distribution using the Shapiro-Wilk test. For the pre-post comparisons of the entire sample, paired t-tests were conducted for normally distributed data (with pooled or Satterthwaite approximations when equal and non-equal variances were encountered, respectively), and signed rank test was conducted for non-normally distributed data.
We also performed comparisons between individuals with and without the BDNF Val66Met polymorphism. We assessed normality using the Shapiro-Wilk test. Then, we assessed baseline equivalence using independent-sample t-tests (with pooled or Satterthwaite approximations when equal and non-equal variances were encountered, respectively). None of the outcome measures in this analysis significantly deviated from a normal distribution. We conducted pre-post comparisons between the two subgroups using independent-sample t-tests. All analyses were two-tailed, the level of significance was set to p < 0.05 and we report outcomes using the mean and standard deviation (SD). Pre-post changes that achieved a p-value of at least 0.1 are reported as statistical trends, and in these instances, the median and interquartile ranges are also reported.
3. Results
Of the 19 participants who enrolled in the study, 14 completed all exercise visits and provided saliva samples for BDNF allelic status determination. All participants who underwent TMS testing and physical exercise tolerated the procedures well, and there were no adverse events. One participant was withdrawn after revealing that she was on medication for clinical depression. Four participants voluntarily withdrew from the study because of lack of interest (n = 1), time constraints (n = 1), or without providing a reason (n = 2). Table 1 presents demographic information and whole-group means for baseline outcome measures.
Table 1.
Characteristics of the sample at baseline
| Mean ± SD | |
|---|---|
| Age (years) | 27 ± 12.3 |
| Handedness (% Right) | 100 |
| Gender (%female) | 63 |
| Height (meters) | 1.72 ± 0.1 |
| Weight (kg) | 75 ± 22.1 |
| Body mass index (kg/m2) | 25.1 ± 5.5 |
| Resting heart rate (bpm) | 73.8 ± 9.7 |
| Mean arterial pressure | 89.4 ± 9.1 |
| BDNF Val66Met Polymorphism (%present) | 40 |
| RAVLT Immediate Recall | 12.9 ± 1.7 |
| RAVLT Delayed Recall | 12.2 ± 2.2 |
| RAVLT Delayed Recognition | 14 ± 1.6 |
| CPT Detectability | 45.2 ± 5.8 |
| CPT Omissions | 45.6 ± 2.8 |
| CPT Commissions | 47.3 ± 7.4 |
| CPT Perseverations | 46.9 ± 2.1 |
| CPT Hit Reaction Time | 50.8 ± 6.6 |
| CPT Hit Reaction Time Standard Deviation | 43.4 ± 7.3 |
| CPT Variability | 44.2 ± 5.5 |
| CPT Hit Reaction Block | 52.5 ± 10.6 |
| CPT Hit Reaction across different inter-stimulus intervals | 48.1 ± 6.2 |
| Stroop color condition | 103.8 ± 16.1 |
| Stroop word condition | 77.3 ± 15.1 |
| Stroop color-word condition | 51.2 ± 8.7 |
| Trails A time | 20.6 ± 5.8 |
| Trails B time | 46.5 ± 16.5 |
| Resting Motor Threshold (biphasic current) | 42 ± 8.4 |
| Corticomotor Reactivity (biphasic current) | 1498.9 ± 742 |
| Active Motor Threshold (biphasic current) | 43.3 ± 10.2 |
| Percent Modulation at T5 | 19.3 ± 30 |
| Percent Modulation at T10 | 33.1 ± 33.3 |
| Percent Modulation at T20 | 29.9 ± 51 |
| Percent Modulation at T30 | 30.7 ± 39.6 |
| Corticomotor Reactivity (monophasic current) | 1424.5 ± 728.4 |
| Short-interval Intracortical Inhibition (%) | −60.5 ± 18.3 |
| Intracortical Facilitation (%) | 29.4 ± 40.7 |
| Long-interval Intracortical Inhibition (%) | −86.5 ± 10.1 |
Abbreviations: kg = kilograms; BDNF = brain-derived neurotrophic factor; Val =Valine; Met =Methionine; RAVLT =Rey Auditory Verbal Learning test; CPT = Conners’ Continuous Performance Test.
Whole-group analysis revealed a statistical trend toward improvement in response inhibition as assessed by performance on the Stroop test (color-word) (pre-test 51.7 ± 8.6, post-test 53.9 ± 9.4, p = 0.059). No other whole-group analyses yielded significant findings or trends (all p’s >0.10). In comparisons between the two subgroups (Val66Val and Met carriers) with regards to the change from pretest to posttest, there was a marginally significant difference in the change in delayed recognition on the RAVLT test (p = 0.056), with the Met carriers exhibiting greater performance (Val66Val –0.37 ± 0.18, Met carriers 0.16 ± 0.16). However, closer inspection of the data demonstrated a ceiling effect at baseline, as the mean score was 13.85 ± 1.46 (92.3% accuracy), and only 2 individuals had scores that feel outside one standard deviation. For this reason, we believe this difference was not clinically meaningful.
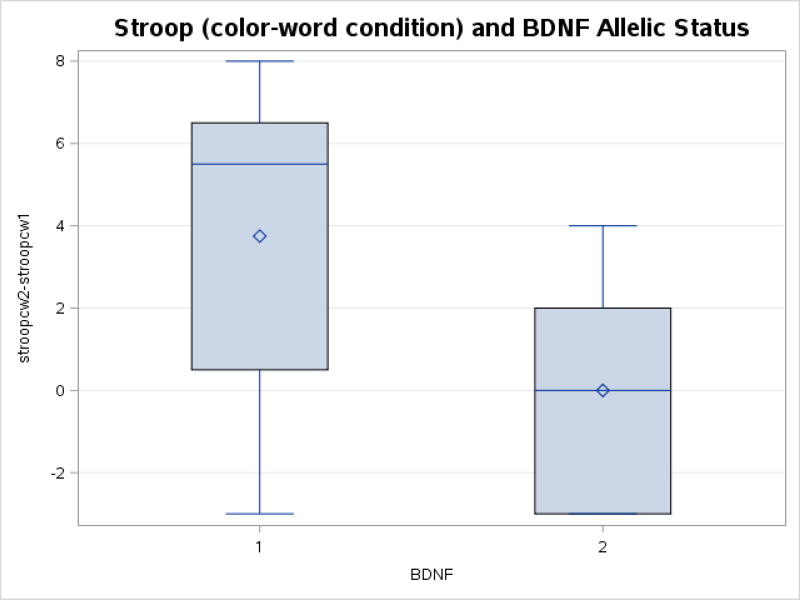
There was a trend toward a difference in the change in Stroop performance (color-word condition) from pre-test to post-test (p = 0.07), with the Val66Val subgroup exhibiting increased performance. Figure 3 depicts the mean, median change ± first and third quartiles for the Stroop (color-word condition) prior to and following exercise. The majority of individuals in the Val66Val subgroup improved their performance (mean 3.75 ± 3.9; median 5.5, first quartile 0.7, third quartile 6.2), whereas most met carriers did not demonstrate a change in performance (mean 0 ± 2.8; median 0, first quartile −2.5, third quartile 1.8).
Figure 3.
Change in Stroop color-word condition (mean, median change ± first and third quartiles) for participants in Val66Val subgroup and Met carriers from pre to post-exercise. There was a trend toward differences in the change in stroop performance (color-word condition) (p=0.07), with the Val66Val group exhibiting increased performance.
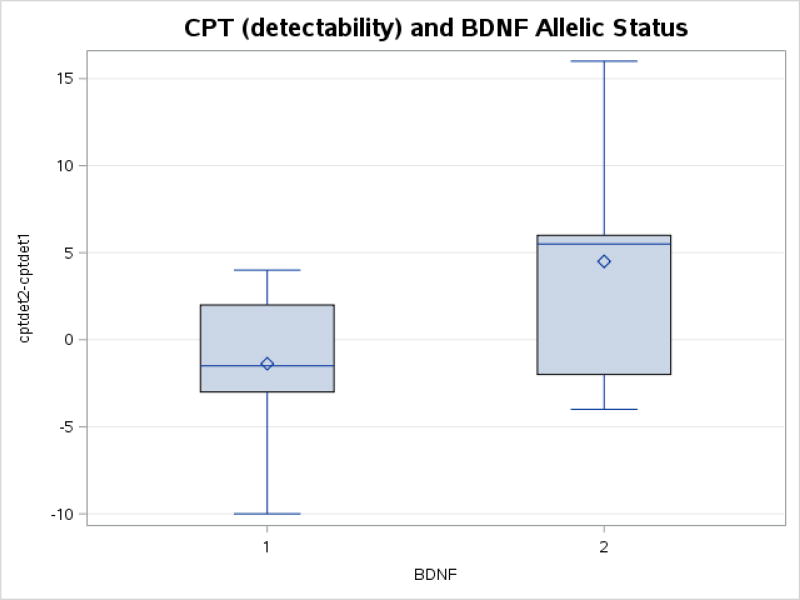
In addition, there was a trend toward a difference in the change in the reaction time (detectability) of the CPT from pre-test to post-test (p = 0.08), with the Val66Val group exhibiting faster reaction times. Figure 4 depicts the mean, median change ± first and third quartiles for the CPT detectability (reaction time) prior to and following exercise. The majority of individuals in the Val66Val subgroup decreased their reaction time (mean −1.3 ± 4.4; median −1.5, first quartile −2.5, third quartile 1.5), whereas most met carriers did not demonstrate a change in performance (mean 4.5 ± 7.0; median 5.5, first quartile −0.2, third quartile 6).
Figure 4.
Change CPT detectability reaction times (mean, median change ± first and third quartiles) for participants in Val66Val subgroup and Met carriers from pre to post-exercise. There was a trend toward differences in the change in stroop performance (color-word condition) (p=0.08), with the Val66Val group exhibiting increased performance.
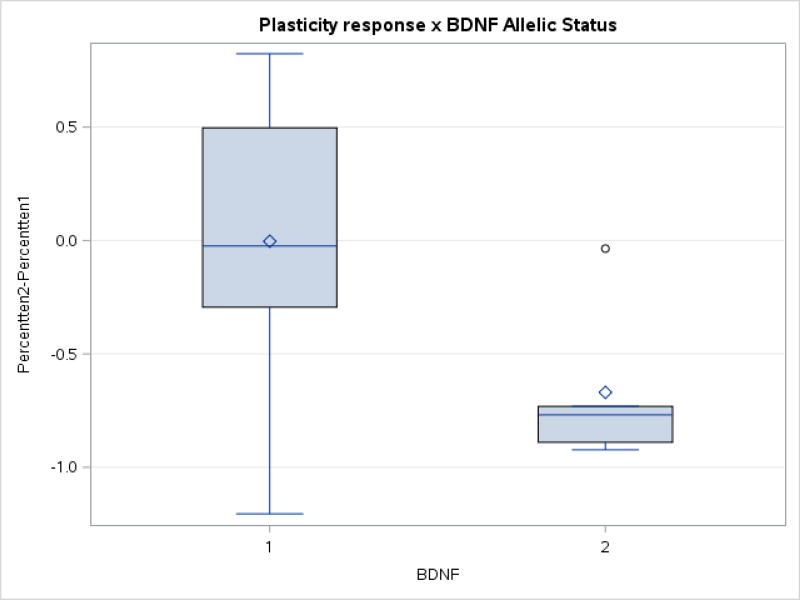
There was a trend toward differences in TMS measures between the two subgroups in percent modulation at T10 (p = 0.07), with the Met carriers demonstrating a negative modulation in TMS responses to TBS. Figure 5 depicts the mean, median change ± first and third quartiles for TBS-induced modulation of TMS responses at minute 10 (T10) prior to and following exercise. The majority of individuals in the Met carrier subgroup showed reduced modulation of TMS responses to TBS at post-test (mean −55.9% ± 42.4; median −75.0%, first quartile −86.0 third quartile −21.0), whereas most Val66Val carriers did not demonstrate a change in TBS-induced modulation of TMS responses (mean 16.9% ± 49.0; median 0, first quartile –6.0, third quartile 50). All other outcomes were not associated with significant changes (all p’s >0.10).
Figure 5.
Change in percent modulation at T10 on the TMS-TBS Platicity test for participants in Val66Val subgroup and Met carriers from pre to post-exercise. There was a trend toward differences in TMS measures between the two subgroups in percent modulation at T10 (p=0.07), with the Met carriers demonstrating a negative modulation in TMS responses to TBS.
4. Discussion
The objective of the present study was to assess the effects of a month of regular aerobic exercise on brain physiology measured with TMS, and relate them to cognitive performance in sedentary young adults. In addition, we aimed to assess the relevance of the BDNF Val66Met polymorphism as a potential modulator of the possible exercise-mediated effects. The whole-group analysis revealed that response inhibition was marginally significantly increased by exercise in all participants. Between-group comparisons revealed that individuals in the Val66Val subgroup exhibited trends toward greater improvements on the Stroop, faster reaction time on the CPT detectability test, and that Met carriers had a trend toward negative change in TBS-induced modulation of TMS responses. These findings suggest that commitment to a moderate lifestyle change (i.e., four weekly 30-minute sessions of treadmill walking over one month) can impact cognitive function and mechanisms of plasticity. In addition, BDNF allelic status may influence the pattern of change in response to a regular exercise regimen.
The finding that commitment to a month of light aerobic regular exercise can improve cognitive function in young healthy adults is encouraging and deserves further examination. Previous studies have shown similar results but typically have done so with a greater exercise dose (in terms of both session time and study length). In the present study, sedentary participants who undertook just 4 weeks of light aerobic exercise demonstrated in improvements in cognition. While cognitive improvements following walking programs have been reported in the literature (Kramer et al., 1999; Leckie et al., 2014), these have been typically delivered over 6 months to 1-year, and have included elderly individuals, who may be experiencing age-related cognitive decline and may have different responsiveness to the exercise-mediated effects on cognition than our young healthy participants.
Furthermore, there have not been adequately powered studies with direct comparisons of different exercise doses on cognition, and therefore strong conclusions cannot be made at this time. Various hypotheses exist regarding how exercise might mediate improvements in cognition. For example, the cardiorespiratory hypothesis suggests that to see improvements in cognition, concomitant increases in cardiovascular fitness are necessary (Etnier et al., 2006). According to this view, the increased efficiency of the oxygen metabolism would promote increased cerebral blood flow, oxygenation and glucose availability (Churchill et al., 2002). This would, in turn, promote the local neurogenic changes at the molecular level, such as a higher availability of neurotrophins and growth factors that would support cognitive processes. Nevertheless, studies assessing non-aerobic physical activity such as yoga (Gothe, Kramer, & McAuley, 2014) and tai-chi (Mortimer et al., 2012) have also led to improvements in cognition, as have non-aerobic resistance training exercise programs (Liu-Ambrose et al., 2010; Liu-Ambrose, Nagamatsu, Voss, Khan, & Handy, 2012). Thus, for the improvement and/or maintenance of cognition function, further research into the optimal exercise dose parameters is encouraged.
The role of the BDNF allelic status in the plasticity induced by exercise interventions warrants further investigations. The Val66Met polymorphism of BDNF influences the intracellular trafficking and packaging of the precursor peptide (pro-BDNF), which is associated with long-term depression (LTD), and the regulated secretion of the mature (m)BDNF, which is involved in long-term potentiation (LTP) (Bramham & Messaoudi, 2005; Egan et al., 2003). While it is conceivable that the BDNF polymorphism alters the activity-dependent secretion of BDNF during exercise, there is also evidence to suggest that Val/Val and Met carriers do not differ in their potential to exhibit plastic changes, but Met carriers may rely more heavily in subcortical than cortical plasticity (Di Pino et al., 2016). A greater understanding of the effects of this polymorphism in studies with larger sample sizes is encouraged.
To our knowledge, this is the first study to examine the pre-post changes in response to a month-long physical exercise regimen, to assess TMS measures of cortical plasticity in relation to cognitive performance, and to explore the influence of BDNF polymorphism. However, this is a feasibility study with limitations. The sample size limits the generalizability of the results. In future studies, differences in baseline outcomes need to be carefully controlled. In addition, we did not collect information regarding the level of education of participants in the sample. Lastly, clinical neuropsychological assessments are subject to practice effects.
5. Conclusion
Four weeks of regular aerobic exercise can influence cognitive performance and cortical plasticity measured by TMS. TMS measures of plasticity may be useful in further elucidating the mechanisms responsible for cognitive-mediated changes and improving the specificity of exercise prescription to maintain or improve cognitive function.
Acknowledgments
The authors thank Chun Lim and Ann Connor (Beth Israel Deaconess Medical Center) for assistance with evaluation of participants’ health/medical history and physical/neurological examination and Bonnie Wong for her assistance with the development of the neuropsychological battery.
A.P.-L. was also supported in part by the Sidney R. Baer Jr. Foundation, the NIH (R01 MH100186, R01 HD069776, R01 NS073601, R21 MH099196, R21 NS085491, R21 HD07616), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). A.J. was supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC PDF 454617). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or any of the listed granting agencies.
A.P.-L. serves on the scientific advisory boards for Magstim, Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging.
Footnotes
Conflict of interests statement
The authors declare no competing interest.
References
- Aguiar AS, Jr, Castro AA, Moreira EL, Glaser V, Santos AR, Tasca CI, Prediger RD. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mechanisms of Ageing and Development. 2011;132(11–12):560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. The Cochrane Database of Systematic Reviews. 2008;(3):CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- Benedict RH. Effects of using same- versus alternate-form memory tests during short-interval repeated assessments in multiple sclerosis. Journal of the International Neuropsychological Society: JINS. 2005;11(6):727–736. doi: 10.1017/S1355617705050782. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Progress in Neurobiology. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. doi:S0301-0082(05)00066-3. [DOI] [PubMed] [Google Scholar]
- Callahan CD, Johnstone B. The clinical utility of the rey auditory-verbal learning test in medical rehabilitation. Journal of Clinical Psychology in Medical Settings. 1994;1(3):261–268. doi: 10.1007/BF01989627. [DOI] [PubMed] [Google Scholar]
- Cargill M, Altshuler D, Ireland J, Sklar P, Ardlie K, Patil N, Lander ES. Characterization of single-nucleotide polymorphisms in coding regions of human genes. Nature Genetics. 1999;22(3):231–238. doi: 10.1038/10290. [DOI] [PubMed] [Google Scholar]
- Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim YH, Pascual-Leone A. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2016;127(8):2892–2897. doi: 10.1016/j.clinph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiology of Aging. 2002;23(5):941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Capone F, Assenza G, Florio L, Falato E, Di Lazzaro V. Val66Met BDNF polymorphism implies a different way to recover from stroke rather than a worse overall recoverability. Neurorehabilitation and Neural Repair. 2016;30(1):3–8. doi: 10.1177/1545968315583721. [DOI] [PubMed] [Google Scholar]
- DNA Genotek Inc. Laboratory protocol for manual purification of DNA from 0.5mL of sample. 2015 Retrieved from http://www.dnagenotek.com/US/pdf/PD-PR-006.pdf.
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. doi:S0092867403000357. [DOI] [PubMed] [Google Scholar]
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Research Reviews. 2006;52(1):119–130. doi: 10.1016/j.brainresrev.2006.01.002. doi:S0165-0173(06)00003-8. [DOI] [PubMed] [Google Scholar]
- George JD, Stone WJ, Burkett LN. Non-exercise VO2max estimation for physically active college students. Medicine and Science in Sports and Exercise. 1997;29(3):415–423. doi: 10.1097/00005768-199703000-00019. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: Normative values from 287 normal adult controls. Italian Journal of Neurological Sciences. 1996;17(4):305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience. 2016;320:205–209. doi: 10.1016/j.neuroscience.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Gothe NP, Kramer AF, McAuley E. The effects of an 8-week hatha yoga intervention on executive function in older adults. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2014;69(9):1109–1116. doi: 10.1093/gerona/glu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homack S, Riccio CA. Conners' continuous performance test (2nd ed.; CCPT-II) Journal of Attention Disorders. 2006;9(3):556–558. doi: 10.1177/1087054705283578. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Davis FC, Vantieghem MR, Whalen PJ, Bucci DJ. Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience. 2012;215:59–68. doi: 10.1016/j.neuroscience.2012.04.056. 10.1016/j.neuroscience.2012.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Iezzi E, Conte A, Suppa A, Agostino R, Dinapoli L, Scontrini A, Berardelli A. Phasic voluntary movements reverse the aftereffects of subsequent theta-burst stimulation in humans. Journal of Neurophysiology. 2008;100(4):2070–2076. doi: 10.1152/jn.90521.2008. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Marsden CD. Corticocortical inhibition in human motor cortex. The Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Erickson KI. BDNF mediates improvements in executive function following a 1-year exercise intervention. Frontiers in Human Neuroscience. 2014;8:985. doi: 10.3389/fnhum.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: A 12-month randomized controlled trial. Archives of Internal Medicine. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: A 12-month randomized controlled trial. Neurobiology of Aging. 2012;33(8):1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Morrow SA. Normative data for the stroop color word test for a north american population. The Canadian Journal of Neurological Sciences.Le Journal Canadien Des Sciences Neurologiques. 2013;40(6):842–847. doi: 10.1017/s0317167100015997. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Ding D, Borenstein AR, DeCarli C, Guo Q, Wu Y, Chu S. Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented chinese elders. Journal of Alzheimer's Disease : JAD. 2012;30(4):757–766. doi: 10.3233/JAD-2012-120079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Oberman L, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, Pascual-Leone A. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile × syndrome and autism spectrum disorder. Frontiers in Synaptic Neuroscience. 2010;2:26. doi: 10.3389/fnsyn.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Rotenberg A. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topography. 2011;24(3–4):302–315. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. an updated report from an I.F.C.N. committee. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherder E, Scherder R, Verburgh L, Konigs M, Blom M, Kramer AF, Eggermont L. Executive functions of sedentary elderly may benefit from walking: A systematic review and meta-analysis. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry. 2014;22(8):782–791. doi: 10.1016/j.jagp.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Sherwood A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppa A, Di Stasio F, Marsili L, Upadhyay N, Belvisi D, Conte A, Berardelli A. Primary motor cortex LTP/LTD-like plasticity in probable corticobasal syndrome. Journal of Neurophysiology. 2016;115(2):717–727. doi: 10.1152/jn.00755.2015. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. Journal of the American College of Cardiology. 2001;37(1):153–156. doi: 10.1016/s0735-1097(00)01054-8. S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Canadian Journal of Sport Sciences = Journal Canadien Des Sciences Du Sport. 1992;17(4):338–345. [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: Past and future directions. Neuromolecular Medicine. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: Something to chew on. Trends in Neurosciences. 2009;32(5):283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischnewski M, Schutter DJ. Efficacy and time course of paired associative stimulation in cortical plasticity: Implications for neuropsychiatry. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology. 2016;127(1):732–739. doi: 10.1016/j.clinph.2015.04.072. [DOI] [PubMed] [Google Scholar]