Abstract
This cross‐sectional survey study evaluated oral hygiene habits in conjunction with whole mouth examinations for dental plaque and gingivitis among adults in India. Subjects across several age groups who provided informed consent [220 male and 158 female (mean age 30.9 years)] were enrolled. All enrolled subjects were interviewed for oral hygiene practices and evaluated by the Turesky modification of the Quigley‐Hein and the Löe‐Silness methods for dental plaque and gingivitis, respectively. Evaluations included oral hygiene parameters, prevalence of dental plaque and gingivitis, and regional differences within the dentition for dental plaque and gingivitis. Results from this study indicate that most subjects (97%) utilized a toothbrush and toothpaste for oral hygiene with a majority (92%) using their right hand to brush their teeth. While 29% reported two or more episodes of daily oral hygiene, a majority (53%) brushed their teeth once daily. Utilization of dental floss and mouthwashes were reported by approximately 1% of this population, and most (73%) reported no dental visits in the preceding 5 years. Whole mouth plaque and gingival scores (average ± standard deviation) for this population were 2.47 ± 0.55 and 1.19 ± 0.31, respectively, with no significant differences between either gender (P > 0.05). Significant correlations (r > 0.44) were observed between plaque and gingival scores for the entire sample, either gender or between age groups (P < 0.001). Analyses indicate that anterior teeth demonstrated lower average scores for dental plaque and gingivitis than posterior and molar regions (P < 0.05). Education was associated with higher plaque and gingival scores: plaque scores [odds ratios; 95% confidence interval; 1.23; 1.01–1.50 and gingival scores odds ratios 1.25; 1.02–1.54]. In summary, results from this study demonstrate the prevalence of dental plaque and gingivitis in the general population and their relationships with demographic characteristics. They reinforce examinations of posterior regions that consistently harbor more plaque and corresponding gingivitis in evaluations of oral health.
Keywords: Dental plaque, dentition, epidemiology, gingivitis, oral hygiene, sociodemographic variables, survey
Introduction
Oral health priorities seek to reduce the negative impacts of oral diseases and their influences on overall health (Dye 2012; Milgrom and Reisine 2000; Petersen 2009). Common oral diseases include caries and inflammatory conditions of the gingiva that affect oral health and may lead to tooth loss (Kornman 2008; Marsh 2012; Milgrom and Reisine 2000). A substantial literature has been instrumental in delineating the etiology and progression of these oral conditions (Petersen 2009; Scannapieco 1998; Socransky and Haffajee 2005). Epidemiological studies demonstrate the global nature of these conditions with a widespread prevalence (Dye 2012). Consequently, efforts to reduce the negative influences of these diseases on oral health represent important priorities for dental health‐care providers (Milgrom and Reisine 2000; Petersen 2009).
Dental plaque is a widely recognized factor in the initiation and progression of a variety of oral diseases (Berezow and Darveau 2011; Marsh 2012). Plaque, a natural biofilm, is commonly recovered from oral surfaces and comprises a diverse array of organisms (Socransky and Haffajee 2005). An unimpeded accumulation of dental plaque on the gingival margin triggers inflammatory effects that can become chronic (Kornman 2008; Rüdiger et al. 2002). Changes in protein profiles and microbial population shifts are reported during the clinical transition from health to inflammatory diseases such as gingivitis and periodontal disease (Rüdiger et al. 2002; Socransky and Haffajee 2005). Based on experimental and epidemiological investigations, dental professionals recommend effective oral hygiene to control the dental plaque and accumulated inflammatory components to maintain optimal oral health (Claydon 2008; Marsh 2012). Whereas the effects of dental plaque on the oral health of individuals are acknowledged, recent studies have assessed influences of poor oral health on overall health (de Oliveira et al. 2010). Taken together, these public health impacts represent important concerns for dental care providers (Scannapieco 1998; Schiavo, 2011).
The global epidemiology of common dental conditions is a reminder for effective dental programs (Dye 2012; Petersen 2009). While a vast literature explains the role of dental plaque in oral diseases (Claydon 2008; Marsh 2012; Milgrom and Reisine 2000; Socransky and Haffajee 2005), fewer studies describe the prevalence of dental plaque and gingivitis in populations. Recent investigations indicate average gingival scores among selected adult groups from different countries ranged from 0.99 to 1.23 (Li et al. 2010; Röthlisberger et al. 2007; Zhang et al. 2010). However, the published literature has few reports describing the prevalence of these common conditions among Indian adults or the distribution of plaque and gingivitis within the dentition. Whereas this information is important from a public health perspective, they are also relevant while evaluating therapeutic strategies to control these common oral conditions.
Accordingly, the present cross‐sectional survey study evaluated the general prevalence of dental plaque and gingivitis among adults (>18 years) in a population from India. Included in this study was an assessment of individual‐level factors, sociodemographic factors, oral health behavior, and dimensions of the home and family environments. Thus, the aims of this investigation were to (1) examine the prevalence of dental plaque and gingivitis among adult subjects; (2) determine distributions of plaque and gingivitis within the dentition; and (3) estimate common oral health practices to evaluate the contributions of these indications on dental plaque and gingivitis in this population.
Materials and Methods
The present cross‐sectional study was conducted among adults (>18 years) after the study protocol was approved by the ethical review board of the SDM Dental College and Hospital, Dharwad, India. Prospective subjects from the local area provided written voluntary informed consent prior to enrollment. Three hundred seventy‐eight adults over the age of 18 years were recruited for this study.
Following enrollment, all subjects were interviewed for household demographics, social and economic characteristics, level of education, residential setting, and disabilities. In addition, an interview evaluated food habits, utilization of dental services, exposure to fluoridated water, oral hygiene habits (use of fluoride toothpaste and toothbrush, dental floss, mouth rinse use, and frequency of toothbrush replacement), frequency of routine and other dental visits, and smoking. Other variables recorded were gender, age in years, and region of residence. All data were collected by dental examiners by questionnaire with multiple choice questions.
Clinical Evaluations
Clinical examinations for dental plaque and gingivitis were conducted by a calibrated examiner under constant lighting conditions. In initial tests and retest assessments among a group of eight subjects, the examiner demonstrated 99% reliability for both indices. Whole‐mouth evaluations for gingivitis and dental plaque were evaluated by the Löe–Silness (Loe 1963) and Turesky Modification of the Quigley–Hein (Turesky et al. 1970), respectively. The scoring scheme for the Löe–Silness gingivitis index is as follows:
0 = absence of inflammation
1 = mild inflammation – slight change in color and little change in texture
2 = moderate inflammation – moderate glazing, redness, edema and hypertrophy. Tendency to bleed upon probing
3 = severe inflammation – marked redness and hypertrophy. Tendency to spontaneous bleeding
The scoring scheme for the Turesky Modification of the Quigley–Hein dental plaque index is as follows:
0 = no plaque
1 = separate flecks of plaque at the cervical margin of the tooth
2 = a thin continuous band of plaque (up to 1 mm) at the cervical margin of the tooth
3 = a band of plaque wider than 1 mm but covering less than one‐third of the crown of the tooth
4 = plaque covering at least one‐third but less than two‐thirds of the crown of the tooth
5 = plaque covering two‐thirds or more of the crown of the tooth
Clinical examinations were conducted by the calibrated examiner, and a dental assistant recorded all results on appropriate forms.
Statistical Analyses
All collected data were entered onto Excel spreadsheets and exported to SAS (Cary, N.C. USA) for statistical analysis. Descriptive statistics reported on collected data along with frequency distribution of demographic results. Frequencies for each evaluation were determined. Statistical analyses by t‐test and analysis of variance determined statistical differences. Statistical analyses were two sided with significance reported at P < 0.05.
Results
A summary of subject demographics and oral hygiene habits from 378 adults [220 men and 158 women; average age 30 years] evaluated is shown in Table 1. A majority of subjects lived in urban or semi‐urban locations, utilized municipal water, and reported a high school education or more. Most used a toothpaste and a toothbrush (97%) for once daily oral hygiene (53%) and were right handed (92%). In this population, 60% used a half head of toothpaste for brushing with many reporting low utilization of mouth rinses and dental floss and 73% reporting no dental visits in the past 5 years.
Table 1.
Sociodemographic variables of study population.
| Characteristics | No. of respondents | % of respondents |
|---|---|---|
| Gender | ||
| Male | 220 | 58.20 |
| Female | 158 | 41.80 |
| Age | ||
| Mean | 30.9 | |
| SD | 10.2 | |
| Marital status | ||
| Unmarried | 155 | 41.01 |
| Married | 223 | 58.99 |
| Residential setting | ||
| Single (lives alone) | 74 | 19.58 |
| Married living with spouse | 117 | 30.95 |
| Family (lives as part of a large family) | 186 | 49.21 |
| Institution | 1 | 0.26 |
| Location | ||
| Rural | 34 | 8.99 |
| Semi‐urban | 158 | 41.80 |
| Urban | 186 | 49.21 |
| Education | ||
| Professional or honors | 22 | 5.82 |
| Graduate or post graduate | 153 | 40.48 |
| Intermediate or post high school diploma | 75 | 19.84 |
| High school certificate | 82 | 21.69 |
| Middle school certificate | 19 | 5.03 |
| Primary school certificate | 17 | 4.50 |
| Illiterate | 10 | 2.65 |
| Occupation | ||
| Professional | 67 | 17.72 |
| Semi‐profession | 24 | 6.35 |
| Clerical, shop owner | 25 | 6.61 |
| Skilled worker | 27 | 7.14 |
| Semi‐skilled worker | 43 | 11.38 |
| Unskilled worker | 49 | 12.96 |
| Unemployed | 143 | 37.83 |
| Disabilities | ||
| None | 372 | 98.41 |
| Physical | 6 | 1.59 |
| Drinking water | ||
| Bottled water | 5 | 1.32 |
| Prepared at home | 22 | 5.82 |
| Tap water | 348 | 92.06 |
| Do not know | 0 | 0.00 |
| Some other sources | 3 | 0.79 |
| Household source drinking | ||
| Town/municipal supply | 263 | 69.31 |
| Well water | 112 | 29.63 |
| Do not know | 3 | 0.79 |
| Oral Health Perceptions (a) (Experienced toothache/oro‐facial pain/food avoidance) | ||
| Yes | 170 | 44.97 |
| No | 208 | 55.03 |
| Oral Health Perceptions (b) (Self‐perceived need for extraction or filling) | ||
| Yes | 194 | 51.32 |
| No | 184 | 48.68 |
| Oral Health Perceptions (c) (Self‐rated oral health) | ||
| Yes | 343 | 90.74 |
| No | 35 | 9.26 |
| Dentist visit | ||
| Problem | 342 | 90.48 |
| Checkup | 20 | 5.29 |
| Other | 16 | 4.23 |
| Frequency of dental visit | ||
| Every 6 months | 18 | 4.76 |
| Every 1–2 years | 84 | 22.22 |
| Every 5+ years or Never/problem only | 276 | 73.02 |
| Last visit to dentist | ||
| Past 12 months | 79 | 20.90 |
| Between 1–2 years | 62 | 16.40 |
| Every 5+ years or never/problem only | 209 | 55.29 |
| Do not know | 28 | 7.41 |
| Avoid dental care | ||
| Yes | 120 | 31.75 |
| No | 258 | 68.25 |
| How do you brush | ||
| Tooth brush | 367 | 97.09 |
| No toothbrush | 9 | 2.38 |
| Natural | 2 | 0.53 |
| Frequency of tooth brushing habits | ||
| Less than once per day | 64 | 16.93 |
| Once per day | 203 | 53.70 |
| Twice per day | 108 | 28.57 |
| More than twice a day | 3 | 0.79 |
| Handedness of brushing | ||
| Right hand | 349 | 92.33 |
| Left hand | 25 | 6.61 |
| Both hands | 4 | 1.06 |
| Brush replacement | ||
| A (<3 months) | 228 | 60.32 |
| B (3–6 months) | 134 | 35.45 |
| C (up to 1 year) | 8 | 2.12 |
| D (>1 year) | 5 | 1.32 |
| E (cannot say) | 3 | 0.79 |
| Dentifrice used (amount of paste used) | ||
| A (full head) | 127 | 33.60 |
| B (half head) | 229 | 60.58 |
| C (unsure) | 6 | 1.59 |
| D (mixed use) | 16 | 4.23 |
| Dental floss | ||
| Yes | 4 | 1.06 |
| No | 374 | 98.94 |
| Mouth rinse | ||
| Yes | 4 | 1.06 |
| No | 371 | 98.15 |
| Occasionally | 3 | 0.79 |
| Grand total | 378 | 100.00 |
SD, standard deviation.
The average whole‐mouth dental plaque and gingival scores of the entire population and among male and female subjects are shown in Table 2. These clinical evaluations were conducted by one dentist who demonstrated 99% reliability [data not shown]. Average scores for dental plaque and gingival index scores for the entire population were 2.47 and 1.19, respectively. Whole‐mouth plaque scores of male and female subjects were 2.48 and 2.46, respectively, while whole‐mouth gingival index scores for men and women were 1.20 and 1.19, respectively, with analyses demonstrating no significant differences between genders for each evaluated parameter (P > 0.05).
Table 2.
Whole mouth clinical scores (mean ± SD).
| Clinical index | Group | Number of subjects | Mean±SD |
|---|---|---|---|
| Dental plaque | All subjects | 378 | 2.47±0.55 |
| Male | 220 | 2.48±0.54‡ | |
| Female | 158 | 2.46±0.57‡ | |
| Gingival scores | All subjects | 378 | 1.19±0.31 |
| Male | 220 | 1.20±0.30‡ | |
| Female | 158 | 1.19±0.32‡ |
SD, standard deviation.
No statistically significant differences between either gender (P > 0.05).
Table 3 provides whole‐mouth clinical scores within each age group. Average plaque scores ranged from 2.45 to 2.5, while gingival scores ranged from 1.17 to 1.27 with no significant differences for each parameter between evaluated age groups (P > 0.05). Significant correlations (r > 0.44) were observed between plaque and gingival scores for the entire sample, either gender or between age groups (P < 0.001) [data not shown].
Table 3.
Whole‐mouth clinical scores within age groups (mean ± SD).
| Age group | Dental plaque scores‡ | Gingival index scores‡ |
|---|---|---|
| 18–27 | 2.49±0.55 | 1.17±0.30 |
| 28–37 | 2.45±0.57 | 1.19±0.31 |
| 38–47 | 2.46±0.54 | 1.24±0.31 |
| 48+ | 2.50±0.53 | 1.27±0.32 |
SD, standard deviation.
No statistically significant differences between each age for either clinical evaluation (P > 0.05).
The distribution of each dental plaque score in the entire population and by gender is shown in Figure 1A. A large number of surfaces registered a dental plaque score of 3 with more than 25,000 observations. Dental plaque scores of 1 and 2 were less frequent in this population, with even fewer sites harboring plaque scores of 4. In this population, sites with a plaque score of 5 were only found in 645 sites with 370 and 275 surfaces in men and women, respectively, with this score. Few surfaces were plaque free.
Figure 1.
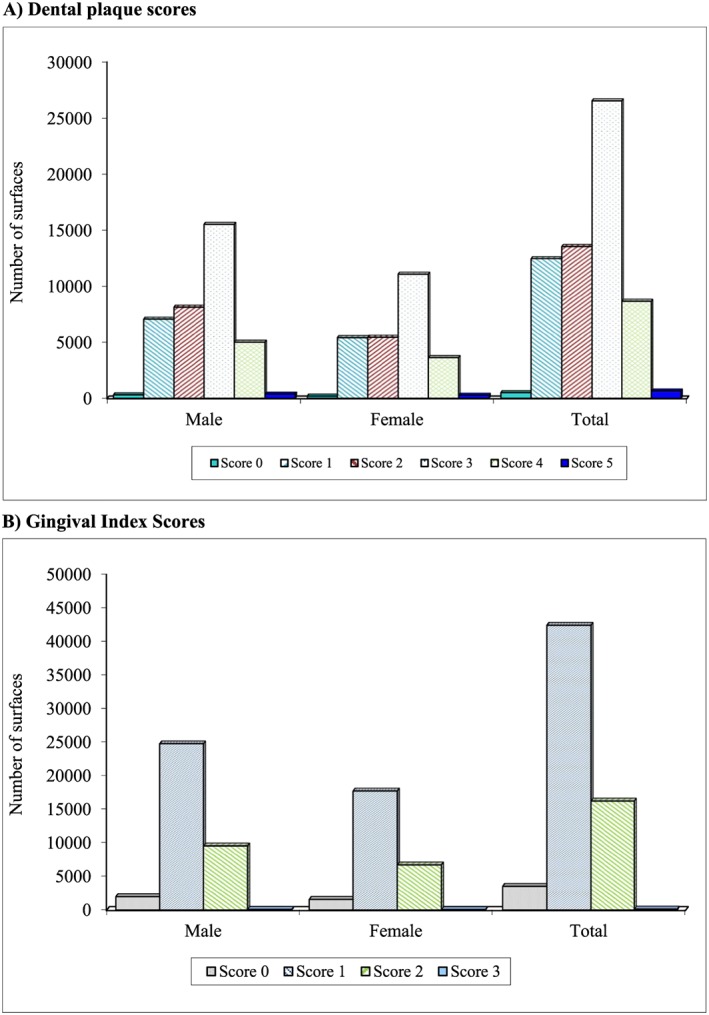
Distribution of individual clinical scores by gender and in the entire population. (A) Dental plaque scores; (B) gingival index scores.
The distribution of gingival index scores in the population and by gender are shown in Figure 1B. Surfaces with scores of 1 were the most common with more than 42,000 surfaces observed in the entire population comprising more than 24,000 and 17,000 among men and women, respectively. Surfaces with gingival scores of 2 were less common than those that registered a score of 1. Clinical observations indicate that a gingival score of 3 was only found on 127 surfaces of the entire population on 77 and 50 surfaces of male and female subjects, respectively. No gingivitis was observed in 5.67% or 3525 surfaces representing 1961 and 1564 surfaces in men and women, respectively.
Frequency distributions of clinical scores (plaque and gingival index) on the anterior and posterior regions of the dentition in each gender and for both clinical parameters are presented in Figures 2A and 2B. Plaque scores of 1–3 were common on anterior teeth and were found on 27–34% of evaluated surfaces. Anterior surfaces with scores of 4 and 5 were less frequent and observed on approximately 5.5% and 0.08% of evaluated surfaces, respectively. Plaque scores on posterior surfaces demonstrated a different frequency distribution than anterior sites. A plaque score of 3 was most common on posterior sites and observed on 49% of evaluated sites. A score of 4 was found on 20% of posterior teeth and were more frequent than scores of 1 and 2 observed in 11–17% of surfaces. Less than 2% of posterior surfaces registered a plaque score of 5. Gingival index scores of anterior regions demonstrated ~9% of sites with a score of 0, while a score of 1 was observed in 72% of sites and ~17% of sites demonstrating a score of 2. Gingival index frequencies of posterior regions indicate ~32% with a score of 2 and ~64% with a score of 1. Less than 3% of posterior sites recorded a gingival index score of 0.
Figure 2.
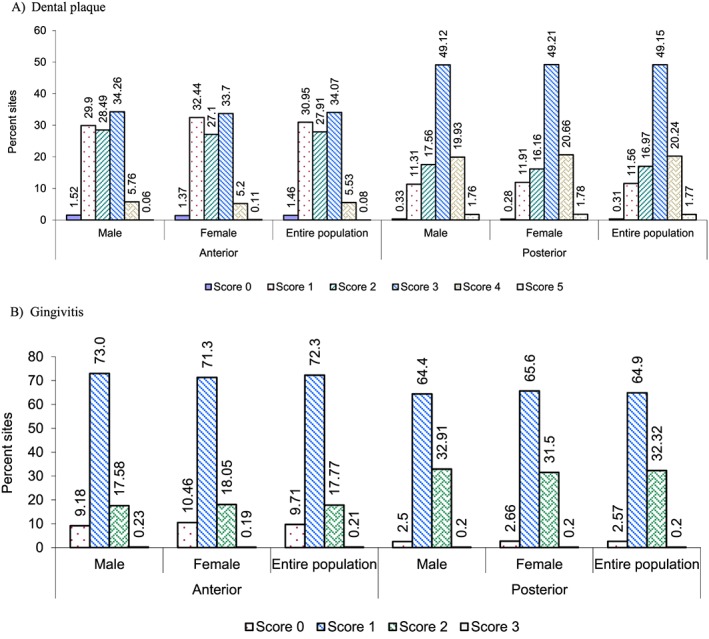
Frequency distribution of individual clinical scores on the anterior and posterior regions of the dentition. (A) Dental plaque; (B) gingivitis.
Dental plaque and gingival index scores from the anterior and posterior regions for the entire population are shown in Table 4. Plaque and gingival scores were 2.12 and 1.09, respectively, for anterior regions, while scores on posterior regions were 2.83 and 1.30 for dental plaque and gingival scores, respectively. Scores for dental plaque were significantly lower on anterior teeth than posterior sites (P < 0.000). Correspondingly, plaque scores on anterior surfaces of male and female subjects were 2.13 and 2.09, respectively, and were significantly lower than corresponding scores on posterior regions, which were 2.82 and 2.84 for male and female subjects, respectively (P < 0.000). For the entire population, average gingival scores on anterior and posterior sites were 1.09 and 1.30, respectively, with significant differences between these sites (P < 0.000). For either gender, significantly lower gingival scores were observed on anterior surfaces (average score of 1.08–1.09) with posterior surfaces registering average scores of 1.31 and 1.29 among male and female subjects, respectively (P < 0.000). An additional analysis compared between gender the scores registered for either the anterior or the posterior regions (Table 4). Within each of these regions, there were no significant differences for dental plaque and gingivitis scores irrespective of gender (P > 0.05).
Table 4.
Clinical scores in anterior and posterior regions of the mouth (average ± SD).
| Clinical Index | Group | Number of subjects | Anterior | Posterior | t‐test (P value) | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Means | SD | ||||
| Dental plaque | All subjects | 378 | 2.12 | 0.64 | 2.83 | 0.57 | 0.0001* |
| Male | 220 | 2.13a | 0.63 | 2.82c | 0.55 | 0.0001* | |
| Female | 158 | 2.09a | 0.65 | 2.84c | 0.58 | 0.0001* | |
| Gingival scores | All subjects | 378 | 1.09 | 0.36 | 1.30 | 0.30 | 0.0001* |
| Male | 220 | 1.09b | 0.35 | 1.31d | 0.30 | 0.0001* | |
| Female | 158 | 1.08b | 0.38 | 1.29d | 0.30 | 0.0001* | |
SD, standard deviation.
Statistically significant differences.
No significant differences between gender for evaluated clinical score (P > 0.05).
Analyses of anterior and posterior teeth within each age group for dental plaque and gingivitis are shown in Tables 5 and 6, respectively. Irrespective of age, analyses indicate significantly higher dental plaque and gingivitis scores on posterior regions than the corresponding anterior regions (P < 0.000).
Table 5.
Plaque scores on anterior and posterior surfaces within age groups.
| Age groups | Number of subjects | Anterior surfaces (Avg±SD) | Posterior surfaces (Avg±SD) | t‐test (P value) |
|---|---|---|---|---|
| 18–27 | 163 | 2.16±0.65 | 2.82±0.55 | 0.0001* |
| 28–37 | 117 | 2.09±0.64 | 2.81±0.58 | 0.0001* |
| 38–47 | 66 | 2.07±0.63 | 2.85±0.55 | 0.0001* |
| 48+ | 32 | 2.10±0.57 | 2.88±0.63 | 0.0001* |
| Entire population | 378 | 2.12±0.64 | 2.83±0.57 | 0.0001* |
Avg, average; SD, standard deviation.
Statistically significant differences
Table 6.
Gingival scores on anterior and posterior surfaces within age groups.
| Age groups | Number of subjects | Anterior surfaces (Avg±SD) | Posterior surfaces (Avg±SD) | t‐test (P value) |
|---|---|---|---|---|
| 18–27 | 163 | 1.07±0.36 | 1.26±0.28 | 0.0001* |
| 28–37 | 117 | 1.08±0.36 | 1.30±0.31 | 0.0001* |
| 38–47 | 66 | 1.11±0.37 | 1.37±0.30 | 0.0001* |
| 48+ | 32 | 1.14±0.36 | 1.4±0.32 | 0.0001* |
| Entire population | 378 | 1.09±0.36 | 1.30±0.30 | 0.0001* |
Avg, average; SD, standard deviation.
Statistically significant differences.
A summary of the average scores with distinct regions of the dentition is shown in Figure 3. Lower plaque and gingival scores were observed in mid‐vestibular and anterior sites, while lingual, posterior, and molar sites registered higher levels. Analyses indicate that anterior teeth demonstrated significantly lower average scores for dental plaque and gingivitis than posterior and molar regions (P < 0.05).
Figure 3.
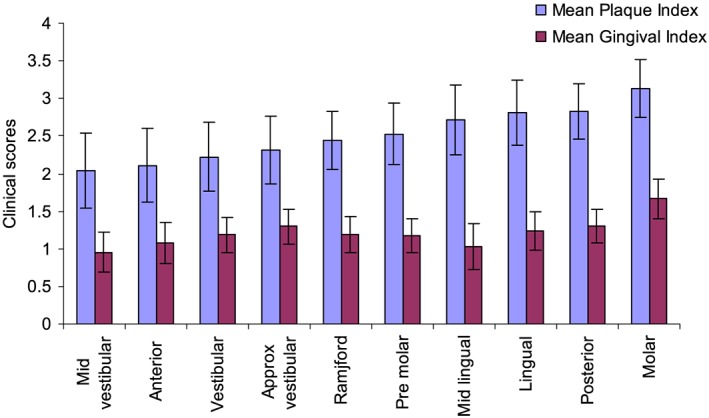
Dental plaque and gingival scores within distinct regions of the dentition (average ± standard deviation).
Using step‐wise logistic regression, education demonstrated a significant association with clinical outcomes for dental plaque and gingivitis. Lower education demonstrated a significant association with higher scores of dental plaque and gingival index (P < 0.05). Similarly, the level of parental education demonstrated a significant relationship with higher gingival scores in the posterior region (P < 0.05). Lower education levels, that is, those with less than middle school education, were associated with higher plaque and gingival index scores: plaque scores [odds ratios (OR); 95% confidence interval (95% CI); 1.23; 1.01–1.50 and gingival index scores OR 1.25; 1.02‐1.54] representing statistically significant relationships.
Discussions
The present study was aimed at gathering data regarding oral health, whole‐mouth evaluations for dental plaque and gingivitis, among a sample of adult subjects in India. While studies on oral health are available from selected groups in India (Ameer et al. 2012; Bharateesh et al. 2012; Bhagyajyothi and Pushpanjali 2011; Chandra Shekar and Reddy 2011; Gupta et al. 2012; Gopinath 2010; Jain et al. 2009; Jain et al. 2012; Mahesh Kumar et al. 2005; Oswal 2013; Poudyal et al. 2010; Singh and Tuli 2013) to our knowledge, outcomes evaluated in this study remain unreported from the general population.
It is important to mention several aspects of this study that were standardized. Whole‐mouth clinical evaluations were conducted with the Turesky modification of Quigley–Hein and the Löe–Silness Index representing well‐recognized approaches to evaluate oral hygiene (Dowsett et al. 2002; Williams et al. 2004; Zhang et al. 2010). Advantages of these indices include their wide application as the “gold standard” for a comprehensive assessment of the entire mouth as presented in many previous studies (Goyal et al. 2005; Poyato‐Ferrera et al. 2003; Williams et al. 2004) in contrast with partial mouth evaluations (Dowsett et al. 2002; Ericsson et al. 2012; Holtfreter et al. 2009; Owens et al. 2003). Clinical evaluations were conducted by a calibrated clinical examiner who demonstrated 99% reliability in the clinical indices.
Subjects evaluated in this study were residents of the local area and drawn from the general population. They were not selected from individuals seeking professional care (Al‐Otaibi et al. 2003b) or individuals belonging to selected groups or subjects of one gender (Bhagyajyothi and Pushpanjali 2011; Jain et al. 2009; Needleman et al., 2013). Unlike other studies, there were no preparatory or washout phases prior to oral examination (Furuichi et al. 1992; Sreenivasan et al. 2010) or any oral hygiene instructions prior to the examination. Subjects did not alter their diet or routine habits to reduce the influences of these parameters on dental plaque (Signoretto et al. 2006). Consistent with other studies, a majority of subjects reported brushing their teeth once daily (Gopinath 2010; Oswal 2013; Singh and Tuli 2013) and during interviews prior to oral examination indicated no prior participation in clinical studies or other investigations to further reduce the influences of these variables on evaluated parameters. While the population was homogeneous for ethnicity, differences in socioeconomic status and habitat were noted. Demographic features of study subjects indicated variations in cultural and dietary practices. A large number of subjects reported no recent dental visits and utilized dental services only in the case of pain or other emergencies, corroborating previous observations (Kumar et al. 2005; Poudyal et al. 2010) representing low utilization of dental services. While several factors such as proximity to dental clinics and affordability remain significant factors, it is also important to highlight the need for dental education. Together, these demographic observations are significant, because the observed results for dental plaque and gingivitis reveal their natural distribution within the mouth with few influencing parameters.
Salient outcomes from this study demonstrate that average whole‐mouth plaque and gingival scores for this population were 2.47 and 1.19, respectively. The average results for gingivitis had similarity to those reported in previous studies of 0.99 from Saudi Arabia (Al‐Otaibi et al. 2003a), 1.23 from Swiss recruits (Röthlisberger et al. 2007), 1.2 from The Gambia (Jordan et al. 2011), 1.05 from USA (Li et al. 2010), and 1.1 from China (Zhang et al. 2010) representing populations from different regions. Included in the present investigation were frequencies of plaque and gingival index scores representing parameters generally not reported. Whole‐mouth scores for dental plaque and gingivitis were ~2.4 and ~1.2, respectively, with no remarkable differences between the age groups or gender. These observations contrast other reports that indicate lower gingivitis scores in female subjects (Idrees et al. 2014) but have similarity with reports suggesting a relationship and education (Ababneh et al. 2012). A majority of sites registered plaque scores between 1 and 3 with a score of 3 being the most common irrespective of gender in the entire mouth. Sites with scores of 4 and 5 were less frequent with few sites free of plaque for the entire population. Gingivitis is prevalent widely in many populations and has been widely reported among adults (Angst et al. 2013; Rebelo et al. 2009), including special populations such as elite athletes (Needleman et al. 2013). Commonly observed gingivitis scores in the present study were 1 and 2. Sites without gingivitis were also observed. Similar to some previous reports, this study demonstrated no differences in gingivitis between gender (Jordan et al. 2011) but were different from several other studies that demonstrate lower scores among female subjects (Furuta et al. 2011; Mizutani et al. 2012). While reasons for these observations remain unclear, it is possible that differences in dental behaviors and attitudes represent likely reasons for these observations. In addition, many in the evaluated population reported atleast a middle school education and comprised community‐dwelling adults who were not seeking dental care during the study period. Analyses indicate that whole‐mouth gingival scores were 1.19 for women and 1.20 for men with no differences noted between genders. Gingival scores within age groups showed minor differences and were between 1.17 and 1.27 similar to previous observations (Lang et al. 2009) and different from others reporting increasing scores with age (Ababneh et al. 2012).
For the entire population, the sites that scored the least for both plaque and gingival indices were the mid‐vestibular regions. Progressive increases in these scores were observed in different areas of the mouth with anterior sites demonstrating one of the least scores. Consistently higher scores were observed in the posterior regions with molar teeth yielding the highest scores for either index. Additional analyses of anterior or posterior regions indicate no significant differences between either gender for either dental plaque or gingivitis. Dental plaque levels from anterior regions were 2.1 and contrasted with 2.8 observed at posterior sites. Similarly, gingivitis scores of anterior sites were 1.09 with posterior sites registering 1.30. Additional analyses indicate no age‐based differences for the indices recorded within the anterior or posterior sites.
Evaluations of the anterior and posterior regions for either gender indicate specific differences between these regions for dental plaque and gingivitis. Approximately 49% of the posterior surfaces demonstrated a plaque score of 3, while a score of 4 was reported in approximately 20% of the sites representing the most common observation. Scores of 1 and 2 were found on 11–17% of the sites and less than 2% of the sites registered a score of 5. Few sites were entirely plaque free in either the anterior or posterior regions representing differences from previous studies (Lang et al. 2009). Analysis of the anterior sites demonstrated a different pattern for dental plaque scores. Scores of 1–3 were most common and observed on 27–34% of sites with less than 6% of surfaces registering a score of 4. Less than 0.1% of the surfaces registered a score of 5 with these observations contrasting results from posterior surfaces. Differences between the regions have been reported previously in studies that included other procedural steps (Angst et al. 2013; Farina et al. 2013; Furuichi et al. 1992; Prasad et al. 2011; Ramberg et al. 1994; Ramberg et al. 1995; Sreenivasan et al. 2010). Results from this investigation also demonstrate differences in gingivitis scores within the mouth. No gingivitis was observed at 9% of anterior sites in comparison with 2.57% of posterior sites. A gingivitis score of 1 was observed at 72% of anterior sites, while 64% of posterior sites registered this score. Sites with a gingivitis score of 2 were observed at a higher frequency in posterior regions and observed in 32% of sites. Recent research reports that sites with persistent and long‐standing gingivitis progress to periodontitis along with sites with gingivitis scores of 2 or more as a clinically relevant risk factor for tooth loss (Lang et al. 2009). In this study, sites with a gingivitis core of 2 were more frequent than those reported previously from a Canadian survey (Health Canada 2010) and other areas (Australian Research Centre for Population Oral Health, The University of Adelaide, South Australia 2009). Furthermore, recent longitudinal research by Soder et al. 2015 indicates an association between years of gingival inflammation and a risk of stroke. Taken together, these observations report a latent or unreported inflammatory burden and a comprehensive survey of community‐dwelling adults from a mid‐size city. These results are relevant from a practical standpoint for prevention of future conditions. At the conclusion of the investigation, all subjects were provided an instructional program on oral health developed by investigators from the dental college. It may be useful to follow up these subjects in a future investigation to evaluate the effect of instructional programs on oral health.
Conclusion
Results from the present study are in congruence with those in previous reports including those that evaluated children (Krisdapong et al. 2012), other populations (Jones et al. 2011), including special populations (Needleman et al. 2013). Furthermore, these observations are noteworthy from the standpoint of health policies and oral health evaluations. Results reaffirm regional differences within the mouth for dental plaque and gingivitis reported previously (Angst et al. 2013; Claydon 2008; Cumming and Löe 1973; Furuichi et al. 1992; Nguyen et al. 2010; Prasad et al. 2011; Ramberg et al. 1994; Ramberg et al. 1995; Sreenivasan et al. 2010). These observations are significant from the stand point of preventative programs and highlight the need for whole‐mouth examinations. In addition, these differences highlight a need for effective oral hygiene in the posterior regions that register higher amounts of dental plaque and gingivitis irrespective of gender.
Conflict of Interest
P.K. Sreenivasan is an employee of Colgate‐Palmolive Company.
Acknowledgment
This work was funded by Colgate‐Palmolive Company.
Sreenivasan, P. K. , Prasad, K. V. V. , and Javali, S. B. (2016) Oral health practices and prevalence of dental plaque and gingivitis among Indian adults. Clinical and Experimental Dental Research, 2: 6–17. doi: 10.1002/cre2.15.
Funding Information
This work was funded by Colgate‐Palmolive Company. P. K. Sreenivasan is an employee of Colgate‐Palmolive Company.
References
- Ababneh, K.T. , Abu Hwaij, Z.M. , Khader, Y.S. , 2012. Prevalence and risk indicators of gingivitis and periodontitis in a multi‐centre study in North Jordan: a cross sectional study. BMC Oral Health 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst, P.D. , Piccinin, F.B. , Oppermann, R.V. , Marcantonio, R.A. , Gomes, S.C. , 2013. Response of molars and non‐molars to a strict supragingival control in periodontal patients. Braz. Oral Res. 27, 55–60. [DOI] [PubMed] [Google Scholar]
- Al‐Otaibi, M. , Al‐Harthy, M. , Söder, B. , Gustafsson, A. , Angmar‐Månsson, B. , 2003a. Comparative effect of chewing sticks and toothbrushing on plaque removal and gingival health. Oral Health Prev. Dent. 1, 301–307. [PubMed] [Google Scholar]
- Al‐Otaibi, M. , Zimmerman, M. , Angmar‐Månsson, B. , 2003b. Prevailing oral hygiene practices among urban Saudi Arabians in relation to age, gender and socio‐economic background. Acta Odontol. Scand. 61, 212–216. [DOI] [PubMed] [Google Scholar]
- Ameer, N. , Palaparthi, R. , Neerudu, M. , Palakuru, S.K. , Singam, H.R. , Durvasula, S. , 2012. Oral hygiene and periodontal status of teenagers with special needs in the district of Nalgonda, India. J Indian Soc Periodontol. 16, 421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Research Centre for Population Oral Health, The University of Adelaide, South Australia , 2009. Periodontal diseases in the Australian adult population. Aust. Dent. J. 54, 390–393. [DOI] [PubMed] [Google Scholar]
- Bhagyajyothi, C.S. , Pushpanjali, K. , 2011. Assessment and comparison of periodontal status among young smokers and nonsmokers of Bangalore, India–a cross sectional study. Community Dent. Health 28, 89–94. [PubMed] [Google Scholar]
- Bharateesh, J. , Ahmed, M. , Kokila, G. , 2012. Diabetes and oral health: a case–control study. Int J Prev Med. 3, 806–809. [PMC free article] [PubMed] [Google Scholar]
- Berezow, A.B. , Darveau, R.P. , 2011. Microbial shift and periodontitis. Periodontol. 2000 55, 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra Shekar, B.R. , Reddy, C. , 2011. Oral health status in relation to socioeconomic factors among the municipal employees of Mysore city. Indian J. Dent. Res. 22, 410–418. [DOI] [PubMed] [Google Scholar]
- Claydon, N.C. , 2008. Current concepts in toothbrushing and interdental cleaning. Periodontol. 2000 48, 10–22. [DOI] [PubMed] [Google Scholar]
- Cumming, B.R. , Löe, H. , 1973. Consistency of plaque distribution in individuals without special home care instruction. J. Periodontal Res. 8, 94–100. [DOI] [PubMed] [Google Scholar]
- Dye, B.A. , 2012. Global periodontal disease epidemiology. Periodontol. 2000 58, 10–25. [DOI] [PubMed] [Google Scholar]
- de Oliveira, C. , Watt, R. , Hamer, M. , 2010. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. BMJ 340, c2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett, S.A. , Eckert, G.J. , Kowolik, M.J. , 2002. The applicability of half‐mouth examination to periodontal disease assessment in untreated adult populations. J. Periodontol. 73, 975–981. [DOI] [PubMed] [Google Scholar]
- Ericsson, J.S. , Östberg, A.L. , Wennström, J.L. , Abrahamsson, K.H. , 2012. Oral health‐related perceptions, attitudes, and behavior in relation to oral hygiene conditions in an adolescent population. Eur. J. Oral Sci. 120, 335–341. [DOI] [PubMed] [Google Scholar]
- Farina, R. , Tomasi, C. , Trombelli, L. , 2013. The bleeding site: a multi‐level analysis of associated factors. J. Clin. Periodontol. 40, 735–742. [DOI] [PubMed] [Google Scholar]
- Furuichi, Y. , Lindhe, J. , Ramberg, P. , Volpe, A.R. , 1992. Patterns of de novo plaque formation in the human dentition. J. Clin. Periodontol. 19, 423–433. [DOI] [PubMed] [Google Scholar]
- Furuta, M. , Ekuni, D. , Irie, K. , Azuma, T. , Tomofuji, T. , Ogura, T. , Morita, M. , 2011. Sex differences in gingivitis relate to interaction of oral health behaviors in young people. J. Periodontol. 82, 558–565. [DOI] [PubMed] [Google Scholar]
- Gopinath, V. , 2010. Oral hygiene practices and habits among dental professionals in Chennai. Indian J. Dent. Res. 21, 195–200. [DOI] [PubMed] [Google Scholar]
- Goyal, C.R. , Qaqish, J.G. , Sharma, N.C. , Warren, P.R. , Cugini, M. , Thompson, M.C. , 2005. Plaque removal efficacy of a novel tooth wipe. J. Clin. Dent. 16, 44–46. [PubMed] [Google Scholar]
- Gupta, T. , Shah, N. , Mathur, V.P. , Dhawan, A. , 2012. Oral health status of a group of illicit drug users in Delhi, India. Community Dent. Health 29, 49–54. [PubMed] [Google Scholar]
- Health Canada , 2010. Summary report on the findings of the oral health component of the Canadian Health Measures Survey, 2007–2009. Health Canada, Ottawa (ON) [Accessed October 2, 2015]. www.fptdwg.ca/English/e‐documents.html. http://www.dal.ca/content/dam/dalhousie/pdf/dentistry/ICOH2010/SummaryCHMS.pdf or http://www.phac‐aspc.gc.ca/publicat/hpcdp‐pspmc/30‐4/preface2‐eng.php [Google Scholar]
- Holtfreter, B. , Schwahn, C. , Biffar, R. , Kocher, T. , 2009. Epidemiology of periodontal diseases in the Study of Health in Pomerania. J. Clin. Periodontol. 36, 114–123. [DOI] [PubMed] [Google Scholar]
- Idrees, M.M. , Azzeghaiby, S.N. , Hammad, M.M. , Kujan, O.B. , 2014. Prevalence and severity of plaque‐induced gingivitis in a Saudi adult population. Saudi Med. J. 35, 1373–1377. [PMC free article] [PubMed] [Google Scholar]
- Jain, N. , Mitra, D. , Ashok, K.P. , Dundappa, J. , Soni, S. , Ahmed, S. , 2012. Oral hygiene‐awareness and practice among patients attending OPD at Vyas Dental College and Hospital, Jodhpur. J Indian Soc Periodontol. 16, 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, M. , Mathur, A. , Kumar, S. , Duraiswamy, P. , Kulkarni, S. , 2009. Oral hygiene and periodontal status among Terapanthi Svetambar Jain monks in India. Braz. Oral Res. 23, 370–376. [DOI] [PubMed] [Google Scholar]
- Jones, D.J. , Munro, C.L. , Grap, M.J. , 2011. Natural history of dental plaque accumulation in mechanically ventilated adults: a descriptive correlational study. Intensive Crit. Care Nurs. 27, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, R.A. , Lucaciu, A. , Fotouhi, K. , Markovic, L. , Gaengler, P. , Zimmer, S. , 2011. Pilot pathfinder survey of oral hygiene and periodontal conditions in the rural population of The Gambia (West Africa). Int. J. Dent. Hyg. 9, 53–59. [DOI] [PubMed] [Google Scholar]
- Kornman, K.S. , 2008. Mapping the pathogenesis of periodontitis: a new look. J. Periodontol. 79(8 Suppl), 1560–1568. [DOI] [PubMed] [Google Scholar]
- Krisdapong, S. , Prasertsom, P. , Rattanarangsima, K. , Sheiham, A. , Tsakos, G. , 2012. The impacts of gingivitis and calculus on Thai children's quality of life. J. Clin. Periodontol. 39, 834–843. [DOI] [PubMed] [Google Scholar]
- Lang, N.P. , Schätzle, M.A. , Löe, H. , 2009. Gingivitis as a risk factor in periodontal disease. J. Clin. Periodontol. 36(Suppl 10), 3–8. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Lee, S. , Hujoel, P. , Su, M. , Zhang, W. , Kim, J. , Zhang, Y.P. , DeVizio, W. , 2010. Prevalence and severity of gingivitis in American adults. Am. J. Dent. 23, 9–13. [PubMed] [Google Scholar]
- Loe, H. , 1963. Silness J: periodontal disease in pregnancy. I. Prevalence and severity. Acta Odont Scand 21, 533–551. [DOI] [PubMed] [Google Scholar]
- Mahesh Kumar, P. , Joseph, T. , Varma, R.B. , Jayanthi, M. , 2005. Oral health status of 5 years and 12 years school going children in Chennai city–an epidemiological study. J. Indian Soc. Pedod. Prev. Dent. 23, 17–22. [DOI] [PubMed] [Google Scholar]
- Marsh, P.D. , 2012. Contemporary perspective on plaque control. Br. Dent. J. 212, 601–606. [DOI] [PubMed] [Google Scholar]
- Milgrom, P. , Reisine, S. , 2000. Oral health in the United States: the post‐fluoride generation. Annu. Rev. Public Health 21, 403–436. [DOI] [PubMed] [Google Scholar]
- Mizutani, S. , Ekuni, D. , Furuta, M. , Tomofuji, T. , Irie, K. , Azuma, T. , Kojima, A. , Nagase, J. , Iwasaki, Y. , Morita, M. , 2012. Effects of self‐efficacy on oral health behaviours and gingival health in university students aged 18‐ or 19‐years‐old. J. Clin. Periodontol. 39, 844–849. [DOI] [PubMed] [Google Scholar]
- Needleman, I. , Ashley, P. , Petrie, A. , Fortune, F. , Turner, W. , Jones, J. , Niggli, J. , Engebretsen, L. , Budgett, R. , Donos, N. , Clough, T. , Porter, S. , 2013. Oral health and impact on performance of athletes participating in the London 2012 Olympic Games: a cross‐sectional study. Br. J. Sports Med. 47, 1054–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T.C. , Witter, D.J. , Bronkhorst, E.M. , Truong, N.B. , Creugers, N.H. , 2010. Oral health status of adults in Southern Vietnam – a cross‐sectional epidemiological study. BMC Oral Health 13 10, 2 DOI: 10.1186/1472-6831-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal, K.C. , 2013. Oral hygiene practice amongst patients visiting visiting Terna Dental College. J Oral Health Comm Dent 7, 33–36. [Google Scholar]
- Owens, J.D. , Dowsett, S.A. , Eckert, G.J. , Zero, D.T. , Kowolik, M.J. , 2003. Partial‐mouth assessment of periodontal disease in an adult population of the United States. J. Periodontol. 74, 1206–1213. [DOI] [PubMed] [Google Scholar]
- Petersen, P.E. , 2009. Global policy for improvement of oral health in the 21st century–implications to oral health research of World Health Assembly 2007, World Health Organization. Community Dent. Oral Epidemiol. 37, 1–8. [DOI] [PubMed] [Google Scholar]
- Poudyal, S. , Rao, A. , Shenoy, R. , Priya, H. , 2010. Utilization of dental services in a field practice area in Mangalore, Karnataka. Indian J Community Med. 35, 424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyato‐Ferrera, M. , Segura‐Egea, J.J. , Bullón‐Fernández, P. , 2003. Comparison of modified Bass technique with normal toothbrushing practices for efficacy in supragingival plaque removal. Int. J. Dent. Hyg. 1, 110–114. [DOI] [PubMed] [Google Scholar]
- Prasad, K.V. , Sreenivasan, P.K. , Patil, S. , Chhabra, K.G. , Javali, S.B. , DeVizio, W. , 2011. Removal of dental plaque from different regions of the mouth after a 1‐minute episode of mechanical oral hygiene. Am. J. Dent. 24, 60–64. [PubMed] [Google Scholar]
- Ramberg, P. , Axelsson, P. , Lindhe, J. , 1995. Plaque formation at healthy and inflamed gingival sites in young individuals. J. Clin. Periodontol. 22, 85–88. [DOI] [PubMed] [Google Scholar]
- Ramberg, P. , Lindhe, J. , Dahlén, G. , Volpe, A.R. , 1994. The influence of gingival inflammation on de novo plaque formation. J. Clin. Periodontol. 21, 51–56. [DOI] [PubMed] [Google Scholar]
- Rebelo, M.A. , Lopes, M.C. , Vieira, J.M. , Parente, R.C. , 2009. Dental caries and gingivitis among 15 to 19 year‐old students in Manaus, AM, Brazil. Braz. Oral Res. 23, 248–254. [DOI] [PubMed] [Google Scholar]
- Röthlisberger, B. , Kuonen, P. , Salvi, G.E. , Gerber, J. , Pjetursson, B.E. , Attström, R. , Joss, A. , Lang, N.P. , 2007. Periodontal conditions in Swiss army recruits: a comparative study between the years 1985, 1996 and 2006. J. Clin. Periodontol. 34, 860–866. [DOI] [PubMed] [Google Scholar]
- Rüdiger, S.G. , Carlén, A. , Meurman, J.H. , Kari, K. , Olsson, J. , 2002. Dental biofilms at healthy and inflamed gingival margins. J. Clin. Periodontol. 29, 524–530. [DOI] [PubMed] [Google Scholar]
- Scannapieco, F.A. , 1998. Position paper of The American Academy of Periodontology: periodontal disease as a potential risk factor for systemic diseases. J. Periodontol. 69, 841–850. [PubMed] [Google Scholar]
- Schiavo, J.H. , 2011. Oral health literacy in the dental office: the unrecognized patient risk factor. J. Dent. Hyg. Fall 85, 248–255. [PubMed] [Google Scholar]
- Singh, M.S. , Tuli, A.K. , 2013. A comparative evaluation of oral hygiene practices, oral health status, and behavior between graduate and post‐graduate dentists of North India: an epidemiological survey. J Int Soc Prev Community Dent. 3, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretto, C. , Burlacchini, G. , Bianchi, F. , Cavalleri, G. , Canepari, P. , 2006. Differences in microbiological composition of saliva and dental plaque in subjects with different drinking habits. New Microbiol. 29, 293–302. [PubMed] [Google Scholar]
- Socransky, S.S. , Haffajee, A.D. , 2005. Periodontal microbial ecology. Periodontol. 2000 38, 135–187. [DOI] [PubMed] [Google Scholar]
- Soder, B. , Meurman, J.H. , Söder, P.Ö. , 2015. Gingival inflammation associates with stroke – a role for oral health personnel in prevention: a database study. PLoS One 10(9e0137142). DOI: 10.1371/journal.pone.0137142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivasan, P.K. , DeVizio, W. , Prasad, K.V. , Patil, S. , Chhabra, K.G. , Rajesh, G. , Javali, S.B. , Kulkarni, R.D. , 2010. Regional differences within the dentition for plaque, gingivitis, and anaerobic bacteria. J. Clin. Dent. 21, 13–19. [PubMed] [Google Scholar]
- Turesky, S. , Gilmore, N.D. , Glickman, I. , 1970. Reduced plaque formation by the chloromethyl analogue of vitamin C. J. Periodontol. 41, 41–43. [DOI] [PubMed] [Google Scholar]
- Williams, K. , Ferrante, A. , Dockter, K. , Haun, J. , Biesbrock, A.R. , Bartizek, R.D. , 2004. One‐ and 3‐minute plaque removal by a battery‐powered versus a manual toothbrush. J. Periodontol. 75, 1107–1113. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Xuan, D. , Fan, W. , Zhang, X. , Dibart, S. , De Vizio, W. , Panagakos, F. , Zhang, Y.P. , 2010. Severity and prevalence of plaque‐induced gingivitis in the Chinese population. Compend. Contin. Educ. Dent. 31, 624–629. [PubMed] [Google Scholar]


