Abstract
A primary caretaker is a potential reservoir of bacteria for an infant child and can be evaluated during a child's caries risk assessment. The aim of this study was to investigate an indirect method for assessing Streptococcus mutans and Streptococcus sobrinus (MS) and lactobacillus (LB) levels in a caretaker's saliva. Thirty‐eight primary caretakers participated in the study to determine whether a 2‐step method to assess the intracellular adenosine triphosphate (ATP) levels in saliva (saliva i‐ATP method) predicted higher MS and LB levels. This method was tested against a 1‐step swab‐based total ATP testing of dental plaque (plaque t‐ATP method). Receiver operating characteristic (ROC) curves were used to examine the relationship between specificity and sensitivity of the two diagnostic tests. Although the area under the ROC curves of both the saliva i‐APT (0.823) and the plaque t‐ATP (0.774) methods were shown to be statistically different (p < .05) than the null hypothesis test of a random coin flip, the diagnostic predictability of the ATP tests to assess high levels of MS and LB remained low. The optimal cutoff, which was defined by the Youden index, for the saliva i‐ATP method produced a sensitivity/specificity of 60.7/100.0 for MS and 78.6/88.9 for LB. Applying these results to populations of low or high bacterial level prevalence produced undesirable positive and negative predictive values for future potential patients. A pair‐wise comparison of both area under the ROC curve values of the saliva i‐ATP and plaque t‐ATP did not find a statistically significant difference in using one test over the other (MS, p = .629; LB, p = .737). The findings of this study can educate dental clinicians that diagnostic tests, such as the 2‐step saliva i‐ATP method, can be found to be statistically significant but not ideal for patient use in terms of diagnostic predictability.
Keywords: bacterial, caries, dentistry, diagnostic, teeth
1. INTRODUCTION
Accurate and time‐efficient methods to assess a primary caretaker's level of cariogenic bacteria may aid clinicians in promptly assessing a young child's risk for developing future dental caries. The rationale for a caretaker assessment is based on numerous studies demonstrating that a primary caretaker's oral bacterial composition strongly influences the bacterial composition of young children they care for (Berkowitz, Jordan, & White, 1975; Chaffee, Gansky, Weintraub, Featherstone, & Ramos‐Gomez, 2014; Kishi et al., 2009; Lindquist & Emilson, 2004; Teanpaisan, Chaethong, Piwat, & Thitasomakul, 2012). The vertical transmission model has been well established and clearly demonstrates that a caretaker can transfer (infect) a young child with acid producing and highly acid‐tolerant bacteria such as Streptococcus mutans and Streptococcus sobrinus (combined as MS), and species of lactobacilli (LB; Chaffee et al., 2014; Lapirattanakul et al., 2008).
Vertical transmission of cariogenic bacteria has been shown to occur over a long time. For this reason, it is important to examine caretaker bacterial levels, because an in‐office examination appointment may predate the time of transmission and colonization, especially for children under 24 months of age (Caufield, Cutter, & Dasanayake, 1993; Teanpaisan et al., 2012). To further confound the diagnostic problem for clinicians, the transmission and colonization can occur from multiple human and exogenous sources (Alves et al., 2009; Caufield, Li, Dasanayake, & Saxena, 2007; Emanuelsson, Li, & Bratthall, 1998; Liu, Zou, Shang, & Zhou, 2007). Although the feasibility of assessing all potential sources is low, there is compelling evidence that a primary caretaker is a highly probable potential reservoir of bacteria for a child and can be evaluated in determining a child's caries risk assessment (Chaffee et al., 2014; Kishi et al., 2009; Lapirattanakul et al., 2008; Li, Caufield, Dasanayake, Wiener, & Vermund, 2005).
Unfortunately, assessing a primary caretaker's MS and LB levels through culture‐based clinical tests is burdensome for clinicians. Clinical culture‐based methods, such as the dip‐slide Caries Risk Test (CRT®; Ivoclar Vivadent, Schaan, Liechtenstein), which use selective agar media, are valuable in determining if cariogenic bacterial levels have reached to a high value threshold (Tanabe et al., 2006; Van Houte, 1993). The main limitations of cultural‐based methods is that the tests require incubation times of several days to assess the level of bacteria (Seppa, Pollanen, & Hausen, 1988). These culture‐based tests are time and space consuming within a dental office, and there is little evidence that these tests are routinely used in practice, outside clinic research and public health initiatives.
The aim of this study was to investigate an indirect method for assessing MS and LB levels in caretaker's saliva. Because saliva is the likely principal source of vertical transmission versus plaque, this study focused on salivary analysis. The approach taken in this study was to have caretakers fast for 1 hr prior to analyzing the intracellular levels of adenosine triphosphate (ATP) within their saliva (saliva i‐ATP method). Although ATP levels within plaque have been investigated, this single step method cannot differentiate between extracellular and intracellular ATP (Fazilat et al., 2010; Hallett & O'Rourke, 2013). In these past studies, ATP derived from dead host and microbial cells impacted the ATP readings.
To achieve analyzing the intracellular ATP levels of intact cells in the saliva, this study examined a salivary assessment assay using a two‐step measurement assay. In this two‐step approach, one reading measured ATP levels after cell lysis in the saliva. This measured the ATP levels of both intracellular and extracellular spaces. Using a second sample of the same collected saliva, a second reading measured the ATP levels of the extracellular space by measuring ATP levels without prior cell lysis. The difference between the two measurement readings was the ATP derived from the intracellular spaces. Although this saliva i‐ATP test is not intended to be a test for a specific bacterial species, if this test is used alongside a pre‐appointment fasting instruction, this test may identify samples that have bacteria with extended metabolic activity (Alcantara, Blasco, Zuniga, & Monedero, 2014; Busuioc, Mackiewicz, Buttaro, & Piggot, 2009), which has the potential to be associated with elevated MS and LB levels.
The possible benefits of using saliva i‐ATP levels include the ability to have a rapid chairside assay with a handheld luminometer. The quantification of ATP levels is based on a chemiluminescence reaction that occurs between a luciferase enzyme, a luciferin substrate and ATP (Harber & Asscher, 1977; Lundin & Thore, 1975). Light emitted during the reaction can be quantitatively measured by a luminometer and correlated with the quantity of ATP extracted from the sample (Schifman et al., 1984). The main objective of the cross‐sectional study was to examine both the statistical relationship and the diagnostic predictability of the intracellular saliva i‐ATP test with traditional culture based methods of assessing MS and LB.
2. METHODS
2.1. Study participants
This study was a diagnostic case‐controlled investigation involving clinical and laboratory procedures. This investigation was approved by the University of Minnesota IRB and recruited potential subjects from both academic and local private practice clinics. Research subjects were primary caregivers with infants between the ages of 12–24 months of age. Potential caregiver subjects were excluded from the study if they possessed significant past or current medical problem history, especially conditions that may affect oral health or oral flora (i.e., diabetes, heart conditions, and other conditions that require antibiotic prophylaxis), enamel hypoplasia or significant fluorosis that would confound clinical examination, professional cleaning or fluoride varnish within 30 days, and medication use that may affect oral flora and salivary flow (i.e., subjects that have had antibiotic therapy within 3 months of analysis, medications associated with xerostomia, medications with added sucrose, and asthma patients that require daily corticosteroids). Additionally, caregiver subjects with removable prosthetic or orthodontic appliances were excluded. Caregiver subjects who fit the inclusion and exclusion criteria for this study were asked to refrain from any oral hygiene practice on the day of the assessment. In addition, they were asked to refrain from the consumption of food or beverage other than water 1 hr prior to participation.
The study's purpose, risks, and benefits were explained to the caregivers, and a written informed consent form authorizing the caregivers' enrollment in the study was obtained. Medical and dental history questionnaires were completed. Participants were informed that participation in the study was not a substitute for a routine dental exam. All assessments took place during a single visit.
2.2. Clinical salivary bacterial testing
CRT (Ivoclar Vivadent), a dip‐slide bacteria test, was used in accordance with manufacturer's recommendations. This test was considered the current clinical standard for assessing the overall bacterial load of MS and LB in saliva of caregivers (Davey & Rogers, 1984). Five milliliters of stimulated saliva (induced by a paraffin wax square) were collected from the caregiver. Each side of the CRT slide was coated with approximately 1.5 ml of saliva. After addition of the sodium bicarbonate tablets to the CRT, the slides were incubated for 72 hr at 37 °C. At 72 hr, a blinded reader assessed the relative bacterial levels using an ordinal scale (0–4 with 4 being the highest level) developed by the manufacturer (Emilson & Krasse, 1986).
2.3. Saliva i‐ATP bioluminescence
A chairside assessment of the intracellular ATP levels were performed using the acquired saliva sample. Using 0.5 ml of saliva of the caregiver subject, a 50X dilution was performed by combining the saliva with 24.75‐ml phosphate‐buffered saline (PBS). The saliva/PBS dilution was vortexed for 10 s. Using a water ATP testing system (Hygiena, Camarillo, CA), approximately 100 ml of saliva/PBS was sampled and subjected to a cell lysis detergent/ATP test vial by the release of a bulb within the vial (AquaSnap ATP Total, Hygiena). A second 100‐ml saliva/PBS sample was sampled and subjected to a nondetergent/ATP test vial by the release of a bulb within the vial (AquaSnap ATP Free, Hygiena). These test vials were stored in 4 °C prior to their use.
The saliva/PBS dilution was allowed to incubate for 25 s before the vial was measured by a handheld luminometer (distributed by Oral Biotech as the CarieScreen but made by Hygiena as the SystemSURE for industrial/medical applications). The luminometer measured the luminescence of the vials and produced a relative light unit (RLU) value of each sample reading. After the saliva/PBS samples that examined the Total ATP and Free ATP were measured, the intracellular ATP levels were determined by subtracting the Free ATP level from the Total ATP level:
Because ATP reaction kinetics are time sensitive, use of a digital timer assured that the RLU was measured at the approximate same time for each patient sample. Immediately after the patient sample was mixed with the luciferase enzyme reagent solution, the digital timer countdown was started with 25 s of mix time. At 0 s, the samples were read by the handheld luminometer.
Throughout the course of the 11‐month study, a monthly calibration was performed on the handheld luminometer. A blank vial was measured as the negative calibration measurement. For the positive calibration measurement, a C14 radioactive source was used to generate a consistent luminescence.
2.4. Plaque total ATP (plaque t‐ATP) and plaque control record
Results of the saliva i‐ATP measurements were compared to plaque t‐ATP values that were obtained using a commercially available surface swab system (CariScreen Testing Swabs, Oral Biotech/Hygiena). The clinical sampling followed the manufacturer's recommended directions of swabbing the mid‐lingual surfaces of the six lower mandibular anterior teeth. The same digital timer countdown, as previously described, was used for these samples.
2.5. Receiver operator curves
Data analysis was performed using MedCalc (version 13.1.2, Belgium). Receiver operating characteristic (ROC) curves that examine the relationship between specificity and sensitivity of the diagnostic tests were plotted. Statistical analysis of the ROC curves was performed in MedCalc. For each diagnostic test, DeLong's method of ROC analysis was used to calculate a p value and measure the probability that the observed area under the ROC curve (AUC) was different than a random or “coin flip” diagnostic test (null hypothesis area = 0.5; DeLong, DeLong, & Clarke‐Pearson, 1988). A summary of ROC analysis is described elsewhere (Christenson, 2007). The optimal sensitivity and specificity combination was determined by the maximum Youden index (Youden, 1950). The statistical methods were based on a previously reported nonparametric approach that compared the areas of two or more correlated ROC curves (DeLong et al., 1988). These methods were also used to calculate sample size estimation for a future study that would compare both the saliva i‐ATP and plaque t‐ATP testing methods based on the results of this initial study and the desired Type I and Type II error rates.
3. RESULTS
Recruitment for this study aimed at enrolling primary caretakers of infant children. A convenience sample of primary caretakers of infant children yielded 38 subjects during an 11‐month period. Thirty‐four subjects identified themselves as female, and four subjects identified themselves as male. The mean age of the research subject was 32 years of age (range of 19–58 years). Thirty‐seven samples were included in the analysis due to one sample not being diagnostic due to the kinetics of the chemical reaction assay. Our calibration measurements for the negative (1.03 ± 1.98 SD) and positive (70 ± 2.45 SD) controls confirmed the consistency of the luminometer readings during the 11‐month study.
From the saliva i‐ATP and plaque t‐ATP diagnostic methods, the relative value units (RLU) of luminescence from each subject's samples were plotted against the corresponding MS and LB bacterial levels that were determined through the CRT clinical‐based culture method (Figure 1). Individual data point measurements show the RLU distribution that corresponds to the bacterial ordinal scale of MS and LB. The data were plotted with MS and LB levels grouped into 0–2, 3, and 4 categorical scale. This was done because readings of 0 (no colonies), 1, and 2 in the visual ordinal scale indicate, based on the manufacturer's instructions for use, a low bacterial level consistent with a colony count of less than 105 CFU/ml. The higher visual ordinal scale of 3 and 4 is indicative of greater than 105 CFU/ml. Using 0–2 and 3–4 to indicate lower and higher levels of bacteria, sensitivity and specificity calculations were performed at different RLU threshold values for both the saliva i‐ATP and plaque t‐ATP diagnostic methods. ROC curves were created by plotting the sensitivity to the false positive rate (100‐specificity) at each threshold value (Figure 2). The diagonal line across each ROC curve graph represents a random 50%/50% test (a null hypothesis area of 0.5). Based on the number of samples in our study and the “AUC,” both the saliva i‐APT and the plaque t‐ATP methods were statistically different than a coin flip test in differentiating lower versus higher levels MS and LB (Table 1).
Figure 1.
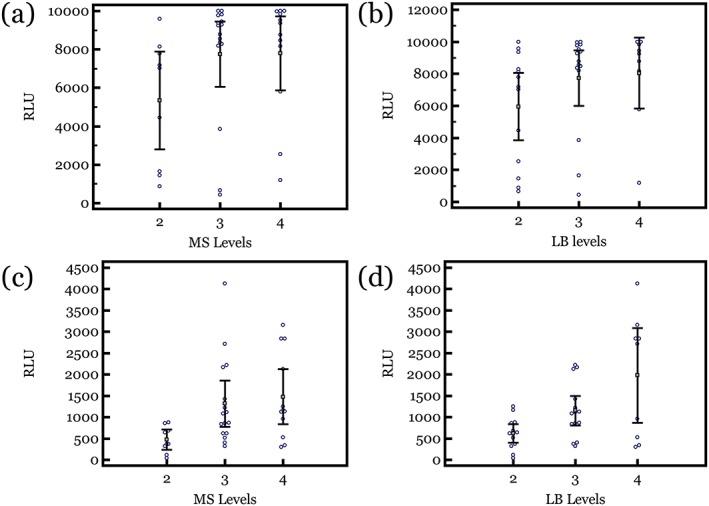
Relative value units (RLUs) of luminescence from each subject's samples were plotted against the corresponding Streptococcus mutans and Streptococcus sobrinus (MS) and lactobacillus (LB) bacterial ordinal scale levels that were determined through the CRT clinical‐based culture method. (a) Plaque t‐ATP RLU versus MS levels. (b) Plaque t‐ATP RLU versus LB levels. (c) Saliva i‐ATP RLU versus MS levels. (d) Saliva i‐ATP RLU versus LB levels. 95% confidence intervals of the means are shown. ATP = adenosine triphosphate
Figure 2.
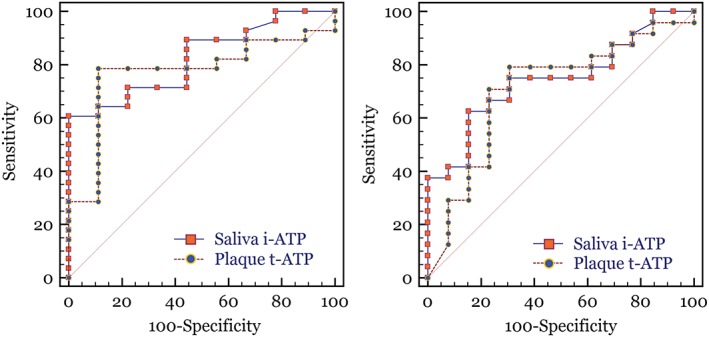
Receiver operator characteristic (ROC) curves of plaque t‐ATP and saliva i‐ATP methods versus MS (left) and LB (right). ROC curves are created by plotting the sensitivity to the false positive rate (100‐specificity) at each relative light unit threshold value. Using the 0–2 to indicate low levels and 3–4 indicating higher levels of bacteria. The diagonal line across the ROC curve graphs represents a random 50%/50% test (a null hypothesis area of 0.5). LB = lactobacillus; MS = Streptococcus mutans and Streptococcus sobrinus
Table 1.
The area under the curve (AUC) of the receiver operator curve analysis (CRT value ≥3) using the full range of cutoff relative light unit (RLU) values of the salivary intercellular ATP (saliva i‐ATP) and plaque total ATP (plaque t‐ATP)
| AUC | p value | Sen | Sp | RLU | |
|---|---|---|---|---|---|
| MS | |||||
| Saliva i‐ATP | 0.823 | <.0001 | 60.7 | 100.0 | >875 |
| Plaque t‐ATP | 0.774 | .0018 | 78.6 | 88.9 | >8,143 |
| LB | |||||
| Saliva i‐ATP | 0.745 | .0028 | 62.5 | 84.6 | >875 |
| Plaque t‐ATP | 0.707 | .029 | 79.2 | 69.2 | >8,143 |
Note. The optimal sensitivity (Sen) and specificity (Sp) combination determined by the maximum Youden index. ATP = adenosine triphosphate.
The optimal sensitivity and specificity values determined by the Youden index were applied to theoretical populations of 1,000 patients with different prevalence of disease in order to estimate the positive and negative predictive values (PPV/NPV) for each test in assessing the MS and LB levels (Table 2).
Table 2.
Applying sensitivity (Sen) and specificity (Sp) to theoretical populations of 1,000 patients
| 10% | 25% | 50% | 75% | |||||
|---|---|---|---|---|---|---|---|---|
| PPV | NPV | PPV | NPV | PPV | NPV | PPV | NPV | |
| MS | ||||||||
| Saliva i‐ATP | [94.0, 100] | [94.3, 97.0] | [97.6, 100] | [86.1, 90.5] | [98.8, 100] | [68.3, 75.1] | [99.2, 100] | [41.7, 50.2] |
| Plaque t‐ATP | [36.6, 51.7] | [96.1, 98.4] | [64.5, 75.5] | [90.4, 94.4] | [84.2, 90.5] | [77.1, 83.8] | [93.7, 97.0] | [52.9, 63.1] |
| LB | ||||||||
| Saliva i‐ATP | [24.8, 40.0] | [93.6, 96.7] | [51.4, 63.5] | [84.5, 89.5] | [75.9, 84.1] | [65.5, 72.9] | [89.8, 94.6] | [38.5, 47.4] |
| Plaque t‐ATP | [18.0, 26.9] | [95.1, 98.0] | [41.4, 51.0] | [88.2, 93.1] | [68.0, 75.7] | [72.7, 80.7] | [85.9, 90.8] | [47.0, 58.1] |
Note. Positive and negative predictive values (PPV and NPV) are dependent on the prevalence of a condition. Potential values (10–75%) of the prevalence of a high CRT threshold value (≥3) within the theoretical population are used. The 95% confidence intervals of the PPV and NPV mean are calculated from Youden index's Sen and Sp at the potential prevalence values. Confidence intervals displayed in bold place the mean values as equal or less predictive than 50%. ATP = adenosine triphosphate.
A pair‐wise comparison of both AUC values of the saliva i‐ATP and plaque t‐ATP did not find a statistically significant difference in using one test over the other (MS, p = .629; LB, p = .737). The AUC values and rank correlation between the two tests in this study were used to calculate the sample size for a future study (Table 3) that attempted to measure if the saliva i‐ATP test was superior (AUC of 0.823 vs. 0.774) to the plaque t‐ATP.
Table 3.
Sample size estimation for a future study that examines salivary intercellular ATP and plaque total ATP assessment
| AUC saliva i‐ATP | AUC plaque t‐ATP | Type I error | Type II error | Expected ratio of samples (pos/neg) | Number of negative samples required | Number of positive sample required | Total number of samples required | |
|---|---|---|---|---|---|---|---|---|
| MS | 0.823 | 0.774 | 0.05 | 0.2 | 2 | 394 | 788 | 1,182 |
Note. This estimation is based on comparing the area under an ROC curve (AUC) with a null hypothesis (0.5) value. The sample size takes into account the required significance level, power (0.80) of the test, and the AUC found in this study. ATP = adenosine triphosphate; ROC = receiver operating characteristic.
The ROC curve was repeated using the threshold of 0–3 versus 4 (Figure 3). A visual assessment of these curves shows how the AUC of these ROC curves is considerably lower than at the previous threshold (Figure 3 vs. Figure 2). In the case of Figure 3, the AUCs are not statistically different than the null hypothesis area. This indicates that neither test could differentiate the highest MS nor LB values versus lower levels better than a random coin flip.
Figure 3.
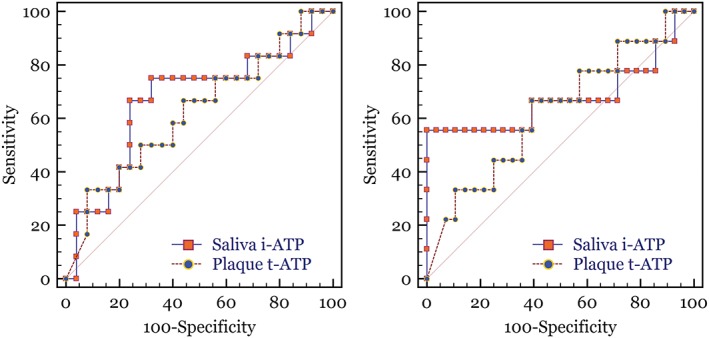
Receiver operator characteristic (ROC) curves of plaque t‐ATP and saliva i‐ATP methods versus MS (left) and LB (right). ROC curves are created by plotting the sensitivity to the false positive rate (100‐specificity) at each relative light unit threshold value. Using the 0–3 to indicate low levels and 4 indicating highest levels of bacteria. The diagonal line across the ROC curve graphs represents a random 50%/50% test (a null hypothesis area of 0.5). LB = lactobacillus; MS = Streptococcus mutans and Streptococcus sobrinus
4. DISCUSSION
While it is not a surprise that saliva i‐ATP levels do not strongly predict specific bacteria level, the study was initiated for several important reasons. First, a previous study showed strong correlation between plaque t‐ATP levels and MS levels. This correlation increased when plaque and saliva were examined together (Fazilat et al., 2010). Confirming and following up on the methodology seemed clinically important given the low commercial availability and phase out of most clinical culture‐based bacterial tests. Secondly, there is substantial evidence that in single bacterial culture experiments, the CFU per milliliter can be correlated to RLU counts (Omidbakhsh, Ahmadpour, & Kenny, 2014). Although a specific bacteria within a mixed community of bacteria cannot be identified by a nonspecific ATP test, the clinical practice of having patients fast (1 hr) prior to the appointment may be a means of testing natural dietary habits prior to the fasting period that produce salivary bacteria with high ATP and with notable metabolic reserve such as intracellular polysaccharides and polyphosphates (Alcantara et al., 2014; Busuioc et al., 2009). MS and LB levels were expected to be potentially correlated to a salivary composition of bacteria that had the potential to have higher metabolic reserve when a research subject had dietary practices with higher carbohydrates. One intention of the 1‐hr fasting guideline was to reduce false positive events where a low risk subject who recently consumed any type of caloric intake had a high ATP‐based reading. We considered adopting a more rigorous fasting guideline to control for exact food intake for several hours prior to the visit, but this approach was deemed impractical for routine clinical practice. We originally speculated that the natural dietary habits of the tested individuals would be indirectly measured by the ATP based tests. For example, an individual with high MS and LB levels would naturally (without instruction) possess longer term deleterious “high carbohydrate” dietary habits prior to the test. In hindsight, it was an error to omit a food frequency questionnaire in the study design. Future studies are needed to determine the longer term effect of dietary intake on the ATP‐based outcomes, especially related to false negative assessments.
This study demonstrates that both the saliva i‐ATP and plaque t‐ATP methods differentiated higher versus lower levels of MS and LB levels using the ROC curve analysis. The fact that the ROC analysis could predict this threshold should be viewed with caution because the ROC analysis at the 0–3 versus 4 threshold did not yield statistically significant AUC values. Even if it can be concluded that the two methods have a statistically significant predictive ability, this does not mean these methods should be advocated for clinical use. Further analysis of the data is important for concluding the diagnostic viability of the test methods.
For example, in this study, one subject out of 38 had a negative value for the salivary i‐ATP assessment. This meant that the subject's ATP Free had a higher value that the ATP Total value. This subject was treated as an outlier and not included in the analysis because the substantial negative value of the test (−239 RLU) would have warranted a retest in clinical practice. For diagnostic predictability, clinicians would not have accepted this negative value as being an accurate value, because in principle, the ATP Total value should always be larger than the ATP Free value. Although the sample was not included in the analysis, it is a relevant observation because it indicates that approximately 3% of subjects may obtain a “nondiagnostic” value (a negative value) and require a retest. Given the complex chemistry in the ATP test, it is not surprising that there is a minority of samples that do not yield a diagnostic value.
Because dental caries is a disease that affects certain populations with greater prevalence than other populations, it is important to view the diagnostic values of sensitivity and specificity determined in this study in the context of disease prevalence within a community. The positive predictive values (PPV) and negative predictive values (NPV) of the saliva i‐ATP and plaque t‐ATP assessment allow clinicians to understand how the sensitivity and specificity of these tests affect the diagnosis of the population that they potentially serve. For example, the PPV is the true positive divided by the total number of positives on a test. A false positive rate (1‐specificity) affects populations that have lower disease prevalence substantially greater than those with higher disease prevalence. The plaque t‐ATP method has a considerably lower specificity than the saliva i‐ATP method. When this test is used in a population that has lower MS and LB, the false positive rate produces results that are discouraging. For example, if a clinician was serving a low caries prevalence population with expected lower MS and LB levels and used the plaque t‐ATP test, only 36–51% of the patients that had a RLU > 8,143 would actually have high levels of MS. For LB, only 18–27% of patients would be true positives. For this same theoretical population where only 10% has high MS and LB levels, the saliva i‐APT method would be more appropriate for predicting MS levels (94–100%) but not for predicting LB levels (24–40%).
The low sensitivity values for both saliva i‐ATP and plaque t‐ATP methods affect the NPVs when applied to populations that are expected to have a higher prevalence of elevated MS and LB levels. It is through the evaluation of PPV/NPV that clinicians can really gain insight on what the ramifications are for the sensitivity and specificity values in this study. In the case of plaque t‐ATP and saliva i‐ATP, these tests do not hold up to predicting bacterial levels at reasonable levels across the entire spectrum of disease (bacterial level) prevalence. The additional information of the two‐step intracellular ATP method did not improve the overall diagnostics defined by the ROC pair‐wise curve analysis, and the sheer sample size needed (based on results of this study; Table 3) for a future study indicates that considerable resources would have to be used to differentiate the two potential diagnostic tests.
Although the real‐time and chairside assessment method of both saliva i‐ATP and plaque t‐ATP tests are not ideal substitutes for the culture‐based strip test methods to assess MS and LB, these tests, especially the saliva i‐ATP method, are able to identify individuals with higher MS levels with acceptable PPV/NPV if used in clinics serving low and moderate disease prevalence populations. The streamlined saliva i‐ATP method test should be considered a significant step in the right direction to advance chairside bacterial assessment of primary caretakers.
This study chose to compare the ATP‐based methods to the clinical gold standard of a dip‐slide culture test rather than the laboratory gold standard of serial plating. This was done in order to assess if these methods could substitute for the clinical test, but future studies may want to increase the sensitivity and the dynamic range of the bacterial assessment with a laboratory gold standard. In order to assess MS and LB in dip‐slide tests, species selective agars are used with the CRT, and the commercially availability of culture based methods will remain limited. Although MS and LB have been recognized for its cariogenic potential, there are other cariogenic bacteria involved in the caries disease process as well. Several other genera of bacteria (Scardovia, Veillonella, Actinomyces, Rothia, and Bifidobacterium) have been significantly associated with caries (Henne et al., 2016; Jagathrakshakan, Sethumadhava, Mehta, & Ramanathan, 2015). There is also a loss or lowering of levels of commensal bacteria within high‐risk individuals. Future diagnostic tests will likely need to better address the genotype and phenotypical changes seen in the diverse bacteria of the oral cavity.
CONFLICT OF INTEREST
The authors themselves declare that they have no competing financial interests.
ACKNOWLEDGEMENTS
This study was partially funded by the University of Minnesota. The Division of Pediatric Dentistry at the University of Minnesota, School of Dentistry, received an unrestricted donation of the handheld luminometer and some materials from Oral Biotech. The authors have not consulted with either company regarding the results of this study. We would like to thank Jill Stoltenberg for her initial co‐mentoring in this study.
Bill C, Danielson JA, Jones RS. Salivary intercellular adenosine triphosphate testing in primary caretakers: An examination of statistical significance versus diagnostic predictability. Clin Exp Dent Res. 2017;3:244–250. https://doi.org/10.1002/cre2.95
REFERENCES
- Alcantara, C. , Blasco, A. , Zuniga, M. , & Monedero, V. (2014. Mar). Accumulation of polyphosphate in Lactobacillus spp. and its involvement in stress resistance. Applied and Environmental Microbiology United States, 80(5), 1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, A. , Nogueira, R. , Stipp, R. , Pampolini, F. , Moraes, A. , Goncalves, R. , … Mattos‐Graner, R. J. (2009. Apr). Prospective study of potential sources of Streptococcus mutans transmission in nursery school children. Journal of Medical Microbiology England, 58(4), 476–481. https://doi.org/10.1099/jmm.0.005777‐0 [DOI] [PubMed] [Google Scholar]
- Berkowitz, R. J. , Jordan, H. V. , & White, G. (1975. Mar). The early establishment of Streptococcus mutans in the mouths of infants. Archives of Oral Biology England, 20(3), 171–174. [DOI] [PubMed] [Google Scholar]
- Busuioc, M. , Mackiewicz, K. , Buttaro, B. A. , & Piggot, P. J. (2009. Dec). Role of intracellular polysaccharide in persistence of Streptococcus mutans . Journal of Bacteriology United States, 191(23), 7315–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufield, P. W. , Cutter, G. R. , & Dasanayake, A. P. (1993. Jan). Initial acquisition of mutans streptococci by infants: Evidence for a discrete window of infectivity. Journal of Dental Research United States, 72(1), 37–45. [DOI] [PubMed] [Google Scholar]
- Caufield, P. W. , Li, Y. , Dasanayake, A. , & Saxena, D. (2007). Diversity of lactobacilli in the oral cavities of young women with dental caries. Caries Research Switzerland, 41(1), 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee, B. W. , Gansky, S. A. , Weintraub, J. A. , Featherstone, J. D. B. , & Ramos‐Gomez, F. J. (2014. Mar). Maternal oral bacterial levels predict early childhood caries development. Journal of Dental Research United States, 93(3), 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson, R. H. , & Committee on Evidence Based Laboratory Medicine of the International Federation for Clinical Chemistry Laboratory Medicine . (2007). Evidence‐based laboratory medicine ‐ a guide for critical evaluation of in vitro laboratory testing. Annals of Clinical Biochemistry 2007/03/17 ed., 44(Pt 2), 111–130. [DOI] [PubMed] [Google Scholar]
- Davey, A. L. , & Rogers, A. H. (1984). Multiple types of the bacterium Streptococcus mutans in the human mouth and their intra‐family transmission. Archives of Oral Biology England, 29(6), 453–460. [DOI] [PubMed] [Google Scholar]
- DeLong, E. R. , DeLong, D. M. , & Clarke‐Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics, 44, 837–845. [PubMed] [Google Scholar]
- Emanuelsson, I. R. , Li, Y. , & Bratthall, D. (1998. Oct). Genotyping shows different strains of mutans streptococci between father and child and within parental pairs in Swedish families. Oral Microbiology and Immunology Denmark, 13(5), 271–277. [DOI] [PubMed] [Google Scholar]
- Emilson, C. G. , & Krasse, B. (1986). Comparison between a dip‐slide test and plate count for determination of Streptococcus mutans infection. Scandinavian Journal of Dental Research [Internet]. 1986/12/01 ed., 94(6), 500–506 Available from: http%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fentrez%2Fquery.fcgi%3Fcmd%3DRetrieve%26amp%3Bdb%3DPubMed%26amp%3Bdopt%3DCitation%26amp%3Blist_uids%3D3544178. [DOI] [PubMed] [Google Scholar]
- Fazilat, S. , Sauerwein, R. , McLeod, J. , Finlayson, T. , Adam, E. , Engle, J. , et al. (2010). Application of adenosine triphosphate‐driven bioluminescence for quantification of plaque bacteria and assessment of oral hygiene in children. Pediatric Dentistry [Internet]. 2010/06/19 ed., 32(3), 195–204 Available from: http%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fentrez%2Fquery.fcgi%3Fcmd%3DRetrieve%26amp%3Bdb%3DPubMed%26amp%3Bdopt%3DCitation%26amp%3Blist_uids%3D20557702. [PubMed] [Google Scholar]
- Hallett, K. B. , & O'Rourke, P. K. (2013). Baseline dental plaque activity, mutans streptococci culture, and future caries experience in children. Pediatric Dentistry United States, 35(7), 523–528. [PubMed] [Google Scholar]
- Harber, M. J. , & Asscher, A. W. (1977. Jan). A new method for antibiotic assay based on measurement of bacterial adenosine triphosphate using the firefly bioluminescence system. The Journal of Antimicrobial Chemotherapy England, 3(1), 35–41. [DOI] [PubMed] [Google Scholar]
- Henne, K. , Gunesch, A.‐P. , Walther, C. , Meyer‐Lueckel, H. , Conrads, G. , & Esteves‐Oliveira, M. (2016). Analysis of bacterial activity in sound and cariogenic biofilm: A pilot in vivo study. Caries Research Switzerland, 50(5), 480–488. [DOI] [PubMed] [Google Scholar]
- Jagathrakshakan, S. N. , Sethumadhava, R. J. , Mehta, D. T. , & Ramanathan, A. (2015). 16S rRNA gene‐based metagenomic analysis identifies a novel bacterial co‐prevalence pattern in dental caries. European Journal of Dentistry. India, 9(1), 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi, M. , Abe, A. , Kishi, K. , Ohara‐Nemoto, Y. , Kimura, S. , & Yonemitsu, M. (2009. Jun). Relationship of quantitative salivary levels of Streptococcus mutans and S. sobrinus in mothers to caries status and colonization of mutans streptococci in plaque in their 2.5‐year‐old children. Community Dentistry and Oral Epidemiology Denmark, 37(3), 241–249. [DOI] [PubMed] [Google Scholar]
- Lapirattanakul, J. , Nakano, K. , Nomura, R. , Hamada, S. , Nakagawa, I. , & Ooshima, T. (2008). Demonstration of mother‐to‐child transmission of Streptococcus mutans using multilocus sequence typing. Caries Research Switzerland, 42(6), 466–474. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Caufield, P. W. , Dasanayake, A. P. , Wiener, H. W. , & Vermund, S. H. (2005. Sep). Mode of delivery and other maternal factors influence the acquisition of Streptococcus mutans in infants. Journal of Dental Research United States, 84(9), 806–811. [DOI] [PubMed] [Google Scholar]
- Lindquist, B. , & Emilson, C. G. (2004). Colonization of Streptococcus mutans and Streptococcus sobrinus genotypes and caries development in children to mothers harboring both species. Caries Research Switzerland, 38(2), 95–103. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Zou, J. , Shang, R. , & Zhou, X. D. (2007. Sep). Genotypic diversity of Streptococcus mutans in 3‐ to 4‐year‐old Chinese nursery children suggests horizontal transmission. Archives of Oral Biology England, 52(9), 876–881. [DOI] [PubMed] [Google Scholar]
- Lundin, A. , & Thore, A. (1975. May). Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Analytical Biochemistry United States, 66(1), 47–63. [DOI] [PubMed] [Google Scholar]
- Omidbakhsh, N. , Ahmadpour, F. , & Kenny, N. (2014. Jun 18). How reliable are ATP bioluminescence meters in assessing decontamination of environmental surfaces in healthcare settings? PLoS One [Internet]. Public Library of Science, 9(6), e99951 Available from: https://doi.org/10.1371/journal.pone.0099951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifman, R. B. , Wieden, M. , Brooker, J. , Chery, M. , Delduca, M. , Norgard, K. , et al. (1984. Oct). Bacteriuria screening by direct bioluminescence assay of ATP. Journal of Clinical MicrobiologyUnited States, 20(4), 644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppa, L. , Pollanen, L. , & Hausen, H. (1988). Streptococcus mutans counts obtained by a dip‐slide method in relation to caries frequency, sucrose intake and flow rate of saliva. Caries Research Switzerland, 22(4), 226–229. [DOI] [PubMed] [Google Scholar]
- Tanabe, Y. , Park, J. H. , Tinanoff, N. , Turng, B. F. , Lilli, H. , & Minah, G. E. (2006). Comparison of chairside microbiological screening systems and conventional selective media in children with and without visible dental caries. Pediatric Dentistry United States, 28(4), 363–368. [PubMed] [Google Scholar]
- Teanpaisan, R. , Chaethong, W. , Piwat, S. , & Thitasomakul, S. (2012). Vertical transmission of mutans streptococci and lactobacillus in Thai families. Pediatric Dentistry United States, 34(2), e24–e29. [PubMed] [Google Scholar]
- Van Houte, J. (1993). Microbiological predictors of caries risk. Advances in Dental Research [Internet]. 1993/08/01 ed., 7(2), 87–96 Available from: http%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fentrez%2Fquery.fcgi%3Fcmd%3DRetrieve%26amp%3Bdb%3DPubMed%26amp%3Bdopt%3DCitation%26amp%3Blist_uids%3D8260016. [DOI] [PubMed] [Google Scholar]
- Youden, W. J. (1950). An index for rating diagnostic tests. Cancer, 3, 32–35. [DOI] [PubMed] [Google Scholar]


