Key Points
Question
Can a single course of topical fluorouracil, 5%, prevent keratinocyte carcinoma?
Findings
In this randomized clinical trial of 932 veterans at high risk for keratinocyte carcinoma, a 2- to 4-week course of topical fluorouracil, 5%, applied twice daily to the face and ears reduced the risk for 1 year of squamous cell carcinoma (SCC) requiring surgery at those sites. No effect was seen on basal cell carcinoma (BCC) in year 1 or on SCC or BCC over 4 years.
Meaning
Effective chemoprevention of cutaneous SCC for a year is achievable with a single 2- to 4-week course of topical fluorouracil, suggesting a possible role for annual use in groups at very high risk.
This randomized, double-blind, placebo-controlled trial examined the use of topical fluorouracil, 5%, to prevent keratinocyte carcinoma.
Abstract
Importance
Keratinocyte carcinoma (ie, cutaneous basal and squamous cell carcinoma) is the most common cancer in the United States.
Objective
To determine whether topical fluorouracil could prevent surgically treated keratinocyte carcinoma.
Design, Setting, and Participants
The Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial was a randomized, double-blind, placebo-controlled trial of topical fluorouracil for chemoprevention of keratinocyte carcinoma. Participants were recruited from May 2009 to September 2011 from 12 Veterans Affairs medical centers and followed until June 30, 2013. Participants were veterans (n = 932) with a history of at least 2 keratinocyte carcinomas in the past 5 years; almost all were white males and the median age was 70 years.
Interventions
Application of fluorouracil, 5%, (n = 468) or vehicle control cream (n = 464) to the face and ears twice daily for 2 to 4 weeks upon randomization.
Main Outcomes and Measures
Surgically treated keratinocyte, basal cell, and squamous cell carcinoma risk on the face and ears in the first year after enrollment; and time to first surgically treated keratinocyte, basal cell, and squamous cell carcinoma. The a priori hypothesis was that fluorouracil would be effective in preventing these cancers.
Results
Of 932 participants (916 men [98%]; 926 white [99%]; median age, 70 years), 299 developed a basal cell carcinoma end point (95 in year 1) and 108 developed a squamous cell carcinoma end point (25 in year 1) over 4 years (median follow-up, 2.8 years). Over the entire study, there was no difference between treatment groups in time to first keratinocyte, basal cell, or squamous cell carcinoma. During the first year, however, 5 participants (1%) in the fluorouracil group developed a squamous cell carcinoma vs 20 (4%) in the control group, a 75% (95% CI, 35%-91%) risk reduction (P = .002). The 11% reduction in basal cell carcinoma risk during year 1 (45 [10%] in the fluorouracil group vs 50 [11%] in the control group) was not statistically significant (95% CI, 39% reduction to 31% increase), nor was there a significant effect on keratinocyte carcinoma risk. However, a reduction in keratinocyte carcinomas treated with Mohs surgery was observed.
Conclusions and Relevance
A conventional course of fluorouracil to the face and ears substantially reduces surgery for squamous cell carcinoma for 1 year without significantly affecting the corresponding risk for basal cell carcinoma.
Trial Registration
clinicaltrials.gov Identifier: NCT00847912
Introduction
Keratinocyte carcinoma (KC), often ambiguously termed nonmelanoma skin cancer (ie, cutaneous basal cell carcinoma [BCC] and squamous cell carcinoma [SCC]), accounts for about three-quarters of all cancers in the United States. Over 5 million KCs were diagnosed in 2012, and incidence is rising.
The standard treatment for KCs is excision, frequently (when on the face or ears) by resource-intensive and expensive Mohs surgery that is associated with tissue conservation and a high cure rate compared with conventional surgery. In the United States, KC treatment cost $4.8 billion annually from 2007 to 2011. In the Veterans Health Administration alone, more than 75 000 veterans were diagnosed with KC in 2012, with costs estimated at almost $200 million.
Additionally, considerable morbidity is associated with KC treatment, as well as destruction and disfigurement that KCs can cause before and after they are treated. Oral isotretinoin and acitretin have been shown to prevent KCs (and are sometimes used in groups at very high risk despite systemic adverse effects), but they are ineffective when treatment ends. Similarly, nicotinamide may decrease risk during treatment. Consistent daily sunscreen use reduces SCC incidence, but the effect after stopping use is unknown. No treatment has been demonstrated to prevent KCs after treatment ends. An effective secondary prevention strategy could dramatically change the way patients at high risk are managed and has the potential to substantially reduce the morbidity and costs associated with surgery (removal and repair) and subsequent care.
Topical fluorouracil reduces the multiplicity of precursors of SCC known as actinic keratoses and can cure superficial BCC (and, in off-label use, SCC in situ). However, no studies have demonstrated efficacy of fluorouracil in preventing BCC and SCC or in preventing lesions requiring surgical treatment. Indeed, it was suggested that fluorouracil might have the opposite effect.
We chose to study veterans because this population includes many individuals who are male, elderly, and have had substantial sun exposure (both during military service and subsequently) and are therefore likely to have a history of multiple KCs and be at high risk for new KCs. We hypothesized that topical fluorouracil would prevent surgically treated skin cancers in this population. To test this hypothesis, we launched the Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) Trial, a randomized trial of topical fluorouracil, 5%, vs vehicle control cream applied to the face and ears.
Methods
Ethics
The VA Central Institutional Review Board approved this trial; all participants gave written informed consent; and Declaration of Helsinki Principles were followed. The trial protocol is available in Supplement 1.
Study Design and Interventions
The VAKCC Trial (Cooperative Studies Program 562) was a double-blind, vehicle-controlled trial for chemoprevention of KCs in veterans at high risk for these cancers. Detailed methods, including sample size calculations, are described elsewhere and in eAppendix 2 in Supplement 2. Participants with a history of at least 2 KCs in the 5 years prior to enrollment, at least 1 of which was on the face or ears, were recruited from 12 Veterans Affairs (VA) medical centers. Veterans who had received solid organ transplants or who had genetic disorders associated with a particularly high skin cancer risk were excluded. Participants were randomized to apply topical fluorouracil, 5%, or vehicle control cream twice daily to the face and ears for 4 weeks—a total of 56 doses. If this dose was intolerable, the medication was stopped and triamcinolone cream, 0.1%, was applied twice daily to the face and ears for 5 days. If this occurred prior to completion of 28 doses, study medication was resumed 3 weeks after stopping and applied once daily to complete 56 doses. If still intolerable, study medication was discontinued. We considered 28 doses the minimum treatment dose for this study. All participants completed treatment within 11 weeks.
After the active treatment phase, participants were evaluated face-to-face semiannually starting with the first 6-month visit until June 30, 2013. In-person visits, telephone interviews, medical record reviews, and full-body skin examinations were conducted to collect information on demographic characteristics, medical history, medication use, adverse events, and study end points.
The primary outcome for this study was patient centered, involving the first occurrence of a primary KC on the face or ears that was surgically removed (ie, primary BCC, primary invasive SCC, and primary SCC in situ). Other key a priori outcomes were risk of BCC, SCC, and KC in the first year after enrollment. Histopathologic specimens were read by local pathologists and later underwent central pathology review by a board-certified dermatopathologist, blind to study group assignment, for final diagnosis. High interrater reliability was documented with 2 other central board-certified dermatopathologists.
Participants received SPF 30 sunscreen and were educated about skin cancer, sun safety, and sunscreen use. Participants were shown photographs of moderate to severe reactions to topical fluorouracil, interviewed about medication reactions, and photographed to document reactions.
Blinding
Participants were blinded to treatment assignment and blinding success was evaluated at 6 months and at the final study visit. Investigators who performed assessments were also blinded to treatment assignment. During the active treatment period, adverse effects were monitored by a designated unblinded investigator at each study site. Blinded investigators assessed participants before they initiated study medication and again starting at 6 months after enrollment, months after the expected resolution of fluorouracil adverse effects.
Statistical Analysis
For each of the key end points (KC, BCC, and SCC) we analyzed time to event (diagnosis) for the entire 4-year trial and risk of event in the first year after randomization as the key prespecified outcomes. We also considered 2 alternative methods of analyzing our data: first, by ignoring the results of our quality control measure; and second, by imputing values for potential end points that were not included in the results otherwise presented because the slides were lost or unreadable.
Results
Study Population
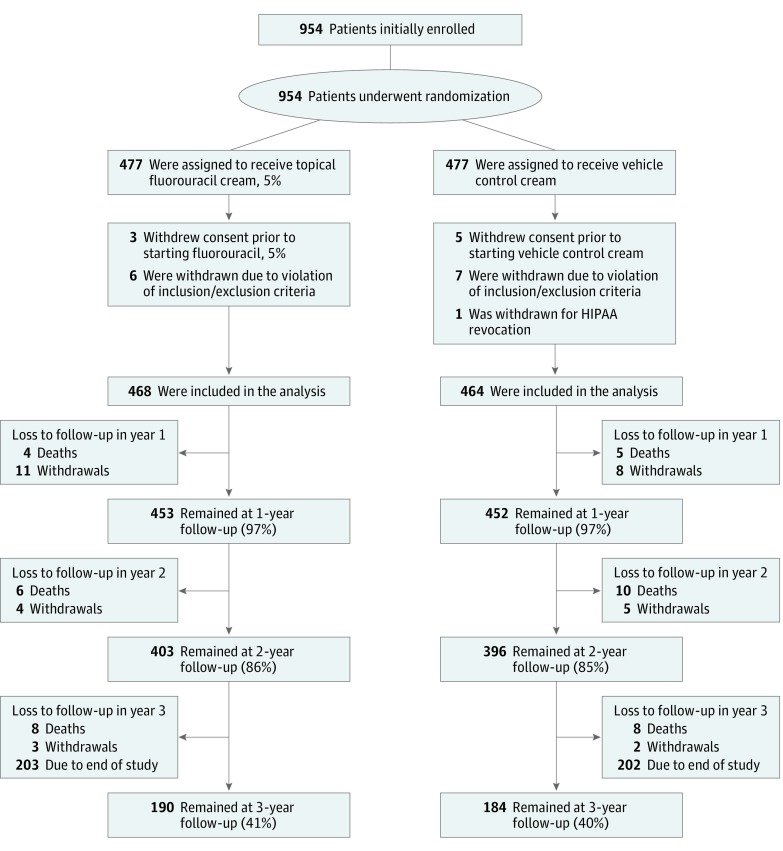
Enrollment occurred from May 2009 through September 2011, and participants were followed through June 2013, the planned study end date. Of 954 enrolled and randomized, 22 were excluded due to Health Insurance Portability and Accountability Act revocations, consent withdrawals, or eligibility violations, so 932 participants were included in the primary analysis: 468 in the fluorouracil group and 464 in the control group (Figure 1).
Figure 1. Randomization, Stratification, and Follow-up of Study Participants.
Participants were recruited from May 2009 to September 2011 and included veterans with a history of at least 2 keratinocyte carcinomas in the past 5 years. Of 954 patients enrolled and randomized, 932 were included in the primary analysis: 468 in the fluorouracil group and 464 in the control group. HIPAA indicates Health Insurance Portability and Accountability Act.
There were 45 deaths during the study: 18 in the fluorouracil group and 23 in the control group (P = .16). An additional 31 participants withdrew before the study ended: 18 in the fluorouracil group and 15 in the control group. Altogether, 36 participants (7.7%) in the fluorouracil group and 38 participants (8.2%) in the control group died or withdrew prior to study completion. Almost all participants were male (916 of 932 [98%]) and the median age was 70 years (mean [SD], 71.1 [9.3] years). In the 5 years prior to enrollment, 870 participants (93%) had a history of at least 1 BCC, 409 (44%) at least 3 BCCs, 361 (39%) at least 1 invasive SCC, and 170 (18%) at least 1 SCC in situ (Table 1). The control and treatment groups were similar, with no statistically significant differences in demographic characteristics, military service periods, sunburn, Fitzpatrick skin type, sun protection use during the study, and medical histories. The median follow-up duration was 2.8 years (mean, 2.7 years) for both groups, and 97% of each group was followed for at least 1 year after randomization.
Table 1. Demographic Information.
| Characteristic | No. (%) | |
|---|---|---|
| Fluorouracil Group (n = 468) |
Control Group (n = 464) |
|
| Demographics | ||
| Age, mean (SD) [range] | 70.7 (9.2) [43-91] | 71.5 (9.4) [43-91] |
| Men | 457 (98) | 459 (99) |
| Racea | ||
| White | 465 (99) | 461 (99) |
| American Indian | 11 (2) | 4 (<1) |
| Native Hawaii/Pacific Islander | 0 | 1 (<1) |
| Other | 1 (<1) | 1 (<1) |
| Self-reported Fitzpatrick skin type | ||
| Type 1 | 123 (26) | 107 (23) |
| Type 2 | 54 (12) | 57 (12) |
| Type 3 | 185 (40) | 200 (43) |
| Type 4 | 103 (22) | 94 (20) |
| Education | ||
| Up to high school | 149 (32) | 131 (28) |
| Some college/technical/trade school | 251 (54) | 262 (57) |
| Graduate school | 68 (15) | 71 (15) |
| Marital status | ||
| Married | 269 (58) | 266 (57) |
| Divorced/separated | 94 (20) | 93 (20) |
| Widowed/single | 105 (22) | 105 (23) |
| Residency | ||
| Northeast | 60 (13) | 54 (12) |
| Midwest | 85 (18) | 96 (21) |
| South | 195 (42) | 188 (41) |
| West | 127 (27) | 126 (27) |
| Enrolled in Medicare | 306 (65) | 315 (68) |
| Weight, mean (SD) [range] | 195.6 (37.1) [81-356] | 201.2 (40.5) [106-416] |
| Height, mean (SD) [range] | 69.6 (3) [57-82] | 69.7 (3) [59-83] |
| History of fluorouracil use | 92 (20) | 74 (16) |
| Median No. of baseline AKs on face/ears | 6 | 6 |
| Keratinocyte Carcinoma History in 5 Years Prior to Enrollment | ||
| Prior BCCs, No. | ||
| 0 | 22 (5) | 40 (9) |
| 1 | 69 (15) | 63 (14) |
| 2 | 171 (37) | 158 (34) |
| 3 | 89 (19) | 80 (17) |
| ≥4 | 117 (25) | 123 (26) |
| Prior invasive SCCs, No. | ||
| 0 | 291 (62) | 280 (60) |
| 1 | 100 (21) | 106 (23) |
| ≥2 | 77 (16) | 78 (17) |
| Prior SCCs in situ, No. | ||
| 0 | 384 (82) | 378 (81) |
| 1 | 65 (14) | 61 (13) |
| ≥2 | 19 (4) | 25 (5) |
| Incidence of sunburn and sun protection during study | ||
| Hat or sunscreen use on face or ears | 374 (80) | 373 (80) |
| Sunburn | 94 (20) | 86 (19) |
Abbreviations: AK, actinic keratosis; BCC, basal cell carcinoma; KC, keratinocyte carcinoma; SCC, squamous cell carcinoma.
Some percentages may not equal 100% owing to participant’s refusal to answer the question, or identification as multiracial.
Outcomes
Study end points were surgically treated BCC or SCC on the face or ears. During the study, 359 participants developed at least 1 KC end point (ie, a KC on the face or ears that was treated surgically). Of these, 299 developed at least 1 BCC and 108 developed at least 1 SCC (48 participants developed both). Within the first year, 111 developed at least 1 KC; 95 developed at least 1 BCC; and 25 developed at least 1 SCC.
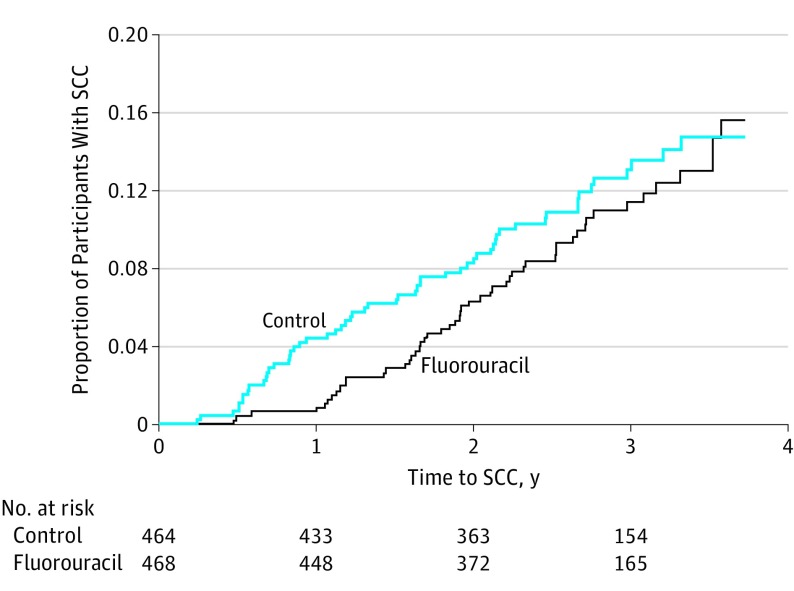
There was no difference between the fluorouracil and control groups in time to first KC, time to first BCC, or time to first SCC for the overall 4-year study period (Table 2). In year 1 there was a 75% reduction in SCC risk in the fluorouracil group compared with the control group (risk ratio [RR], 0.25; 95% CI, 0.09-0.65; P = .002) (Table 2 and Figure 2). The year 1 BCC risk was reduced by 11%, which was not statistically significant (RR, 0.89; 95% CI, 0.61-1.31; P = .56). There was no difference in year 1 KC risk (Table 2).
Table 2. Risk of Keratinocyte Carcinoma in Year 1 and Time to Outcome During Overall Study Perioda.
| Type of Lesion | Fluorouracil Group: Participants With ≥1 Lesion, No. (%) | Control Group: Participants With ≥1 Lesion, No. (%) | Hazard Ratio (95% CI) | Risk Ratio (95% CI) | P Value of Risk Ratio |
|---|---|---|---|---|---|
| Year 1 | |||||
| KC | 48 of 468 (10) | 63 of 464 (14) | 0.74 (0.51-1.08) |
0.76 (0.53-1.08) |
.12 |
| BCC | 45 of 468 (10) | 50 of 464 (11) | 0.89 (0.59-1.33) |
0.89 (0.61-1.31) |
.56 |
| SCC | 5 of 468 (1) | 20 of 464 (4) | 0.24 (0.09-0.65) |
0.25 (0.09-0.65) |
.002 |
| Overall Study Period | |||||
| KC | 182 of 468 (39) | 177 of 464 (38) | 1.01 (0.83-1.25) |
1.02 (0.87-1.20) |
.82 |
| BCC | 151 of 468 (32) | 148 of 464 (32) | 1.02 (0.81-1.28) |
1.01 (0.84-1.22) |
.85 |
| SCC | 52 of 468 (11) | 56 of 464 (12) | 0.90 (0.62-1.31) |
0.92 (0.65-1.31) |
.65 |
Abbreviations: BCC, basal cell carcinoma; KC, keratinocyte carcinoma (ie, BCC or SCC); SCC, squamous cell carcinoma.
The median follow-up was 2.8 years (mean, 2.7 years) for both groups. In the fluorouracil group, 317 KCs (246 BCCs and 71 SCCs) were treated surgically during the overall study; 62 of these (57 BCCs and 5 SCCs) in year 1. In the control group, 330 KCs (251 BCCs and 79 SCCs) were treated surgically during the overall study; 91 of these (68 BCCs and 23 SCCs) in year 1.
Figure 2. Proportion of Participants With SCC by Treatment Group.
Participants who completed a 2- to 4-week course of topical fluorouracil, 5%, applied twice daily to the face and ears reduced the risk of SCC requiring surgery at those sites by 75% for 1 year. No effect was seen over 4 years. SCC indicates squamous cell carcinoma.
During the study, 95 of 299 participants (32%) who developed BCC, and 25 of 108 participants (23%) who developed SCC, did so during the first year. We examined the BCC and SCC RRs between groups within each year of follow-up. The only difference in BCC risk was in year 2 of the study, in which the risk was higher in the fluorouracil group (87 vs 59 tumors; P = .01), but there were no differences in years 1, 3, or 4. The only difference in SCC risk was the year 1 difference noted above.
Sensitivity analyses were conducted to (1) include nonsurgically treated KCs in analyses (18 participants [38 lesions, 9 in year 1] had a nonsurgically treated KC); (2) include only participants who had completed the minimum dose of study medication per protocol analyses (28 applications); and (3) ignore the results of our quality control review of all outcomes. Results were not substantially changed in any of these analyses.
We examined risk of BCC and SCC treated with Mohs surgery, the most resource-intensive and expensive of the common treatments for KC, and the most common treatment used in the study participants. The decision to use Mohs surgery was determined by local treating dermatologists. In year 1, 36 BCCs were treated by Mohs among 27 participants in the control group and 17 BCCs among 14 participants in the fluorouracil group (RR, 0.51; 95% CI, 0.27-0.97; P = .045). The number of SCCs treated with Mohs surgery (5 in the control group and 3 in the fluorouracil group) was too small to further analyze. For KCs in year 1, the RR was 0.51 (95% CI, 0.28-0.92; P = .02) after accounting for clustering within individuals. There were no differences between groups for the overall study period (Table 3).
Table 3. Mohs Surgery Performed on Keratinocyte Carcinomas During the Triala.
| Time Period | Fluorouracil | Control | Risk Ratio (95% CI) | P Value |
|---|---|---|---|---|
| KCs Treated With Mohs Surgery | ||||
| Year 1 | 20 | 41 | 0.72 (0.48-1.10) | .11 |
| Overall study period | 153 | 149 | 1.07 (0.91-1.26) | .43 |
| Participants With ≥1 KC Treated With Mohs Surgery | ||||
| Year 1 | 16 | 31 | 0.51 (0.28-0.92) | .02 |
| Overall study period | 101 | 92 | 1.09 (0.85-1.40) | .51 |
| BCCs Treated With Mohs Surgery | ||||
| Year 1 | 17 | 36 | 0.56 (0.36-0.89) | .01 |
| Overall study period | 120 | 118 | 1.04 (0.86-1.25) | .69 |
| Participants With ≥1 BCC Treated With Mohs Surgery | ||||
| Year 1 | 14 | 27 | 0.51 (0.27-0.97) | .045 |
| Overall study period | 87 | 79 | 1.09 (0.84-1.44) | .53 |
Abbreviations: BCC, basal cell carcinoma; KC, keratinocyte carcinoma; SCC, squamous cell carcinoma.
In the fluorouracil group, 149 KCs (120 BCCs and 29 SCCs) were treated with Mohs surgery during the overall study; 20 of these (17 BCCs and 3 SCCs) in year 1. In the control group, 149 KCs (118 BCCs and 31 SCCs) were treated with Mohs surgery during the overall study; 41 of these (36 BCCs and 5 SCCs) in year 1. The number of SCCs treated with Mohs in year 1 was too small to further analyze. In the overall study, there was no difference in risk of SCC treated with Mohs in the fluorouracil vs the control group (data not shown).
Tolerability and Toxic Effects of Fluorouracil
Adverse effects of fluorouracil represent a barrier to its general use for KC risk reduction. To assess tolerability and toxic effects, we evaluated participants’ completion of the study medication course, occurrence of adverse effects, and the resulting impact on participants’ willingness to use this intervention in the future.
Cumulative dosing of study medication was calculated in 2 ways that yielded similar results. Overall, 85% of participants (397 of 468) in the fluorouracil group and 96% of participants (445 of 464) in the control group completed at least 28 doses of the study medication; and 31% (144 of 468) of the fluorouracil group and 81% (375 of 464) in the control group completed 56 doses.
We analyzed 3 measures of study medication adverse effects: (1) a photograph-based toxic effect score at 2 weeks; (2) self-reported symptoms at 2 weeks; and (3) a retrospective self-report of adverse effect severity at the 6-month visit. The first 2 measures were highly correlated (Pearson r = 0.8), and the photograph-based score also correlated well with the 6-month recollection of adverse effect severity (Pearson r = 0.6).
Two weeks into the intervention, 92% of participants (368 of 402) in the fluorouracil group reported erythema (82% [329 of 402] had erythema on photographs at that visit), and 61% (245 of 402) demonstrated mild-to-moderate crusting on photographs. Six months after starting the study medication, 21% (92 of 439) in the fluorouracil group retrospectively rated treatment adverse effects as “severe,” 40% (176 of 439) rated them as “moderate,” 25% (111 of 439) rated them as “mild,” and 14% (60 of 439) said they had “none.” In contrast, 76% (329 of 432) in the control group retrospectively reported no adverse effects at the 6-month visit (eTable in Supplement 2). Detailed analysis of treatment adverse effects is described elsewhere.
At 6 months and at the end of the study we asked participants about perceived study group assignment and willingness to repeat treatment if shown to be effective in reducing future KC risk.
In the fluorouracil group, 91% of participants (397 of 435) correctly guessed their study group at 6 months, and 87% (335 of 386) correctly guessed at the final visit. In the control group, however, only 81% (344 of 424) and 75% (296 of 393) guessed their study group correctly at the 6-month and final visits, respectively.
In the fluorouracil group, 87% of participants were willing to repeat treatment if shown to be effective in reducing future skin cancers, when surveyed at both 6 months (378 of 436) and the final visit (337 of 386). In the control group, 94% (406 of 433) were willing to repeat treatment when surveyed at 6 months, and 95% (376 of 397) when surveyed at the final visit.
Discussion
We have reported a randomized trial demonstrating that, in a population at high risk, a single 2- to 4-week course of topical fluorouracil, 5%, twice daily to the face and ears is effective in reducing the risk of SCC requiring surgery by 75% (95% CI, 35%-91%) for the first year after use. However, fluorouracil did not have an effect on BCC in the first year and did not result in a reduction of SCC or BCC risk over the 4-year duration of the trial. We found no “rebound” effect of increased SCCs in the fluorouracil group in year 2 or subsequently. Despite the absence of a statistically significant reduction in BCC risk in year 1 after the intervention (an 11% reduction in BCC vs a 75% reduction in SCC), we did note a large reduction in participants who had Mohs surgery treatments of BCCs and KCs (both 49%) in the fluorouracil group compared with the control group.
While we documented the expected adverse effects of fluorouracil in these participants, the vast majority (87% [715 of 822]) indicated they would be willing to do this treatment again if it were found to be effective, indicating that this is a viable chemoprevention option.
The protective effect of fluorouracil on KCs in year 1 was similar to the presumed protective effect on which the overall study was powered (0.767 vs 0.704, respectively). However, because most end points (surgically treated KCs) occurred after the first year of follow-up, the primary end point of the study did not achieve statistical significance. Hence, researchers may wish to focus future trials on the first year after intervention.
Limitations
Study limitations include the potential unblinding of participants due to the adverse effects of treatment with fluorouracil (but not the blinded investigators). Additionally, because 916 participants (98%) were men and their mean age was 71 years, generalizability to women and younger individuals is limited, although we do not expect major differences in younger or female populations. Special populations at high risk for KC due to rare genetic syndromes (eg, basal cell nevus syndrome or xeroderma pigmentosum) were excluded, as were patients who had received transplants and were taking immunosuppressants, so generalizability to these groups is also limited. Because we studied a high-risk population, the effect on those at lower risk is unknown. The high-risk population is also a strength of the study because they are the ones who are at greatest risk of substantial morbidity, and inclusion of this group allowed the trial to be completed with a realistic sample size and duration of follow-up. This was a randomized trial with 932 participants in the intention-to-treat analysis and excellent compliance with the intervention and follow-up. The intervention is one that has been used for decades for the treatment of actinic keratosis. It is readily available, and many practitioners will be experienced in its use for that purpose. This trial incorporated counseling and appropriate materials, including images of potential reactions, in the pretreatment preparation so that compliance was enhanced. In our clinical experience, failure to use these measures may result in overuse or underuse of the medication or misunderstandings that may frighten or confuse patients.
Squamous cell carcinoma is more aggressive than basal cell carcinoma, and patients with multiple SCCs tend to have more over time. Until now the typical approach has been “wait and cut”: after the SCC has been removed, simply wait until the next occurs and then surgically remove it, and then wait for the next to occur. This trial demonstrates a proactive approach that is effective. Because the effect only lasts for the first year, annual application may be required. Clinical experience has suggested that the second and third application may have fewer adverse effects owing to improvement in the keratinocytic dysplasia in the sun-damaged areas to which it is applied.
Other methods to reduce SCC risk have been evaluated. Consistent use of sunscreen over several years was demonstrated in a randomized trial in the general population to lower SCC risk by 40%. It is clear that such use of sunscreen is difficult to maintain, and the effect of sunscreen after stopping use is unknown. The advantage of sunscreen is the absence or infrequency of adverse effects, but the disadvantage is the need for consistent, sustained use as opposed to a single 2- to 4-week course, and the lesser reduction of SCC risk. However, the 2 approaches can be combined. Neither sunscreen nor fluorouracil have been shown to be effective for reduction in BCC risk, and the combination has not been rigorously studied. Oral isotretinoin and acitretin have been shown to prevent KCs during active treatment, but this effect is lost upon stopping treatment. Topical fluorouracil is the first agent we know of that demonstrates extended posttreatment effects in SCC chemoprevention.
Daily ingestion of a large dose of nicotinamide has recently been shown in a randomized trial to reduce the risk of SCC and BCC by 23%, an effect that disappears promptly when the nicotinamide dosing ceases. This also offers potential promise, although that same trial suggested the possibility that it may simultaneously increase the risk of the most aggressive types of BCC and SCC.
Conclusions
Further study is needed to better define the effect of fluorouracil on BCC risk and risk of BCC that requires Mohs surgery, as well as SCC risk in patients who have received transplants and other special high-risk populations. It is reasonable at this point to consider the use of a standard and perhaps annual course of topical fluorouracil, 5%, to the face and ears for the reduction of SCC risk in high-risk populations, and potentially for a reduction in need for Mohs surgery; more detailed study could define precisely the groups that would most benefit.
Trial Protocol
eAppendix 1. Key personnel of the VAKCC Trial
eAppendix 2. Detailed methods of the VAKCC Trial
eTable 1. Study medication side effects reported by participants in the 5-fluorouracil (5-FU) and control groups at the six-month and final visits
eReferences
References
- 1.Karimkhani C, Boyers LN, Dellavalle RP, Weinstock MA. It’s time for “keratinocyte carcinoma” to replace the term “nonmelanoma skin cancer.” J Am Acad Dermatol. 2015;72(1):186-187. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Cancer facts & figures 2012. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2012.html. Accessed May 24, 2016.
- 3.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151(10):1081-1086. [DOI] [PubMed] [Google Scholar]
- 4.Guy GP Jr, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48(2):183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047. [DOI] [PubMed] [Google Scholar]
- 6.DiGiovanna JJ. Retinoid chemoprevention in the high-risk patient. J Am Acad Dermatol. 1998;39(2 Pt 3):S82-S85. [DOI] [PubMed] [Google Scholar]
- 7.Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373(17):1618-1626. [DOI] [PubMed] [Google Scholar]
- 8.Green A, Williams G, Nèale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354(9180):723-729. [DOI] [PubMed] [Google Scholar]
- 9.Pomerantz H, Hogan D, Eilers D, et al. ; Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) Trial Group . Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151(9):952-960. [DOI] [PubMed] [Google Scholar]
- 10.Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39(9):1306-1316. [DOI] [PubMed] [Google Scholar]
- 11.Kurtis B, Rosen T. Squamous-cell carcinoma arising in a basal-cell epithelioma treated with 5-fluorouracil. J Dermatol Surg Oncol. 1979;5(5):394-396. [DOI] [PubMed] [Google Scholar]
- 12.Cobb MW, Pellegrini AE. Squamous cell carcinoma following fluorouracil-responsive ‘keratoacanthoma’. Arch Dermatol. 1987;123(8):987-988. [PubMed] [Google Scholar]
- 13.Korgavkar K, Firoz EF, Xiong M, et al. ; VAKCC Trial Group . Measuring the severity of topical 5-fluorouracil toxicity. J Cutan Med Surg. 2014;18(4):229-235. [DOI] [PubMed] [Google Scholar]
- 14.Chen SC, Hill ND, Veledar E, Swetter SM, Weinstock MA. Reliability of quantification measures of actinic keratosis. Br J Dermatol. 2013;169(6):1219-1222. [DOI] [PubMed] [Google Scholar]
- 15.Lee KC, Lew R, Weinstock MA. Improvement in precision of counting actinic keratoses. Br J Dermatol. 2014;170(1):188-191. [DOI] [PubMed] [Google Scholar]
- 16.Pomerantz H, Korgavkar K, Lee KC, Lew R, Weinstock MA; VAKCC Trial Group . Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20(5):458-466. [DOI] [PubMed] [Google Scholar]
- 17.Siegel JA, Chren MM, Weinstock MA; Department of Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial Group . Correlates of skin-related quality of life (QoL) in those with multiple keratinocyte carcinomas (KCs): a cross-sectional study. J Am Acad Dermatol. 2016;75(3):639-642. [DOI] [PubMed] [Google Scholar]
- 18.Siegel JA, Luber AJ, Weinstock MA; Department of Veterans Affairs Topical Tretinoin Chemoprevention Trial Group and Keratinocyte Carcinoma Chemoprevention Trial Group . Predictors of actinic keratosis count in patients with multiple keratinocyte carcinomas: a cross-sectional study. J Am Acad Dermatol. 2017;76(2):346-349. [DOI] [PubMed] [Google Scholar]
- 19.Walker JL, Siegel JA, Sachar M, et al. 5-fluorouracil for actinic keratosis treatment and chemoprevention: a randomized controlled trial. J Invest Dermatol. 2017;137(6):1367-1370. [DOI] [PubMed] [Google Scholar]
- 20.Pomerantz H, Chren MM, Lew R, Weinstock MA; VA Keratinocyte Carcinoma Chemoprevention (VAKCC) Trial Group . Validation and comparison of quality-of-life measures for topical 5-fluorouracil treatment: results from a randomized controlled trial. Clin Exp Dermatol. 2017;42(5):488-495. [DOI] [PubMed] [Google Scholar]
- 21.Xiong MY, Rizzo AE, Cohen TS, et al. ; Veterans Affairs Topical Tretinoin Chemoprevention (VATTC) Trial Group . Predictors of squamous cell carcinoma in high-risk patients in the VATTC trial. J Invest Dermatol. 2013;133(6):1521-1532. [DOI] [PubMed] [Google Scholar]
- 22.van der Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2546-2548. [DOI] [PubMed] [Google Scholar]
- 23.Yélamos O, Halpern AC, Weinstock MA. Reply to “A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients.” Br J Dermatol. 2017;176(2):551-552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Key personnel of the VAKCC Trial
eAppendix 2. Detailed methods of the VAKCC Trial
eTable 1. Study medication side effects reported by participants in the 5-fluorouracil (5-FU) and control groups at the six-month and final visits
eReferences