Key Points
Question
What is the effect of prophylactic haloperidol on survival among critically ill adults?
Findings
In this multicenter randomized trial involving 1789 critically ill patients at high risk of delirium, the median number of days patients survived in 28 days was 28 days in the 2-mg haloperidol group vs 28 days in the placebo group, a nonsignificant difference.
Meaning
The use of prophylactic haloperidol therapy did not improve survival among critically ill adults at high risk of delirium.
Abstract
Importance
Results of studies on use of prophylactic haloperidol in critically ill adults are inconclusive, especially in patients at high risk of delirium.
Objective
To determine whether prophylactic use of haloperidol improves survival among critically ill adults at high risk of delirium, which was defined as an anticipated intensive care unit (ICU) stay of at least 2 days.
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled investigator-driven study involving 1789 critically ill adults treated at 21 ICUs, at which nonpharmacological interventions for delirium prevention are routinely used in the Netherlands. Patients without delirium whose expected ICU stay was at least a day were included. Recruitment was from July 2013 to December 2016 and follow-up was conducted at 90 days with the final follow-up on March 1, 2017.
Interventions
Patients received prophylactic treatment 3 times daily intravenously either 1 mg (n = 350) or 2 mg (n = 732) of haloperidol or placebo (n = 707), consisting of 0.9% sodium chloride.
Main Outcome and Measures
The primary outcome was the number of days that patients survived in 28 days. There were 15 secondary outcomes, including delirium incidence, 28-day delirium-free and coma-free days, duration of mechanical ventilation, and ICU and hospital length of stay.
Results
All 1789 randomized patients (mean, age 66.6 years [SD, 12.6]; 1099 men [61.4%]) completed the study. The 1-mg haloperidol group was prematurely stopped because of futility. There was no difference in the median days patients survived in 28 days, 28 days in the 2-mg haloperidol group vs 28 days in the placebo group, for a difference of 0 days (95% CI, 0-0; P = .93) and a hazard ratio of 1.003 (95% CI, 0.78-1.30, P=.82). All of the 15 secondary outcomes were not statistically different. These included delirium incidence (mean difference, 1.5%, 95% CI, −3.6% to 6.7%), delirium-free and coma-free days (mean difference, 0 days, 95% CI, 0-0 days), and duration of mechanical ventilation, ICU, and hospital length of stay (mean difference, 0 days, 95% CI, 0-0 days for all 3 measures). The number of reported adverse effects did not differ between groups (2 [0.3%] for the 2-mg haloperidol group vs 1 [0.1%] for the placebo group).
Conclusions and Relevance
Among critically ill adults at high risk of delirium, the use of prophylactic haloperidol compared with placebo did not improve survival at 28 days. These findings do not support the use of prophylactic haloperidol for reducing mortality in critically ill adults.
Trial Registration
clinicaltrials.gov Identifier: NCT01785290
This randomized clinical trial compares the effects of prophylactic haloperidol on 28-day survival in patients in the intensive care unit at high risk of developing delirium.
Introduction
Delirium is an acute brain disorder characterized by an acute onset of confusion, inattention, and a change in level of consciousness, the symptoms of which fluctuate during the day.1 Delirium occurs frequently among patients in intensive care units (ICUs) with incidence rates of approximately 30% to 50% and a prevalence rate of up to 80%.2,3 It is associated with deleterious clinical outcomes, including prolonged duration of mechanical ventilation, increased ICU- and hospital length of stay (LOS),2,3 and increased mortality.3,4 Moreover, impaired cognitive functioning may persist when delirium resolves.5,6
Best practice delirium management includes treating or removing delirium risk factors, such as avoiding any excess use of sedatives or benzodiazepines and use of early mobilization. Haloperidol is not only often prescribed as a pharmacological treatment for delirium7 but also sometimes prescribed as a prophylaxis to prevent delirium.8,9 Prophylactic haloperidol treatment for patients who were not critically ill has had reportedly beneficial effects on delirium outcomes10,11; however, in ICU patients, its role is inconsistent.12,13,14,15
The primary aim of this study was to determine the effects of 2 different doses of haloperidol given as prophylactic agent compared with placebo on 28-day survival among ICU patients cared for in a setting in which nonpharmacological prevention strategies had been adopted already. Secondary aims included determining the effects on the 90-day survival, delirium incidence, delirium- and coma-free days, other delirium-related outcomes, and possible adverse effects of prophylactic haloperidol.
Methods
Design and Setting
The Prophylactic Haloperidol Use for Delirium in ICU Patients at High Risk for Delirium (REDUCE) study, was a 3-group, randomized, double-blind, placebo-controlled multicenter investigator-driven study involving ICU patients with a high risk of delirium. Twenty-one centers, including university hospitals and teaching and nonteaching hospitals, in the Netherlands participated. This study was conducted from July 2013 to December 2016, and follow-up was conducted at 90 days with the final follow-up on March 1, 2017. The medical ethical committee of Arnhem-Nijmegen, the Netherlands (CMO) approved this study including the deferred consent procedure (CMO-number 2012/424). The complete study protocol has been published previously (Supplement 1).16
Study Population and Ethics
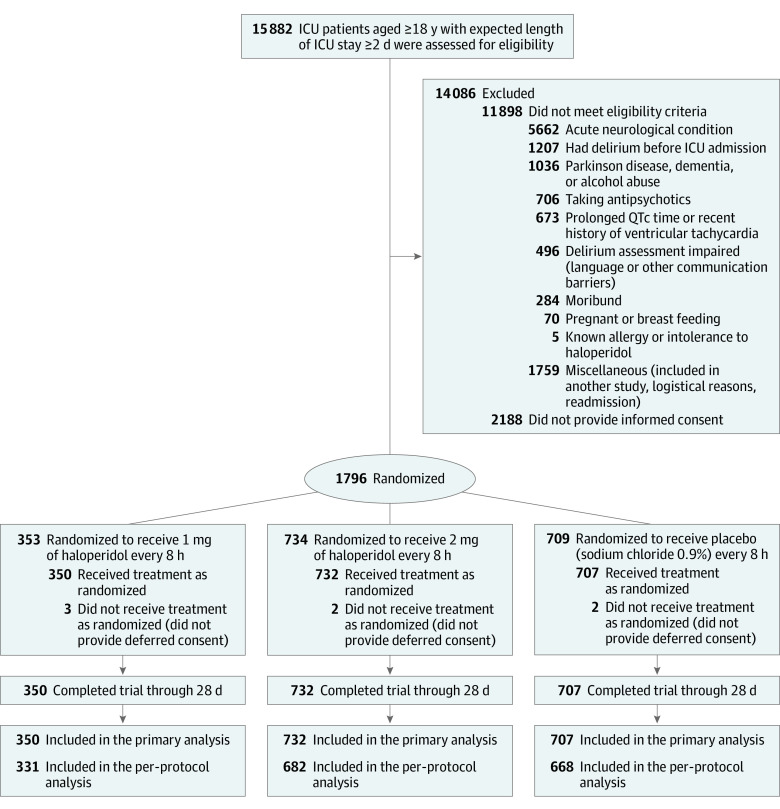
Patients aged 18 years or older who were delirium free, with an anticipated ICU stay of at least 2 days as estimated by the attending intensivist, were eligible for study participation. Exclusion criteria were delirium prior to inclusion, Parkinson disease, dementia, alcohol abuse, an acute neurological condition, history of a psychiatric disease and use of antipsychotic agents, history of clinically relevant ventricular arrhythmia in the last 12 months, QTc time of at least 500 milliseconds, pregnant or breastfeeding, expected death within 2 days, known allergy or intolerance to haloperidol, and inability to give or have no one able to give informed consent.
The informed consent process was initiated immediately after ICU admission. If obtaining informed consent was not possible, a deferred consent procedure was used. Written consent was then necessary within 24 hours after the first administration of the study medication. If the patient or the family member refused to give deferred informed consent, study medication was stopped immediately, and the patient was excluded from further analyses. Patients who provided consent and received at least 1 dose of study medication remained in the study. Patients for whom study medication had to be reduced or stopped, eg, due to adverse effects, remained in the study and were included in the intention-to-treat analysis.
Randomization and Study Medication
Eligible patients were randomly assigned receive either 1 mg or 2 mg of haloperidol or placebo (Figure 1). Randomization was applied by the pharmacist of the Radboudumc using a permuted block randomization. Patients were allocated to each group in a 1:1:1 ratio. The pharmacist who kept the randomization code and the members of the data and safety management board were the only people who were unblinded for this study. The pharmacist was not involved in clinical management of the patients. Each participating center had a stock of study medication that was divided equally by group. The study medication was accompanied with a randomization list. Following randomization, the numbers printed on the medication box were coupled with the randomization numbers. The numbered boxes consisted of 12 ampoules of the study drug. If necessary, when a patient was admitted to the ICU for more than 4 days and did not develop delirium, a follow-up study medication box was assigned to this patient consisting of the same study regime as the previous box. The follow-up study medication was always delivered by a researcher or pharmacist who was not involved in the study, using a shadow list with the group code to ensure that the patients remained in the same study group.
Figure 1. Patient Enrollment and Flow Through Study.
All study medication was prepared by the pharmacy department at Radboudumc according to good manufacturing practice regulations. All ampoules of the study medication had a total volume of 1 mL. The ampoules and drug boxes were identical in appearance and in labeling.
End Points
The primary end point was the number of days patients survived in 28 days following inclusion. Prespecified secondary end points were the number of days survived in 90 days following inclusion, delirium incidence, number of delirium-free and coma-free days over 28 days, duration of mechanical ventilation, and length of ICU and hospital stay. Additional prespecified secondary end points included incidence of unplanned removal of tubes and catheters, incidence of ICU readmission, and the long-term quality-of-life outcome (the results of which will be reported elsewhere). Furthermore, the incidence of all adverse effects was monitored for which study medication was reduced or stopped. Exact definitions of the end points are shown in the first eMethods section of Supplement 2.
Intervention and Control Group
Nonpharmacological delirium interventions focusing specifically on delirium risk factors were part of their daily ICU care. The reported implementation of early mobilization, improving patient circadian rhythm, noise reduction, reduction of sedation and benzodiazepines and awakening trials, and use of hearing and visual aids is depicted in the second eMethods section of Supplement 2. Implementation rates of these nonpharmacological interventions reached close to 90% in the participating center, and the prophylactic pharmacological study was conducted in addition to this.
The intervention groups received either 1 mg or 2 mg of haloperidol and the placebo group received 0.9% of sodium chloride, all of which were administered intravenously 3 times daily. To decrease the likelihood of adverse effects in specific cohorts, the dose of the study medication was reduced by 50% for patients who were 80 years or older, had a body weight of 50 kg or less, or had liver failure (serum bilirubin level >2.9 mg/dL; to convert to μmol/L, multiply by 17.104) present at the time of inclusion or during the study.
The first dose of the study medication was administered as soon as possible, always within 24 hours after ICU admission. Study medication was continued through day 28, until ICU discharge (whichever came first), or until delirium occurred. In the latter case, study medication was stopped and patients could be treated with open-label haloperidol. In agitated patients, 2 mg of haloperidol was prescribed intravenously 3 times daily, other delirium subtypes received 1 mg intravenously every 8 hours. Dosage could be increased up to a maximum of 5 mg every 8 hours in cases of serious agitation or anxiety due to delirium. Rescue medication was midazolam, clonidine, propofol, or dexmedetomidine at the discretion of the attending physician. The wards followed a similar treatment protocol as that used in the ICU: administering haloperidol intravenously, intramuscularly, or orally to transferred patients. For patients who were treated for more than 3 days, the haloperidol dose was reduced by 50% once delirium resolved, the dose was reduced by 50% again after the second delirium-free day. Haloperidol was stopped when the patient remained nondelirious. In case delirium recurred, the original dose was restarted. Study medication was not restarted once delirium subsided and therapeutic haloperidol was stopped or if a patient had been readmitted to the ICU within 28 days.
Data Collection
Demographic data including age, sex, delirium prediction scores17,18; Acute Physiology and Chronic Health Evaluation II (APACHE-II) score; and diagnosis group were collected, together with data on all end points.
Patients were diagnosed with delirium if they had at least 1 positive confusion assessment method–ICU (CAM-ICU)19,20 or the intensive care delirium screening checklist (ICDSC)21 result during the 28-day study period. A CAM-ICU screening tool is scored as either positive or negative for delirium. The ICDSC scores patients from 0 to 8. A score of 4 or higher indicates that patient has delirium. We defined the number of delirium- and coma-free days as days a patient was alive without delirium and as a Richmond Agitation Sedation Scores (RASS) of negative 4 or higher.13,22 The RASS ranges from 4 to negative 5. Positive scores reflect a level of agitation, and negative scores reflect a certain level of drowsiness, sedation.
If a patient with delirium was discharged to the ward, a delirium day was defined according to the validated delirium observation scale score23 of 3 or more. We considered the days following a patient’s discharge from the ICU to the wards delirium free. Clinicians collecting data on delirium at participating centers were all experienced in delirium assessment using either the CAM-ICU or, at 1 center, the ICDSC. Both validated assessment tools are recommended by critical care societies and have nearly the same diagnostic performance.24 In 14 of the 21 participating centers, data on delirium and coma days were collected; mortality, safety data, and delirium incidence were collected in all centers. From 7 centers no data on delirium and coma days could be retrieved because of limited availability of research personnel.
QTc time prolongation and drowsiness were specifically evaluated safety issues. Furthermore, the occurrence of extrapyramidal symptoms, such as dystonia, tremor, myoclonus, tics, rigidity, and akathisia,25 were determined daily by physical examination by the attending intensivist. If an adverse event occurred, the dose could be reduced or stopped, depending on the severity of the adverse event and at the discretion of the attending physician. Strict medication stopping rules applied only for prolonged QTc time, in which case the study medication was temporarily stopped until the patient’s QTc time was normalized, after which study medication resumed.
Sample Size Calculation and Statistical Analysis
Sample size was calculated based on the previous finding in a before-after study,14 with a hazard ratio (HR) of 0.80 for 28-day mortality. In that study, the median survival time in the control group was 18 days. To be conservative, the effect size was set on a smaller effect size using an HR of 0.85, resulting in a total of 715 patients per group needed to achieve a power of 0.80, with an α of .05.
Patients with informed consent and who received at least 1 dose of the study medication were included in the intention-to-treat analysis. We then conducted a per-protocol analysis to compare the intervention groups with the placebo group for those who received study medication according to the study protocol and as defined in the statistical analysis plan (both in Supplement 1).
In cases of missing values, the mean normal value of the whole group of patients was used for imputation or the value not present (see the statistical analysis plan in Supplement 1 for a more extensive description). Missing data for calculating the early delirium prediction model and 24 hours for the delirium prediction model scores were imputed as in previous studies17,18,26 and as described in the statistical analysis plan. In total, there were 10 missing values of the APACHE-II score and 39 missing blood urea values, which were subsequently imputed.
For the descriptive statistics, continuous variables were presented as mean (SD) or median (interquartile range [IQR]), depending on their distribution. Normally distributed variables were tested using the t test for comparison and the Mann-Whitney U test for nonnormally distributed variables. Confidence intervals for the difference between 2 medians were calculated using Hodges-Lehmann estimates. Categorical (and binary) variables were presented as numbers with percentages and analyzed using χ2 test. Confidence intervals of the differences between proportions were calculated using the Yates correction for continuity.
Survival analyses with Kaplan-Meier curves were used for graphical presentation. Cox proportional hazard regression analyses were used to estimate the HRs for 28-day and 90-day survival with the use of haloperidol vs placebo. In addition to unadjusted comparisons, adjusted analyses were performed using prior set relevant covariates (APACHE-II score, age, sex, diagnosis group, sepsis, urgent admission, and center). Although confounding is unlikely in a randomized clinical trial of this size, the power of the study may increase by adjustments for covariates, which were chosen prior to the study because these are all related to the primary end point.27,28 Therefore, we performed the Cox regression analyses both without and with these covariates.
Prior to the study, several subgroups were defined for sensitivity analyses. The prespecified subgroups included patients with a predicted delirium risk of less than 20%, 20% to 30%, and more than 30%; patients who were in different admission diagnosis groups, patients in severity of illness groups with an APACHE score of 20, 20 to 25, and more than 25; patients who were receiving study medication up to 2 days, more than 2 days, 3 days, or 5 days; and patients who developed or did not develop delirium. Interaction was tested by subgroup. The secondary end points and the sensitivity analyses were not adjusted for multiple testing; therefore, the results of these analyses should be considered exploratory.
An independent data and safety management board, consisting of 3 members (a psychiatrist, an anesthesiologist, and a statistician) performed unblinded safety, futility, and superiority interim analyses after the inclusion of 175, 350, 500 (safety and futility), and 1000 (safety and superiority) patients. For safety analyses, the incidence of adverse and serious adverse events in the intervention and the placebo groups was compared. For assessing futility or superiority of the intervention or placebo, 28-day survival, the primary end point, was used. Per analysis, first, the difference between the highest dose of haloperidol vs placebo was tested, and second, the difference between the lowest dose and placebo was tested. Differences in adverse events between the groups were reviewed.
Early stopping rules were prespecified regarding safety issues and futility during each interim analysis. The advice to discontinue a treatment group because of safety concerns or futility was given on discretion of the data and safety management board, a time sequential to illustrate the increasing power of the study, leading to a trend toward benefit, harm, or futility. The adaptive design allowed for discontinuation of 1 therapy group based on predefined futility definitions. After inclusion of 1000 patients, superiority was determined with a proven superiority (P < .003, 2-sided) of any dose of haloperidol over placebo resulting in an α of .049 (2-sided) for the final analysis. This α distribution was calculated by an independent statistician according to the method of the Lan-DeMets cumulative α spending function of the O’Brien-Fleming α spending.29 All statistical tests were 2-sided and statistical significance was defined as P <.05. Statistical analyses were performed using SPSS version 23 (SPSS) and R version 3.4.2. (R Foundation for Statistical Computing).
Results
During the study period, 15 882 eligible ICU adult patients who were expected to stay in the ICU for at least 2 days in the participating ICUs. A total of 11 898 patients were excluded, most frequently due to an acute neurological condition (35.7%). For 2188 (13.8%) of the eligible patients, no informed consent was provided, and for 7 patients (0.3%), no confirmation of a deferred consent was obtained (Figure 1). A total of 732 patients were included in the 2-mg haloperidol group, of which 46 patients (6.3%) were included via deferred consent, and 707 were included in the placebo group, of which 52 (7.4%) were included via deferred consent. No safety issues occurred in all interim analyses. Upon recruitment of 1000 patients in the study, the data and safety management board while still blinded conducted its fourth interim analysis and decided to discontinue 1 of the study groups, which it later learned was the 1-mg haloperidol group, due to futility, as reflected in the time sequential analysis (eFigure 1 in Supplement 2). For this reason, only 350 patients were included the 1-mg group analysis. A post hoc power calculation was performed, the power for the 1-mg haloperidol group to demonstrate a significant effect, after the inclusion of 1000 patients was 6.1%. As a consequence, and per the statistical analysis plan, only the effects of the 2-mg haloperidol group were compared with the placebo group in the primary analyses. The demographic and patient characteristics between both groups were comparable (Table 1).
Table 1. Demographic and Characteristics of Included Patients.
| No. (%) of Patients | |||
|---|---|---|---|
| 1-mg Haloperidol (n = 350) | 2- mg Haloperidol (n = 732) | Placebo (n = 707) | |
| Age, mean (SD), y | 66.1 (12.6) | 66.7 (12.7) | 67.0 (12.6) |
| Men | 206 (58.9) | 459 (62.7) | 434 (61.4) |
| Admission type | |||
| Surgical | 163 (46.6) | 337 (46.0) | 328 (46.4) |
| Medical | 171 (48.9) | 365 (49.9) | 357 (50.5) |
| Trauma | 16 (4.6) | 30 (4.1) | 22 (3.1) |
| Urgent admission | 285 (81.4) | 600 (82.0) | 572 (80.9) |
| Mechanically ventilated | 247 (70.6) | 498 (68) | 457 (64.6) |
| APACHE-II score, mean (SD)a | 20.1 (7.1) | 19.2 (6.9) | 19.0 (6.8) |
| History of cognitive disturbance | 6 (1.7) | 12 (1.6) | 17 (2.4) |
| Use of corticosteroids before ICU admission | 69 (19.7) | 186 (25.4) | 194 (27.4) |
| Acute respiratory failure | 136 (38.9) | 304 (41.5) | 296 (41.9) |
| Blood urea level at time of ICU admission, median (IQR), mg/dL | 43.2 (30.0-72.0) | 46.8 (33.0-74.4) | 46.2 (33.0-78) |
| Mean arterial blood pressure, mean (SD), mm Hg | 75 (30) | 78 (27) | 79 (26) |
| Sepsis | 107 (30.6) | 274 (37.4) | 234 (33.1) |
| PRE-DELIRIC score, mean (SD), %17,18,b | 26.3 (12.4) | 26.1 (11.9) | 25.6 (11.8) |
| Early PRE-DELIRIC 26 score, median (IQR), %26,b | 18 (11-31) | 19 (11-29) | 19 (12-30) |
| QTc time at time of inclusion, median (IQR), ms | 440 (410-469) | 447 (422-466) | 443 (420-468) |
Abbreviations: APACHE-II: Acute Physiology and Chronic Health Evaluation-II; ICU, intensive care unit; IQR, interquartile range; PRE-DELIRIC, prediction of delirium in ICU patients.
SI conversion factor: to convert uric acid from mg/dL to mmol/L, multiply by 6.
APACHE-II score ranges from 0 to 71; the higher the score, the more severely ill the patient and the higher the hospital mortality risk.
Early PRE-DELIRIC score ranges from 0 to 100, representing the percentage chance that delirium may occur during the complete ICU length of stay.
Primary Outcome
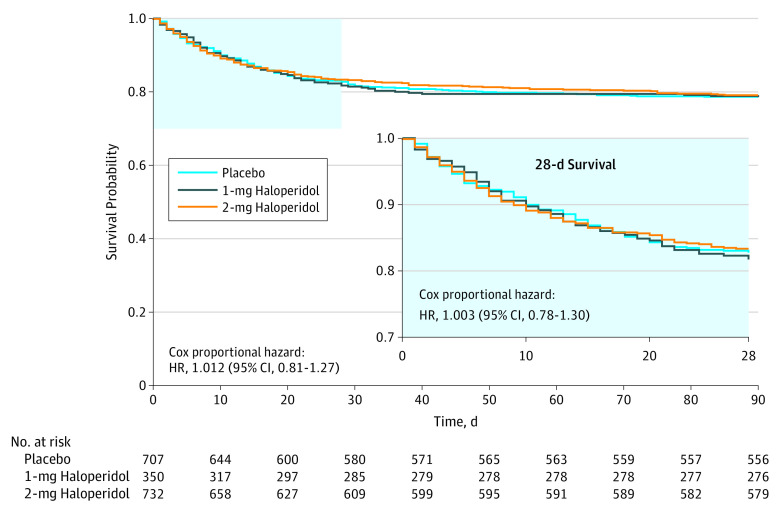
The median number of days patients that survived in 28 days was 28 in the 2-mg haloperidol group vs 28 days in the placebo group (difference, 0 days; 95% CI, 0-0; P = .93; HR, 1.003; 95% CI, 0.78-1.30)(Figure 2). These survival times translated into 28-day survival rates of 83.3% (610 of 732) for the 2-mg haloperidol group and 82.7% (585 of 707) for the placebo group (proportion difference, 0.6; IQR, −3.4 to 4.6; Table 2). For both groups the median survival was 28 days (IQR, 28-28 days).
Figure 2. Survival Analysis at 28 and 90 Days.
For the 28-day end point, follow-up for the 1-mg haloperidol group was a median of 28 days (interquartile range [IQR], 28-28 days); for the 2-mg group, 28 days (IQR, 28-28 days); and for the placebo group, 28 days (IQR, 28-28 days). For the 90-day end point, follow-up for the 1-mg haloperidol group was 90 days (IQR, 90-90 days), for the 2-mg haloperidol group, 90 days (IQR, 90-90 days); and for the placebo group, 90 days (IQR, 90-90 days).
Table 2. Primary and Secondary Outcomes for the Intention-to-Treat Analysis.
| 2-mg Haloperidol (n = 732) | Placebo (n = 707) | 2-mg Haloperidol vs Placebo, Difference (95% CI)a | 1-mg Haloperidol (n = 350) | |
|---|---|---|---|---|
| Primary analysis, days alive at 28 days, median (IQR) | 28 (28 to 28) | 28 (28 to 28) | 0 (0 to 0) | 28 (28 to 28) |
| Survival, No. (%) | ||||
| 28 d | 610 (83.3) | 585 (82.7) | 0.6 (−3.4 to 4.6) | 286 (81.7) |
| 90 d | 579 (79.1) | 556 (78.6) | 0.5 (−3.9 to 4.8) | 275 (78.6) |
| 28-Day end points | ||||
| Incidence of delirium, No. (%) | 244 (33.3) | 233 (33.0) | 0.4 (−4.6 to 5.4) | 139 (39.7) |
| No. of delirium- and coma-free, median (IQR), db | 26 (17 to 28) | 26 (19 to 28) | 0.0 (0 to 0)a | 26 (17 to 28) |
| No. of delirium-free, median (IQR), db | 28 (22 to 28) | 28 (23 to 28) | 0.0 (0 to 0)a | 28 (21 to 28) |
| No. of coma-free, median (IQR), db | 27 (22 to 28) | 27 (23 to 28) | 0.0 (0 to 0)a | 27 (21 to 28) |
| No. of days to occurrence of delirium, median (IQR)b | 3 (2 to 6) | 3 (2 to 6) | 0.0 (0 to 0)a | 4 (2 to 6) |
| Duration of mechanical ventilation, median (IQR), d | 2 (0 to 6) | 2 (0 to 5) | 0.0 (0 to 0)a | 2 (0.3 to 7) |
| Length of stay, median (IQR), d | ||||
| ICU | 5 (2 to 9) | 4 (2 to 9) | 0 (−0.0 to 1.0)a | 4 (2 to 9) |
| Survivors | 4 (2 to 4) | 4 (2 to 8) | 0 (0 to 1.0)a | 4 (2 to 9) |
| Nonsurvivors | 17 (10 to 32) | 16 (10 to 30) | 0 (−1.0 to 1.0)a | 18 (9 to 34) |
| Hospital | 15 (9 to 28) | 15 (9 to 26) | 1.0 (0 to 2.0)a | 16 (9 to 31) |
| Survivors | 6 (2 to 9) | 5 (2 to 10) | 1.0 (0 to 2.0)a | 7 (2 to 11) |
| Nonsurvivors | 9 (5 to 15) | 10 (4 to 17) | 11 (6 to 22) | |
| Incidence, No. (%) | ||||
| ICU readmission, No. (%) | 65 (8.9) | 68 (9.6) | 0.7 (−3.4 to 2.4) | 36 (10.3) |
| Physical restraints, No. (%) | 191 (27.0) | 169 (24.8) | 2.2 (−2.4 to 6.8) | 102 (30.0) |
| Unplanned removal of tubes or catheters, No. (%) | 81 (11.1) | 73 (10.3) | 0.7 (−2.5 to 4.1) | 42 (12.0) |
| Reintubation, No. (%) | 71 (9.7) | 62 (8.8) | 0.9 (−0.2 to 4.1) | 32 (9.1) |
| No. of days treated with open-label haloperidol, median (IQR) | 2.0 (1.0 to 5.0) | 2.0 (1.0 to 5.0) | 0 (0 to 0)a | 2.0 (1.0 to 5.0) |
| Open-label haloperidol dose, median (IQR), mg/d | 3.0 (2.0 to 4.6) | 3.0 (3.0-4.6) | 0 (−0.4 to 0.3)a | 3.0 (2.0 to 4.3) |
| Safety issues | ||||
| Maximum QTc time, median (IQR), ms | 465 (446 to 483) | 463 (440 to 486) | 1.0 (−2.0 to 5.0)a | 465 (440 to 489) |
| No. of QTc time prolongations, No. (%) | 33 (4.5) | 36 (5.1) | −0.5 (−2.9 to 1.8) | 31 (8.9) |
| Incidence of extrapyramidal symptoms, No. (%) | ||||
| Dystonia | 1 (0.1) | 3 (0.4) | 0.3 (−0.1 to 4.0) | 3 (0.9) |
| Tremor | 6 (0.8) | 7 (1.0) | −1.7 (−1.2 to 0.9) | 6 (1.7) |
| Myoclonus | 4 (0.5) | 4 (0.6) | −0.1 (−0.8 to 0.8) | 4 (1.1) |
| Tics | 4 (0.5) | 6 (0.8) | −0.3 (−1.3 to 0.6) | 4 (1.1) |
| Rigidity | 3 (0.4) | 6 (0.8) | −0.5 (−1.4 to 5.2) | 3 (0.9) |
| Akathisia | 4 (0.5) | 4 (0.6) | −0.0 (−0.8 to 0.7) | 6 (1.7) |
| Reported serious adverse events, No. (%) | 2 (0.3) | 1 (0.1) | 0.1 (−0.5 to 0.7) | 2 (0.6) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
Differences between medians are described as absolute difference in ranking following order, calculated using Hodges-Lehmann estimates. For further explanation, see the Methods section.
Data collected in smaller group of patients: 608 (83.1%) in 2-mg haloperidol group, 599 (84.7%) in placebo group, and 299 (85.4%) in 1-mg haloperidol group.
Secondary Outcomes
90-Day Survival, Delirium Incidence, Delirium- and Coma-Free Days
The median number of days patients survived in 90 days in the 2-mg haloperidol group was 90 days vs 90 days in the placebo group (difference, 0 days; 95% CI, 0-0; P = .86; Figure 2).
The delirium incidence between the haloperidol and placebo groups was not statistically different, 33.3% vs 33.0% (proportion difference, 0.4%; 95% CI, −4.6 to 5.4). The number of days until delirium developed also did not differ between the groups. Patients who developed delirium and subsequently received open-label haloperidol according to the study protocol, were treated for a similar duration. In both groups, the median number of days of open label delirium treatment was 2 days (IQR, 1-5 days). The dose of open-label haloperidol was also not significantly different between groups. Both had a median dose of 3.0 mg (IQR, 2.0-4.6 mg). Data on delirium incidence and coma days could be retrieved from a total of 1506 patients (84.2%). There were no significant differences in number of delirium free-days, coma free-days, and delirium- and coma-free days among those who survived 28 days (Table 2).
Delirium-Related Outcome Measures
No significant differences were found between groups regarding the duration of mechanical ventilation, incidence of unplanned removal of tubes, incidence of ICU readmission, length of ICU stay and in-hospital stay, and other delirium-related outcomes (Table 2). The proportion of patients who required physical restraints was not statistically different between groups: 191 patients (27.0%) in the 2-mg haloperidol group vs 169 patients (24.8%) in the placebo group, for a proportion difference of 2.2% (95% CI, −2.4% to 6.8%).
Per-Protocol Analysis
All patients who did not receive the study medication according to the study protocol16 (6.8% in 2-mg haloperidol group and 5.5% in the placebo group) were excluded from the per-protocol analysis. Again, no statistically significant differences were found between groups in the per-protocol analysis for any of the reported outcome measures (eTable in Supplement 2).
Safety Issues
Five serious adverse events were reported. Three patients died, 1 in each of the 3 groups (Table 2). None of the serious adverse events were likely related to the study medication. Two patients in the 1-mg haloperidol group and 1 patient in the 2-mg haloperidol group had a monomorphic ventricular tachycardia. One patient in the 2-mg haloperidol group developed refractory shock. One patient in the placebo group had a suspected malignant neuroleptic syndrome event. The number of reported adverse events was not statistically different between groups.
Subgroup Analysis
Sensitivity analyses were performed for all predefined subgroups in strata according to delirium prediction scores, admission diagnosis, severity of illness, duration of prophylactic therapy, and patients who developed delirium and those who did not develop delirium. No significant interaction between any of the subgroups and treatment were found (Table 3; eFigure 2A-C in Supplement 2). Twenty-eight- and 90-day survival, as well as delirium incidence, across all tested subgroups showed no significant differences between patients who received 2 mg of haloperidol and those who received placebo (Table 3).
Table 3. Sensitivity Analyses for the Intention-to-Treat Analysis.
| No. (%) of Patients | 2-mg Haloperidol vs Placebo, Difference (95% CI) | Interaction Effects, P Value | No. (%) of Patients Taking 1-mg Haloperidol (n = 350) | ||
|---|---|---|---|---|---|
| 2-mg Haloperidol (n = 732) | Placebo (n = 707) | ||||
| Admission Group | |||||
| Surgical (n = 828) |
|
||||
| Survival | |||||
| At 28 d | 295 (87.5) | 285 (86.9) | 0.6 (–4.7 to 6.0) | 142 (87.1) | |
| At 90 d | 280 (83.1) | 273 (83.2) | 0.1 (–6.0 to 5.7) | 135 (82.8) | |
| Delirium incidence | 139 (41.2) | 122 (37.2) | 4.1 (–3.4 to 11.8) | 72 (44.2) | |
| Medical Group (n = 893) | |||||
| Survival | |||||
| At 28 d | 288 (78.9) | 279 (78.2) | 0.7 (–5.5 to 7.0) | 131 (76.6) | |
| At 90 d | 272 (74.5) | 262 (73.4) | 1.1 (–5.5 to 7.8) | 127 (74.3) | |
| Delirium incidence | 97 (26.6) | 103 (28.9) | 2.3 (–9.0 to 4.5) | 59 (34.5) | |
| Trauma (n = 68) | |||||
| Survival | |||||
| At 28 d | 27 (90.0) | 21 (95.5) | 5.5 (–23.0 to 12.3) | 13 (81.3) | |
| At 90 d | 27 (90.0) | 21 (95.5) | 5.5 (–23.2 to 12.3) | 13 (81.3) | |
| Delirium incidence | 8 (26.7) | 8 (36.4) | 9.7 (–39.2 to 19.8) | 8 (50.0) | |
| APACHE-II Score | |||||
| <20 (n = 1006) |
|
||||
| Survival | |||||
| At 28 d | 376 (91.5) | 367 (90.0) | 1.6 (–2.7 to 5.8) | 169 (90.4) | |
| At 90 d | 364 (88.6) | 355 (87.0) | 1.6 (–3.1 to 6.3) | 166 (88.8) | |
| Delirium incidence | 119 (29.0) | 111 (27.2) | 1.7 (–4.7 to 8.1) | 62 (33.2) | |
| 20-25 (N = 398) | |||||
| Survival | |||||
| At 28 d | 129 (79.6) | 121 (78.6) | 1.1 (–8.5 to 10.7) | 61 (74.4) | |
| At 90 d | 122 (75.3) | 114 (74.0) | 1.2 1.3 (–8.9 to 11.5) | 58 (70.7) | |
| Delirium incidence | 63 (38.9) | 56 (36.4) | 1.3 2.5 (–8.8 to 13.8) | 38 (46.3) | |
| >25 (n = 385) | |||||
| Survival | |||||
| At 28 d | 105 (66.0) | 97 (66.9) | 1.0 (–12.3 to 10.2) | 56 (69.1) | |
| At 90 d | 93 (58.5) | 87 (60.0) | 2.0 –1.8(–13.5 to 9.9) | 51 (63.0) | |
| Delirium incidence | 62 (39.0) | 66 (45.5) | 3.0 –6.2 (–17.9 to 5.5) | 39 (48.1) | |
| PRE-DELIRIC Score | |||||
| <20 (n = 600) |
|
||||
| Survival | |||||
| At 28 d | 220 (95.2) | 233 (92.5) | 2.8 (–1.9 to 7.5) | 108 (92.3) | |
| At 90 d | 217 (93.9) | 227 (90.1) | 3.9 (–1.3 to 9.1) | 107 (91.5) | |
| Delirium incidence | 45 (19.5) | 49 (19.4) | –0.0 (–7.2 to 7.1) | 26 (22.2) | |
| 20-30 (n = 578) | |||||
| Survival | |||||
| At 28 d | 213 (85.9) | 198 (88.0) | 2.1 (–8.6 to 4.4) | 87 (82.9) | |
| At 90 d | 202 (81.5) | 186 (82.7) | –1.2 (–8.6 to 6.1) | 85 (81.0) | |
| Delirium incidence | 85 (34.3) | 71 (31.6) | 2.7 (–6.2 to 11.6) | 46 (43.8) | |
| >30 (n = 611) | |||||
| Survival | |||||
| At 28 d | 177 (70.0) | 154 (67.0) | 2.9 (–5.8 to 11.6) | 91 (71.1) | |
| At 90 d | 160 (63.2) | 143 (62.2) | 1.0 (–8.1 to 9.9) | 83 (64.8) | |
| Delirium incidence | 114 (45.1) | 113 (49.1) | –4.0 (–13.2 to 5.5) | 67 (52.3) | |
| Duration of Preventive Treatment | |||||
| ≤2 d (n = 967) |
|
||||
| Survival | |||||
| At 28 d | 333 (85.6) | 320 (82.5) | 3.1 (–2.2 to 8.5) | 162 (85.3) | |
| At 90 d | 315 (81.0) | 303 (78.1) | 2.9 (–3.0 to 8.8) | 157 (82.6) | |
| Delirium incidence | 121 (31.1) | 130 (33.5) | –2.4 (–9.2 to 4.4) | 68 (35.8) | |
| >2 d (n = 822) | |||||
| Survival | |||||
| At 28 d | 277 (80.8) | 265 (83.1) | –2.3 (–8.5 to 3.8) | 124 (77.5) | |
| At 90 d | 264 (77.0) | 253 (79.3) | –2.3 (–8.9 to 4.3) | 118 (73.8) | |
| Delirium incidence | 123 (35.9) | 103 (32.3) | 3.6 (–3.9 to 11.1) | 71 (44.4) | |
| ≤3 d (n = 1176) |
|
||||
| Survival | |||||
| At 28 d | 412 (86.4) | 396 (83.5) | 2.8 (–1.9 to 7.6) | 192 (85.3) | |
| At 90 d | 394 (82.6) | 376 (79.3) | 3.3 (–1.9 to 8.5) | 187 (83.1) | |
| Delirium incidence | 151 (31.7) | 147 (31.0) | 0.6 (–5.5 to 6.7 | 80 (35.6) | |
| >3 d (n = 613) | |||||
| Survival | |||||
| At 28 d | 198 (77.6) | 189 (81.1) | –3.5 (–11.0 to 4.1) | 94 (75.2) | |
| At 90 d | 185 (72.5) | 180 (77.3) | –4.7 (–12.8 to 3.4) | 88 (70.4) | |
| Delirium incidence | 93 (36.5) | 86 (36.9) | –0.4 (–9.4 to 8.5) | 59 (47.2) | |
| ≤5 d (n = 1431) |
|
||||
| Survival | 495 (85.2) | 483 (83.6) | 1.6 (–2.7 to 6.0) | 225 (82.7) | |
| At 28 d | 470 (80.9) | 461 (79.8) | 1.1 (–3.6 to 5.9) | 220 (80.9) | |
| At 90 d | 187 (32.2) | 184 (31.8) | 0.4 (–5.2 to 5.9) | 104 (38.2) | |
| >5 d (n = 358) | |||||
| Survival | |||||
| At 28 d | 115 (76.2) | 102 (79.1) | –2.9 (–13.4 to 7.6) | 61 (78.2) | |
| At 90 d | 109 (72.2) | 95 (73.6) | –1.5 (–12.6 to 9.7) | 55 (70.5) | |
| Delirium incidence | 57 (37.7) | 49 (38.0) | –0.2 (–11.9 to 11.4) | 35 (44.9) | |
| Nondelirium Patients (n = 1173) | |||||
| Survival |
|
||||
| At 28 d | 407 (83.4) | 396 (83.5) | –0.1 (–5.0 to 4.7) | 175 (82.9) | |
| At 90 d | 389 (79.7) | 377 (79.5) | 0.2 (–5.1 to 5.4) | 172 (81.5) | |
| No. of delirium- and coma-free days in 28 d, median (IQR) | 27 (25-28) | 28 (26-28) | 0.0 (0 to 0)a | 27 (26-28) | |
| Delirium patients (n = 616) | |||||
| Survival |
|
||||
| At 28 d | 203 (83.2) | 189 (81.1) | 2.1 (–5.2 to 9.4) | 111 (79.9) | |
| At 90 d | 190 (77.9) | 179 (76.8) | 1.0 (–6.9 to 9.0) | 103 (74.1) | |
| No. of delirium- and coma-free days in 28 d, median (IQR) | 20 (13-24) | 21 (13-25) | 0 (–19.9 to 9.9)a | 20 (8-24) | |
Abbreviation: APACHE-II: Acute Physiology and Chronic Health Evaluation II; IQR, interquartile range; NA, not applicable; PRE-DELIRIC, Prediction delirium in ICU patients.
Differences between medians are described as absolute difference in ranking following order.
Discussion
In this large multicenter double-blind randomized clinical trial involving critically ill adults at high risk of delirium, there was no significant difference in the number of days survived at 28 days following inclusion between patients who received prophylactic haloperidol therapy and patients who received placebo. Also, no significant differences were found in the number of days patients survived at 90 days between the haloperidol group and the placebo group. No differences were found for any other reported secondary end points. Furthermore, across predefined subgroups, the lack of a prophylactic effect was very consistent. Prophylactic haloperidol therapy was not associated with haloperidol-induced adverse effects.
The pathophysiological mechanism of delirium is poorly understood. Delirium is considered a multifactorial disorder and many different pathways to its occurrence have been postulated30,31 resulting in many hypotheses.31 The fact that the average of 11 risk factors are present at the same time in ICU patients with delirium2 suggests the involvement of multiple pathways in its development. Therefore, it seems plausible that a mediator that alters causal pathways of delirium may be helpful. Haloperidol is an antipsychotic agent with antidopaminergic, antiadrenergic, limited anticholinergic properties and possibly has antiinflammatory effects32 that potentially antagonizes multiple pathways of delirium.
Haloperidol has been the first-line drug of choice to treat delirium7 for decades despite the lack of evidence that haloperidol is effective. For this reason, the Society of Critical Care Medicine in its last guideline on pain, agitation, and delirium33 did not recommend the use of haloperidol for treatment or for delirium prevention for critically ill adults. However, several ICU studies have evaluated possible prophylactic effects of haloperidol but demonstrate contradictory effects.12,13,14,15 In one randomized clinical trial, postoperative ICU patients received a maximum of 1.2 mg of haloperidol and showed a reduced delirium incidence and more delirium-free days.12 Another randomized clinical trial involving severely ill medical ICU patients receiving 2.5 mg 3 times daily, showed no beneficial effect,13 and no effect was found in reducing subsyndromal delirium with prophylactic haloperidol.15 Although a previous before-after study14 showed clinical relevant and favorable effects in a similar group of high-risk and critically ill adults, these beneficial effects could not be replicated in the current randomized clinical trial. However, the findings of this study corroborate the findings of other randomized clinical trials involving critically ill adults.13,15 The large sample size of the current study allowed us to perform several sensitivity analyses, confirming the lack of effect across the different subgroups.
Limitations
This study has several limitations. First, the 1-mg haloperidol group was terminated early, as a predefined consequence of the adaptive design, which is considered a strength of our study. This discontinuation did not affect the presented study findings. Second, the duration of prophylactic therapy (median, 2 days) could be too short to prevent delirium and its deleterious outcome. It cannot be excluded that longer exposure to haloperidol may be needed to influence patient outcome. However, subgroup analysis in patients treated for more than 2 days also did not show any beneficial effect. Third, the dose may have been too low. In a before-after study14 a 1-mg dose every 8 hours demonstrated beneficial effects without relevant adverse effects. For this reason, using a higher dose was included as part of the current study, similar to the study of Page et al.13 In both haloperidol dosage groups no beneficial effects were found. Fourth, it was not feasible to collect data for all secondary outcome measures in some centers due to research staff limitations. However, the median number of delirium- and coma-free days between both groups did not differ; therefore, collecting these data in a somewhat smaller group did not affect the results.
Fifth, the study population included severely ill ICU adults, whose brains may have been too seriously affected for haloperidol to exert a prophylactic effect, since in non-ICU adults, prophylactic haloperidol may have beneficial effects.10,11 But the subgroup of patients with a low severity of illness score also demonstrated no beneficial effects. Nevertheless, it cannot be ruled out that delirium may be more easily and favorably affected in non-ICU adults than critically ill ICU adults. Sixth, the results of the long-term quality of life are not included herein, and therefore the effects of haloperidol on quality of life remain to be determined.
Conclusions
Among critically ill adults with high-risk of delirium, the use of prophylactic haloperidol compared with placebo did not improve 28-day survival. These findings do not support the use of prophylactic haloperidol in critically ill adults.
Study Protocol and Statistical Analysis Plan
eMethods 1. Definition of study objectives
eMethods 2. The use of nonpharmacologic delirium preventive interventions in the participating centers
eFigure 1. Time Sequential Analysis
eTable 1. Primary and secondary outcomes [per protocol analysis]
eFigure 2a. Forest plot with interaction for 28days
eFigure 2b. Forest plot with interaction for 90days
eFigure 2c. Forest plot with interaction for delirium incidence
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Vol. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Boogaard M, Schoonhoven L, van der Hoeven JG, van Achterberg T, Pickkers P. Incidence and short-term consequences of delirium in critically ill patients: a prospective observational cohort study. Int J Nurs Stud. 2012;49(7):775-783. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. [DOI] [PubMed] [Google Scholar]
- 5.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Boogaard M, Schoonhoven L, Evers AW, van der Hoeven JG, van Achterberg T, Pickkers P. Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit Care Med. 2012;40(1):112-118. [DOI] [PubMed] [Google Scholar]
- 7.Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burry LD, Williamson DR, Mehta S, et al. Delirium and exposure to psychoactive medications in critically ill adults: a multi-centre observational study. J Crit Care. 2017;42:268-274. [DOI] [PubMed] [Google Scholar]
- 9.Mo Y, Zimmermann AE, Thomas MC. Practice patterns and opinions on current clinical practice guidelines regarding the management of delirium in the intensive care unit. J Pharm Pract. 2017;30(2):162-171. [DOI] [PubMed] [Google Scholar]
- 10.Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko T, Cai J, Ishikura T, Kobayashi M, Naka T, Kaibara N. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Med. 1999;42(3):179-184. [Google Scholar]
- 12.Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial. Crit Care Med. 2012;40(3):731-739. [DOI] [PubMed] [Google Scholar]
- 13.Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2013;1(7):515-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Boogaard M, Schoonhoven L, van Achterberg T, van der Hoeven JG, Pickkers P. Haloperidol prophylaxis in critically ill patients with a high risk for delirium. Crit Care. 2013;17(1):R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Qadheeb NS, Skrobik Y, Schumaker G, et al. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med. 2016;44(3):583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Boogaard M, Slooter AJ, Brüggemann RJ, et al. Prevention of ICU delirium and delirium-related outcome with haloperidol: a study protocol for a multicenter randomized controlled trial. Trials. 2013;14:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Boogaard M, Pickkers P, Slooter AJ, et al. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICU patients) delirium prediction model for intensive care patients: observational multicentre study. BMJ. 2012;344:e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Boogaard M, Schoonhoven L, Maseda E, et al. Recalibration of the delirium prediction model for ICU patients (PRE-DELIRIC): a multinational observational study. Intensive Care Med. 2014;40(3):361-369. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. [DOI] [PubMed] [Google Scholar]
- 21.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859-864. [DOI] [PubMed] [Google Scholar]
- 22.Simons KS, Laheij RJ, van den Boogaard M, et al. Dynamic light application therapy to reduce the incidence and duration of delirium in intensive-care patients: a randomised controlled trial. Lancet Respir Med. 2016;4(3):194-202. [DOI] [PubMed] [Google Scholar]
- 23.Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The delirium observation screening scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31-50. [DOI] [PubMed] [Google Scholar]
- 24.Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16(4):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdo WF, van de Warrenburg BP, Burn DJ, Quinn NP, Bloem BR. The clinical approach to movement disorders. Nat Rev Neurol. 2010;6(1):29-37. [DOI] [PubMed] [Google Scholar]
- 26.Wassenaar A, van den Boogaard M, van Achterberg T, et al. Multinational development and validation of an early prediction model for delirium in ICU patients. Intensive Care Med. 2015;41(6):1048-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hauck WW, Anderson S, Marcus SM. Should we adjust for covariates in nonlinear regression analyses of randomized trials? Control Clin Trials. 1998;19(3):249-256. [DOI] [PubMed] [Google Scholar]
- 28.Hernández AV, Steyerberg EW, Habbema JD. Covariate adjustment in randomized controlled trials with dichotomous outcomes increases statistical power and reduces sample size requirements. J Clin Epidemiol. 2004;57(5):454-460. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. [PubMed] [Google Scholar]
- 30.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375(9716):773-775. [DOI] [PubMed] [Google Scholar]
- 31.Flacker JM, Lipsitz LA. Neural mechanisms of delirium: current hypotheses and evolving concepts. J Gerontol A Biol Sci Med Sci. 1999;54(6):B239-B246. [DOI] [PubMed] [Google Scholar]
- 32.Milbrandt EB, Kersten A, Kong L, et al. Haloperidol use is associated with lower hospital mortality in mechanically ventilated patients. Crit Care Med. 2005;33(1):226-229. [DOI] [PubMed] [Google Scholar]
- 33.Barr J, Pandharipande PP. The pain, agitation, and delirium care bundle: synergistic benefits of implementing the 2013 Pain, Agitation, and Delirium Guidelines in an integrated and interdisciplinary fashion. Crit Care Med. 2013;41(9)(suppl 1):S99-S115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol and Statistical Analysis Plan
eMethods 1. Definition of study objectives
eMethods 2. The use of nonpharmacologic delirium preventive interventions in the participating centers
eFigure 1. Time Sequential Analysis
eTable 1. Primary and secondary outcomes [per protocol analysis]
eFigure 2a. Forest plot with interaction for 28days
eFigure 2b. Forest plot with interaction for 90days
eFigure 2c. Forest plot with interaction for delirium incidence