Key Points
Question
Is there an association between lymph node density (LND)—the ratio of the number of positive lymph nodes to the total number of nodes excised—and survival in patients with papillary thyroid cancer?
Findings
In a cohort of 2542 patients, central neck LND reliably stratified patients according to risk of death. Incorporating LND into the current American Joint Committee on Cancer staging system successfully stratified risk groups compared with the traditional TNM staging system.
Meaning
Lymph node density can be used as a complementary prognostic tool to assess risk of disease-related death in patients with papillary thyroid cancer.
This cohort study assesses the association of lymph node density—the ratio of the number of positive lymph nodes to the total number of nodes excised—with survival in patients with papillary thyroid cancer.
Abstract
Importance
Lymph node metastases are common in papillary thyroid cancer (PTC), yet the impact of nodal metastases on survival remains unclear. Lymph node density (LND) is the ratio between the number of positive lymph nodes excised and the total number of excised lymph nodes. Lymph node density has been suggested as a prognostic factor in many types of cancer.
Objective
To evaluate the prognostic role of LND in PTC.
Design, Setting, and Participants
This cohort study reviewed medical records of patients with PTC who were treated at the University of Texas MD Anderson Cancer Center between January 1, 2000, and December 31, 2015. Survival and recurrence outcomes were calculated by using the Kaplan-Meier method. Significant variables on univariate analysis were subjected to a Cox proportional hazards regression multivariate model.
Main Outcomes and Measures
Primary study outcome was disease-specific survival (DSS); other measurements included overall survival (OS).
Results
The study cohort included data for 2542 patients (1801 [71%] male; median age, 48 years [range, 18-97 years]) with a median follow-up of 55 months (range, 4-192 months). The 10-year disease-specific survival rate was 98% for patients with LND of 0.19 or less, compared with 90% for those with LND greater than 0.19 (effect size, 8%; 95% CI, 4%-15%). The 10-year overall survival was 87% for patients with LND of 0.19 or less, compared with 79% for patients with LND greater than 0.19 (effect size, 8%; 95% CI, 3%-15%). Multivariable analysis revealed that LND greater than 0.19 was independently associated with an adverse DSS (hazard ratio [HR], 4.11; 95% CI, 2.11-8.97) and OS (HR, 1.96; 95% CI, 1.24-4.11). Subgroup analysis of patients with 18 or more lymph nodes analyzed revealed that LND greater than 0.19 remained a significant marker for DSS (HR, 2.94; 95% CI, 1.36-9.81) and OS (HR, 2.26; 95% CI, 1.12-5.34). Incorporating LND into the current American Joint Committee on Cancer staging system successfully stratified risk groups compared with the traditional TNM staging system.
Conclusions and Relevance
This single-institute study demonstrates the reproducibility of LND as a predictor of outcomes in PTC. Lymph node density can potentially assist in identifying patients with poorer survival who may benefit from more aggressive adjuvant therapy.
Introduction
The incidence of papillary thyroid cancer (PTC) has increased steadily since the 1970s; however, the 10-year overall survival (OS) rate remains greater than 90%. Approximately half of patients have cervical lymph node metastases at presentation, with more than 90% of patients having occult micrometastases in the lymph nodes. Still, the prognostic significance of nodal metastases, as well as that of concurrent predictors such as size and number of involved nodes and extracapsular spread (ECS), remains unclear, particularly as prophylactic central compartment dissection has become more prevalent.
In the American Joint Committee on Cancer (AJCC) TNM staging system, the presence of lymph node metastases results in upstaging, but the prognostic role of lymph node metastases is controversial.
Lymph node density (LND) is the ratio of the number of positive lymph nodes excised to the total number of excised lymph nodes. It is used to minimize the risk of pathological understaging caused by limited lymph node dissection or inadequate specimen processing. Lymph node density has been shown to play a predictive role after surgery for pancreatic, gastric, colon, oral cavity, and thyroid cancers. This ratio controls for bias in both the sampling method (the extent of surgical lymph node clearance, ie, the total number of nodes removed during surgery) and the specimen processing (meticulousness of the histopathologic processing of the neck dissection specimen).
In this study, we evaluated the role of LND as a prognostic predictor for disease-specific survival (DSS) in patients with PTC according to the location of the involved lymph nodes (ie, lateral and central neck nodes). We also assessed the validity of incorporating LND into the AJCC staging system.
Methods
Patients and Methods
This retrospective study was approved by the institutional review board at MD Anderson Cancer Center, and informed consent was waived due to the retrospective nature of the study. The study cohort included anonymized data for 2542 patients with a median follow-up of 55 months (range, 4-192 months). No patients had prior treatment, and all had received their primary surgical treatment for PTC at MD Anderson between January 1, 2000, and December 31, 2015. Demographic, clinical, and pathological data were retrospectively collected for all patients by using a uniform database template. Inclusion criteria consisted of a confirmed histopathological diagnosis of PTC, an available neck dissection specimen, and greater than 6 months of follow-up data unless death or disease recurrence occurred before 6 months of follow-up. Table 1 presents the demographic and clinical characteristics of the cohort.
Table 1. Demographic Characteristics and Clinical Data for Surgical Patients With Papillary Thyroid Carcinoma.
| Variable | No. (%) (N = 2542) |
|---|---|
| Age, median (range), y | 48 (18-97) |
| Sex | |
| Male | 1801 (71) |
| Female | 741 (29) |
| Treatment | |
| Surgery | 1138 (45) |
| Surgery + radioactive iodine treatment | 1404 (55) |
| Extent of neck dissection | |
| Central | 787 (31) |
| Central and lateral | 619 (2) |
| Type of neck dissection | |
| Electivea | 635 (44) |
| Therapeutic | 811 (56) |
| T classification | |
| 1 | 1116 (44) |
| 2 | 272 (10) |
| 3 | 1020 (41) |
| 4 | 125 (5) |
| N classification | |
| N0 | 1487 (56) |
| N1a | 429 (20) |
| N1b | 626 (24) |
| Overall TNM stage | |
| I | 1591 (62) |
| II | 107 (4) |
| III | 499 (20) |
| IV | 345 (14) |
| Follow-up, mo | |
| Mean (SD) | 63.1 (43) |
| Median (range) | 55 (4-192) |
All elective neck dissections were central neck dissections.
Histopathological Analysis
All tissues were evaluated by a certified head and neck pathologist according to the guidelines for the examination and reporting of head and neck cancer specimens. A total of 33 796 lymph nodes were evaluated, 9439 (28%) of which were positive.
Statistical Analysis
A log-rank test was used to compare OS, DSS, disease-free survival, locoregional control, and distant metastasis rates calculated with use of the Kaplan-Meier method. Overall survival was defined as the interval between the surgery date and the date of last follow-up or death. Disease-specific survival was defined as the interval between the diagnosis time and PTC-related death. Recurrence was determined to calculate disease-free survival according to the response categories in the 2015 Guidelines of the American Thyroid Association.
Factors with prognostic potential as indicated by univariate analyses were included in a multivariate analysis by using the Cox proportional hazards regression model. Continuous variables were compared by using the t test and categorical variables with the χ2 test. All tests were 2 sided with P < .05 as the limit of statistical significance. All data were analyzed with use of the JMP 12.1 software package (SAS Institute Inc). To determine the threshold for LND, we used time-dependent receiver operating characteristic curves to calculate sensitivity, specificity, and likelihood ratios and to define various risk groups of patients with PTC according to LND. We calculated the LND cutoff by using time-dependent receiver operating characteristic curve analysis for disease-specific death. The value of 0.19 was selected with an area under the curve (c index) of 0.84 (95% CI, 0.59-0.94), and specificity and sensitivity for 10-year DSS of 71.6% and 79.1%, respectively. Finally, we compared the current eighth edition TNM staging system with a novel LND-based staging system (eTable in the Supplement).
Results
In this study of 2542 patients with PTC treated at MD Anderson, therapeutic neck dissection was performed in 811 (32%) who had clinical evidence of neck nodal metastasis. Elective neck dissection was performed in 635 (37%) of 1731 patients without clinical evidence of nodal metastasis. All elective neck dissection included the central neck alone. Pathological assessment of neck dissection specimens revealed that 750 of 811 specimens (92%) from those who underwent therapeutic neck dissection were positive for PTC in the lymph nodes. Of the 635 patients who underwent elective neck dissection, specimens from 305 (48%) were positive for PTC in the lymph nodes. Of the 1055 patients who had pN1 disease, 429 (41%) had pN1a disease, and 626 (59%) had pN1b disease.
The 5-year DSS and OS rates for all study patients were 98% (2488 of 2542) and 94% (2384 of 2542), respectively. The 5-year DSS was 100% for patients with pN0 disease and 98% for patients with pN1 disease (effect size, 2%; 95% CI, 0.6%-12%) (eFigure in the Supplement). The 5-year OS was 95% for patients with pN0 and 92% for those with pN1 disease (effect size, 3%; 95% CI, 1.3%-13%) (eFigure in the Supplement). Extracapsular spread had a significant effect on DSS (effect size, 6%; 95% CI, 1%-12%; n = 417) and on OS (effect size, 4%; 95% CI, 1%-15%; n = 417) on univariate analysis.
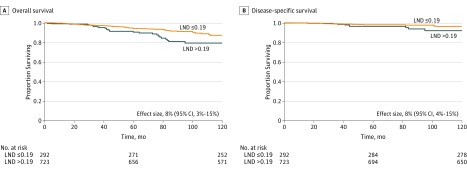
Next, we analyzed the patients with pN1 disease, excluding 40 patients who had only a lateral neck dissection (n = 1015). The median number of lymph nodes in each neck dissection specimen was 23 (range, 4-163 nodes), and the median number of positive nodes was 6 (range, 1-57 nodes). The 10-year DSS was 98% in patients with an LND of 0.19 or less, compared with 90% in patients with an LND greater than 0.19 (effect size, 8%; 95% CI, 4%-15%) (Figure 1). The 10-year OS in patients with an LND of 0.19 or less was 87%, compared with 79% in patients with an LND greater than 0.19 (effect size, 8%; 95% CI, 3%-15%). Of note, a subgroup analysis of patients with central neck disease revealed that central neck LND was a predictor of 10-year DSS (effect size, 4%; 95% CI, 2%-11%) and 10-year OS (effect size, 6%; 95% CI, 2%-10%).
Figure 1. Kaplan-Meier 10-Year Overall and Disease-Specific Survival Curves for Patients With Neck Lymph Nodes Positive for Papillary Thyroid Cancer.
Lymph node density (LND) with a cutoff point of 0.19 (P < .01) was used.
We then assessed the effect of clinicopathological variables on survival in a multivariate model without LND. The variables included in the model were sex, age, tumor morphology, tumor size, extrathyroid extension invasion to the strap muscles, pathological T classification, pN classification, ECS, M classification, overall TNM stage, total number of excised lymph nodes, and radioactive iodine treatment. Pathological N (hazard ratio [HR], 1.28; 95% CI, 1.05-8.36) and M (HR, 2.73; 95% CI, 1.54-8.16) classifications significantly predicted DSS. Pathological T stage (HR, 1.12; 95% CI, 1.01-7.21), the presence of distant metastasis (HR, 1.96; 95% CI, 1.25-7.41), and nodal ECS (HR, 1.19; 95% CI, 1.02-5.41) were significant predictors of OS.
Next, we used the same multivariate model with LND and excluded the total number of lymph nodes excised to eliminate co-linearity between the LND and the total number of lymph nodes. The results showed that an LND greater than 0.19 was independently associated with an adverse DSS (HR, 4.11; 95% CI, 2.11-8.97) and OS (HR, 1.96; 95% CI, 1.24-4.11). Other significant predictors for DSS were tumor size, extrathyroid extension to the strap muscles, nodal classification, distant metastasis, and nodal ECS; variables that were significant for OS were sex, age, presence of distant metastasis, nodal ECS, and radioactive iodine treatment (Table 2).
Table 2. Multivariate Analysis of Prognostic Factors for Overall and Disease-Specific Survival .
| Variable | Adjusted Hazard Ratio (95% CI) (n = 1015) |
|
|---|---|---|
| Overall Survival | Disease-Specific Survival | |
| Sex | ||
| Male | 1 [Reference] | 1 [Reference] |
| Female | 2.07 (1.42-2.96) | 1.14 (0.40-3.16) |
| Age, y | ||
| <45 | 1 [Reference] | 1 [Reference] |
| ≥45 | 3.12 (1.54-7.03) | 1.31 (0.72-1.41) |
| Tumor morphologic type | ||
| Papillary | 1 [Reference] | 1 [Reference] |
| Papillary, follicular variant | 0.94 (0.61-1.38) | 1.09 (0.04-11.3) |
| Papillary, follicular poorly differentiated | 4.03 (0.71-8.43) | 3.08 (0.08-13.6) |
| Change in the risk of death per 1-cm tumor size | 1.71 (0.41-7.79) | 1.37 (1.07-1.81) |
| Extrathyroid extension to the strap muscles | ||
| Absent | 1 [Reference] | 1 [Reference] |
| Present | 1.01 (0.52-1.89) | 3.25 (1.08-10.11) |
| Pathologic T classification | ||
| T1 | 1 [Reference] | 1 [Reference] |
| T2 | 1.46 (0.55-4.51) | 1.51 (1.1-3.9) |
| T3 | 1.91 (0.91-1.14) | 2.67 (5.71-1.72) |
| T4 | 1.94 (0.93-4.21) | 12.6 (1.72-4.73) |
| Pathologic N classification | ||
| N1 | 1 [Reference] | 1 [Reference] |
| N1a | 0.94 (0.35-2.43) | 0.95 (0.18-6.61) |
| Nb | 1.36 (0.49-4.06) | 6.21 (0.96-44.61) |
| M classification | ||
| 0 | 1 [Reference] | 1 [Reference] |
| 1 | 2.12 (1.12-3.7) | 3.14 (1.1-9.81) |
| Extracapsular spread | ||
| Absent | 1 [Reference] | 1 [Reference] |
| Present | 2.28 (1.12-4.44) | 4.36 (1.2-20.61) |
| Treatment group | ||
| Surgery | 1 [Reference] | 1 [Reference] |
| Surgery + radioactive iodine | 2.15 (1.31-3.17) | 3.14 (0.64-13.5) |
| Lymph node density | ||
| ≤0.19 | 1 [Reference] | 1 [Reference] |
| >0.19 | 1.96 (1.24-4.11) | 4.11 (2.11-8.97) |
To control for nodal yield, we first analyzed the distribution of the LND compared with the total number of lymph nodes. The mean (SD) numbers of lymph nodes removed from patients’ central and lateral necks in our cohort were 10.1 (5.4) and 45.2 (24.1), respectively. Nodal yield of 18 lymph nodes was selected because its prognostic implication was previously described. Previous studies showed that once 18 nodes are surgically removed and pathologically analyzed, the neck is likely to be correctly staged and occult microscopic disease adequately treated, with subsequent increases in nodal yield yielding negligible or no further advantage. After including only patients with at least 18 lymph nodes resected (n = 741), we repeated the multivariate analysis, which revealed that LND greater than 0.19 remained a significant marker for DSS (HR, 2.94; 95% CI, 1.36-9.81) and OS (HR, 2.26; 95% CI, 1.12-5.34).
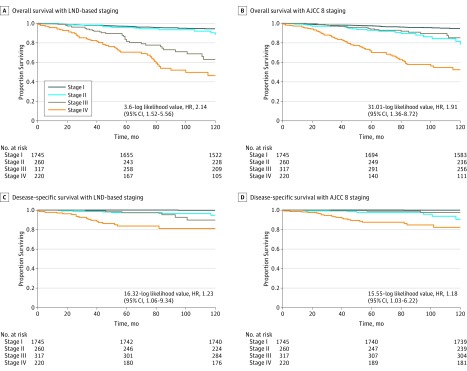
Finally, we compared the current AJCC eighth edition TNM staging system with a novel LND-based TNM staging system (eTable in the Supplement). The multivariate model showed that the fit proportional hazard models for DSS were 36.31 (–log likelihood value, HR, 2.14; 95% CI, 1.52-5.56) and 31.01 (–log likelihood value, HR, 1.91; 95% CI, 1.36-8.72) for the LND-based TNM staging system and the current AJCC eighth edition TNM staging system; and for OS, 16.32 (–log likelihood value, HR, 1.23; 95% CI, 1.06-9.34) and 15.55 (–log likelihood value, HR, 1.18; 95% CI, 1.03-6.22) for the LND-based TNM staging system and the current AJCC eighth edition TNM staging system. Figure 2 shows the DSS and OS Kaplan-Meier curves according to the disease stage groups of the current AJCC staging system and the LND incorporated into the staging system.
Figure 2. Kaplan-Meier Overall and Disease-Specific Survival Curves for Patients With Papillary Thyroid Cancer According to the Eighth Edition American Joint Committee on Cancer (AJCC) TNM Staging System and a Lymph Node Density (LND)-Based Staging System .
Hazard ratios (HRs) and log-likelihood values for each model are presented.
Discussion
Therapeutic neck dissection is performed in patients with PTC who have clinically involved nodes, and elective central compartment neck dissection should be considered in patients with cN0 PTC who have locally advanced primary tumors or clinically involved lateral neck nodes (cN1b), or if information can be obtained from this procedure that can be used to guide further treatment.
Most patients with papillary carcinomas present with lymph node metastases at the time of diagnosis. Some authors have reported no significant association between PTC lymph node metastases and outcome in low-risk patients; however, a recent Surveillance, Epidemiology, and End Results (SEER) database study found that lymph node metastases predicted poor OS. In addition, patients with PTC and cervical lymph node metastasis (particularly central lymph node metastasis) have been shown to have higher rates of locoregional recurrence.
In head and neck cancer, LND has been shown to be superior to conventional nodal staging for predicting outcome. Lymph node density entails 3 factors that influence nodal staging: (1) surgical factors (the yield of the neck dissection), (2) tumor factors (the degree of nodal spread), and (3) sampling factors (the comprehensiveness of the pathological processing). Patients with a similar N classification can have different LNDs. We hypothesized that among patients with similar nodal staging, those with a higher LND would have a worse prognosis than would those with a lower LND.
Previous studies investigated the utility of LND in thyroid cancer using small single-institute cohorts of specific patient groups (eg, only patients with N1a disease) or SEER database mixed thyroid cancer histologic types. These studies and others were designed to determine the association of LND with survival in patients with well-differentiated PTC but showed conflicting evidence about the value of LND in node-positive patients. In the current long-term, single-institute, large nonnational database cohort, we investigated the predictive value of LND in patients with PTC. This study conducted the first large-scale analysis of LND as a prognostic factor for survival in patients with PTC. Subgroup analysis of patients with central neck disease showed that central neck LND was associated with OS and DSS. A recent study by Wang et al showed that LND of the lateral neck is predictive of recurrence using a lymph node ratio of 0.17. These findings suggest that the LND of each neck compartment (central and lateral) might be used separately as a prognostic factor. Taken together, our data might suggest a modification to the AJCC staging classification that includes incorporation of LND into the current nodal classification as a complementary measure for patients with nodal metastases.
Limitations
We recognized that the interplay with other factors related to nodal status, such as the volume of the lymph node and the extent of ECS, needed elaboration. Given the retrospective nature of the pathology reports, we could not differentiate between lymph nodes with micrometastatic foci and grossly involved lymph nodes. We also realized that a limitation of this study was the potential inconsistency in surgical technique that may have introduced potential errors. In addition, our data were from a single comprehensive cancer center and might thus have been biased toward a surgical practice with relatively more advanced disease presentation and treatment. However, all of our patients had either selective or comprehensive neck dissection with a nodal yield that correlated with previously reported mean lymph node yield in a neck dissection accompanying total thyroidectomy, which ranged from 6 to 64 nodes. The variation in the number of lymph nodes retrieved from our specimens (ie, the denominator of the LND) was therefore similar to that in other studies. A repeated analysis using an established number of lymph nodes resected resulted in results similar to those in the general population. We also recognized that more accurate LND calculation using molecular methods to detect occult micrometastases may have affected the LND cutoff point. Due to the retrospective nature of the study, data regarding the indication for prophylactic neck dissection were not reliably available. Conversely, the significance of LND as a predictor of outcome in our heterogeneous cohort across multiple surgeons ensured the broad applicability of LND as a prognosticator into standard practice in diverse patient populations.
Conclusions
We have validated the importance of LND in a large single-institution study by demonstrating its reproducibility as a predictor of outcome in PTC, both as an independent factor and as a useful adjunct to the current eighth edition TNM staging system. Moreover, we have shown that LND may be used to identify patients at high risk of PTC disease–related death.
eTable. Lymph node density–based TNM staging
eFigure. Kaplan-Meier overall and disease-specific survival curves for patients with neck lymph nodes positive (red) and negative (blue) for papillary thyroid cancer
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164-2167. [DOI] [PubMed] [Google Scholar]
- 2.Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996;5(1):43-63. [PubMed] [Google Scholar]
- 3.Nam-Goong IS, Kim HY, Gong G, et al. . Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf). 2004;60(1):21-28. [DOI] [PubMed] [Google Scholar]
- 4.Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002;131(3):249-256. [DOI] [PubMed] [Google Scholar]
- 5.Leboulleux S, Rubino C, Baudin E, et al. . Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90(10):5723-5729. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya N. A population-based analysis of survival factors in differentiated and medullary thyroid carcinoma. Otolaryngol Head Neck Surg. 2003;128(1):115-123. [DOI] [PubMed] [Google Scholar]
- 7.Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71(9):731-734. [DOI] [PubMed] [Google Scholar]
- 8.Agrama MT, Reiter D, Cunnane MF, Topham A, Keane WM. Nodal yield in neck dissection and the likelihood of metastases. Otolaryngol Head Neck Surg. 2003;128(2):185-190. [DOI] [PubMed] [Google Scholar]
- 9.Patel SG, Amit M, Yen TC, et al. ; International Consortium for Outcome Research (ICOR) in Head and Neck Cancer . Lymph node density in oral cavity cancer: results of the International Consortium for Outcomes Research. Br J Cancer. 2013;109(8):2087-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SR, Kim HO, Son BH, Shin JH, Yoo CH. Prognostic significance of the metastatic lymph node ratio in patients with gastric cancer. World J Surg. 2012;36(5):1096-1101. [DOI] [PubMed] [Google Scholar]
- 11.Partelli S, Fernandez-Del Castillo C, Bassi C, et al. . Invasive intraductal papillary mucinous carcinomas of the pancreas: predictors of survival and the role of lymph node ratio. Ann Surg. 2010;251(3):477-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu N, Shi RL, Lu ZW, et al. . Metastatic lymph node ratio can further stratify risk for mortality in medullary thyroid cancer patients: a population-based analysis. Oncotarget. 2016;7(40):65937-65945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu IS, Song CI, Choi SH, Roh JL, Nam SY, Kim SY. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol. 2014;21(1):277-283. [DOI] [PubMed] [Google Scholar]
- 14.Jiang T, Huang C, Xu Y, et al. . Ratio of positive lymph nodes: the prognostic value in stage IV thyroid cancer. Oncotarget. 2017;8:79462-79468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins KT, Shaha AR, Medina JE, et al. ; Committee for Neck Dissection Classification, American Head and Neck Society . Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008;134(5):536-538. [DOI] [PubMed] [Google Scholar]
- 16.Pathology Group Guidelines for the examination and reporting of head and neck cancer specimens. Leeds, UK: Yorkshire Cancer Network; 2010. http://www.angcn.nhs.uk/download/1ac80ad8-643d-4721-a7a3-06dfde2fb219. Accessed October 11, 2017.
- 17.Peto R, Pike MC, Armitage P, et al. . Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haugen BR, Alexander EK, Bible KC, et al. . 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox D. Regression models and life-tables. J R Stat Soc [Ser A]. 1982;34(2):187-220. [Google Scholar]
- 20.Xiao LB, Yu JX, Wu WH, Xu FF, Yang SB. Superiority of metastatic lymph node ratio to the 7th edition UICC N staging in gastric cancer. World J Gastroenterol. 2011;17(46):5123-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espín F, Bianchi A, Llorca S, et al. . Metastatic lymph node ratio versus number of metastatic lymph nodes as a prognostic factor in gastric cancer. Eur J Surg Oncol. 2012;38(6):497-502. [DOI] [PubMed] [Google Scholar]
- 22.Etzioni R, Kooperberg C, Pepe M, Smith R, Gann PH. Combining biomarkers to detect disease with application to prostate cancer. Biostatistics. 2003;4(4):523-538. [DOI] [PubMed] [Google Scholar]
- 23.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337-344. [DOI] [PubMed] [Google Scholar]
- 24.Heaton CM, Chang JL, Orloff LA. Prognostic implications of lymph node yield in central and lateral neck dissections for well-differentiated papillary thyroid carcinoma. Thyroid. 2016;26(3):434-440. [DOI] [PubMed] [Google Scholar]
- 25.Robinson TJ, Thomas S, Dinan MA, Roman S, Sosa JA, Hyslop T. How many lymph nodes are enough? assessing the adequacy of lymph node yield for papillary thyroid cancer. J Clin Oncol. 2016;34(28):3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M, Kim WG, Oh HS, et al. . Comparison of the 7th and 8th editions of the AJCC/UICC TNM staging system for differentiated thyroid cancer. Thyroid. 2017;27(9):1149-1155. [DOI] [PubMed] [Google Scholar]
- 27.Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148(6):1100-1106. [DOI] [PubMed] [Google Scholar]
- 28.Randolph GW, Duh QY, Heller KS, et al. ; American Thyroid Association Surgical Affairs Committee’s Taskforce on Thyroid Cancer Nodal Surgery . The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22(11):1144-1152. [DOI] [PubMed] [Google Scholar]
- 29.Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106(3):524-531. [DOI] [PubMed] [Google Scholar]
- 30.Slidell MB, Chang DC, Cameron JL, et al. . Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15(1):165-174. [DOI] [PubMed] [Google Scholar]
- 31.Baek SK, Jung KY, Kang SM, et al. . Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. 2010;20(2):147-152. [DOI] [PubMed] [Google Scholar]
- 32.Beasley NJ, Lee J, Eski S, Walfish P, Witterick I, Freeman JL. Impact of nodal metastases on prognosis in patients with well-differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg. 2002;128(7):825-828. [DOI] [PubMed] [Google Scholar]
- 33.Amar A, Rapoport A, Curioni OA, Dedivitis RA, Cernea CR, Brandão LG. The density of metastatic lymph node as prognostic factor in squamous cell carcinoma of the tongue and floor of the mouth. Braz J Otorhinolaryngol. 2012;78(3):86-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SY, Nam SY, Choi SH, Cho KJ, Roh JL. Prognostic value of lymph node density in node-positive patients with oral squamous cell carcinoma. Ann Surg Oncol. 2011;18(8):2310-2317. [DOI] [PubMed] [Google Scholar]
- 35.Rudra S, Spiotto MT, Witt ME, Blair EA, Stenson K, Haraf DJ. Lymph node density—prognostic value in head and neck cancer. Head Neck. 2014;36(2):266-272. [DOI] [PubMed] [Google Scholar]
- 36.Chen CC, Lin JC, Chen KW. Lymph node ratio as a prognostic factor in head and neck cancer patients. Radiat Oncol. 2015;10:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beal SH, Chen SL, Schneider PD, Martinez SR. An evaluation of lymph node yield and lymph node ratio in well-differentiated thyroid carcinoma. Am Surg. 2010;76(1):28-32. [PubMed] [Google Scholar]
- 38.Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol. 2013;20(6):1906-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LY, Palmer FL, Nixon IJ, et al. . Lateral neck lymph node characteristics prognostic of outcome in patients with clinically evident N1b papillary thyroid cancer. Ann Surg Oncol. 2015;22(11):3530-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vas Nunes JH, Clark JR, Gao K, et al. . Prognostic implications of lymph node yield and lymph node ratio in papillary thyroid carcinoma. Thyroid. 2013;23(7):811-816. [DOI] [PubMed] [Google Scholar]
- 41.Trimble EL, Abrams JS, Meyer RM, et al. . Improving cancer outcomes through international collaboration in academic cancer treatment trials. J Clin Oncol. 2009;27(30):5109-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Lymph node density–based TNM staging
eFigure. Kaplan-Meier overall and disease-specific survival curves for patients with neck lymph nodes positive (red) and negative (blue) for papillary thyroid cancer