Key Points
Question
What is the effect of a healthy low-fat (HLF) diet vs a healthy low-carbohydrate (HLC) diet on weight change at 12 months and are these effects related to genotype pattern or insulin secretion?
Findings
In this randomized clinical trial among 609 overweight adults, weight change over 12 months was not significantly different for participants in the HLF diet group (−5.3 kg) vs the HLC diet group (−6.0 kg), and there was no significant diet-genotype interaction or diet-insulin interaction with 12-month weight loss.
Meaning
There was no significant difference in 12-month weight loss between the HLF and HLC diets, and neither genotype pattern nor baseline insulin secretion was associated with the dietary effects on weight loss.
Abstract
Importance
Dietary modification remains key to successful weight loss. Yet, no one dietary strategy is consistently superior to others for the general population. Previous research suggests genotype or insulin-glucose dynamics may modify the effects of diets.
Objective
To determine the effect of a healthy low-fat (HLF) diet vs a healthy low-carbohydrate (HLC) diet on weight change and if genotype pattern or insulin secretion are related to the dietary effects on weight loss.
Design, Setting, and Participants
The Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) randomized clinical trial included 609 adults aged 18 to 50 years without diabetes with a body mass index between 28 and 40. The trial enrollment was from January 29, 2013, through April 14, 2015; the date of final follow-up was May 16, 2016. Participants were randomized to the 12-month HLF or HLC diet. The study also tested whether 3 single-nucleotide polymorphism multilocus genotype responsiveness patterns or insulin secretion (INS-30; blood concentration of insulin 30 minutes after a glucose challenge) were associated with weight loss.
Interventions
Health educators delivered the behavior modification intervention to HLF (n = 305) and HLC (n = 304) participants via 22 diet-specific small group sessions administered over 12 months. The sessions focused on ways to achieve the lowest fat or carbohydrate intake that could be maintained long-term and emphasized diet quality.
Main Outcomes and Measures
Primary outcome was 12-month weight change and determination of whether there were significant interactions among diet type and genotype pattern, diet and insulin secretion, and diet and weight loss.
Results
Among 609 participants randomized (mean age, 40 [SD, 7] years; 57% women; mean body mass index, 33 [SD, 3]; 244 [40%] had a low-fat genotype; 180 [30%] had a low-carbohydrate genotype; mean baseline INS-30, 93 μIU/mL), 481 (79%) completed the trial. In the HLF vs HLC diets, respectively, the mean 12-month macronutrient distributions were 48% vs 30% for carbohydrates, 29% vs 45% for fat, and 21% vs 23% for protein. Weight change at 12 months was −5.3 kg for the HLF diet vs −6.0 kg for the HLC diet (mean between-group difference, 0.7 kg [95% CI, −0.2 to 1.6 kg]). There was no significant diet-genotype pattern interaction (P = .20) or diet-insulin secretion (INS-30) interaction (P = .47) with 12-month weight loss. There were 18 adverse events or serious adverse events that were evenly distributed across the 2 diet groups.
Conclusions and Relevance
In this 12-month weight loss diet study, there was no significant difference in weight change between a healthy low-fat diet vs a healthy low-carbohydrate diet, and neither genotype pattern nor baseline insulin secretion was associated with the dietary effects on weight loss. In the context of these 2 common weight loss diet approaches, neither of the 2 hypothesized predisposing factors was helpful in identifying which diet was better for whom.
Trial Registration
clinicaltrials.gov Identifier: NCT01826591
This randomized clinical trial compares the effects of a healthy low-fat vs a healthy low-carbohydrate diet on 12-month weight change among adults aged 18 to 50 years with a body mass index of 28 to 40 without diabetes, and investigates modification of the diet effect by genotype pattern or insulin secretion.
Introduction
Obesity is a 21st-century major public health challenge.1,2 Among many strategies studied for weight loss, a common contrast has been low-fat diets vs low-carbohydrate diets.3,4,5 Most diet trials have reported modest (ie, <5%) mean weight loss after 12 months and negligible mean weight loss differences between diet groups.6 In contrast, individual weight losses have varied widely within diet groups in these studies, ranging from approximately 25 kg lost to approximately 5 kg gained.3,4,5
The substantial variability of weight loss response suggests some strategies may work better for some individuals than others, and that no one diet should be recommended universally.7 Yet, interindividual differences in response to diet are poorly understood. Some studies have reported that genotype variation could predispose individuals to differential weight loss that varies by diet type.8,9
In a preliminary retrospective study, a 3-fold difference was observed in 12-month weight loss for initially overweight women who were determined to have been appropriately matched (mean weight loss of 6 kg) vs mismatched (mean weight loss of 2 kg) to a low-fat or low-carbohydrate diet based on multilocus genotype patterns with single-nucleotide polymorphisms (SNPs) from 3 genes (PPARG, ADRB2, and FABP2) relevant to fat and carbohydrate metabolism (a putative low-fat–responsive genotype and a low-carbohydrate–responsive genotype). The participants with the low-fat–responsive genotype were observed to lose more weight when assigned to a low-fat diet than those assigned to a low-carbohydrate diet, and vice versa for those with the low-carbohydrate–responsive genotype.9,10
Similarly, several studies11,12,13,14 have reported that baseline insulin dynamics may explain differential weight loss success obtained via a low-fat diet vs a low-carbohydrate diet. For example, individuals with greater insulin resistance may have better success with low-carbohydrate diets due to the decreased demand on insulin to clear a lower amount of dietary carbohydrate delivered to the circulation. However, these studies were limited by relatively small sample sizes or post hoc analyses of the results.
The primary objective of the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) study was to test whether (1) a set of 3 SNP genotype patterns or (2) baseline differences in insulin secretion (the blood insulin concentration at 30 minutes after a glucose challenge; INS-30),12,13 or both, predisposed individuals to differential success in 12-month weight change while on a low-fat diet vs a low-carbohydrate diet.
Methods
The Stanford University human subjects committee approved the study. All study participants provided written informed consent.
Study Design
This single-site, parallel-group, weight loss diet trial randomized individuals to a healthy low-fat diet or a healthy low-carbohydrate diet for 12 months. Participant enrollment began on January 29, 2013, and continued through April 14, 2015. The date of final follow-up was May 16, 2016. Interventions consisted primarily of class-based instruction. Five waves of recruitment (cohorts) had staggered start dates between March 2013 and March 2015. The primary outcome was 12-month weight change.
The first primary hypothesis was that there is a significant diet × genotype pattern interaction for weight loss. The second primary hypothesis was that there is a significant diet × insulin secretion interaction for weight loss. Secondary outcomes included anthropometric measures, plasma lipid levels, insulin and glucose levels, and blood pressure levels. The protocol update and statistical analysis plan are included in Supplement 1 and the full study protocol was published previously10 (the protocol included details regarding blood sampling, storage, and specific laboratory assays).
Participants
We aimed to recruit 600 adults from the Stanford and San Francisco Bay areas of California using media advertisements and email lists from previous recruitment for nutrition studies conducted by our laboratory group. We considered men and premenopausal women aged 18 to 50 years with a body mass index (calculated as weight in kilograms divided by height in meters squared) of 28 to 40.
The major criteria for exclusion were having uncontrolled hypertension or metabolic disease; diabetes; cancer; heart, renal, or liver disease; and being pregnant or lactating. Individuals were excluded if taking hypoglycemic, lipid-lowering, antihypertensive, psychiatric, or other medications known to affect body weight or energy expenditure. Any medication type not noted was allowed if the individual had been stable while taking such medication for at least 3 months prior to baseline data collection.
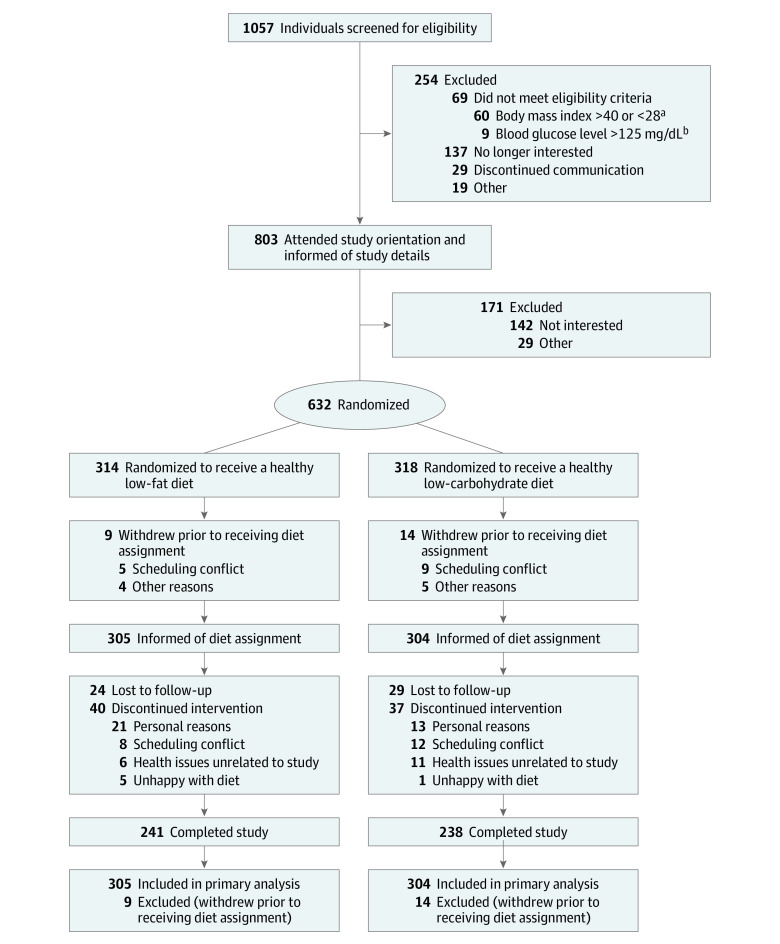
Randomization to a healthy low-fat diet or a healthy low-carbohydrate diet was performed using an allocation sequence determined by computerized random-number generation (Blockrand in R version 3.4.0; R Project for Statistical Computing) in block sizes of 8 (with 4 individuals going to each diet) by a statistician not involved in intervention delivery or data collection. Participants did not learn of their diet group assignment until they completed all baseline measures and attended their first intervention class (Figure 1).
Figure 1. Flow of Participants Through the Diet Intervention Examining The Factors Interacting with Treatment Success Trial.
aBody mass index is calculated as weight in kilograms divided by height in meters squared.
bTo convert glucose to mmol/L, multiply by 0.0555.
The original study design was a 2 × 2 factorial design (diet × genotype-pattern matching). However, near the onset of the study, the initial funding was more than doubled, allowing for a 50% increase in sample size, the addition of a second primary hypothesis for the assessment of a diet × insulin secretion interaction, and an expanded set of measurements. To test for both primary hypotheses, the study was changed to a simple parallel group design with testing for 2 interactions (described in further detail in eAppendix 1 in Supplement 2).
Weight Loss Intervention
The protocol included a 1-month run-in period during which participants were instructed to maintain their habitual diet, physical activity level, and body weight. The intervention involved 22 instructional sessions held over 12 months in diet-specific groups of approximately 17 participants per class. Sessions were held weekly for 8 weeks, then every 2 weeks for 2 months, then every 3 weeks until the sixth month, and monthly thereafter. Classes were led by 5 registered dietitian health educators who each taught 1 healthy low-fat class and 1 healthy low-carbohydrate class per cohort. Dietitians were blinded to all laboratory measures and genotype.
The dietary interventions were described previously.10 Briefly, the main goals were to achieve maximal differentiation in intake of fats and carbohydrates between the 2 diet groups while otherwise maintaining equal treatment intensity and an emphasis on high-quality foods and beverages. Thus, participants were instructed to reduce intake of total fat or digestible carbohydrates to 20 g/d during the first 8 weeks. Higher priorities for reduction were given to specific foods and food groups that derived their energy content primarily from fats or carbohydrates. For example, the reduction of edible oils, fatty meats, whole-fat dairy, and nuts was prioritized for the healthy low-fat group, whereas the reduction of cereals, grains, rice, starchy vegetables, and legumes was prioritized for the healthy low-carbohydrate group.
Then individuals slowly added fats or carbohydrates back to their diets in increments of 5 to 15 g/d per week until they reached the lowest level of intake they believed could be maintained indefinitely. No explicit instructions for energy (kilocalories) restriction were given. Both diet groups were instructed to (1) maximize vegetable intake; (2) minimize intake of added sugars, refined flours, and trans fats; and (3) focus on whole foods that were minimally processed, nutrient dense, and prepared at home whenever possible. Other components of the emphasis on high-quality food for both diet groups are described elsewhere.10
Participants were encouraged to follow current physical activity recommendations.15 Health educators emphasized emotional awareness and behavior modification to support dietary adherence and weight loss. Behavioral modification strategies included empirically supported principles of self-regulatory behavior change (eg, goal setting, self-efficacy building, supportive environments, and relapse prevention) based on social cognitive theory and the transtheoretical model.10,16,17,18
Outcome Measurements
All data were collected at baseline and at months 3, 6, and 12 for all cohorts unless noted otherwise. Staff who measured outcomes were blinded to diet assignment, genotype pattern, INS-30, and diet assignment. Dietary intake at each time point was assessed using 3 unannounced 24-hour multiple-pass recall interviews (2 on weekdays and 1 on a weekend day).19
Total energy expenditure was assessed using the Stanford Seven-Day Physical Activity Recall questionnaire.20 Both the dietary intake and physical activity recall were self-reported measures. Weight was measured by digital scale at the Stanford Clinical Translational Research Unit and 12-month weight change was the primary outcome.
Genotype pattern and insulin secretion were assessed for interaction testing. The Affymetrix UK Biobank Axiom microarray was used for analysis of 820 967 SNPs and insertions or deletions. The array included 2 of the SNPs from the original study design: PPARG (rs1801282) and ADRB2 (rs1042714). FABP2 (rs1799883) was imputed with an imputation quality score (r2 = 0.99). Additional details appear in eAppendix 2 in Supplement 2. The 3 SNP multilocus genotype patterns have been explored previously.9
Of 27 possible 3-locus genotypes that could arise from the combination of the 3 SNPs, 15 were observed with 1% or greater genotype frequency in previously studied samples of adults. The multilocus genotypes were grouped into those predicted to be more sensitive to fat (low-fat genotype; patterns 1-5), more sensitive to carbohydrates (low-carbohydrate genotype; patterns 6-14), or sensitive to neither genotype (pattern 15). Additional details are available in eAppendix 3 in Supplement 2.
Before randomization and at months 6 and 12, each participant completed an oral glucose tolerance test of 75 g. This included measurement of insulin concentration 30 minutes after glucose consumption (ie, INS-30, which is a proxy measure of insulin secretion).10,21,22 When this study was first designed, insulin sensitivity was to be measured and used as a predictor of differential weight loss success. After the study was initiated, reports were published12,13,23,24 indicating INS-30 was a successful predictor of weight loss in the context of low-carbohydrate diets or similar diets. There also was evidence25 that early-phase insulin secretion differed markedly between diets that were similar to those tested in the DIETFITS study. Prior to examining any data, we modified the primary hypothesis of our study and tested baseline INS-30 rather than a measure of insulin sensitivity as the putative effect modifier. No other glucose or insulin variables were tested for effect modification.
A set of related secondary outcomes was assessed. Concentrations of plasma lipids, glucose, and insulin were measured in fasting samples, waist circumference was assessed by measuring tape, blood pressure was measured via automated device, and all of these were assessed using standard assessment techniques.10
Body composition was assessed by dual-energy x-ray absorptiometry and both respiratory exchange ratio (bounded by 0.7 [using solely fat for fuel] and 1.0 [using solely glucose for fuel]) and resting energy expenditure were assessed by metabolic cart (ie, measures respiratory exchange of oxygen and carbon dioxide while a participant is supine and resting) at baseline and at months 6 and 12 in cohorts 2 through 5. Adequate funding became available for dual-energy x-ray absorptiometry, respiratory exchange ratio, and resting energy expenditure only after cohort 1 was enrolled. The metabolic syndrome was determined using Adult Treatment Panel III guidelines from the National Cholesterol Education Program.26
Statistical Analysis
Based on the original study design, assuming 100 participants in each of the 4 relevant groups (genotype and dietary assignment), and normally distributed values of weight change at 12 months, there was 80% power to detect clinically meaningful differences in treatment effect by genotype (eg, whether dietary assignment had an effect on weight change at 12 months except for those assigned to the low-carbohydrate diet who have the low-carbohydrate genotype because such individuals lose 3.2 kg on average). This calculation was based on simulations, and assumed a 2-sided Wald test conducted at the .05 level of significance.
Under similar assumptions regarding the statistical testing and type I error, and assuming a sample size of only 400 participants (200 in each treatment group), there was greater than 80% power to detect differences in the treatment effect with insulin secretion, including for example, if for every 1-unit increase in insulin secretion, weight loss at 12 months increases by 0.8 lb (0.36 kg). These power calculations were performed a priori for the originally planned sample size of 400. As described in greater detail in eAppendix 1 in Supplement 2, after initially being funded by the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases in 2012, additional funding was received to augment the trial, which involved, among other modifications, increasing the sample size from 400 to 600, and adding INS-30 as a second variable for interaction testing. With the larger sample size, the study had even greater statistical power, estimated at 90% based on post hoc calculations.
The main hypotheses addressed 12-month weight change by diet, diet and genotype, and diet and baseline INS-30. All hypotheses were addressed using generalized, linear mixed-effects models.27,28 We applied modified intent-to-treat principles. This means that all participants who were randomized and had baseline information were included in the analysis and analyzed according to original treatment assignment, regardless of adherence or loss to follow-up (Figure 1). For the hypothesis about the effect of diet group on 12-month weight change, a linear mixed-effects model for weight that accounted for missing data under flexible assumptions regarding missingness was used with fixed effects for diet, time (baseline, 3, 6, and 12 months), and their interaction, along with a random effect for participant. For the hypotheses involving diet and genotype (or diet and baseline INS-30), an additional fixed effect was added for genotype (or baseline INS-30), along with all 2- and 3-way interactions (model appears in eAppendix 4 in Supplement 2).
The validity of such an analysis relies on the assumption that the missing outcome data measured at follow-up are unrelated to unobserved values of weight conditional on observed variables such as treatment assignment and baseline and intermittent values of weight. The hypothesis about diet was tested using a Wald test for the 2-way interaction between the 12-month time point and diet. The hypothesis about genotype (or baseline INS-30) was tested using a Wald test for the 3-way interaction between the 12-month time point, diet, and genotype (or baseline INS-30). Genotype was defined as matched for those participants with a 3-SNP combination suggesting success on a low-carbohydrate diet who were randomized to the low-carbohydrate diet, or for those participants with a 3-SNP combination suggesting success on a low-fat diet who were randomized to the low-fat diet. Genotype was otherwise defined as mismatched and is described in eAppendices 2 and 3 in Supplement 2.
There were 185 individuals who were not classified as having either the low-fat genotype pattern or a low-carbohydrate genotype pattern (146 individuals with other 3-SNP patterns and 39 with missing or compromised genotyping data) who were excluded from the genotype analysis for the first hypothesis as originally planned.10 An additional diet-genotype analysis was performed, restricting the study population to whites only and focusing on only 1 ancestry group as originally planned.10 The second hypothesis was tested using a Wald test for the interaction among diet, 12-month time point, and baseline INS-30. The INS-30 variable was analyzed as a continuous variable, but is presented as tertiles for ease of presentation in parallel to the presentation of genotype pattern data. The cutoffs for the tertiles were determined using the baseline insulin concentrations of all 609 participants.
A Satterthwaite approximation for denominator degrees of freedom was used in all Wald tests.29 All tests were 2-sided and conducted at the .05 level of significance. Formal hypothesis testing was performed only for the 2 primary hypotheses. All other P values that were generated were purely descriptive in nature and correspond to secondary and exploratory analyses. Statistical analyses were performed using R version 3.4.0 (R Project for Statistical Computing). Specifically, the lme430 package was used for mixed-effects models and the lmerTest29 package was used for hypothesis tests in the mixed-effects models.
Results
Among 609 participants randomized (mean age, 40 [SD, 7] years; 57% women; mean body mass index, 33 [SD, 3]; 244 [40%] had a low-fat genotype; 180 [30%] had a low-carbohydrate genotype; mean baseline INS-30, 93 μIU/mL), 481 (79%) completed the trial. The flow of the participants through the trial appears in Figure 1. Baseline characteristics by diet group appear in Table 1. Among participants in the healthy low-fat diet group, 130 (42.6%) had the low-fat genotype and 83 (27.2%) had the low-carbohydrate genotype, whereas in the healthy low-carbohydrate group, 114 (37.5%) had the low-fat genotype and 97 (31.9%) had the low-carbohydrate genotype.
Table 1. Baseline Demographics and Anthropometric and Metabolic Variables.
| Healthy Low-Fat Diet (n = 305) | Healthy Low-Carbohydrate Diet (n = 304) | |
|---|---|---|
| Sex, No. (%) | ||
| Women | 167 (54.8) | 179 (58.9) |
| Men | 138 (45.2) | 125 (41.1) |
| Age, mean (SD), y | 39.3 (6.8) | 40.2 (6.7) |
| Highest level of education achieved, No. (%)a | ||
| <High school degree | 2 (0.6) | 2 (0.6) |
| High school degree | 5 (1.6) | 11 (3.6) |
| Some college | 63 (20.7) | 67 (22.0) |
| College degree | 102 (33.4) | 106 (34.9) |
| Some postgraduate school | 25 (8.2) | 12 (3.9) |
| Postgraduate degree | 107 (35.1) | 103 (33.9) |
| Race/ethnicity, No. (%)b | ||
| White | 176 (57.7) | 182 (59.9) |
| Hispanic | 67 (22.0) | 61 (20.1) |
| Asian | 30 (9.8) | 30 (9.9) |
| African American | 10 (3.3) | 13 (4.3) |
| American Indian, Alaskan Native, or Pacific Islander | 3 (1.0) | 0 |
| Other | 19 (6.2) | 18 (5.9) |
| Weight, mean (SD), kg | ||
| Women | 90.7 (11.5) | 88.9 (12.5) |
| Men | 105.7 (13.9) | 106.8 (13.7) |
| Both sexes | 97.5 (14.7) | 96.3 (15.7) |
| Body mass index, mean (SD)c | ||
| Women | 33.3 (3.4) | 32.9 (3.4) |
| Men | 33.5 (3.4) | 33.8 (3.4) |
| Both sexes | 33.4 (3.4) | 33.3 (3.4) |
| Body fat %, mean (SD)d | ||
| Women | 41.0 (3.9) | 40.4 (4.0) |
| Men | 29.9 (4.5) | 30.3 (4.7) |
| Both sexes | 36.3 (6.9) | 36.5 (6.6) |
| Waist circumference, mean (SD), cme | ||
| Women | 103.5 (10.4) | 102.6 (10.5) |
| Men | 111.8 (9.7) | 112.7 (9.9) |
| Both sexes | 107.2 (10.9) | 106.7 (11.4) |
| Blood lipid level, mean (SD), mmol/L | ||
| High-density lipoprotein cholesterol | 1.28 (0.23) | 1.29 (0.24) |
| Low-density lipoprotein cholesterolf | 2.89 (0.79) | 2.94 (0.68) |
| Triglycerides | 1.45 (0.80) | 1.45 (1.03) |
| Blood pressure, mean (SD), mm Hgg | ||
| Systolic | 122.9 (12.5) | 122.9 (12.4) |
| Diastolic | 81.0 (7.3) | 81.2 (7.8) |
| Fasting glucose, mean (SD), mg/dL | 98.6 (8.6) | 98.5 (9.7) |
| Fasting insulin, mean (SD), μIU/mL | 15.9 (13.5) | 15.5 (8.0) |
| Insulin-30, mean (SD), μIU/mLh | 95.1 (67.5) | 91.8 (61.7) |
| Metabolic syndrome, No. (%)i | 106 (34.8) | 100 (32.9) |
| Respiratory exchange ratio, mean (SD)j,k | 0.861 (0.065) | 0.862 (0.058) |
| Resting energy expenditure, mean (SD), kcalj,k | 1651 (283) | 1629 (293) |
| Energy expenditure, mean (SD), kcal/kg/dj,l | 32.6 (1.7) | 32.5 (2.2) |
| Genotype, No. (%)m | ||
| Low fat | 130 (42.6) | 114 (37.5) |
| Low carbohydrate | 83 (27.2) | 97 (31.9) |
| Neither | 70 (23.0) | 76 (25.0) |
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555; high-density and low-density lipoprotein cholesterol to mg/dL, divide by 0.0259; insulin to pmol/L, multiply by 6.945; triglycerides to mg/dL, divide by 0.0113.
There were missing data for 1 participant in the low-fat group and 3 participants in the low-carbohydrate group.
Determined by self-report using fixed categories.
Calculated as weight in kilograms divided by height in meters squared.
Available only for cohorts 2 through 5 because additional funding became available for use of dual-energy x-ray absorptiometry and technician time. There were missing data for 77 participants in the low-fat group and 66 participants in the low-carbohydrate group. In addition, 37 participants in the low-fat group and 28 participants in the low-carbohydrate group declined being measured.
There were missing data for 3 participants in the low-fat group and 2 participants in the low-carbohydrate group.
There were missing data for 1 participant in the low-carbohydrate group.
There were missing data for 2 participants in the low-fat group and 1 participant in the low-carbohydrate group.
Indicates the blood concentration of insulin at the 30-minute time point of an oral glucose tolerance test. There were missing data for 3 participants in the low-fat group.
Defined by Adult Treatment Panel III guidelines from the National Cholesterol Education Program.26
Available only for cohorts 2 through 5 because additional funding became available for metabolic cart and technician time.
There were missing data for 41 participants in the low-fat group and 40 participants in the low-carbohydrate group.
There were missing data for 23 participants in the low-fat group and 33 participants in the low-carbohydrate group.
There were missing data for 22 participants in the low-fat group and 17 participants in the low-carbohydrate group.
Of 22 assigned intervention instruction sessions for the full study sample, the mean number of sessions attended was 14.4 (SD, 5.3) for the healthy low-fat diet group and 14.6 (SD, 5.1) for the healthy low-carbohydrate diet group, which includes dropouts. Retention at 12 months, which was defined as participants who provided any data at 12 months, was 79% for both groups. Participant ratings for health educator enthusiasm and knowledge of material was high and similar between diet groups. The mean ratings were 4.6 to 5.0 on a scale of 1 to 5, with 5 as the highest rating.
Total energy intake was not different between diet groups at baseline or at any subsequent time point (P ≥ .10 for all; Table 2). Despite not being instructed to follow a specific energy (kilocalorie) intake restriction, the mean reported energy intake reduction relative to baseline was approximately 500 to 600 kcal/d for both groups at each time point after randomization.
Table 2. Dietary Intake by Time Point.
| Healthy Low-Fat Diet | Healthy Low-Carbohydrate Diet | Mean Between-Group Difference (95% CI)a | |||
|---|---|---|---|---|---|
| No. of Participants | Mean (SD) | No. of Participants | Mean (SD) | ||
| Total Energy Intake, kcal | |||||
| Baseline | 304 | 2148.1 (39.4) | 304 | 2222.8 (37.5) | −76.3 (−166.1 to 13.4) |
| 3 mo | 274 | 1515.0 (27.7) | 275 | 1580.8 (29.1) | −56.9 (−150.2 to 36.4) |
| 6 mo | 240 | 1624.4 (37.3) | 251 | 1621.3 (33.2) | 0.2 (−96.9 to 97.3) |
| 12 mo | 225 | 1716.1 (34.5) | 224 | 1697.1 (32.1) | 2.9 (−97.2 to 103.0) |
| Carbohydrates, g | |||||
| Baseline | 304 | 241.8 (5.0) | 304 | 246.5 (4.5) | −4.9 (−16.6 to 6.9) |
| 3 mo | 274 | 205.2 (4.3) | 275 | 96.6 (3.4) | 109.0 (96.8 to 121.2) |
| 6 mo | 240 | 211.2 (5.3) | 251 | 113.2 (4.1) | 95.6 (83.0 to 108.3) |
| 12 mo | 225 | 212.9 (5.0) | 224 | 132.4 (4.2) | 74.2 (61.2 to 87.2) |
| Carbohydrates, % kcal | |||||
| Baseline | 304 | 44.5 (0.5) | 304 | 44.0 (0.4) | 0.5 (−1.1 to 2.1) |
| 3 mo | 274 | 52.6 (0.6) | 275 | 23.1 (0.7) | 29.4 (27.8 to 31.0) |
| 6 mo | 240 | 50.8 (0.7) | 251 | 26.5 (0.7) | 24.1 (22.4 to 25.8) |
| 12 mo | 225 | 48.4 (0.7) | 224 | 29.8 (0.7) | 17.8 (16.0 to 19.5) |
| Fat, g | |||||
| Baseline | 304 | 87.0 (2.0) | 304 | 92.6 (1.9) | −5.6 (−10.4 to −0.8) |
| 3 mo | 274 | 42.0 (1.2) | 275 | 88.8 (2.0) | −46.2 (−51.2 to −41.2) |
| 6 mo | 240 | 50.3 (1.8) | 251 | 86.6 (2.0) | −36.0 (−41.2 to −30.8) |
| 12 mo | 225 | 57.3 (1.7) | 224 | 86.2 (2.0) | −28.4 (−33.8 to −23.0) |
| Fat, % kcal | |||||
| Baseline | 304 | 34.8 (0.4) | 304 | 36.0 (0.3) | −1.2 (−2.4 to 0.1) |
| 3 mo | 274 | 24.0 (0.5) | 275 | 49.0 (0.5) | −24.9 (−26.2 to −23.6) |
| 6 mo | 240 | 26.4 (0.6) | 251 | 46.8 (0.6) | −20.3 (−21.7 to −18.9) |
| 12 mo | 225 | 28.7 (0.5) | 224 | 44.6 (0.6) | −15.4 (−16.8 to −14.0) |
| Protein, g | |||||
| Baseline | 304 | 92.1 (1.7) | 304 | 93.1 (1.6) | −1.1 (−5.8 to 3.6) |
| 3 mo | 274 | 79.5 (1.6) | 275 | 96.9 (2.0) | −17.1 (−22.0 to −12.2) |
| 6 mo | 240 | 81.9 (1.9) | 251 | 93.8 (1.9) | −11.6 (−16.6 to −6.5) |
| 12 mo | 225 | 84.5 (1.8) | 224 | 93.3 (2.0) | −8.5 (−13.8 to −3.3) |
| Protein, % kcal | |||||
| Baseline | 304 | 17.9 (0.3) | 304 | 17.3 (0.2) | 0.6 (−0.4 to 1.5) |
| 3 mo | 274 | 21.5 (0.4) | 275 | 25.9 (0.4) | −4.4 (−5.4 to −3.5) |
| 6 mo | 240 | 20.8 (0.4) | 251 | 24.3 (0.4) | −3.5 (−4.5 to −2.5) |
| 12 mo | 225 | 20.6 (0.4) | 224 | 22.9 (0.4) | −2.1 (−3.2 to −1.1) |
| Saturated Fat, g | |||||
| Baseline | 304 | 28.9 (0.7) | 304 | 30.8 (0.7) | −1.9 (−3.7 to −0.1) |
| 3 mo | 274 | 12.7 (0.4) | 275 | 29.1 (0.7) | −16.2 (−18.1 to −14.3) |
| 6 mo | 240 | 15.3 (0.7) | 251 | 27.9 (0.7) | −12.4 (−14.4 to −10.5) |
| 12 mo | 225 | 18.2 (0.6) | 224 | 28.2 (0.8) | −9.8 (−11.8 to −7.8) |
| Saturated Fat, % kcal | |||||
| Baseline | 304 | 11.5 (0.2) | 304 | 11.9 (0.2) | −0.4 (−0.9 to 0.2) |
| 3 mo | 274 | 7.2 (0.2) | 275 | 16.1 (0.3) | −8.8 (−9.4 to −8.2) |
| 6 mo | 240 | 8.0 (0.2) | 251 | 15.0 (0.3) | −7.0 (−7.6 to −6.4) |
| 12 mo | 225 | 9.0 (0.2) | 224 | 14.5 (0.3) | −5.3 (−5.9 to −4.7) |
| Fiber, g | |||||
| Baseline | 304 | 22.0 (0.6) | 304 | 21.6 (0.5) | 0.4 (−1.2 to 1.9) |
| 3 mo | 274 | 24.2 (0.7) | 275 | 16.7 (0.8) | 7.5 (5.9 to 9.1) |
| 6 mo | 240 | 23.7 (0.7) | 251 | 17.2 (0.5) | 6.5 (4.8 to 8.2) |
| 12 mo | 225 | 23.0 (0.6) | 224 | 18.6 (0.5) | 4.1 (2.3 to 5.8) |
| Fiber, g/1000 kcal | |||||
| Baseline | 304 | 10.8 (0.2) | 304 | 10.3 (0.2) | 0.4 (−0.5 to 1.4) |
| 3 mo | 274 | 16.5 (0.4) | 275 | 11.3 (0.6) | 5.3 (4.3 to 6.3) |
| 6 mo | 240 | 15.4 (0.4) | 251 | 11.2 (0.3) | 4.2 (3.1 to 5.2) |
| 12 mo | 225 | 14.2 (0.4) | 224 | 11.6 (0.3) | 2.5 (1.4 to 3.5) |
| Added Sugars, g | |||||
| Baseline | 304 | 49.3 (2.0) | 304 | 52.2 (2.0) | −3.0 (−7.3 to 1.3) |
| 3 mo | 274 | 28.5 (1.3) | 275 | 16.2 (1.2) | 12.4 (8.0 to 16.9) |
| 6 mo | 240 | 31.5 (1.7) | 251 | 18.9 (1.3) | 12.2 (7.5 to 16.9) |
| 12 mo | 225 | 33.1 (1.7) | 224 | 22.8 (1.6) | 9.6 (4.7 to 14.5) |
| Sugar, g/1000 kcal | |||||
| Baseline | 304 | 22.4 (0.7) | 304 | 23.2 (0.7) | −0.8 (−2.7 to 1.1) |
| 3 mo | 274 | 18.4 (0.7) | 275 | 9.8 (0.6) | 8.6 (6.6 to 10.6) |
| 6 mo | 240 | 18.9 (0.8) | 251 | 11.2 (0.7) | 7.6 (5.5 to 9.7) |
| 12 mo | 225 | 18.7 (0.9) | 224 | 12.9 (0.8) | 5.6 (3.5 to 7.8) |
| Glycemic Indexb | |||||
| Baseline | 304 | 57.8 (0.3) | 304 | 58.2 (0.3) | −0.4 (−1.4 to 0.6) |
| 3 mo | 274 | 56.0 (0.3) | 275 | 50.1 (0.4) | 5.9 (4.9 to 7.0) |
| 6 mo | 240 | 56.2 (0.4) | 251 | 51.4 (0.5) | 4.7 (3.6 to 5.8) |
| 12 mo | 225 | 56.1 (0.4) | 224 | 52.7 (0.5) | 3.1 (2.0 to 4.3) |
| Glycemic Loadc | |||||
| Baseline | 304 | 128.2 (2.9) | 304 | 132.4 (2.7) | −4.3 (−11.1 to 2.6) |
| 3 mo | 274 | 102.1 (2.3) | 275 | 43.1 (2.0) | 59.0 (51.9 to 66.2) |
| 6 mo | 240 | 107.0 (3.1) | 251 | 52.4 (2.5) | 53.0 (45.6 to 60.4) |
| 12 mo | 225 | 108.0 (2.8) | 224 | 62.9 (2.6) | 41.5 (33.8 to 49.1) |
Healthy low-fat diet minus healthy low-carbohydrate diet from linear mixed-effects model.
Indicates ranking of foods according to the potential of 50 g of carbohydrates from that food to raise blood glucose relative to 50 g of glucose (scale of 0-100; a score of 100 refers to the same rate as glucose).
Indicates the actual amount of carbohydrates multiplied by the glycemic index.
At baseline, there were no significant between-group differences for any nutrients examined. In contrast, there were significant between-group differences after randomization at every time point (all P ≤ .001) for percentage of energy; intakes of carbohydrates, fat, protein, saturated fat, fiber, and added sugars; and glycemic index and glycemic load (Table 2). In the healthy low-fat diet vs the healthy low-carbohydrate diet, respectively, the mean 12-month macronutrient distributions were 48% vs 30% for carbohydrates, 29% vs 45% for fat, and 21% vs 23% for protein.
Primary Outcome
The mean 12-month weight change was −5.3 kg (95% CI, −5.9 kg to −4.7 kg) for the healthy low-fat diet group and −6.0 kg (95% CI, −6.6 kg to −5.4 kg) for the healthy low-carbohydrate diet group, which was not statistically different (Table 3). There was a similar range for weight change of approximately 40 kg within each group (−30 kg to 10 kg; eFigure 1 in Supplement 2).
Table 3. 12-Month Change Estimates for Anthropometric Variables by Diet.
| 12-mo Change Estimate (95% CI)a | Between-Group Difference (95% CI)b |
||
|---|---|---|---|
| Healthy Low-Fat Diet (n = 305) |
Healthy Low-Carbohydrate Diet (n = 304) |
||
| Weight, kg | −5.29 (−5.93 to −4.65) | −5.99 (−6.63 to −5.35) | 0.70 (−0.21 to 1.60) |
| Body mass indexc | −1.75 (−1.97 to −1.52) | −2.07 (−2.30 to −1.85) | 0.33 (0.01 to 0.64) |
| Body fat %d | −1.97 (−2.38 to −1.56) | −2.15 (−2.54 to −1.75) | 0.18 (−0.40 to 0.75) |
| Waist circumference, cm | −3.74 (−4.64 to −2.84) | −4.41 (−5.31 to −3.51) | 0.67 (−0.60 to 1.94) |
| Lipid level, mg/dL | |||
| High-density lipoprotein cholesterol | 0.40 (−0.37 to 1.18) | 2.64 (1.87 to 3.41) | −2.24 (−3.33 to −1.15) |
| Low-density lipoprotein cholesterol | −2.12 (−4.70 to 0.47) | 3.62 (1.04 to 6.19) | −5.74 (−9.38 to −2.09) |
| Triglycerides | −9.95 (−17.46 to −2.44) | −28.20 (−35.67 to −20.72) | 18.25 (7.65 to 28.84) |
| Blood pressure, mm Hg | |||
| Systolic | −3.18 (−4.33 to −2.03) | −3.72 (−4.86 to −2.58) | 0.54 (−1.07 to 2.16) |
| Diastolic | −1.94 (−2.65 to −1.22) | −2.64 (−3.34 to −1.93) | 0.70 (−0.31 to 1.71) |
| Fasting glucose, mg/dL | −3.67 (−4.90 to −2.44) | −2.10 (−3.32 to −0.87) | −1.58 (−3.31 to 0.16) |
| Fasting insulin, μIU/mL | −2.64 (−3.79 to −1.49) | −2.33 (−3.48 to −1.19) | −0.31 (−1.93 to 1.31) |
| Insulin-30, μIU/mLe | −15.38 (−21.13 to −9.62) | −11.48 (−17.18 to −5.78) | −3.90 (−12.00 to 4.20) |
| Metabolic syndrome, No. (%)f | |||
| Had metabolic syndrome at baseline but not at 12 mo | 36 (11.8) | 36 (11.8) | |
| Had metabolic syndrome at baseline and 12 mo | 39 (12.8) | 36 (11.8) | |
| Did not have metabolic syndrome at baseline or 12 mo | 128 (42.0) | 137 (45.1) | |
| Did not have metabolic syndrome at baseline but had metabolic syndrome at 12 mo | 13 (4.3) | 11 (3.6) | |
| Respiratory exchange ratiog | −0.008 (−0.018 to 0.002) | −0.027 (−0.037 to −0.018) | 0.020 (0.006 to 0.033) |
| Resting energy expenditure, kcalg | −66.45 (−96.65 to −36.26) | −76.93 (−106.68 to −47.19) | 10.48 (−31.91 to 52.87) |
| Energy expenditure, kcal/kg/d | 0.55 (0.20 to 0.90) | 0.49 (0.13 to 0.84) | 0.06 (−0.44 to 0.56) |
SI conversion factors: To convert glucose to mmol/L, multiply by 0.0555; high-density and low-density lipoprotein cholesterol to mmol/L, multiply by 0.0259; insulin to pmol/L, multiply by 6.945; triglycerides to mmol/L, multiply by 0.0113.
Data were missing for 91 participants in the healthy low-fat diet group and 86 in the healthy low-carbohydrate diet group (almost exclusively due to dropout).
Healthy low-fat diet minus healthy low-carbohydrate diet.
Calculated as weight in kilograms divided by height in meters squared.
There were missing data for 138 participants in the healthy low-fat diet group and 123 in the healthy low-carbohydrate diet group. This was due to a combination of dropout and not having any data for cohort 1.
Indicates the blood concentration of insulin at the 30-minute time point of an oral glucose tolerance test.
Defined by Adult Treatment Panel III guidelines from the National Cholesterol Education Program.26
There were missing data for 125 participants in the healthy low-fat diet group and 121 in the healthy low-carbohydrate diet group due to a combination of dropout and not having any data for cohort 1.
Interaction Testing
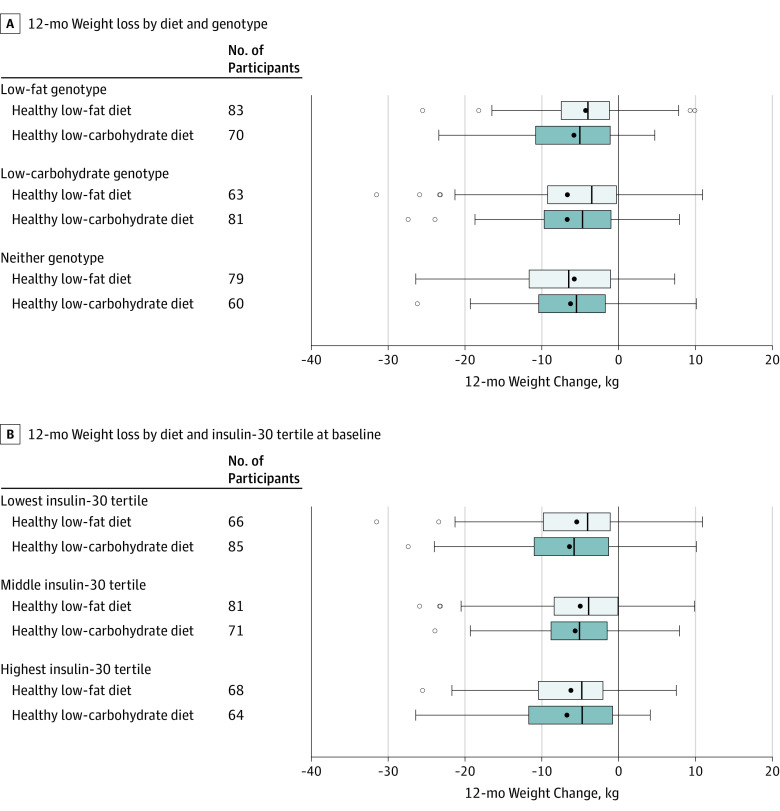
The test for the interaction among diet, genotype pattern, and the 12-month time point was not statistically significant. The interpretation of the beta coefficient for the 3-way interaction (beta coefficient, 1.38 [95% CI, −0.72 to 3.49], P = .20) is that 12-month weight change increases (estimated as 1.38 kg) when switching from a healthy low-carbohydrate diet and a low-carbohydrate genotype to a healthy low-fat diet and low-fat genotype beyond the main effects of switching from a healthy low-carbohydrate diet to a healthy low-fat diet and from a low-carbohydrate genotype to a low-fat genotype (Figure 2A). This indicates that there was no significant difference in weight change among participants matched vs mismatched to their diet assignment based on their 3-SNP genotype pattern. In analyses restricted to participants of European descent only, no significant interaction was observed by genotype pattern (the 3-way interaction for the main diet, genotype, and time yielded a beta coefficient of 2.58 [95% CI, −0.18 to 5.34]; P = .07).
Figure 2. Interaction Among Diet and Genotype and Diet and Insulin-30 Tertile at Baseline and 12-Month Weight Loss.
The black solid circle indicates the mean, the left and right borders of the box mark the first and third quartiles, the black vertical line indicates the median, the error bars indicate the 5th and 95th percentiles, and the hollow circles indicate the individuals whose values were outside the 5th or 95th percentiles. The No. of participants reflect data for the individuals who had weight data at both baseline and 12 months. Statistical analyses include data from all individuals randomized (described in the Statistical Analysis section).
A, Three-way interaction term among diet, genotype, and the 12-month time point was not statistically significant (beta coefficient, 1.38 [95% CI, −0.72 to 3.49]; P = .20). As described in Stanton et al,10 of all the possible combinations of variance in 3 single-nucleotide polymorphism multilocus genotype patterns, some were considered consistent with the low-fat genotype pattern, some with the low-carbohydrate genotype pattern, and some with neither of these 2 genotype patterns. By design, as described in the initial National Institutes of Health grant application, those individuals with neither of the main 2 genotype patterns were not included in the main analyses. There were 39 participants who had compromised or missing DNA.
B, Three-way interaction term among diet, insulin, and the 12-month time point was not statistically significant (beta coefficient for 10-μIU/mL increase in insulin, 0.08 [95% CI, −0.13 to 0.28]; P = .47). Insulin-30 is the blood concentration of insulin 30 minutes after consuming 75 g of glucose as part of a standard oral glucose tolerance test. Insulin-30 was treated as a continuous variable in the statistical model. Tertiles were used in this Figure for ease of presentation. The mean for the lowest tertile was 40.8 μIU/mL (range, 7.3-60.6 μIU/mL); middle, 80.1 μIU/mL (range, 60.7-103.1 μIU/mL); and highest, 159.6 μIU/mL (range, 103.4-562.5 μIU/mL).
Similarly, the test for interaction among diet, baseline insulin secretion (INS-30), and the 12-month time point was not statistically significant. The interpretation of the beta coefficient for the 3-way interaction (beta coefficient, 0.08 [95% CI, −0.13 to 0.28], P = .47) is that 12-month weight change increases (estimated as 0.08 kg) when switching from a healthy low-carbohydrate diet and x units of baseline INS-30 to a healthy low-fat diet and x + 10 units of baseline INS-30 beyond the effects of changing from a healthy low-carbohydrate diet to a healthy low-fat diet and increasing baseline INS-30 by 10 μIU/mL (Figure 2B). Weight change trajectories for the diet-genotype pattern subgroups are presented in eFigure 2A and for diet and INS-30 tertile subgroups in eFigure 2B in Supplement 2.
Secondary Outcomes
There were improvements in the secondary outcomes for both diet groups. However, there were no significant between-group differences observed for body mass index, body fat percentage, and waist circumference (Table 3). At 12 months relative to baseline, both diets improved lipid profiles and lowered blood pressure, insulin, and glucose levels, with the exception of low-density lipoprotein cholesterol concentrations, which increased for participants in the healthy low-carbohydrate group (Table 3). The 12-month changes in low-density lipoprotein cholesterol concentrations significantly favored a healthy low-fat diet. High-density lipoprotein cholesterol concentrations increased significantly more and concentrations of triglycerides decreased significantly more for the healthy low-carbohydrate diet group than for the healthy low-fat diet group. The decrease in the prevalence of the metabolic syndrome was not significantly different between the diet groups.
Respiratory exchange ratio was not significantly different between the groups at baseline, but was lower for the healthy low-carbohydrate diet group than for the healthy low-fat diet group at each time point after randomization (P < .001; eTable 1 in Supplement 2). Resting energy expenditure was not significantly different between groups at baseline or at 6 months or 12 months, but decreased significantly from baseline in both diet groups. Total energy expenditure was not significantly different between groups at baseline or any other time point. Relative to baseline, there was a small absolute mean increase in energy expenditure for both diet groups that was not significantly different than baseline.
Adverse Events
During the trial, there were 7 serious adverse events, all requiring hospitalization; 2 of these could have been related to the study (kidney stones and diverticulitis requiring surgery). There were 11 adverse events; 9 of these were related to the study or possibly related (eg, hypoglycemia following oral glucose tolerance test). Combined serious adverse events and adverse events were evenly distributed across the 2 diet groups.
Discussion
In this clinical trial of 609 generally healthy overweight or obese adults without diabetes who were randomly assigned to a healthy low-fat vs a healthy low-carbohydrate diet, there was no significant difference in weight loss at 12 months. In addition, there were no significant interactions between diet and 3 SNP multilocus genotype patterns or diet and baseline insulin secretion on 12-month weight loss. These results were observed in the context of similar mean 12-month weight loss in both diet groups that was greater than 5% of baseline body weight, and a similar and substantial range of weight change, reflecting approximately 40 kg within each diet group (from losing approximately 30 kg to gaining approximately 10 kg).
Dietary intake of fats and carbohydrates was well differentiated between the 2 diet groups, as confirmed by diet assessment, and corroborated by changes in blood lipid parameters and respiratory exchange ratio, indicating strong treatment fidelity. With the large sample size, good retention, substantial weight loss and weight loss variability, and good adherence to and differentiation of diets, the study was well positioned to detect significant interactions by the primary variables of interest if they existed. However, no such effects were observed. Differences in weight loss between the 2 groups were nonsignificant and not clinically meaningful.
Among the secondary outcomes, the clinical variables that were significantly different between the diet groups were the blood lipid results, which were more favorable in the healthy low-fat diet group for changes in low-density lipoprotein cholesterol and were more favorable in the healthy low-carbohydrate diet group for changes in high-density lipoprotein cholesterol and triglycerides. The magnitude of the between-group differences were 5% for low-density lipoprotein cholesterol, 5% for high-density lipoprotein cholesterol, and 15% for triglycerides.
There is considerable scientific interest in identifying genetic variants that help explain interindividual differences in weight loss success in response to diet interventions,31,32 particularly diets with varying macronutrient compositions. Multiple secondary analyses of low-fat and low-carbohydrate weight loss diet trials, including the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) and the Nutrient-Gene Interactions in Human Obesity (NUGENOB) trials,8,32,33,34 have reported effect modification by SNPs on associations of dietary fat and carbohydrates with weight loss.
For example, Qi et al8 reported that individuals with the IRS1 rs2943641 CC genotype were more successful with weight loss than those without this genotype when assigned to a low-fat and high-carbohydrate diet vs a low-carbohydrate and high-fat diet. Grau et al32 reported that individuals with the FTO rs9939609 TT genotype had greater decreases in the homeostatic assessment model of insulin resistance on low-fat vs low-carbohydrate diets; however, the diet-genotype interaction for weight loss was not statistically significant. Most prior studies examined single SNPs, with few replication attempts. The intent in the current study was to replicate the post hoc findings from the A TO Z (Atkins, Traditional, Ornish, Zone) Weight Loss Study.3
The finding of no significant difference in weight loss in genotype-matched vs mismatched groups in the current study highlights the importance of conducting large, appropriately powered trials such as DIETFITS for validating early exploratory analyses. Analyses of all the genomic data obtained are under way to evaluate whether other genetic signatures may demonstrate effect modification.
Several research groups previously reported observing a differential effect of low-fat vs low-carbohydrate diets on weight loss by baseline insulin status. In both a 6-month feeding study with 32 participants and an 18-month free-living study with 56 participants, effect modification between diet assignment (low-fat vs low-carbohydrate or low glycemic load) and INS-30 was reported.12,13 Using fasting insulin cutoffs in a 4-month feeding study involving 20 participants, Cornier et al11 observed a significant diet × fasting insulin interaction for weight loss. A post hoc analysis from the A TO Z Study revealed a significant diet × fasting insulin interaction on 12-month weight loss among a subset of 81 overweight and obese women.14
However, in a recent pilot study conducted in preparation for the DIETFITS study, a significant effect modification was not detected for INS-30 status.35 In each case in which a significant interaction was reported, investigators proposed a mechanism involving insulin secretion status, insulin sensitivity, or insulin resistance interacting with glycemic load to differentially affect weight loss response with low-fat diets high in carbohydrates vs high-fat diets low in carbohydrates.12,36
In these studies, the consistent direction of the finding was that a lower carbohydrate diet was superior for those individuals with higher insulin secretion or higher insulin resistance; the putative mechanism involves a lower demand or burden on insulin-mediated glucose disposal for those with impaired insulin metabolism while maintaining a lower carbohydrate and higher fat diet. Despite mechanistic plausibility, studies to date have involved relatively small sample sizes.
Effect modification claims observed in single randomized trials are often spurious and this result is even more frequent when small sample sizes and post hoc analyses are involved; validation of such claims is infrequent.37,38,39 The current study with a larger sample, a low-carbohydrate diet that was also a low glycemic load diet, and using INS-30 could not replicate findings from prior studies using smaller numbers of patients or those studies with a shorter duration. We consider the differences between the current findings and the studies cited to potentially involve diet quality beyond simply differentiating fat and carbohydrate intake. In this regard, refined grains are low in fat but considered of poor nutritional quality due to low-nutrient density relative to energy content. In contrast, vegetables are high in nutrient density, and relatively high in proportional carbohydrate content, but low in calories. Both diet groups in the current study were instructed to minimize or eliminate refined grains and added sugars and maximize intake of vegetables. We conclude that when equal emphasis is given to high dietary quality for both low-fat and low-carbohydrate eating plans, it is not helpful to preferentially direct an individual with high insulin secretion status who is seeking weight loss to follow a lower-carbohydrate eating plan instead of a lower-fat eating plan.
This study had several strengths. Study design strengths included the similarly intensive demands on both diet groups in making changes to baseline diets, similar focus on dietary quality, repeated major time points of data collection, and the extensive range of types of data collected. Strengths in study conduct included meeting and exceeding the sample size target of 600 participants, the nearly equal proportions of women and men enrolled, high and equivalent retention for both diet groups, and comparability of change between groups in potentially important outcomes related to weight loss, such as physical activity. In addition, the collective loss of approximately 3000 kg among study participants, and the wide individual variability of weight loss, provided the opportunity to meaningfully test for effect modification.
Limitations
The study also has several limitations. First, generalizability of the findings may be limited by the conduct of the study in a geographic area with individuals who have attained relatively high education levels, and have personal resources and high accessibility to high-quality food options. To address this, the study was broadly advertised and successfully enrolled participants with relatively good ethnic and racial diversity, and a range, albeit limited, of educational attainment.
Second, in regard to the possible role of insulin-glucose dynamics as an effect modifier in low-fat vs low-carbohydrate studies, there are many possible indices to consider other than INS-30,36 a proxy measure of insulin secretion selected for reasons described elsewhere.12,13 But others have reported finding significant effect modification according to prestudy fasting insulin concentrations.11,14
Third, there were 3 missing secondary anthropometric and metabolic variables (percentage of body fat, resting energy expenditure, and respiratory exchange ratio) for the first 78 participants enrolled in the study due to inadequate initial funding. This funding situation subsequently changed (described in eAppendix 1 in Supplement 2), which allowed the addition of these measurements for the remaining participants enrolled.
Fourth, the Stanford 7-day Physical Activity Recall tool (which was used to determine total energy expenditure) provides only a relatively crude assessment of total energy expenditure. Another method of measuring energy expenditure, such as the doubly labeled water method, would have provided greater accuracy; however, the overall cost and added participant burden were determined to be beyond the scope of the study. In addition, self-reported diet assessment methods are all known to have limited accuracy; therefore, we chose to use the Nutrition Data System for Research, which is recognized as a top method.
Fifth, even though insulin sensitivity was well assessed in this study, assessment of genetic characteristics as effect modifiers of diet response need better and increased study in the future because there has been much progress in understanding the genetic architecture of metabolic phenotypes such as obesity since the current trial was designed. Other explanations for heterogeneity besides insulin dynamics and genetic characteristics also need to be assessed.
Sixth, by not randomizing or conducting stratification according to genotype or insulin secretion status, the level of causal inference to be drawn from the analyses of interactions was limited; however, this allowed us to test for 2 primary interaction associations in the same study.
Conclusions
In this 12-month weight loss diet study, there was no significant difference in weight change between a healthy low-fat diet vs a healthy low-carbohydrate diet, and neither genotype pattern nor baseline insulin secretion was associated with the dietary effects on weight loss. In the context of these 2 common weight loss diet approaches, neither of the 2 hypothesized predisposing factors was helpful in identifying which diet was better for whom.
Trial protocol
eAppendix 1. Chronology of funding for DIETFITS – increased sample size, additional 10 primary analysis
eAppendix 2. Additional details on genotype assessment
eAppendix 3. Additional rationale for the 3-SNP multilocus genotype as written at the time of 13 the original grant submission, in 2011
eAppendix 4. Statistical model
eTable 1. Anthropometric and metabolic variables at all four time points
eTable 2A. Baseline characteristics of the 6 subgroups of Diet X Genotype pattern
eTable 2B. Baseline characteristics of the 6 subgroups of Diet X INS-30 tertiles
eFigure 1. Waterfall plot of weight loss by diet group
eFigure 2A. Participant flow, by Diet X INS-30 Tertile groups
eFigure 2B. Participant flow, by Diet X Genotype Group
eFigure 3A. Mean weight for 6 Diet X Genotype subgroups at four time points: Baseline, 3-, 6- 32 and 12-months
eFigure 3B. Mean weight for 6 Diet X Ins-30 Tertile subgroups at four time points: Baseline, 3-, 35 6- and 12-months
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zylke JW, Bauchner H. The unrelenting challenge of obesity. JAMA. 2016;315(21):2277-2278. [DOI] [PubMed] [Google Scholar]
- 3.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969-977. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shai I, Schwarzfuchs D, Henkin Y, et al. ; Dietary Intervention Randomized Controlled Trial (DIRECT) Group . Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229-241. [DOI] [PubMed] [Google Scholar]
- 6.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA. 2014;312(9):923-933. [DOI] [PubMed] [Google Scholar]
- 7.Field AE, Camargo CA Jr, Ogino S. The merits of subtyping obesity: one size does not fit all. JAMA. 2013;310(20):2147-2148. [DOI] [PubMed] [Google Scholar]
- 8.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation. 2011;124(5):563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dopler Nelson M, Prabakar P, Kondragunta V, Kornman KS, Gardner CD. Genetic phenotypes predict weight loss success: the right diet does matter. Paper presented at: joint conference of the 50th Cardiovascular Disease Epidemiology and Prevention and Nutrition, Physical Activity, and Metabolism; March 2-3, 2010; San Francisco, CA. [Google Scholar]
- 10.Stanton MV, Robinson JL, Kirkpatrick SM, et al. DIETFITS study (Diet Intervention Examining The Factors Interacting With Treatment Success): study design and methods. Contemp Clin Trials. 2017;53:151-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornier MA, Donahoo WT, Pereira R, et al. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes Res. 2005;13(4):703-709. [DOI] [PubMed] [Google Scholar]
- 12.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092-2102. [DOI] [PubMed] [Google Scholar]
- 13.Pittas AG, Das SK, Hajduk CL, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28(12):2939-2941. [DOI] [PubMed] [Google Scholar]
- 14.McClain AD, Otten JJ, Hekler EB, Gardner CD. Adherence to a low-fat vs low-carbohydrate diet differs by insulin resistance status. Diabetes Obes Metab. 2013;15(1):87-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Physical Activity Guidelines Advisory Committee, US Office of Disease Prevention and Health Promotion . 2008 physical activity guidelines for Americans. https://health.gov/paguidelines/guidelines/. Accessed November 21, 2017.
- 16.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman and Co; 1997. [Google Scholar]
- 17.Foreyt JP, Goodrick GK. Impact on behavior therapy on weight loss. Am J Health Promot. 1994;8(6):466-468. [DOI] [PubMed] [Google Scholar]
- 18.King AC, Frey-Hewitt B, Dreon DM, Wood PD. Diet vs exercise in weight maintenance: the effects of minimal intervention strategies on long-term outcomes in men. Arch Intern Med. 1989;149(12):2741-2746. [DOI] [PubMed] [Google Scholar]
- 19.Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47-57. [DOI] [PubMed] [Google Scholar]
- 20.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91-106. [DOI] [PubMed] [Google Scholar]
- 21.Chiu KC, Martinez DS, Yoon C, Chuang LM. Relative contribution of insulin sensitivity and beta-cell function to plasma glucose and insulin concentrations during the oral glucose tolerance test. Metabolism. 2002;51(1):115-120. [DOI] [PubMed] [Google Scholar]
- 22.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11(3):286-292. [DOI] [PubMed] [Google Scholar]
- 23.Hron BM, Ebbeling CB, Feldman HA, Ludwig DS. Relationship of insulin dynamics to body composition and resting energy expenditure following weight loss. Obesity (Silver Spring). 2015;23(11):2216-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr. 2008;87(2):303-309. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103(3):E26. [DOI] [PubMed] [Google Scholar]
- 26.Goni L, Cuervo M, Milagro FI, Martínez JA. Future perspectives of personalized weight loss interventions based on nutrigenetic, epigenetic, and metagenomic data. J Nutr. 2016;146(4):905S-912S. [DOI] [PubMed] [Google Scholar]
- 27.Diggle P. Analysis of Longitudinal Data. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 28.Batterham MJ, Tapsell LC, Charlton KE. Analyzing weight loss intervention studies with missing data: which methods should be used? Nutrition. 2013;29(7-8):1024-1029. [DOI] [PubMed] [Google Scholar]
- 29.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: tests in linear mixed effects models. R package version 2.0-33. https://cran.r-project.org/. Accessed January 10, 2018.
- 30.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. [Google Scholar]
- 31.Bray MS, Loos RJ, McCaffery JM, et al. ; Conference Working Group . NIH working group report-using genomic information to guide weight management: from universal to precision treatment. Obesity (Silver Spring). 2016;24(1):14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grau K, Cauchi S, Holst C, et al. TCF7L2 rs7903146-macronutrient interaction in obese individuals’ responses to a 10-wk randomized hypoenergetic diet. Am J Clin Nutr. 2010;91(2):472-479. [DOI] [PubMed] [Google Scholar]
- 33.Heianza Y, Ma W, Huang T, et al. Macronutrient intake-associated FGF21 genotype modifies effects of weight-loss diets on 2-year changes of central adiposity and body composition: the POUNDS Lost trial. Diabetes Care. 2016;39(11):1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi Q, Bray GA, Hu FB, Sacks FM, Qi L. Weight-loss diets modify glucose-dependent insulinotropic polypeptide receptor rs2287019 genotype effects on changes in body weight, fasting glucose, and insulin resistance: the Preventing Overweight Using Novel Dietary Strategies trial. Am J Clin Nutr. 2012;95(2):506-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner CD, Offringa LC, Hartle JC, Kapphahn K, Cherin R. Weight loss on low-fat vs low-carbohydrate diets by insulin resistance status among overweight adults and adults with obesity: a randomized pilot trial. Obesity (Silver Spring). 2016;24(1):79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittas AG, Roberts SB. Dietary composition and weight loss: can we individualize dietary prescriptions according to insulin sensitivity or secretion status? Nutr Rev. 2006;64(10 pt 1):435-448. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: Users’ Guide to the Medical Literature. JAMA. 2014;311(4):405-411. [DOI] [PubMed] [Google Scholar]
- 38.Wallach JD, Sullivan PG, Trepanowski JF, Steyerberg EW, Ioannidis JPA. Sex based subgroup differences in randomized controlled trials: empirical evidence from Cochrane meta-analyses. BMJ. 2016;355:i5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol. 2004;57(3):229-236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eAppendix 1. Chronology of funding for DIETFITS – increased sample size, additional 10 primary analysis
eAppendix 2. Additional details on genotype assessment
eAppendix 3. Additional rationale for the 3-SNP multilocus genotype as written at the time of 13 the original grant submission, in 2011
eAppendix 4. Statistical model
eTable 1. Anthropometric and metabolic variables at all four time points
eTable 2A. Baseline characteristics of the 6 subgroups of Diet X Genotype pattern
eTable 2B. Baseline characteristics of the 6 subgroups of Diet X INS-30 tertiles
eFigure 1. Waterfall plot of weight loss by diet group
eFigure 2A. Participant flow, by Diet X INS-30 Tertile groups
eFigure 2B. Participant flow, by Diet X Genotype Group
eFigure 3A. Mean weight for 6 Diet X Genotype subgroups at four time points: Baseline, 3-, 6- 32 and 12-months
eFigure 3B. Mean weight for 6 Diet X Ins-30 Tertile subgroups at four time points: Baseline, 3-, 35 6- and 12-months