Summary
The ketogenic diet (KD) is an effective treatment for children with drug‐resistant epilepsy and has been widely used in young children. Adult patients with intractable epilepsy would also benefit from this dietary treatment. However, only a few studies have been published, and the use of the KD in intractable epilepsy in adults has been limited. This meta‐analysis summarized the findings of the relevant published studies to identify the efficacy of the KD for the treatment of intractable epilepsy in adults. In this meta‐analysis, PubMed, Embase, and Cochrane Library were used for searching studies concerning the effects of the KD and its major subtypes with intractable epilepsy in adults published up to January 10, 2017. The primary outcomes were seizure freedom, seizure reduction by 50% or more, and seizure reduction by <50%. The quality of the methodology of the observational studies was reviewed by using the Newcastle‐Ottawa Scale. We identified 402 articles, of which, 16 studies including 338 patients met the inclusion criteria. The results of the meta‐analysis showed that the combined efficacy rates of all the symptoms of seizure freedom, seizure reduction by 50% or more, and seizure reduction below 50% in adults with intractable epilepsy were 13%, 53%, and 27%, respectively. The adverse reactions of the KD were mild, whereas low glycemic index diet (LGID) and low‐dose fish oil diet (LFOD) may have fewer side effects. Weight loss, high level of low‐density lipoprotein, and elevated total cholesterol were most frequent. The meta‐analysis indicates that the KD for refractory epilepsy in adults is a well‐tolerated treatment and that its side effects are acceptable, which show that the KD is a promising treatment in adult intractable epilepsy. Further research is needed to assess which type of diet or ratio is more effective in the KD treatment.
Keywords: Ketogenic diet, Dietary treatment, Intractable epilepsy, Efficacy, Adult
Key Points.
There is limited evidence for the effectiveness of the KD and its major subtypes in the prevention or treatment of seizures in adults with intractable epilepsy
Promising evidence was shown for the use of the dietary treatment in assisting the management of intractable epilepsy in adults to reduce seizures
More effective comprehensive evaluation of the dietary treatment is necessary for the management of intractable epilepsy
Epilepsy is a serious and common neurologic disease, which can be controlled successfully in most patients with one or more antiepileptic drugs. However, about 20%‐30% of patients still have intractable epilepsy despite all available antiepileptic drug options.1, 2 For nearly a century, the ketogenic diet (KD) has been used to treat intractable seizures in children because of its beneficial role in seizure control.3, 4, 5, 6 The KD for intractable epilepsy is a high‐fat, low‐protein, and low‐carbohydrate diet.7 It could mimic the starvation state, which may induce a shift away from glycolytic energy production (restriction of carbohydrate intake) toward energy generation through oxidative phosphorylation (fatty acid β‐oxidation and ketone body production).8 Increasing γ‐aminobutyric acid (GABA) synthesis, limiting reactive oxygen species generation, and boosting energy production may be possible anticonvulsant mechanisms of the KD.8 The diet therapies of the classical ketogenic diet (CKD), modified Atkins diet (MAD), low glycemic index diet (LGID), and low‐dose fish oil diet (LFOD) have been applied in the treatment of intractable epilepsy in recent years.3, 9, 10, 11
The efficacy of the KD was evaluated with the use of the original research and evaluation system, which showed that the diet was an effective and well‐tolerated treatment for refractory childhood epilepsy.12, 13 Many pediatric epilepsy centers offered this therapy in addition to surgery and medications. In recent years, interest in the KD as treatment for intractable epilepsy has spread to adults. The KD and its variants may lead to reductions in seizure frequency in adults similar to those seen in children.14, 15 However, the studies on the efficacy and tolerability of the KD in adults with refractory epilepsy are still lacking. To a certain extent, this may restrict the clinical application for adult intractable epilepsy. Therefore, we collected the relevant studies about KD therapy in adults with refractory epilepsy and conducted a meta‐analysis to evaluate the efficacy and adverse reactions of the dietary therapy.
Materials and Methods
Search strategy
The following electronic databases were searched for studies reporting on the efficacy of KD treatment for intractable epilepsy in adults: PubMed, Embase, and Cochrane Library. The keywords “ketogenic diet,” “intractable epilepsy,” “dietary treatment,” and “refractory epilepsy” were used. The final search was carried out on January 10, 2017.
Inclusion and exclusion criteria
The inclusion criteria were designed according to the PICOS (Participants, Intervention, Comparator, Outcome, Study design) principle. Published studies were considered appropriate for the meta‐analysis when they met the following criteria: (1) research objects were adult patients (aged ≥16) years with refractory partial or symptomatic generalized epilepsy; (2) dietetic treatment (the subtype of KD) was the basic treatment method; (3) the outcome index needed for the meta‐analysis was seizure frequency of epilepsy, mainly including seizure freedom, seizure reduction by 50% or more, and seizure reduction below 50%; (4) other basic information required for meta‐analysis, such as demographic characteristics of the subjects, duration of the trial, ratio or dosage of KD, number of enrolled patients, and numbers of adherents and dropouts; (5) the preceding 4 main points were included without limitations to geographical region or language.
Studies were considered unsuitable for meta‐analysis if the following factor were identified: systematic reviews.
Literature screening, data extraction, and methodological quality assessment
All the included articles were carefully reviewed by 2 independent review authors to extract relevant information, and the filtered information was crosschecked to ensure the integrity, objectivity, and correctness of the data. Discrepancies were resolved by consensus. When articles provided insufficient data, we contacted the authors for the required details or sought help from others to determine if data were missing at random or not. The quality of the methodology of the observational studies was reviewed by using the Newcastle‐Ottawa Scale.16 The following aspects were used: selection and comparability of study groups and exposure/outcome of interest. The semi‐quantitative principle of star system was adopted, and the full score is 9 stars. Across studies, high‐quality papers reached 70% or more of the maximum number of stars.
Statistical analysis
Stata software (version 13) was utilized for statistical analysis. The heterogeneity of similar studies was evaluated with the Ι 2 test. A random‐effects model of meta‐analysis was used for quantitatively combined analysis on research literature about KD treatment for intractable epilepsy. The efficacy rate of each included study was calculated on the numbers of completers (those patients who did not withdraw) and patients achieving therapeutic success. Then, the meta‐analysis was made to calculate the combined efficacy rate of KD. When the raw efficacy rate in a certain study is 0 (when incidence number = 0) or 1 (when incidence number = total number), the efficacy rate should be converted with the following formula before being used for meta‐analysis. If the incidence number = 0, and meanwhile the total number <50, compute efficacy rate as r = 1/(4*total number); if incidence number = total number, and meanwhile total number <50, compute efficacy rate as r = (total number‐0.25)/total number. Efficacy was based on a comparison of the average monthly baseline seizure frequency (1 or 3 months before diet initiation) to the seizure frequency during every month after initiation of the KD.17 The percentage change in seizure frequency to baseline seizure frequency was calculated after the initiation of diet. Efficacy was rated using the following categories10: (1) seizure freedom (100% seizure remission); (2) seizure reduction by 50% or more (50–99% reduction in seizure frequency); (3) seizure reduction below 50% (1–49% reduction in seizure frequency); (4) unchanged; (5) worsened when the seizure rate increased. Seizure reduction by 50% or more was established as the proportion of patients with ≥50% reduction in the monthly frequency of seizures compared to baseline during the treatment periods. Seizure freedom was defined as the percentage of intent‐to‐treat patients who did not withdraw and were free of seizures during the treatment periods.25, 27 We planned to present seizure freedom, seizure reduction by over 50%, and seizure reduction below 50% as odds ratios (ORs) and 95% confidence intervals (CIs). Publication bias was analyzed by funnel plot.
Results
Search results
A total of 402 potentially relevant publications from inception through January 10, 2017, were systematically identified in the electronic databases (PubMed, 153 studies; Embase, 207 studies; and Cochrane Library, 42 studies). A total of 16 studies met the inclusion criteria, including 338 patients (209 patients on sticking to the diet and 129 patients in dropping a diet) included in the meta‐analysis written in English and focusing on intractable epilepsy. The study excluded and included processes, as listed in Figure 1.
Figure 1.
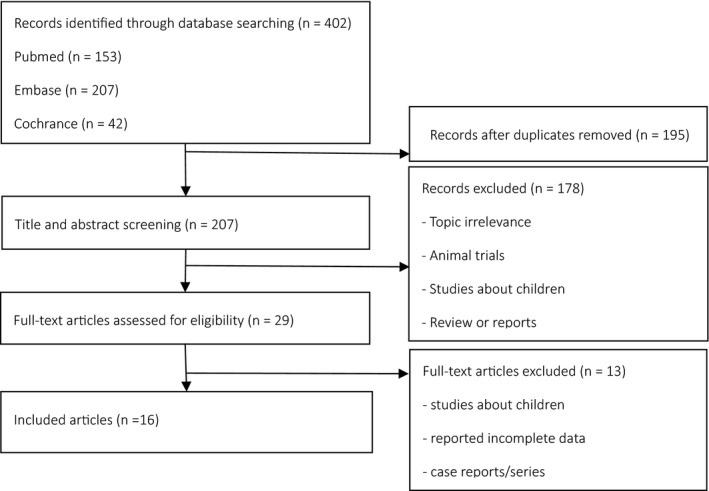
Literature selection. n: Number of articles.
The 16 studies from 8 countries were all open‐label and prospective. Strict inclusion and exclusion criteria were used in all studies. A total of 338 patients (including two children aged 15 and 11 years) aged 16–86 years with symptomatic generalized, focal, and idiopathic generalized epilepsy experienced evidence of at least 2 or 3 seizures per month during the 4‐week baseline. Furthermore, one or 2 concomitant antiepileptic drugs were included in the population. At the same time, adults with hypercholesterolemia, cardiovascular diseases, or renal diseases were excluded, or patients who were able to use the KD were included. In all studies, KD was used as a complementary therapy to the previously prescribed anticonvulsant drugs. KD was used in 5 studies (n = 76 patients); the MAD was used in 8 (n = 209 patients); the LFOD was used in one (n = 24 patients); and the LGID was used in one (n = 6 patients). The studies of Schoeler (n = 23 patients) used both KD and MAD. Table 1 shows the baseline information and relevant data of all the included studies.
Table 1.
Study characteristics of this meta‐analysis
| Author (year) | Total subjects (n) | Completion (n) | Dropout (n) | Nation | Sex (female, male) | Age range (year) | Duration of baseline, treatment (moon) | Ratio (fat: protein, carbohydrate) (g) or dosage (carbohydrates) | Treatment type | > 50% sz ↓ (n), % (95% CI)a | < 50% sz ↓ (n), % (95% CI)a | Seizure free (n), % (95% CI)a | Study type | Blind methods |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sirven J (1999)17 | 11 | 7 | 4 | USA | 9, 2 | 19–45 | 3, 8 | 4:1 | KD | 6, 0.86 (0.60, 1.12) | 1, 0.14 (−0.12, 0.40) | b | Prospective | Open‐label |
| Nei M (2014)18 | 29c | 20 | 9 | USA | 13, 16 | 16–51 | 3, 6–24 | 4:1 | KD | 13, 0.65 (0.44, 0.86) | 4, 0.20 (0.02, 0.38) | b | Prospective | Open‐label |
| Mosek A (2009)19 | 9 | 2 | 7 | Israel | 2, 7 | 18–45 | 2, 2–3 | 3:1 | KD | 2, 0.88 (0.42, 1.33) | 0, 0.12 (−0.33, 0.58) | b | Prospective | Open‐label |
| Klein P (2010)20 | 12 | 9 | 3 | USA | 8, 4 | 25–65 | 2, 4–26 | 3:1, 1600 kcal/day | KD | 5, 0.56 (0.23, 0.88) | 5, 0.56 (0.23, 0.88) | b | Prospective | Open‐label |
| Lambrechts DA (2012)21 | 15 | 5 | 10 | Netherlands | 8, 7 | 18–40 | 3, 12 | 3:1 | KD | 2, 0.40 (−0.03, 0.83) | 3, 0.60 (0.17, 1.03) | b | Prospective | Open‐label |
| Carrette E (2008)9 | 8 | 3 | 5 | Belgium | 3, 5 | 30–54 | 1, 6 | 20/g | MAD | 1, 0.33 (−0.20, 0.87) | 2, 0.67 (0.13, 1.20) | b | Prospective | Open‐label |
| Kossoff EH (2008)22 | 30 | 14 | 16 | USA | 19, 11 | 18–53 | 1, 6 | 15/g | MAD | 10, 0.71 (0.48, 0.95) | 5, 0.36 (0.11, 0.61) | b | Prospective | Open‐label |
| Smith M (2011)23 | 18 | 14 | 4 | USA | 11, 7 | 18–55 | 1, 12 | 20/g | MAD | 3, 0.21 (−0.00, 0.43) | 4, 0.29 (0.05, 0.52) | b | Prospective | Open‐label |
| Cervenka MC (2012)24 | 22 | 14 | 8 | USA | b | 18–66 | 1, 3 | 20/g | MAD | 6, 0.43 (0.17, 0.69) | b | 1, 0.07 (−0.06, 0.21) | Prospective | Open‐label |
| Kossoff EH (2013)25 | 8 | 7 | 1 | USA | 6, 2 | 15–44 | 1, 3 | 10/g | MAD | 4, 0.57 (0.20, 0.94) | 0, 0.04 (−0.10, 0.17) | 2, 0.29 (−0.05, 0.62) | Prospective | Open‐label |
| Vaccarezza MM (2014)26 | 4 | 4 | 0 | Spain | 2, 2 | 17–21 | 1, 6–36 | 6:3:1 | MAD | 2, 0.50 (0.01, 0.99) | 2, 0.50 (0.01, 0.99) | b | Prospective | Open‐label |
| Cervenka, MC (2016)27 | 106 | 59 | 47 | USA | 12, 1 | 18–86 | 1, 3 | 20/g | MAD | 38, 0.64 (0.52, 0.77) | 15, 0.25 (0.14, 0.37) | 17, 0.29 (0.17, 0.40) | Prospective | Open‐label |
| Kverneland M (2015)28 | 13 | 6 | 7 | Norway | 12, 1 | 16–58 | 3, 2–3 | 15–20/g | MAD | 4, 0.67 (0.29, 1.04) | 1, 0.17 (−0.13, 0.46) | b | Prospective | Open‐label |
| Schoeler, NE (2014)29 | 23 | 19 | 4 | Britain | 13, 10 | 16–65 | 1, 3 | 2:1 to 3.5:1 | KD+MAD | 9, 0.47 (0.25, 0.70) | 5, 0.26 (0.07, 0.46) | 0, 0.01 (−0.04, 0.06) | Prospective | Open‐label |
| DeGiorgio CM (2015)30 | 24 | 20 | 4 | USA | 16, 8 | 18–56 | 1, 9–10 | 1080/mgd | LFOD | 5, 0.25 (0.06, 0.44) | b | 2, 0.10 (−0.03, 0.23) | Prospective | Open‐label |
| Coppola, G (2011)10 | 6 | 6 | 0 | Italy | 13, 10 | 16–22 | 1, 1–60 | 40–60/g | LGID | 2, 0.33 (−0.04, 0.71) | 3, 0.50 (0.10, 0.90) | b | Prospective | Open‐label |
KD, ketogenic; MAD, modified Atkins diet; LFOD, low‐dose fish oil diet; LGID, low glycemic index diet; sz, seizures.
Percentage based on completed sample size (those patients who did not withdraw).
Not reported.
29 studies including the 1 children aged 11.
3 capsules/day, 1080 mg eicosapentaenoic acid+docosahexaenoic acid; dropout including people under the age of 16.
Meta‐analysis of efficiency in patients with intractable epilepsy
During the analysis, 209 of the original 338 participants who began KD remained on the diet. The meta‐analysis revealed that the combined efficacy rates of the diet achieved seizure freedom of 13% (random effect model) (r = 0.13, 95% CI = 0.01–0.25, p < 0.05; Fig. 2). Evidence suggested a significant heterogeneity among the studies (I 2 = 80.2%, p = 0.000). The combined efficacy rate of seizure reduction by 50% or more and no seizure freedom was 53% (r = 0.53, 95% CI = 0.42–0.63, p < 0.05; Figure 3). Evidence indicated a significant heterogeneity among the studies (I 2 = 57.4%, p = 0.002). The combined efficacy rate of seizure reduction below 50% was 27% (r = 0.27, 95% CI = 0.18–0.35, p < 0.5; Figure 4). Low heterogeneity was detected for the outcome of seizure reduction below 50% (I 2 = 41.6%, p = 0.051).
Figure 2.
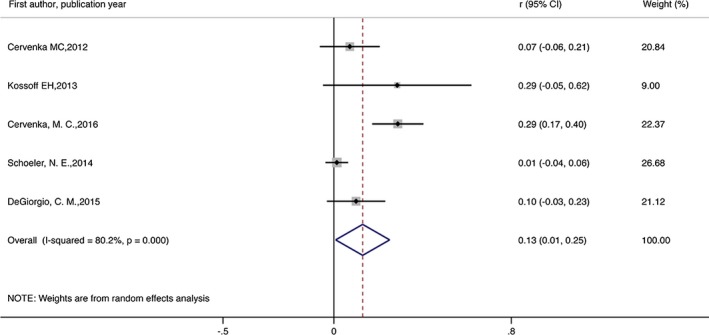
Meta‐analysis of seizure freedom in patients with intractable epilepsy.
Figure 3.
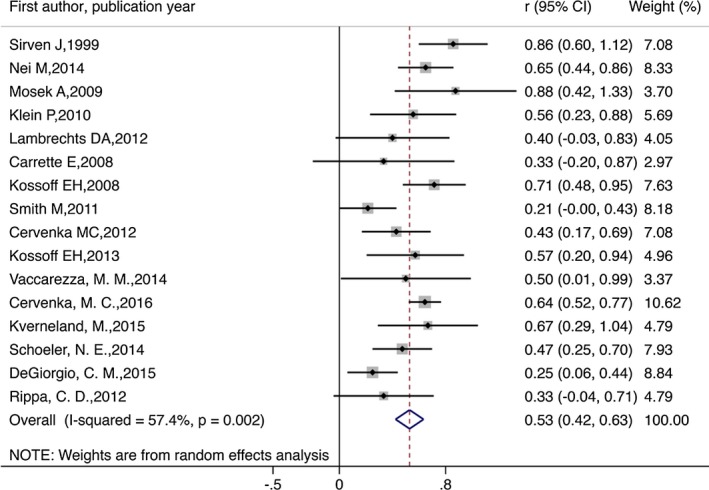
Meta‐analysis of seizure reduction by 50% or more in patients with intractable epilepsy.
Figure 4.
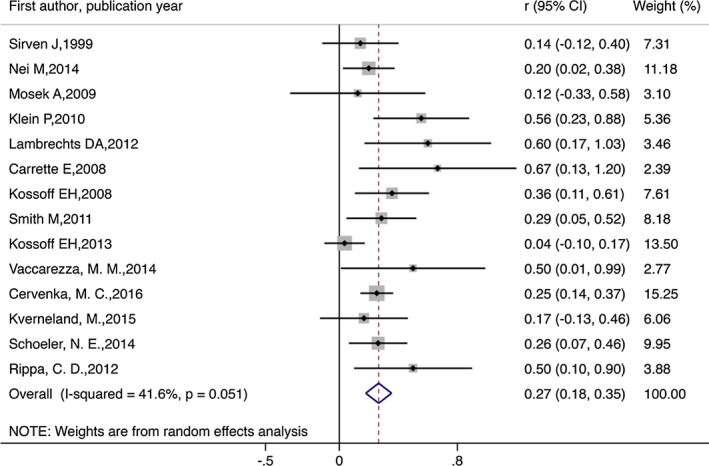
Meta‐analysis of seizure reduction by below 50% in patients with intractable epilepsy.
Summary of common adverse effects in ketogenic diet
A meta‐analysis was not conducted to pool the results because a particular complication cannot be identified from each included study. Instead, a summary of these common adverse effects in KD was created, as shown in Table 2. This table shows the various adverse effects that occurred with the KD. Among them, the most common were weight loss, high level of low‐density lipoprotein, and elevated total cholesterol.
Table 2.
Summary of common adverse effects in ketogenic diet
| Adverse effects | Numbers | Adverse effects | Numbers |
|---|---|---|---|
| Weight loss | 63 | Gastroesophageal reflux | 1 |
| None | 29 | Acne | 2 |
| Low‐density lipoprotein(LDL) ≥ 130 mg/dL | 27 | Carnitine deficiency | 2 |
| Elevated total cholesterol | 20 | Osteoporosis | 2 |
| Menstrual irregularities (female patients) | 10 | Lethargy or drowsiness | 2 |
| Hunger | 8 | Lipid disorder | 2 |
| Diarrhea | 6 | Flatulence | 1 |
| Constipation | 8 | Abdominal pain | 1 |
| Worse seizures | 8 | Increased seizure frequency | 1 |
| Fatigue | 7 | Metabolic acidosis | 1 |
| Triglycerides ≥150 mg/dL | 7 | A temporary increase in serum cholesterol | 1 |
| Vomiting | 5 | Alopecia | 1 |
| Weakness | 4 | Cholecystitis | 1 |
| Mild intermittent constipation | 4 | Ecchymosis | 1 |
| Weight gain | 3 | Halitosis | 1 |
| Nausea | 3 | Leg cramps | 1 |
| Nephrolithiasis | 3 | Symptoms of gallstones | 1 |
| Abdominal cramps | 2 | Thirst | 1 |
| Headache | 2 | Psychosis/hallucinations | 1 |
| High‐density lipoprotein B 40 mg/dL | 2 |
Sensitivity analysis
Sensitivity analysis was used to determine the seizure frequency of intractable epilepsy, and the quantitatively combined analysis of each trial had a little change after one article and was excluded individually. Then the data were analyzed by the random‐effects model to see the irreversible meta‐analysis results, which illustrated that the results were reliable.
Publication bias analysis
The funnel plot revealed a fairly symmetrical distribution of the efficacy rates, with three studies were excluded from 95% CI. The results of the funnel plot showed that the points on both sides of the fitted curve had better symmetry, which suggested no significant publication bias (Figure 5).
Figure 5.
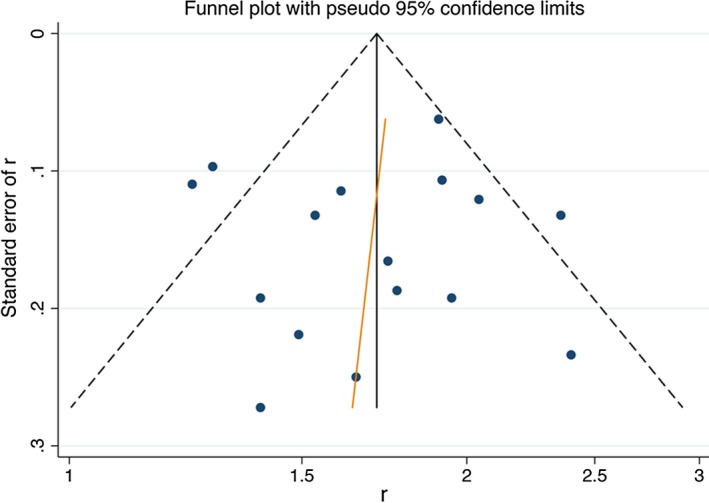
Funnel plot.
Discussion
The KD has been of increasing interest for intractable epilepsy in adults in recent years. The aim of this meta‐analysis was to establish whether adult patients with drug‐resistant epilepsy might benefit from the KD and assess to its side effects. The analysis showed that the KD was effective for intractable epilepsy in adults, with 13% of patients becoming seizure free, 53% with a seizure reduction by 50% or more, and 27% had a reduction of less than 50% among completers. The adverse reactions of the KD were mild and acceptable. Therefore, the KD is an effective therapy for adult intractable epilepsy. Effectiveness was mainly reflected in the reduction of seizure frequency. Our findings are similar to those of previous reports.31 The KD treatment should be further investigated in the future.
This meta‐analysis evaluated all the published studies that focused on the KD in adults with intractable epilepsy and aimed at providing a comprehensive evaluation of current evidence on dietary treatment. Different from those of previous studies, in our study, 4 kinds of dietary therapy (CKD, MAD, LGID, and LGIT) were included and referred to as the KD. Although the energy proportion from the 3 main nutrient groups (carbohydrates, protein, and fat) differed in the 4 kinds of dietary therapy, all these diets were essentially high in fat and low in carbohydrates, mimicked the metabolic state of starvation, and forced the body to utilize fat as its primary source of energy for the treatment of intractable epilepsy. Our study showed that the efficacy rates of all the symptoms of seizure freedom, seizure reduction by over 50%, and seizure reduction below 50% in adults with intractable epilepsy were 13%, 53%, and 27%, respectively. Related research reported that the rates of seizure freedom reached as high as 55%, and that the rates of seizure reduction reached as high as 85% when the KD was applied in refractory childhood and adolescent epilepsy.12 Other KD trials in children have reported similar results.4, 5, 6 The efficacy in adult was less than that in children and adolescents. Lack of compliance (Table 1) may be one of the causes of low efficacy of the KD therapy. The chief signs were ineffectiveness, loss to follow‐up, lack of motivation, and poor compliance.
The relevant side effects that were summarized would be minimized by interventions and monitoring. All adverse effects were reported in 12 of the 16 articles. A total of 26 patients had no adverse effects in the LFOD and the LGID for the treatment of intractable epilepsy.10, 30 Exactly 29 patients experienced no side effects in all studies. This finding showed that further study of the efficacy of the LFOD and LGID for the treatment of intractable epilepsy in adults is necessary. The adverse effects in 2 articles22, 23 were not reported due to abnormal laboratory results or increased seizure frequency. Immediate, short‐term, and long‐term side effects need to be differentiated. Immediate side effects (acidosis, lethargy, and others) may reduce the enthusiasm and optimism of patients. The most common short‐term side effects are gastrointestinal symptoms (vomiting, diarrhea, constipation, and so on), which may lead to poor compliance, affecting efficacy. Long‐term side effects (kidney stones, excessive weight loss, and others) could occur with higher prevalence than short‐term side effects. However, most of the adverse effects, including metabolic abnormalities, gastrointestinal symptoms, and nutritional deficiencies, are preventable and have been proven to be treatable.32 Given the few reports of severe or fatal side effects, increasing the efficacy and availability of the dietary therapy is possible. Moreover, the prevention and control of side effects should be considered during KD treatment; for example, it is necessary to be cautious and careful supervision of the patient by an experienced dietitian and neurologist while on the KD treatment.33
This meta‐analysis provides clinical application and support for the efficacy of the KD for the treatment of intractable epilepsy in adults. Despite nearly a century of use, the mechanisms underlying the clinical efficacy of the KD remain unknown.8 A recent study showed a novel epigenetic mechanism of the diet and indicated that the KD also affects DNA methylation.34 Although the number of patients using the KD has increased, numbers remain low due to lack of referrals and funding or different centers according to the level of expertise, experience, and knowledge regarding the diet as a therapeutic option.32, 35 Relatively few reports about the effects of the KD on epilepsy exist. Several studies showed that the KD has effectively improved the intractable epilepsy caused by various causes, including viral encephalitis, autoimmune encephalitis, febrile infection‐related epilepsy syndrome and dravet syndrome.36, 37, 38 Because the cause of epilepsy is different, and the degree of attack is inconsistent, the KD has not become a routine treatment for intractable epilepsy. Therefore, the KD could be a promising complementary therapy in adult intractable epilepsy, which is consistent with those of previous studies.39 In addition, a larger sample of studies is needed to further confirm the above findings.
Nevertheless, some limitations were encountered in our analysis. First, our analysis was conducted in a subpopulation of patients (completers), which may introduce a bias, since it is possible that completers are those who had a better response. The main reason that we use per protocol (PP) is that subjects with unknown outcome were missing data in some of the included literature. For example, reasons that some participants quit the trial are unknown or unexplained, or outcome indicators of respondents who completed the trial were not fully described. Second, only 16 studies were included in this meta‐analysis, and the sample size was relatively small, which may have affected the accuracy of the results. Third, low‐dose fish oil diet and low glycemic index diet were assessed in only one study and 2 studies, respectively. This assessment might not be a representative. Moreover, eligible trials varied in several respects, including differences in trial populations, test sites, and treatment regimens. Methodological differences might have confounded the differences recorded across the data of trials.
Conclusion
This meta‐analysis indicates that the KD for refractory epilepsy in adults is a well‐tolerated and that its side effects are acceptable. These findings show that the KD is a promising treatment for intractable epilepsy in adults. Further research is needed to assess the therapeutic effects of the KD between adult intractable focal epilepsy and adult intractable generalized epilepsy, as well as which type of diet or ratio is more effective in KD treatment.
Disclosure
We declare no competing interests. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgments
We would like to thank our team members for their support and contributions to this study. We would also like to thank Professor L.H. Lumey and Dr. Chihua Li at Columbia University Medical Center for their support; Guoqiang Zhang at Children's Hospital of Chongqing Medical University; and Ruixue Bai, Tingting Wu, and Tingting Li at the Chongqing Medical University for their help.
Biography
Hongyan Liu is a postgraduate at the School of Public Health and Management, Chongqing Medical University, Chongqing, China.

References
- 1. Schmidt D, Schachter SC. Drug treatment of epilepsy in adults. BMJ 2014;348:g254. [DOI] [PubMed] [Google Scholar]
- 2. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314–319. [DOI] [PubMed] [Google Scholar]
- 3. Wilder RM. The effect of ketonemia on the course of epilepsy. Mayo Clin Bull 1921;2:307–308. [Google Scholar]
- 4. Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 2008;7:500–506. [DOI] [PubMed] [Google Scholar]
- 5. Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics 2000;105:E46. [DOI] [PubMed] [Google Scholar]
- 6. Hemingway C, Freeman JM, Pillas DJ, et al. The ketogenic diet: a 3‐ to 6‐year follow‐up of 150 children enrolled prospectively. Pediatrics 2001;108:898–905. [DOI] [PubMed] [Google Scholar]
- 7. Cervenka MC, Kossoff EH. Dietary treatment of intractable epilepsy. Continuum 2013;19:756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 2007;48:43–58. [DOI] [PubMed] [Google Scholar]
- 9. Carrette E, Vonck K, de Herdt V, et al. A pilot trial with modified Atkins’ diet in adult patients with refractory epilepsy. Clin Neurol Neurosurg 2008;110:797–803. [DOI] [PubMed] [Google Scholar]
- 10. Coppola G, D'Aniello A, Messana T, et al. Low glycemic index diet in children and young adults with refractory epilepsy: first Italian experience. Seizure 2011;20:526–528. [DOI] [PubMed] [Google Scholar]
- 11. Karimzadeh P, Sedighi M, Beheshti M, et al. Low Glycemic Index Treatment in pediatric refractory epilepsy: the first Middle East report. Seizure 2014;23:570–572. [DOI] [PubMed] [Google Scholar]
- 12. Martin K, Jackson CF, Levy RG, et al. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev 2016;2:CD001903. [DOI] [PubMed] [Google Scholar]
- 13. Sharma S, Jain P. The ketogenic diet and other dietary treatments for refractory epilepsy in children. Ann Indian Acad Neurol 2014;17:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Payne NE, Cross JH, Sander JW, et al. The ketogenic and related diets in adolescents and adults—A review. Epilepsia 2011;52:1941–1948. [DOI] [PubMed] [Google Scholar]
- 15. Klein P, Tyrlikova I, Mathews GC. Dietary treatment in adults with refractory epilepsy: a review. Neurology 2014;83:1978–1985. [DOI] [PubMed] [Google Scholar]
- 16. Stang A. Critical evaluation of the newcastle‐ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 17. Sirven J, Whedon B, Caplan D, et al. The ketogenic diet for intractable epilepsy in adults: preliminary results. Epilepsia 1999;40:1721–1726. [DOI] [PubMed] [Google Scholar]
- 18. Nei M, Ngo L, Sirven JI, et al. Ketogenic diet in adolescents and adults with epilepsy. Seizure 2014;23:439–442. [DOI] [PubMed] [Google Scholar]
- 19. Mosek A, Natour H, Neufeld MY, et al. Ketogenic diet treatment in adults with refractory epilepsy: a prospective pilot study. Seizure 2009;18:30–33. [DOI] [PubMed] [Google Scholar]
- 20. Klein P, Janousek J, Barber A, et al. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav 2010;19:575–579. [DOI] [PubMed] [Google Scholar]
- 21. Lambrechts DA, Wielders LH, Aldenkamp AP, et al. The ketogenic diet as a treatment option in adults with chronic refractory epilepsy: efficacy and tolerability in clinical practice. Epilepsy Behav 2012;23:310–314. [DOI] [PubMed] [Google Scholar]
- 22. Kossoff EH, Rowley H, Sinha SR, et al. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia 2008;49:316–319. [DOI] [PubMed] [Google Scholar]
- 23. Smith M, Politzer N, Macgarvie D, et al. Efficacy and tolerability of the modified Atkins diet in adults with pharmacoresistant epilepsy: a prospective observational study. Epilepsia 2011;52:775–780. [DOI] [PubMed] [Google Scholar]
- 24. Cervenka MC, Terao NN, Bosarge JL, et al. E‐mail management of the modified Atkins Diet for adults with epilepsy is feasible and effective. Epilepsia 2012;53:728–732. [DOI] [PubMed] [Google Scholar]
- 25. Kossoff EH, Henry BJ, Cervenka MC. Efficacy of dietary therapy for juvenile myoclonic epilepsy. Epilepsy Behav 2013;26:162–164. [DOI] [PubMed] [Google Scholar]
- 26. Vaccarezza MM, Toma MV, Ramos Guevara JD, et al. Treatment of refractory epilepsy with the modified Atkins diet. Arch Argent Pediatr 2014;112:348–351. [DOI] [PubMed] [Google Scholar]
- 27. Cervenka MC, Henry BJ, Felton EA, et al. Establishing an Adult Epilepsy Diet Center: experience, efficacy and challenges. Epilepsy Behav 2016;58:61–68. [DOI] [PubMed] [Google Scholar]
- 28. Kverneland M, Selmer KK, Nakken KO, et al. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav 2015;53:197–201. [DOI] [PubMed] [Google Scholar]
- 29. Schoeler NE, Wood S, Aldridge V, et al. Ketogenic dietary therapies for adults with epilepsy: feasibility and classification of response. Epilepsy Behav 2014;37:77–81. [DOI] [PubMed] [Google Scholar]
- 30. DeGiorgio CM, Miller PR, Harper R, et al. Fish oil (n‐3 fatty acids) in drug resistant epilepsy: a randomised placebo‐controlled crossover study. J Neurol Neurosurg Psychiatry 2015;86:65–70. [DOI] [PubMed] [Google Scholar]
- 31. Ye F, Li XJ, Jiang WL, et al. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta‐analysis. J Clin Neurol 2015;11:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pasca L, De Giorgis V, Macasaet JA, et al. The changing face of dietary therapy for epilepsy. Eur J Pediatr 2016;175:1267–1276. [DOI] [PubMed] [Google Scholar]
- 33. Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol 2006;35:1–5. [DOI] [PubMed] [Google Scholar]
- 34. Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol 2017;30:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lord K, Magrath G. Use of the ketogenic diet and dietary practices in the UK. J Hum Nutr Diet 2010;23:126–132. [DOI] [PubMed] [Google Scholar]
- 36. Chiusolo F, Diamanti A, Bianchi R, et al. From intravenous to enteral ketogenic diet in PICU: a potential treatment strategy for refractory status epilepticus. Eur J Paediatr Neurol 2016;20:843–847. [DOI] [PubMed] [Google Scholar]
- 37. Farias‐Moeller R, Bartolini L, Pasupuleti A, et al. A Practical Approach to Ketogenic Diet in the Pediatric Intensive Care Unit for Super‐Refractory Status Epilepticus. Neurocrit Care 2016;26:267–272. [DOI] [PubMed] [Google Scholar]
- 38. Appavu B, Vanatta L, Condie J, et al. Ketogenic diet treatment for pediatric super‐refractory status epilepticus. Seizure 2016;41:62–65. [DOI] [PubMed] [Google Scholar]
- 39. Thakur KT, Probasco JC, Hocker SE, et al. Ketogenic diet for adults in super‐refractory status epilepticus. Neurology 2014;82:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]


