Summary
Objective
Functional neurological disorders (FNDs) and psychogenic nonepileptic seizures (PNES) are likely as common in Sub‐Saharan Africa (SSA) as in the rest of the world, but there is a dearth of literature on the epidemiology and clinical presentation of these disorders in Africa. The purpose of this paper is to describe a case series of FNDs presenting to a referral hospital in SSA. In addition, we review the existing literature on FNDs in Africa.
Methods
A hospital‐based retrospective cross‐sectional study was conducted to determine the prevalence, epidemiology, and clinical phenotype of FNDs and PNES in a referral hospital in Northern Tanzania over a 6‐year period (2007–2013).
Results
Of 2,040 patients presenting with neurological complaints, 44 (2.2%) were diagnosed with FNDs. Half (n = 22) had the clinical presentation of PNES. Age of presentation for FNDs and PNES peaked in the teen years 12–19 (n = 21 48%; and n = 14, 63%, respectively), and the majority were female (n = 30, 68%; and n = 14, 63%, respectively). The majority presented acutely with short‐lived and self‐limiting symptoms (only 2 recurrent cases). Literature review revealed multiple reports of “mass hysteria” in SSA often meeting the clinical criteria of epidemic FNDs.
Significance
FNDs and PNES occur in Africa with age and gender distribution comparable to that found elsewhere. Although the percentage of FND cases overall was relatively low (2.2%), it is likely to be an underestimate because not all cases were recorded, and cases may be appropriately managed locally before patients are referred to a hospital. PNES was the most common phenotype of FNDs reported, and the African phenotype may be short‐lived and self‐limiting rather than chronic and recurrent, as reported elsewhere in the world. PNES presentations may also occur in clusters, which may have cultural significance in Africa. FNDs in Africa appear to be underreported, particularly over the last 30 years.
Keywords: Africa, Tanzania, Psychogenic nonepileptic seizure, Epilepsy, Functional neurological disorder
Key Points.
Psychogenic nonepileptic seizures (PNES) constitute a major part of functional neurological disorders in Sub‐Saharan Africa
Their phenotype is different from that described in high‐income countries
The 30‐year gap in PNES reports from Africa reflects a changed prioritization since the era of HIV/AIDS
Introduction
Functional neurological disorders (FNDs) are conditions in which patients experience neurological symptoms that are not due to underlying neurological disease. FNDs occur worldwide and descriptions date back to Hippocrates in 700 B.C.1 The terminology for FNDs has evolved over the centuries, and includes the following: hysteria, psychogenic complaints, dissociative states, neurologically unexplained symptoms, somatoform, and conversion disorder. The currently favored term functional neurological disorder2 emphasizes how the condition is a disorder of function, not of structure or disease. Major risk factors for FNDs include psychosocial stressors and posttraumatic stress disorder (PTSD).3, 4
The main clinical presentations of FNDs include the following: loss or impairment of speech, vision, power, sensation, consciousness, or gain of involuntary movements or spells concerning for seizures. A thorough clinical and neurological assessment, often with additional investigations (eg, imaging, lab work), is necessary to exclude underlying disease. Reassuringly, a British 18‐month follow‐up study of neurologically unexplained symptoms showed that only 0.4% ultimately received disease diagnoses that plausibly explained the patient's original symptoms.5 Treatment of FNDs requires careful, empathetic counseling on the diagnosis, recognition of psychosocial or posttraumatic stress as a trigger, and reassurance regarding the absence of organic disease.3, 4 In practice, inadequate attention to psychosocial stress factors is common due to limited clinician awareness, understanding, or time to spend counseling the patient.
There is scant current medical literature (ie, publications from the 21st century) on the incidence, prevalence, and characteristics of somatization and FNDs in Sub‐Saharan Africa (SSA). High rates of somatization or psychogenic complaints have been reported in a handful of studies of certain subpopulations in SSA, often occurring in association with traumatic events such as war, displacement, human immunodeficiency virus (HIV) infection, and gender‐based violence.6, 7, 8, 9 Moreover, several studies of African immigrant populations conducted in North America, Europe, and Australia, report that FNDs are as common in the African immigrant population10, 11, 12 as in other subgroups. A 1996 World Health Organization (WHO) collaborative study among 15 primary care centers in 14 countries, including a center in Nigeria, used reliable instruments to study the phenomenon and epidemiology of somatization in different cultures in a uniform way13; they found that frequency of unexplained somatic symptoms did not clearly vary according to geography or level of economic development. The authors’ conclusion was that somatization is a common problem in primary care across cultures and is associated with significant health problems and disability.13
In 2011, a working group of the International League Against Epilepsy (ILAE) produced an international consensus‐based clinical practice statement on the management of neuropsychiatric disorders associated with epilepsy14; this statement identified psychogenic nonepileptic seizures (PNES) as among the top 3 neuropsychiatric problems that needed to be addressed around the world. Subsequently, an ILAE PNES Task Force was founded to summarize the current evidence and guidelines on the diagnosis and treatment of PNES. Recently, this Task Force published a report on “PNES around the world: where we are now and how we can close the diagnosis and treatment gaps.”15 In the report, they provide a knowledge update about the etiology, epidemiology, subgroups of PNES, and treatment gaps. In addition, the report described different approaches to clinical management and unique obstacles for the disorder in 6 different countries, including Zambia. In Zambia, as in Tanzania, regional health centers have limited access to electroencephalography (EEG), especially overnight EEG, cranial imaging, and individuals trained in PNES identification and management; the authors’ conclusions were that there is a strong need to increase awareness about PNES, develop infrastructure for diagnosis and management, develop proper teaching and training modules for clinical officers and nonspecialists, develop a strong referral system, and increase the number of health professionals trained in the management of PNES.15
In SSA, profound treatment gaps exist for neurological and psychiatric conditions.15, 16, 17 For example, studies have shown that up to 70% of patients with epilepsy and Parkinson's disease do not get diagnosed or treated.18 The treatment gaps are largely due to inadequate resources and a shortage of trained neurologists and psychiatrists, but other barriers to treatment include cultural stigma and traditional beliefs regarding patients with neuropsychiatric conditions.15, 16 The stigma may in part be based on traditional cultural beliefs regarding the etiology of neurological or psychiatric symptoms as “spirit disorders,” such as the Malawian disorder “vimbuza” characterized by various neuropsychiatric complaints.19 In the ILAE PNES Task Force report, the authors noted that in Zambia, epilepsy is not considered a “normal medical condition” by the population, but instead a mental disorder15; this likely contributes to the medical treatment gap for both epileptic seizures and nonepileptic seizures. FNDs straddle the boundary between neurological and psychiatric conditions, so they can be challenging to recognize and treat.
PNES are defined as paroxysmal, time‐limited disturbances of body function that mimic epileptic seizures, but that are not associated with abnormal electrical discharges in the brain seen in epileptic seizures. Thus the definitive way to distinguish PNES from epileptic seizures is with EEG testing, although there are multiple clinical clues that help distinguish the conditions.20, 21 For example, diagnosis can be made based on positive features of the examination (eg, Hoover's sign, resistance to eye opening) or attack (eg, prolonged sudden motionless unresponsiveness with eyes closed). One major risk factor for PNES is PTSD from a history of emotional, physical, or sexual abuse.4, 20 PNES can be acute and self‐limited or chronic and protracted, and may occur in case clusters, such as recently described in a Tanzanian school.22 Although many studies describe and characterize PNES in high‐income countries, only a few studies come from SSA and these are mostly from the Republic of South Africa and in select populations.23, 24 Most published references on FNDS and PNES in SSA predate the arrival of HIV in Africa during the early 1980s.19, 25, 26
FNDs and PNES are an underreported and potentially underrecognized challenge in SSA. This retrospective hospital‐based study reports a case series of patients presenting to a hospital in Tanzania in East Africa with FNDs, half of which had the clinical phenotype of PNES. This study is the first to report the prevalence and clinical presentation of FNDs presenting to a tertiary referral center in SSA, and the first dedicated literature review of FNDs and PNES in SSA in the 21st century.
Methods
The purpose of the study was to determine the prevalence, epidemiology, and clinical phenotype of FNDs presenting to a major tertiary care hospital in Northern Tanzania. This hospital‐based retrospective cross‐sectional study was conducted at the Kilimanjaro Christian Medical Centre (KCMC) in Moshi, Tanzania,27 which has had its own medical school since 1999, training both physicians and ancillary healthcare providers. Medical ethical clearance for the study was obtained from the ethics committee at KCMC College. A 600‐bed teaching hospital and tertiary referral center with outpatient clinics, KCMC covers a catchment population of over 16 million people. Northern Tanzania has limited diagnostics and interventions available: one computed tomography (CT) scanner and one EEG machine to facilitate routine studies, and the closest magnetic resonance imaging (MRI) scanner is ~80 km away in Arusha, Tanzania. Imaging studies are costly for patients (CT 75 Euro, MRI 315 Euro), which limits their use to patients and families who can afford them, since there is not a national healthcare system. Two (MD, WH) of Tanzania's seven practicing internationally boarded neurologists are based at KCMC in the medicine (1) and pediatric (1) departments; there is no stand‐alone neurology department, and these 2 neurologists, as well as a neurologist‐in‐training (SU), evaluate all the patients presenting with neurological complaints.
The study population consisted of all patients aged 12 years and older who presented for initial evaluation of neurological complaints to the adult neurology outpatient clinic or were admitted to KCMC adult wards during the 6‐year study period from April 2007 to March 2013. Each patient was seen and examined by specialty‐trained neurologists who determined the primary underlying diagnosis for the neurological complaints. Exclusion criteria included prior history of neurological disease, clinical or investigational evidence of preexisting neurological disease (unrelated to the presenting complaints), or previous presentations to KCMC for the complaints.
A total of 2,040 subjects who met inclusion and exclusion criteria were entered into a database. Data collected for each subject included age, sex, primary neurological diagnosis, and disease category. Disease categories included stroke, infection, paraplegia, confusion/coma, space‐occupying lesion, neurodegenerative disease, and “other.” Figure 1 illustrates the prevalence of each disease category in the study population. FNDs fell into the category of “other.” Laboratory and radiological investigations were performed at the KCMC main laboratory and radiology department. EEG and CT scanning were obtained as clinically indicated if patients could afford the cost. Data were analyzed and graphed using Open Source software.28 A literature review was conducted using PubMed.http://www.ncbi.gov.
Figure 1.
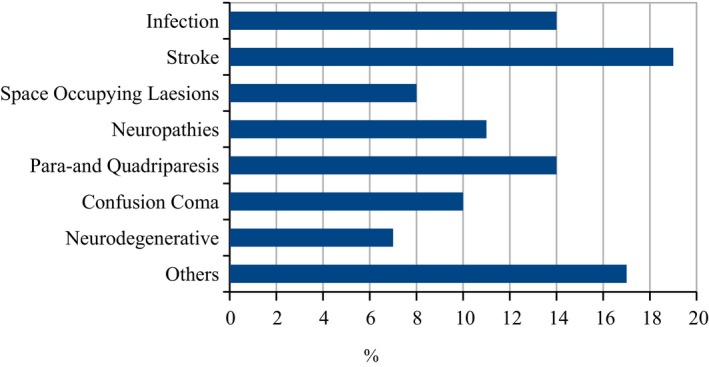
Neurological Disorders in Northern Tanzania, Kilimanjaro Christian Medical Centre 2007–2013 (n = 2,040), W. Howlett, unpublished data.
Results
Of the 2,040 patients presenting for evaluation of neurological complaints, 44 (2.2%) were diagnosed with an FND, and formed the primary study group. Each of the 44 patients presented with neurological complaints and had examination findings consistent with FND (such as Hoover's sign, distractibility), but had no evidence of underlying organic disease on history, examination, or investigations. Thirty (68%) of the 44 patients with FND were female and 21 (48%) were under the age of 20 years.
The most common clinical phenotype of FND was PNES: half (22) of the 44 patients presented with PNES. Other common clinical phenotypes included an inability to walk (n = 9, 20.5%), functional movement disorder (n = 6, 13.6%), stroke‐like presentation (n = 4, 9.1%) such as hemiplegia, loss of vision (n = 2, 4.5%), and confusion/coma (n = 1, 2.3%) with evidence of preserved consciousness on examination. Figure 2 illustrates the prevalence of each phenotype. Of the 22 patients with PNES, 63% (n = 14) were female. Figure 3 illustrates the skewed age distribution of patients presenting with PNES, with most patients being younger: 63% (n = 14) were under 20‐years‐old and 82% (n = 18) were under 30‐years‐old. Two (9.1%) of the 22 patients with PNES returned to KCMC to report recurrence of their PNES.
Figure 2.
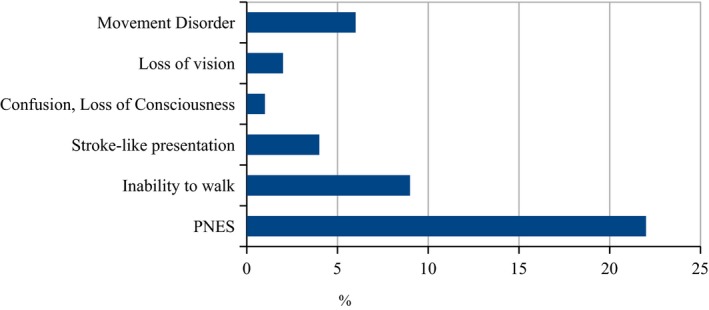
Functional Neurological Disorders in Kilimanjaro Christian Medical Centre 2007–2013 (n = 44), W. Howlett, unpublished data.
Figure 3.

Psychogenic nonepileptic seizures (n = 22), age distribution.
Discussion
In keeping with the worldwide epidemiology of FNDs and PNES,4, 15, 20 a striking female predominance and younger age of onset (teenage years) was noted in this study. In the experience of one of the authors (MD), a similar gender distribution is seen in children under 12‐years‐old in the pediatric neurological population at this referral center (M. C. J. Dekker/W. P. Howlett: unpublished preliminary data). In contrast to reports on high recurrence rates of up to 70% from Europe and North America,20, 29 this study's data suggest that patients with PNES have lower rates of re‐presenting to a tertiary referral center for reevaluation (2/22 or 9.1%). The 2 patients who repeatedly presented with a recurrent form of PNES (one male and one female) were teenagers with concomitant depression and PTSD, respectively. In the experience of the authors, most cases of PNES had short‐lived and self‐limiting symptoms (M. C. J. Dekker/W. P. Howlett: unpublished preliminary data). The reasons for the infrequent re‐presentation of PNES cases to a single medical center in this population are unclear, but this pattern has been observed in the past in SSA.26 However, a limitation of this cross‐sectional study is that it is uncertain what percentage of the patients had recurrent PNES and did not return to KCMC; instead, they may have presented to local healers or different healthcare centers.
Although the percentage of reported FND cases in the total case series of neurological presentations is very low (n = 44/2040, 2.2%), this is likely an underestimate of the true prevalence of FNDs. A limitation to the study was that not all cases were recorded due to incomplete or lost paper‐based records, inability to run necessary tests to rule out organic disease (eg, cost, access to necessary equipment), and a tendency for general hospital‐based physicians without formal neurology or psychiatry training to rule out organic disease without further mentioning any suspicion of FND. As evidence of probable underreporting, 4 patients (all women) presenting with FNDs were admitted to the KCMC Medical Department in January 201530; although this cluster may have represented a statistical anomaly, if half that number of FND cases (2) presented monthly over the 6‐year study period, there would have been 144 subjects with FND admitted to the hospital alone, not including the clinic evaluations. In addition, because clinical workups were performed in a resource‐poor setting, patients may not have been given the definitive diagnosis of FND because they were unable to access or afford the necessary diagnostic studies (eg, EEG, CT, and MRI) to definitively rule out neurological disease. It is probable that a higher percentage of the “other” category of neurological diagnoses represented unconfirmed FND. Finally, a critical limitation to the study that reduces its broad applicability to the Tanzanian population as a whole, is that the cost of medical evaluation likely deterred many patients from presenting to the hospital. Furthermore, other factors such as religion, age, and literacy may also have deterred certain subpopulations from presenting to the hospital. For example, the younger average age of patients in this study may be because younger patients are more comfortable with newer Western medicine approaches; and, the shorter life expectancy of people in Tanzania (men 60 and women 64, per WHO 2015 data31) may have contributed to the lower average age of presentation.
On the other hand, referral bias may be a significant reason for the low percentage of FND cases presenting to KCMC, a tertiary care hospital and major referral center in Northern Tanzania. As described in Peltzer's 1989 article on the “Nosology and etiology of a spirit disorder (Vimbuza) in Malawi,”19 there may be an existing traditional African healer or community health worker approach to recognizing and managing mental health presentations and FNDs. In Pelzer's study, the most common reasons cited for the etiology of vimbuza are “bad ancestral spirits,” “naturally” or “by itself” where predispositions such as family history are recognized; “too much thinking over personal problems” such as death of a child, divorce, or other trauma; and “witchcraft.” Consequently, FNDs in traditional SSA communities may not utilize as many hospital resources as this population does in the West.32 We hypothesize that the traditional African community approach to management may be more cost‐effective than the Western approach, since the few existing studies report lower rates of recurrent presentations to specialized medical centers. However, there is no robust evidence to support this hypothesis and further research in this area is merited.
In high‐income countries, the reported phenotypes of FNDs include less well‐defined phenomena such as pain syndromes and diffuse weakness,5 and FNDs are frequently associated with extended sick leave and high healthcare costs.32 FNDs in high‐income countries can have delayed diagnosis for prolonged periods, often due to clinician fears of misdiagnosis and possible litigation, poor social acceptance of mental health disorders and FNDs, and insufficient access to mental health treatment. In a South African study of life after PNES diagnosis (in which 6/10 subjects were white), most subjects (6/10) were employed at follow‐up; although most (9/10) were still experiencing PNES recurrences episodically,24 delayed diagnosis of greater than 1 year had occurred in 4/10 subjects. Regardless of country of origin, if the psychosocial aspects of FNDs are overlooked or unaddressed then functional outcomes are poor, and may lead to ongoing psychological suffering and stigmatization.15, 20, 23, 24 Perhaps Western medicine has something to learn from the African approach to FND management.
PNES appears to be a very common clinical phenotype of FNDs in SSA,23, 24, 26 as supported by this study (Figure 2). The most recent medical literature on PNES in SSA comes from the Republic of South Africa,23, 24 with epidemiology similar to that of the present study. Since as early as the 1950s, there were reports of FNDs in Africa.33 In Osuntokun's unique large‐scale study (9,359 patients) from 1957 to 1968, only 23 patients were reported to have FND (often PNES) with an age and gender distribution similar to that of our study.25 In Spillane's book on “Tropical Neurology” from 1973, authors from various countries in Southern, Eastern, and Western Africa report on the spectrum of neurological disorders in the respective African regions.26 They refer to FNDs as a disease category regularly encountered, albeit under the terms hysteria and neurosis. The authors suggested that occurrence rates were comparable to those in Europe. Numerous epidemic outbreaks of FNDs or “mass hysteria” have been reported as recently as 2017,22 often occurring in grade schools.33, 34 Such epidemics were reported in South Africa, Tanzania, Malawi, Zimbabwe, Zambia, and Uganda. In a literature review of the topic, Kokota suggests that “mass hysteria” may be more common in SSA than in other parts of the world,33 although there is no clear evidence to support this hypothesis. The apparent increase in cases may be the result of reporting bias, therefore mass hysteria is likely just as prevalent in Western countries as in SSA.
There was a large gap in reporting of FNDs and PNES in Africa from the 1970s to the present, except for case reports cited above and the literature from South Africa.23, 24 The gap in literature may be secondary to the scarcity of practicing neurologists and the perception of what falls in the neurological domain, a virtual absence of mental health services, and traditional medicine practices. In addition, since the HIV/AIDS epidemic emerged in Africa in the 1980s, the reporting of neurological disorders in SSA has been dominated by the neurological sequelae of HIV/AIDs.15, 21
However, FNDs have always existed and continue to pose a clinical challenge worldwide. This study's data are largely in line with the epidemiology of other countries and cultures: there is female predominance, the proportion of FNDs that present as PNES are similar, and there is a younger age of presentation (which must be taken in the context of local life expectancy).15, 35, 36 The fact that the epidemiology is similar across countries and cultures suggests that the spectrum and demography of FNDs and PNES may not be very different between Tanzania and wealthier countries, and that relatively fewer patients with FNDs are seen (or rather diagnosed) at specialized medical centers in Tanzania for the reasons discussed earlier. Fortunately, progress is being made, as evidenced by the recent ILAE Commission on the Neuropsychiatric Aspects of Epilepsy14 and the ILAE PNES Task Force,15 although there remains a great need for further research on the causes and optimal treatments, education of healthcare providers, and patient advocacy.
Conclusion
FNDs and PNES commonly occur in SSA and have been underreported in last half century. This retrospective cross‐sectional study of FNDs and PNES notes a marked female predominance (71% and 63%, respectively) and younger age of presentation (48% and 67% aged 12–19 years, respectively), which is consistent with worldwide epidemiology. This hospital‐based study noted a low rate of re‐presentation of cases to the hospital (9.1%); however, we cannot draw firm conclusions about recurrence rates, since patients may have re‐presented elsewhere. Although this study reported a relatively low number of cases over the 6‐year period (2.2%), the study was limited by incomplete records and referral bias. Increased knowledge and awareness are critical to the successful diagnosis and management of FNDs and PNES worldwide.
Disclosure
The authors have no funding and have nothing to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Biography
Marieke C. J. Dekker is a neurologist in KCMC, a tertiary referral hospital in Kilimanjaro, Tanzania.

References
- 1. Adams F. The genuine works of Hippocrates. Translated from the Greek Hippocrates: on the sacred disease. Baltimore, MD: Williams and Wilkins; 1939. [Google Scholar]
- 2. Ding JM, Kanaan RAA. Conversion disorder: a systematic review of current terminology. Gen Hosp Psychiatry 2017;45(Suppl C):51–55. [DOI] [PubMed] [Google Scholar]
- 3. Carson A, Lehn A, Ludwig L, et al. Explaining functional disorders in the neurology clinic: a photo story. Pract Neurol 2016;16:56–61. [DOI] [PubMed] [Google Scholar]
- 4. Asadi‐Pooya AA. Psychogenic nonepileptic seizures: a concise review. Neurol Sci 2017;38:935–940. [DOI] [PubMed] [Google Scholar]
- 5. Stone J, Carson A, Duncan R, et al. Symptoms ‘unexplained by organic disease’ in 1144 new neurology out‐patients: how often does the diagnosis change at follow‐up? Brain 2009;132:2878–2888. [DOI] [PubMed] [Google Scholar]
- 6. Carey PD, Stein DJ, Zungu‐Dirwayi N, et al. Trauma and posttraumatic stress disorder in an urban Xhosa primary care population: prevalence, comorbidity, and service use patterns. J Nerv Ment Dis 2003;191:230–236. [DOI] [PubMed] [Google Scholar]
- 7. Yeomans PD, Herbert JD, Forman EM. Symptom comparison across multiple solicitation methods among Burundians with traumatic event histories. J Trauma Stress 2008;21:231–234. [DOI] [PubMed] [Google Scholar]
- 8. Zacarias AE, Svanström L, Antai D. Symptoms of depression, anxiety, and somatization in female victims and perpetrators of intimate partner violence in Maputo City, Mozambique. Int J Womens Health 2012;4:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edwards SD, Jainarain M, Randeree FA, et al. Conversion disorders in Zulu patients. South Afr Med J 1982;62:97–99. [PubMed] [Google Scholar]
- 10. Pfortmueller CA, Graf F, Tabarra M, et al. Acute health problems in African refugees. Wien Klin Wochenschr 2012;124:647–652. [DOI] [PubMed] [Google Scholar]
- 11. Aragona M, Rovetta E, Pucci D, et al. Somatization in a primary care service for immigrants. Ethn Health 2012;17:477–491. [DOI] [PubMed] [Google Scholar]
- 12. Bragazzi NL, Puente GD, Natta WM. Somatic perception, cultural differences and immigration: results from administration of the Modified Somatic Perception Questionnaire (MSPQ) to a sample of immigrants. Psychol Res Behav Manag 2014;7:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gureje O, Simon GE, Ustun TB, et al. Somatization in cross‐cultural perspective: a World Health Organization study in primary care. Am J Psychiatry 1997;154:989–995. [DOI] [PubMed] [Google Scholar]
- 14. Kerr MP, Mensah S, Besag F, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. Epilepsia 2011;52:2133–2138. [DOI] [PubMed] [Google Scholar]
- 15. Kanemoto K, LaFrance WC, Duncan R, et al. PNES around the world: where we are now and how we can close the diagnosis and treatment gaps‐an ILAE PNES Task Force report. Epilepsia Open 2017;2:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer A‐C, Dua T, Ma J, et al. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ 2010;88:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chin JH, Vora N. The global burden of neurologic diseases. Neurology 2014;83:349–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dotchin C, Msuya O, Kissima J, et al. The prevalence of Parkinson's disease in rural Tanzania. Mov Disord 2008;23:1567–1672. [DOI] [PubMed] [Google Scholar]
- 19. Peltzer K. Nosology and etiology of a spirit disorder (Vimbuza) in Malawi. Psychopathology 1989;22:145–151. [DOI] [PubMed] [Google Scholar]
- 20. Reuber M, Elger CE. Psychogenic nonepileptic seizures: review and update. Epilepsy Behav 2003;4:205–216. [DOI] [PubMed] [Google Scholar]
- 21. Howlett WP. Neurology in Africa. Neurology 2014;83:654–655. [DOI] [PubMed] [Google Scholar]
- 22. Kanenda S. Hysteria in Secondary School. Mwananchi 2017.
- 23. Cronje G, Pretorius C. The coping styles and health‐related quality of life of South African patients with psychogenic nonepileptic seizures. Epilepsy Behav 2013;29:581–584. [DOI] [PubMed] [Google Scholar]
- 24. Pretorius C, Sparrow M. Life after being diagnosed with psychogenic non‐epileptic seizures (PNES): a South African perspective. Seizure 2015;30:32–41. [DOI] [PubMed] [Google Scholar]
- 25. Osuntokun BO. The pattern of neurological illness in tropical Africa. Experience at Ibadan, Nigeria. J Neurol Sci 1971;12:417–442. [DOI] [PubMed] [Google Scholar]
- 26. Spillane JD. Tropical neurology. Proc Biol Sci 1969;62:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dekker MCJ, Urasa SJ, Howlett WP. Neurological letter from Kilimanjaro. Pract Neurol 2017;17:412–416. [DOI] [PubMed] [Google Scholar]
- 28. Savannah: the software forge for people committed to free software. Available at: http://savannah.gnu.org/. Accessed May 3, 2017.
- 29. Betts T, Boden S. Diagnosis, management and prognosis of a group of 128 patients with non‐epileptic attack disorder. Part I. Seizure 1992;1:19–26. [DOI] [PubMed] [Google Scholar]
- 30. Kellogg M, Howlett W, Dekker MCJ. The underreported challenge of disabling psychogenic neurological disorders in Sub‐Saharan Africa: a case series of patients presenting to a regional hospital in Tanzania. Poster presentation at the American Academy of Neurology Meeting: Vancouver, BC, 16 April 2016; 2016. [Google Scholar]
- 31. WHO . WHO | United Republic of Tanzania [Internet]. Available at: http://www.who.int/countries/tza/en/. Accessed October 11, 2017.
- 32. Salinsky M, Storzbach D, Goy E, et al. Health care utilization following diagnosis of psychogenic nonepileptic seizures. Epilepsy Behav 2016;60:107–111. [DOI] [PubMed] [Google Scholar]
- 33. Kokota D. Episodes of mass hysteria in African schools: a study of literature. Malawi Med J 2011;23:74. [PMC free article] [PubMed] [Google Scholar]
- 34. Govender I. Mass hysteria with possible pseudoseizures at a primary school. S Afr Med J 2008;93:10. [PubMed] [Google Scholar]
- 35. Lempert T, Dieterich M, Huppert D, et al. Psychogenic disorders in neurology: frequency and clinical spectrum. Acta Neurol Scand 1990;82:335–340. [DOI] [PubMed] [Google Scholar]
- 36. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—The diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010;112:747–751. [DOI] [PubMed] [Google Scholar]


