Summary
SUDEP is the sudden unexpected death of a person with epilepsy, when no structural or toxicological cause of death can be found. The majority of witnessed cases are reported to be preceded by a convulsive seizure and postictal hypoventilation. Here, we report an 8‐year‐old girl with drug‐resistant focal seizures secondary to a focal cortical dysplasia type IIb. While undergoing invasive intracranial monitoring with subdural and depth electrodes, she had a clinical apnea event recorded on video, followed by bradycardia, which required resuscitation. Her intracranial electroencephalogram (EEG) during the event showed diffuse slowing and attenuation of cortical activity, with bradycardia that responded to positive pressure ventilation with oxygen. This near SUDEP event was not preceded by either an electroclinical or electrographic seizure. This is the first report of a witnessed, near‐SUDEP event during intracranial monitoring. It emphasizes the fact that near‐SUDEP can occur without a preceding seizure.
Keywords: Drug‐resistant epilepsy, Apnea, Near‐SUDEP, Intracranial EEG
Brief Communication
We present an 8‐year‐old, right‐handed girl with drug‐resistant focal epilepsy since the age of 3 years. All seizures were right‐sided focal motor seizures. The seizure semiology consisted of fisting of her right hand, followed by drooling from the right side of the mouth. Seizures were resistant to three antiepileptic drugs. Medications at the time of surgical evaluation were oxcarbazepine, clobazam, and lacosamide.
Past medical history, including pre‐ and perinatal history, was otherwise unremarkable. Developmental history was significant for a learning disability. Neuropsychology evaluation demonstrated overall verbal and perceptual reasoning abilities in the Borderline range. She demonstrates relative strengths in verbal abilities. Family history was negative for epilepsy, sudden death, or cardiac arrhythmia. Her father has obstructive sleep apnea.
Presurgical evaluation suggested seizures arising from the left Rolandic region. Three‐tesla magnetic resonance imaging (MRI) demonstrated subtle focal cortical dysplasia in the depth of the left central sulcus. Positron emission tomography (PET) demonstrated a hypometabolic area in the anterior aspect of the left parietal lobe, including the postcentral gyrus, and magnetoencephalography (MEG) demonstrated 45 spike sources clustered in the left Rolandic region. Scalp video electroencephalography (EEG) recording confirmed the seizure onset was from the left Rolandic region.
She was determined to be a candidate for invasive monitoring. The placement of the subdural and depth electrodes lasted 4 h between 8 am and 12 pm. Sixty‐four subdural electrodes were placed over the left frontoparietal region, and two (four contact) depth electrodes were inserted into the MRI lesion. The procedure was performed under general anesthesia with intravenous propofol, remifentanil, fentanyl, and inhalational gas sevoflurane. She received one dose of morphine at the end of the procedure. She was admitted to the pediatric intensive care unit postoperatively. She received acetaminophen and did not require opioids as postoperative analgesia. Maintenance doses of oxcarbazepine and lacosamide were reduced to induce seizures. During the first 15 hours of recording, from 2:35 pm to 5:38 am, she had 14 habitual focal motor seizures lasting for 20 seconds without secondary generalized tonic‐clonic seizures. Her seizure semiology consisted of head deviation to the left with tonic posturing of the right arm, followed by clonic twitching of the right hand. The patient remained responsive during the seizures and was able to speak directly afterward. Each seizure lasted between 18 and 35 s. At midnight she received her regular maintenance doses of oxcarbazepine and lacosamide owing to the frequent seizures. The sequence of events is described and complemented by the video EEG as supporting information.
At 5:38:32 am she had a habitual seizure, concluding at 5:39:01. Intracranial EEG returned to the baseline interictal pattern just after 5:39:01. At 5:58:03 am, the bedside nurse talked to her and she responded. At 5:58:12 the nurse noted a drop in her oxygen saturation to 60% and asked her to take a deep breath; however, her oxygen saturation continued to decrease in association with a reduced respiratory rate and visible reduction in chest excursion. At 5:58:40, she completely stopped breathing. At 5:58:52, the intracranial EEG began to show diffuse polymorphic slow waves, and heart rate was 96 bpm. At 5:59:17, the nurse started tactile stimulation. At 5:59:32, the EEG background voltage was gradually attenuated, and heart rate was 81 bpm. At 5:59:45, the EEG background activity became completely flat, and heart rate was 60 bpm. There was no change with vigorous stimulation at 5:59:47 when she became unconscious. At 6:00:25, resuscitation was initiated. She required oxygen with positive pressure ventilation via bag‐valve‐mask and rapid sequence intubation. At 6:00:46, the heart rate slowed to 42 bpm. At 6:00:58, the heart rate became 60 bpm; however, the EEG was still markedly attenuated. At 6:01:06 when the heart rate was back to 92 bpm, diffuse polymorphic slow waves returned on the EEG. At 6:01:40, she had a bilateral convulsive seizure lasting 34 s. This seizure had the same ictal EEG distribution as her habitual seizures. Postresuscitation, she woke up and returned to her baseline. Within 1 h, brain computerized tomography (CT) scan and MRI demonstrated no acute changes. There did not appear to be a lesion or pressure effect from the depth electrodes or subdural grid on the primary motor cortex. The positions of the electrodes are depicted in Fig. 1. She was extubated the following day. A timeline of the events is depicted in Fig. 2.
Figure 1.

A three‐dimensional fusion image was constructed by combining preoperative MRI with magnetoencephalographic (MEG) dipoles and CT after the insertion of intracranial electrodes, using BrainLab software. The location of the 64 subdural electrodes (pink), 2 (four contact) depth electrodes (black lines), MEG dipoles (green), and epileptogenic MRI lesion (orange) are marked.
Figure 2.
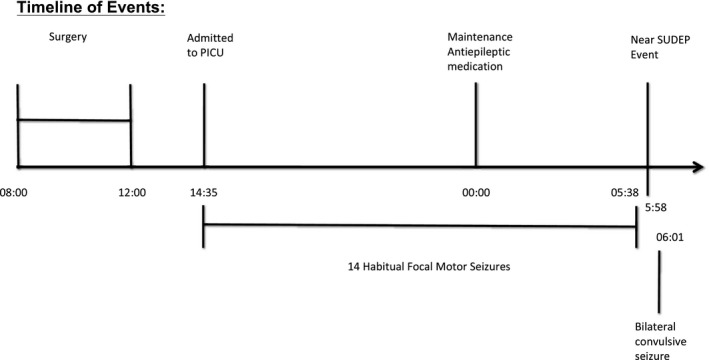
The events leading up to the Near SUDEP event are depicted in the timeline.
Intracranial EEG and cardiorespiratory monitoring during this near‐SUDEP event showed initial apnea followed by diffuse slowing, and attenuation of cortical activity, with bradycardia that responded to positive pressure ventilation with oxygen. The intracranial EEG then demonstrated a focal ictal pattern that clinically manifested as a bilateral convulsive seizure. Invasive monitoring was continued for 3 more days. The ictal onset zone, the symptomatogenic zone, and the irritative zone were shown to originate from the left Rolandic region. She underwent cortical excision of left Rolandic MRI lesion including sensory cortex. The pathology confirmed a focal cortical dysplasia type IIb.
Cardiology and respirology evaluations were performed with no abnormality found. She is seizure free for 2 years following surgery. Paresis of the right arm was present postoperatively, as expected owing to the surgical resection, and rehabilitation was undertaken. Prior to discharge, her parents completed a basic life support course.
Discussion
Near SUDEP is defined as a sudden, unexpected, nontraumatic, and nondrowning cardiorespiratory arrest in a person with epilepsy without a structural cause identified after investigation.1 It occurs in benign circumstances, with or without evidence for a seizure, excluding documented status epilepticus, where the patient survived resuscitation for more than 1 h after the cardiorespiratory arrest. Despite an increasing interest in SUDEP, a single underlying cause of death in these cases has not been identified. Many studies of SUDEP have focused on the final pathophysiological mechanisms in which a seizure could lead to cardiorespiratory arrest and death.2
We report a near‐SUDEP event during intracranial monitoring, with unprovoked hypoventilation while lying in a supine position, followed by bradycardia without a preceding generalized tonic‐clonic seizure or cardiac arrhythmia. Intracranial EEG demonstrated that the last seizure occurred 19 min prior to onset of hypoventilation. EEG suppression occurred in all the electrode contacts after being apneic for 65 s with no evidence of a preceding ictal EEG change. It is possible that a focal onset electrographical seizure may have occurred in regions not sampled by the intracranial electrodes.
This case emphasizes the fact that near SUDEP can occur without a seizure. People with epilepsy may be at risk for apnea, in the absence of a preceding convulsive seizure, suggesting an alternate pathway to SUDEP without preceding seizure. Other cases of SUDEP and near SUDEP without a preceding seizure have been reported. Recently, Lhatoo et al. reported a case series of 3 adults with definite or probable SUDEP without electroclinical evidence of a seizure preceding death. A primary brainstem etiology affecting respiration was hypothesized as a result of the abnormal respiration patterns and generalized EEG suppression without documented cardiac tachyarrhythmias.3 Langan et al. reported 3 SUDEP deaths not associated with a convulsive seizure, among a cohort of 15 witnessed SUDEP cases. One person collapsed 5 min after a generalized seizure, another collapsed after an aura only, and one died while in a probable postictal state.4 Donner et al. reported 10 witnessed cases among 27 cases of SUDEP in children. Of these 10 witnessed cases, only 5 were associated with a convulsive seizure. The authors hypothesized that an electrographic seizure may have occurred prior to death.5
In contrast, a review of 29 cardiorespiratory arrests occurring in epilepsy monitoring units included 16 SUDEP and 9 near‐SUDEP cases6; all except 2 out of the 9 near‐SUDEP cases were preceded by a generalized tonic‐clonic seizure. Cardiorespiratory data available for 10 SUDEP cases identified a consistent pattern prior to arrest, beginning with postictal tachypnea, followed by apnea, bradycardia, and postictal generalized EEG suppression (PGES). In some patients, a period of transient ineffective gasping breaths, associated with QRS complexes on electrocardiogram (ECG), occurred followed by terminal apnea and then terminal asystole.6
A case report of SUDEP occurring during invasive monitoring did not have detailed respiratory or cardiac monitoring for review, although pulse artifact on the EEG recording allowed for assessment of heart rate.7 The terminal event began with a secondarily generalized temporal lobe seizure followed by postictal flattening of brain activity, bradycardia, and cardiorespiratory arrest.7 A near SUDEP occurring in an adult epilepsy monitoring unit (EMU) patient with intractable temporal lobe epilepsy was reported. In this case, the patient developed postictal laryngospasm, requiring intubation.8 There was no evidence of laryngospasm in our patient, and intubation following the apneic episode was performed with ease.
The mechanism of apnea in our patient remains unclear. Several brain regions, including the cingulate gyrus, amygdala, hippocampus, and thalamic nuclei, have been associated with ventilation and cardiac dysfunction and hypothesized to precipitate SUDEP.9 Our patient's MRI lesion and ictal onset zones were over the left Rolandic region, suspected to be involving the depth of the left central sulcus, but away from the above‐mentioned areas, which were not sampled by subdural or depth electrodes. Therefore, a seizure originating within one of these deeper structures cannot be excluded. Central, mixed, and obstructive apnea, O2 desaturation, and hypoventilation have been documented to occur during or after certain focal or generalized epileptic seizures.4, 10 However, the incidence of primary apnea in people with epilepsy, occurring without a seizure, is not known. Further, although ictal asystole itself is rare, peri‐ictal hypoventilation may lead to potentially fatal cardiac arrhythmias related to short or prolonged QT intervals.11, 12, 13
Several factors may have contributed to our patient's SUDEP risk profile. She had received a general anesthetic for the placement of intracranial electrodes less than 24 h prior to the near‐SUDEP event and was weaned off her anticonvulsant medications. The intracranial EEG demonstrated very frequent interictal discharges and 14 electroclinical seizures over 15 h.
The roles of the anesthetic, the frequent interictal discharges, and the frequent habitual seizures should be considered. Delayed postoperative respiratory depression may occur secondary to oversedation from anesthetic and analgesia medications, including opioids.14 Although this is a plausible explanation, it is less likely because our patient had two previous anesthetics for MRI without complications of respiratory depression. Another possible explanation is that the general anesthesia may have triggered an apneic event associated with late‐onset central hypoventilation syndrome occurring beyond the neonatal period owing to variable penetrance of the PHOX2B mutation.15 This scenario is unlikely because this syndrome usually results in the need for permanent nocturnal ventilator support, which was not the case. Another hypothesis to consider is that this near‐SUDEP event may have been precipitated by the release of endogenous opioids induced by frequent habitual seizures. This theory is supported by animal studies that suggested seizures release endogenous opioids to assist with seizure termination, and human studies that demonstrated opioid receptor availability increases following spontaneous seizures.16, 17 It is therefore hypothetically possible that a cluster of seizures occurring within the first 24 h after general anesthesia might have increased the risk of opioid‐related delayed postoperative respiratory depression in this patient.
Though there are several potential contributing factors to this near‐SUDEP case, the physiological events are clear. Our patient presented with a hypoventilation and apnea, unrelated to an ictal event that would have resulted in SUDEP if not for aggressive resuscitation. As the literature on SUDEP continues to grow, more information is coming forward to increase our understanding of the mechanism of death. This case confirms the role of primary apnea in SUDEP and suggests that there will not be a single identified mechanism for SUDEP.
Disclosure
The authors have no conflict of interests with regard to the manuscript submission. All coauthors were involved in the manuscript preparation, approved the final version, and accept responsibility for its content. The authors confirm they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. This report did not receive any source of funding.
Supporting information
Video S1. Video EEG of the Near SUDEP event
Acknowledgments
We would like to thank our patient and her family and acknowledge further members of the Epilepsy surgery team, including Nurse Practitioner Vera Nenadovic, J.P. Appendino, and our EEG technologists Elysa Widjaja and Ms. Stephanie Holowka.
Biography
Duaa M. Ba‐Armah is a consultant in pediatric neurology and epilepsy at King Abdullah Specialist Children Hospital—National Guards, Riyadh, Saudi Arabia.

References
- 1. Nashef L, So EL, Ryvlin P, et al. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012;53:227–233. [DOI] [PubMed] [Google Scholar]
- 2. Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet 2011;378:2028–2038. [DOI] [PubMed] [Google Scholar]
- 3. Lhatoo SD, Nei M, Raghavan M, et al. Nonseizure SUDEP: sudden unexpected death in epilepsy without preceding epileptic seizures. Epilepsia 2016;57:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langan Y, Nashef L, Sander JW. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry 2000;68:211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donner EJ, Smith CR, Snead OC 3rd. Sudden unexplained death in children with epilepsy. Neurology 2001;57:430–434. [DOI] [PubMed] [Google Scholar]
- 6. Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 7. Bird JM, Dembny KAT, Sandeman D, et al. Sudden unexplained death in epilepsy: an intracranially monitored case. Epilepsia 1997;38:S52456. [Google Scholar]
- 8. Tavee J, Morris H 3rd. Severe postictal laryngospasm as a potential mechanism for sudden unexpected death in epilepsy: a near‐miss in an EMU. Epilepsia 2008;49:2113–2117. [DOI] [PubMed] [Google Scholar]
- 9. Terra VCSFA, Arida RM, Cavalheiro EA, et al. Sudden unexpected death in epilepsy. Fut Neurol 2010;5:691–699. [Google Scholar]
- 10. Bateman LM, Spitz M, Seyal M. Ictal hypoventilation contributes to cardiac arrhythmia and SUDEP: report on two deaths in video‐EEG‐monitored patients. Epilepsia 2010;51:916–920. [DOI] [PubMed] [Google Scholar]
- 11. Massey CA, Sowers LP, Dlouhy BJ, et al. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol 2014;10:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seyal M, Pascual F, Lee CY, et al. Seizure‐related cardiac repolarization abnormalities are associated with ictal hypoxemia. Epilepsia 2011;52:2105–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Surges R, Thijs RD, Tan HL, et al. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol 2009;5:492–504. [DOI] [PubMed] [Google Scholar]
- 14. Weingarten TN, Herasevich V, McGlinch MC, et al. Predictors of delayed postoperative respiratory depression assessed from naloxone administration. Anesth Analg 2015;121:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahfouz AK, Rashid M, Khan MS, et al. Late onset congenital central hypoventilation syndrome after exposure to general anesthesia. Can J Anaesth 2011;58:1105–1109. [DOI] [PubMed] [Google Scholar]
- 16. Benarroch EE. Endogenous opioid systems: current concepts and clinical correlations. Neurology 2012;79:807–814. [DOI] [PubMed] [Google Scholar]
- 17. Hammers A, Asselin MC, Hinz R, et al. Upregulation of opioid receptor binding following spontaneous epileptic seizures. Brain 2007;130:1009–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Video EEG of the Near SUDEP event


