Abstract
The neuroendocrine brain or hypothalamus has emerged as one of the most highly sexually dimorphic brain regions in mammals, and specifically in rodents. It is not surprising that hypothalamic nuclei play a pivotal role in controlling sex-dependent physiology. This brain region functions as a chief executive officer or master regulator of homeostatic physiological systems to integrate both external and internal signals. In this review, we describe sex differences in energy homeostasis that arise in one area of the hypothalamus, the ventrolateral subregion of the ventromedial hypothalamus (VMHvl) with a focus on how male and female neurons function in metabolic and behavioral aspects. Because other chapters within this book provide details on signaling pathways in the VMH that contribute to sex differences in metabolism, our discussion will be limited to how the sexually dimorphic VMHvl develops and what key regulators are thought to control the many functional and physiological endpoints attributed to this region. In the last decade, several exciting new studies using state-of-the-art genetic and molecular tools are beginning to provide some understanding as to how specific neurons contribute to the coordinated physiological responses needed by male and females. New technology that combines intersectional spatial and genetic approaches is now allowing further refinement in how we describe, probe, and manipulate critical male and female neurocircuits involved in metabolism.
Introduction
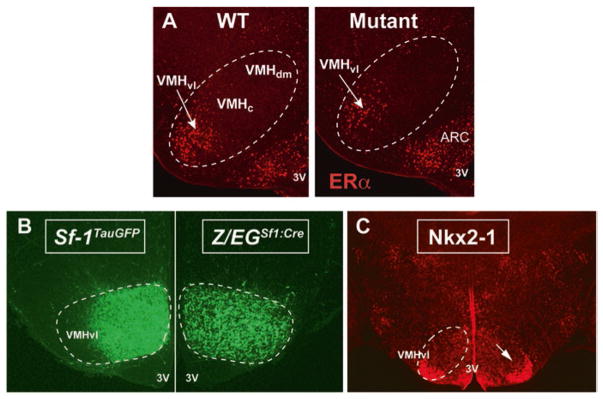
If one asks the general question as to why sex differences are needed in metabolic circuits, the obvious answer is to preserve and enhance reproductive capacity. Embedded in this conclusion is the overall objective in terms of evolutionary pressure on males and females to reproduce. This topic was recently reviewed by Torre and Maggi (Della Torre and Maggi 2017), which describes how in females, but not males, evolutionary pressure in a changing environment is needed to optimize energy intake and expenditure with reproduction. This pressure is evident in invertebrates as reviewed by (Mccall 2004), with demands increasing in placental and lactating vertebrates. Over the last two decades, works from several labs have created an overall narrative as to how neurons in the VMHvl help to control the sexually dimorphic male and female behavioral and metabolic responses (Fig. 1). The VMHvl is one of the many brain regions that expresses a major detector of the sex steroid estrogen, estrogen receptor alpha (ERα, encoded by Esr1), where expression increases in females postnatally (Fig. 2a). Other ERα-expressing regions within the medial basal hypothalamus (MBH) include the nearby arcuate nucleus (ARC), as well as the medial preoptic area (MPOA), and the bed nucleus of the stria terminalis (BnST) (Brock et al. 2015). In the VMHvl, this nuclear receptor is predominantly nuclear, and thus, one can reasonably assume that nearly all estrogen signaling in the VMHvl occurs through genomic rather than non-genomic actions. One of the striking features of ERα is the sexually distinct pattern in rodents (Koch 1990), with both transcripts and protein levels much higher in the female VMHvl; refer to (Correa et al. 2015) and references within. Contribution by the two other estrogen receptors is assumed to be minimal as evidenced by the exceeding low transcript levels for both estrogen receptor beta (ERβ) and the 7-transmembrane G-protein-coupled estrogen receptor 1 (GPER-1), formerly referred to as GPR30 (Chimento et al. 2014; Prossnitz et al. 2008). Indeed, expression of ERβ (Zuloaga et al. 2014) and GPER (Brailoiu et al. 2007) is sparse or undetectable in the adult VMHvl. The VMHvl also expresses Cyp19A1 that encodes aromatase, an essential enzyme needed for local conversion of androgens to estrogens (Stanic et al. 2014; Wu et al. 2009). In males, expression of aromatase in the VMHvl region is thought to be critical for the early masculinization of the male brain via estrogen, as reviewed in (Yang and Shah 2014). Indeed, the loss of aromatase in males impairs aggression as measured by the frequency and duration of attacks in a standard resident/intruder assays.
Fig. 1.
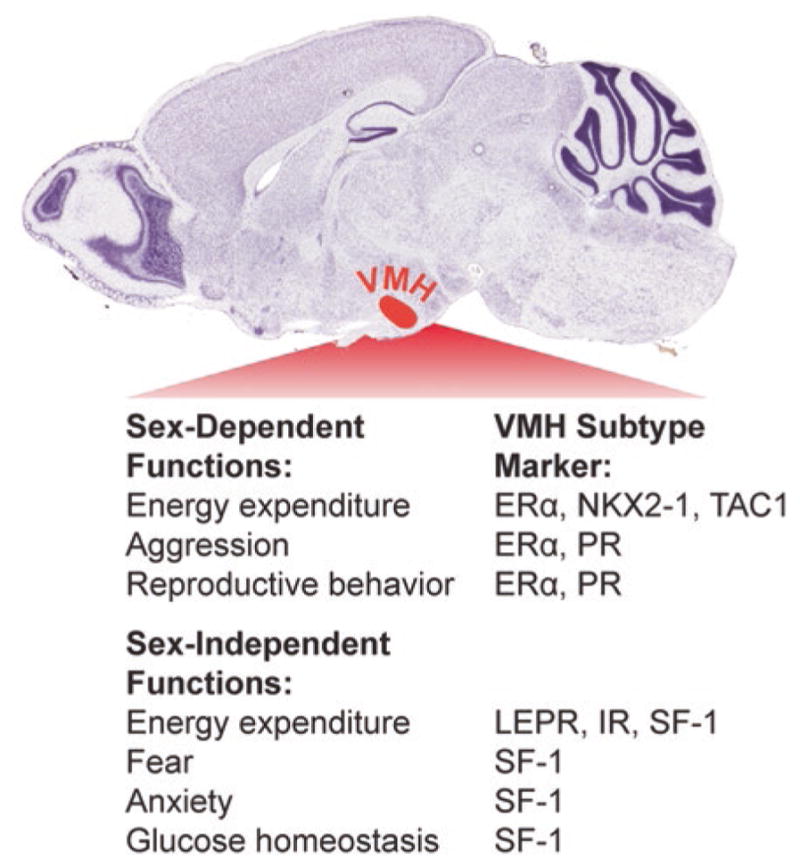
Sex-dependent VMH functions are mediated by ERα-expressing VMHvl neurons. Within the hypothalamus, the VMH (red-shaded region) controls multiple aspects of metabolism and behavior. Whereas leptin receptor (LEPR) and insulin receptor (IR) expression overlap with SF-1 and regulate metabolism in both males and females, sex-dependent functions of the VMH are mediated by ERα-expressing VMH neurons (Nissl-stained image adapted from the Allen Brain Atlas)
Fig. 2.
Development of the VMHvl module that modulates energy homeostasis in females. a) ERα immunostaining demonstrates that expression is restricted to the VMHvl in female mice as well as the arcute nucleus (ARC). Fewer VMHERα neurons are born in the Nkx2-1Sf1-Cre mutant females (Mutant) compared to Nkx2-1fl/fl control females (WT), as described in text. Third ventricle (3V). b) VMHvl neurons do not express SF-1, as illustrated by the lack of GFP-positive neurons using a knock-in reporter (Sf-1)TauGFP. However, Cre-mediated lineage tracing (Z/EG)Sf1:Cre reveals that most VMHvl neurons derive from SF1-expressing precursors. c) Postnatal NKX2-1 expression is largely restricted to ERα-positive and SF-1-negative VMHvl neurons
Another nuclear receptor, the progesterone receptor (PR), is also expressed in the VMHvl. The PR gene is a well-established transcriptional target of ERα. Interestingly, while PR has emerged as one of the sexually dimorphic transcripts detected in the adult VMHvl (Yang et al. 2013), at earlier stages prenatally, PR expression is not confined to the VMHvl but instead is unrestricted and found throughout the entire VMH (Correa et al. 2015). Thus, its pattern of expression in the VMH is not necessarily identical to that of ERα at all developmental stages. Indeed, while one observes a clear sex-dependent pattern of ERα expression beginning postnatally, the same cannot be said for PR. In this instance, PR transcripts are not differentially expressed until adulthood (Hagihara et al. 1992; Simerly et al. 1990).
The VMH Mediates Sex Differences in Energy Expenditure
This chapter will focus primarily on estrogen effects on the VMHvl, with emphasis on female metabolism. Others have reviewed some of the more recent work on the VMHvl in male sexual behavior (Yang and Shah 2014). When appropriate, we will highlight general statements inferred from studies in both rats and mice. Much of the differences noted for females emerged over a century ago with the seminal observations by Strominger. Using methods of the day and challenged by their limited access to reagents post-WWII, they noticed a tight correlation between the estrus cycle and peaks of activity concomitant with decreased food intake (Brobeck et al. 1947). If we fast forward to the next century, genetic manipulations achieved by either SiRNA- or Cre-mediated disruption of estrogen signaling clearly highlight the VMHvl as a center for sex differences in female metabolism. Indeed, ShRNA knockdown of ERα in the rat VMH increases food intake and decreases diet-induced thermogenesis and physical activity, resulting in obesity (Musatov et al. 2007). Conditional knockout of ERα in the VMH using Sf1Cre also lowers brown adipose tissue (BAT) thermogenesis in female mice and yields a mild transient weight gain in females due to increased size of gonadal fat pads (Xu et al. 2011). It should be mentioned that some of the earlier work using stereotaxic delivery of SiRNA directed against ERα to the entire VMH region showed pronounced increase in food intake (Musatov et al. 2007). However, three different genetic models generated to date using different Cre drivers, including Esr1Sf1-Cre, Esr1Nkx2–1Cre, and Nkx2-1Sf1-Cre, fail to support the notion that estrogen signaling in the murine VMHvl directly controls food intake (Xu et al. 2011, Correa et al. 2015), and unpublished data H.A.I.). It is worth noting that within the VMHvl, the Cre-based recombination efficiency is substantially higher using Nkx2-1Cre versus Sf1Cre, with the former effecively eliminating all ERα in this hypothalamic subregion. Table 1 lists the phenotypes for these three different mouse models that eliminate ERα in the VMHvl.
Table 1.
Comparison of mouse models that eliminate ERα in the VMHvl
| Phenotype | Nkx2-1Sf1-Cre | Esr1Sf1-Cre | Esr1Nkx2-1Cre |
|---|---|---|---|
| ERα expression | ++ | + | − |
| Tac1 expression | ++ | +++ | +++ |
| # of VMHvl neurons | ++ | +++ | +++ |
| Reproduction | Fertile | Infertile | Infertile |
| Body weight (chow) | Increase | Transient increase | No change |
| BAT thermogenesis | Normal | Lower | Lower |
| Food intake | Normal | Normal | Normal |
| Locomotion | Decreased | Trends lower | Decreased |
= Wild-type levels. References for the table: Xu et al. (2011), Correa et al. (2015) and unpublished data (H.A.I)
VMHvl Development
VMH projections are visible as early as embryonic day (E) 10.5 when few postmitotic neurons have been born, suggesting that formation of VMH circuitry begins at the onset of neurogenesis (Cheung et al. 2013) and also reviewed in (McClallan et al. 2006). One can follow in time the original migration from the ventricular zone to the VMHvl by BrdU labeling (Tran et al. 2003). One of the earliest molecular markers expressed throughout the VMH is the nuclear receptor steroidogenic factor 1 (SF-1, NR5A1), which appears at E9 (Ikeda et al. 2001). Although SF-1 is not required for the initial organization and migration of neurons in the developing VMH nucleus (Ikeda et al. 1995; Tran et al. 2003), this transcription factor is essential for terminal differentiation and maintenance of VMH neuronal populations (Davis et al. 2004; McClallan et al. 2006). The loss of SF-1 also results in diminished efferent projections to the amygdala (Tran et al. 2003) and altered afferent projections from the preoptic area to the VMH (Budefeld et al. 2011).
Earlier descriptive studies based on immunofluorescence staining and in situ hybridization of SF-1 protein and transcripts, respectively, supported the idea that SF-1 marks all embryonic neurons that would give rise to the VMH proper. However, comparison of the VMH neurons derived from the SF-1 lineage versus those that express SF-1 shows that the cluster of VMHvl neurons and, by association, ERα neurons in the VMH evolve into a distinct neuronal subpopulation within the VMH (Cheung et al. 2013).
Specifically, two approaches that exploit the widespread expression of SF-1 in the VMH were used to trace SF-1 expression and the major VMH axonal projections during embryonic and postnatal stages (Fig. 2b). The first relied on tandem reporters, the wheat germ agglutinin (WGA) (Braz et al. 2002) and tau-green fluorescent protein (tauGFP), knocked into the 3′-untranslated region (UTR) of the Sf-1 (Nr5a1) locus. In this knockin line, referred to as Sf-1TauGFP, WGA and GFP are under the control of intact regulatory elements and are thus coexpressed and coregulated with SF-1 expression. In the second, more standard Cre-mediated labeling line, the transgenic Sf1:Cre mouse was crossed with a Z/EG reporter mouse, referred to as Z/EGSf1:Cre, which results in constitutive expression of eGFP (enhanced GFP) after Cre-mediated recombination (Dhillon et al. 2006). One conclusion from this work was that SF-1 neurons appear in the presumptive VMH area by E10.5 but only begin to coalesce into the conventional oval-shaped nucleus later in the development at E14.5. Even at this very early developmental stage, prominent neuronal VMH projections are evident. Further, while it has been generally assumed that SF-1 marks the entire VMH because of its early and broad expression, striking differences were observed if neurons are marked by the endogenous SF-1 promoter or instead marked by Cre recombination (Cheung et al. 2013). By directly comparing GFP+ labeling in all subregions of the VMH in the Sf-1TauGFP and Z/EGSf1-Cre lines, one can conclude that SF-1 is transiently expressed at early embryonic stages and then silenced in neurons that populate the VMHvl. These findings imply that developmental signals must exist to silence SF-1 expression in the VMHvl prior to E14.5. By this developmental point, ERα expression has already begun (Brock et al. 2015; Correa et al. 2015), but the interplay between SF-1 and ERα transcriptional response remains undefined at this juncture. Transcriptome profiling has only been done at later stages or using the entire VMH (Kurrasch et al. 2007), and the field is still awaiting data from comprehensive single cell sequencing. Ultimately, this detailed transcriptome profiling of both male and female VMHvl should provide a glimpse into the molecular complexity that exists in the VMHvl. Currently, based on different phenotypic outcomes, it is assumed that unique molecular modules will help constitute distinct neural circuits that result in different physiological endpoints. It should be noted that there are a limited number of ERα neurons in the vicinity of the VMHvl that do coexpress SF-1; this is especially true for cell bodies more dorsal to the VMHvl proper. That the VMHvl has a unique molecular signature from the other two subregions of the VMH, the central and dorsal medial, fits well with the notion that distinct SF-1-negative neurons in the VMHvl are dedicated to elaborating sex-dependent physiological and behavioral responses.
Sex Differences in Energy Expenditure
Recent studies show that aside from BAT thermogenesis, physical activity or locomotion is mediated by estrogen-responsive neurons in the VMHvl. This was discovered by manipulating a prominent developmental factor, the homeobox transcription factor Nkx2-1, which is required for proper development of several major organs, including the pancreas, lung, thyroid, and brain. NKX2-1 is also required for patterning in many brain regions including the rostroventral hypothalamus (Kimura et al. 1996; Marin et al. 2002; Shimamura and Rubenstein 1997), which gives rise to the VMH. In adult male and female mice, Nkx2-1, as with ERα, is highly restricted to the VMHvl (Davis et al. 2004; Tran et al. 2003) and Fig. 2c. However, not all NKX2-1 neurons in the VMHvl express ERα. Earlier in development, NKX2-1 is expressed throughout the presumptive MBH (Marin et al. 2002; Shimamura and Rubenstein 1997; Yee et al. 2009) and appears earlier and is more broadly expressed than SF-1 as judged by immunofluorescence (Correa et al. 2015) and by detailed lineage tracing using the Nkx2-1Cre and the reporter mouse (Salvatierra et al. 2014). Global deletion of NKX2-1 impairs the development of the VMH and other hypothalamic nuclei leading to diabetes (Sussel et al. 1998, 1999). However, if NKX2-1 is eliminated late in development (E9-10) using the Sf1-Cre, the VMH remains largely intact. A similar result is observed if Synapsin-Cre is used to delete NKX2-1 (Mastronardi et al. 2006). From these and other birthdating studies, it is concluded that NKX2-1 marks the earliest born or oldest neurons in the VMH, with the majority of these cells eventually residing in the VMHvl with ERα-expressing neurons. Further, conditional deletion of NKX2-1 in the VMHvl eliminates a sizable fraction (30 %) of ERα-expressing VMHvl neurons (Correa et al. 2015). These data are consistent with the fact that development or migration of some but not all estrogen-responsive neurons in the VMHvl depends on NKX2-1.
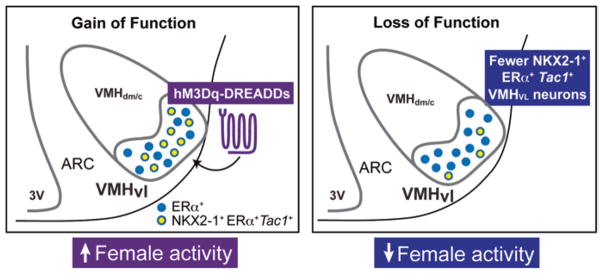
Ablating NKX2-1 in this VMHvl subpopulation (Nkx2-1Sf1-Cre) results in a sex-specific decrease in spontaneous physical activity without affecting BAT thermogenesis or fertility, resulting in female-specific obesity independent of diet (Fig. 3 and Table 1). Recall that eliminating some but not all ERα in the VMH using the Esr1Sf1-Cre has a minimal effect on activity but does impair BAT thermogenesis and reproduction. In both models, male mice fail to show any signs of metabolic or reproductive deficits. As is true with many Cre lines, it is rare that they exhibit the temporal and spatial specificity that one would desire. In fact, this is the case for the Sf1-Cre transgenic line, which is active in peripheral endocrine organs as well as the VMH. Indeed, SF-1 is robustly expressed in the adrenal, the anterior pituitary, the gonads, and the spleen beginning early in development (Ikeda et al. 1994). Given that SF1-Cre will alter expression in the early primordial bipotential gonad and the adult ovary (Ikeda et al. 1994; Ingraham et al. 1994; Shen et al. 1994), it remains possible that the sex-dependent phenotypes described in these mouse models may partially result from a disruption of feedback loops in the hypothalamic-pituitary-gonadal axis.
Fig. 3.
A molecularly distinct subset of VMHERα neurons are necessary and sufficient to drive physical activity in female mice. Chemogenetic activation of VMHvl neurons increases energy expenditure via physical activity in females and requires ERα and TAC1 (left panel). In contrast, reducing the number of VMHERα,TAC1 neurons decreases physical activity and results in female-specific obesity (right panel)
To circumvent the limited spatial specificity of the Cre-Lox system and more definitively demonstrate the role of VMH neurons in promoting locomotion, viral vectors carrying designer receptors exclusively activated by designer drugs (DREADDs) were expressed in the VMH by stereotaxic delivery. Indeed, ERα is required for the full increase in pharmacogenetic-mediated or DREADD-induced locomotion (Correa et al. 2015). Male mice fail to show the same DREADD-induced responses with respect to locomotion (Fig. 3) but do exhibit a modest increase in oxygen consumption. Although increased oxygen consumption was not associated with higher locomotion or heat generation, we cannot exclude the possibility that VMHvl neurons play a minor role in male locomotion. Indeed, pharmacologic activation in the VMHvl induces running in male rats (Narita et al. 1993). DREADD-induced activation of locomotion in females appears much more sensitive when compared to DREADD- or ChR-induced activation of behaviors in males (Lee et al. 2014; Silva et al. 2013). Indeed, increased movement is observed in females even after unilateral or limited infection of DREADDs into VMHvl neurons. The ability to blunt DREADD-induced movement by genetic deletion of ERα, as shown here, establishes that ERα signaling is the main mediator of hormone-induced movement, as previously reported for female sexual behavior (Musatov et al. 2006, Lunahn et al. 1993). As mentioned above, hormone dependency has yet to be shown for experimentally induced male behaviors, including social fear, mating, and aggression (Lee et al. 2014, Silva et al. 2013).
One limitation with mouse models is the difficulty in showing a tight link between hormonal variation in cycling females and changes in energy expenditures. DREADD-induced locomotion in female mice appears to be insensitive to normal fluctuations in estrogen. Only after eliminating all gonadal hormones or all ERα signaling does one observe the dramatic influence of estrogen signaling on DREADD-induced locomotion. These findings are consistent with little to no effect of the estrous cycle on locomotion in mice (Kopp et al. 2006) and imply that the estrous cycle on physical activity is far stronger in rats than in mice. These and other data (Prendergast et al. 2014) also show that the assumption that the estrus cycle has a dramatic and robust effect on measured parameters in female mice is highly overstated, thus directly challenging the historical aversion to the use of both sexes.
The striking phenotypic differences in reproduction and metabolism observed between Nkx2-1Sf-1-Cre and Esr1Sf-1-Cre mouse models are instructive for dissecting out the complex and coordinated metabolic and reproductive functions of the VMHvl in females. One would like to define the signaling events and targets of ERα in the VMHvl that ultimately drive sex-dependent physiological endpoints. Within the VMHvl, there is a subpopulation of NKX2-1-positive VMHvl neurons coexpressing ERα and Tac1, which appears important for female activity (Fig. 3), is enriched in females compared to males, and is largely absent in Nkx2-1Sf1-Cre mutant females. Tac1 is enriched in the VMHvl, as previously reported for rats (Dornan et al. 1990). Eliminating ERα neurons results in diminished Tac1 transcripts in Nkx2-1Sf1-Cre mice, suggesting that this neuropeptide might participate in mediating female-specific physiology. However, Tac1 is not directly regulated by ERα (Correa et al. 2015). It is possible that these ERα+, Tac1+ VMH neurons may project to MPOA neurons, which have been linked to estrogen-induced running in rats (Spiteri et al. 2012; Fahrbach et al. 1985). Other VMH projections relevant to locomotion might include those to the subthalamic and mesencephalic locomotor regions (Cheung et al. 2012), areas that when activated increase controlled movement in rats (Skinner and Garciarill 1984) or when lesioned in humans lead to deficits in locomotion (Hathout and Bhidayasiri 2005). Given that the neuropeptide-encoding gene Tachykinin 1 (Tac1) is associated with estrogen-responsive VMHvl neurons and is enriched in females raises the question as to its role in mediating sex-dependent behaviors. Unfortunately, while mice deleted globally for Tac1, as well as its receptor (NK-1), exhibit improved glucose homeostasis (Karagiannides et al. 2011a) and resistance to diet-induced obesity (Karagiannides et al. 2011b), both studies only report data from male mice. Nonetheless, this might suggest that neurokinin A (formerly substance P), encoded by Tac1, normally counteracts estrogen, opposing a negative energy state.
Excitatory Activity in VMH Neurons and Sex Differences
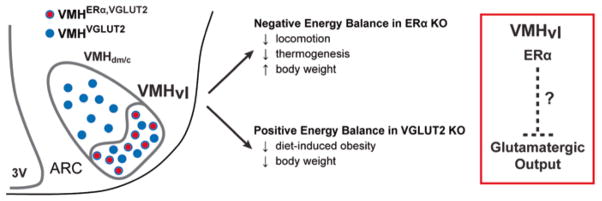
Nearly all VMH neurons express two markers, steroidogenic factor 1 (SF-1 encoded by Nr5a1) and vesicular glutamate transporter 2 (VGLUT2 encoded by Slc17a6). The prominent expression of Vglut2 in the VMH (Ziegler et al. 2002; Fremeau et al. 2001) suggests that excitatory, glutamatergic neurotransmission mediates multiple aspects of VMH function, including those associated with the sexually dimorphic VMHvl (Fig. 4). In both males and females, expression of the glutamate decarboxylase (Gad67) that marks inhibitory neurons is for the most part completely excluded from the entire VMH as well as the VMHvl. Prior studies might predict that disrupting VMH excitatory neurotransmission would alter food intake and susceptibility to diet-induced obesity, especially given the established glutamatergic connections between the VMH and other metabolic brain centers, such as the arcuate nucleus (Fu and Van Den Pol 2008; Sternson et al. 2005). The initial work by Tong et al. examined this question by generating the VMH knockout of Vglut2 using Sf1-Cre (Vglut2Sf1-Cre), which for future reference was done in a mixed genetic background (Tong et al. 2007). Despite the fact that the VMHvl should be targeted using this approach, no sex-dependent metabolic changes were noted in their published work. However, they did detect lowered serum glucose in the fasted but not fed state in both sexes, suggesting that the VMH excitatory output is needed for the counterregulatory response to hypoglycemia. This was recently reexamined in a pure C57BL/6 background (Cheung et al. 2015): a strain with increased DIO induced weight gain and hyperglycemia (Montgomery et al. 2013; Collins et al. 2004). In this pure strain setting, sex differences emerged. When compared to Vglut2fl/fl controls, weight gain in Vglut2Sf1-Cre females was notably lower when placed on high-fat diet (HFD) at 10 weeks of age, whereas C57BL/6 mutant males showed no body weight differences (Cheung et al. 2015). Consistent with the female-specific resistance to DIO, glucose homeostasis was improved in Vglut2Sf1-Cre females as measured by an intraperitoneal glucose tolerance test (IP GTT). This result is somewhat at odds with the report that Vglut2Sf1-Cre males and females are heavier when fed a high-fat, high-sucrose diet (Tong et al. 2007), perhaps reflecting strain and dietary differences.
Fig. 4.
Silencing glutamatergic VMH neurons promotes negative energy balance in females. Summary of the relative distribution of VMHVGLUT2 and VMHERα,VGLUT2 neurons and the metabolic consequences resulting from genetic knockout of either ERα or VGLUT2. Opposing metabolic phenotypes observed in mutant female mice following deletion of ERα or VGLUT2 in the VMH suggest that ERα signaling reduces rather than enhances glutamaterigic output from the VMHvl
The reduced body weight in the female Vglut2Sf1-Cre mice, whose origins remain unclear, mimics the metabolic consequence of elevated estrogen signaling. In other words, the loss of excitatory output from the VMHvl has a negative effect on energy balance, rather than a positive effect. This result undermines the simple assumption that estrogen signaling potentiates VMH neurotransmitter output, suggesting instead that ERα signaling inhibits circuits that otherwise promote energy storage (Fig. 4). Because this brain region is tightly linked to reproductive behavior (Ogawa et al. 1998), one might speculate that in females, regulation of glutamatergic VMH neurons by estrogen maximizes fuel reserves in states of overnutrition (HFD), to ultimately improve reproductive fitness in times of undernutrition.
Interestingly, behavioral sex differences are also apparent after the loss of all excitatory output. Indeed, while both male and female Vglut2Sf1-Cre mice exhibit less anxiety in standard assays such as open field or elevated maze, mutant Vglut2Sf1-Cre males do exhibit greater exploratory drive (Cheung et al. 2015). As predicted from prior literature cited above, male resident Vglut2Sf1-Cre mice are less aggressive and attack far less frequently than Vglut2fl/fl controls. In summary, the phenotypes exhibited by Vglut2Sf1-Cre mice are consistent with emerging evidence that the VMHvl regulates sex-dependent metabolic responses and social behaviors. The ability to specifically target the excitatory output of different molecular modules in the VMHvl will be important to dissect and map the circuitry that controls male and female metabolic and behavioral endpoints.
Future Directions
One of the most critical and pressing questions raised by current data is how steroid signaling regulates the behavioral or physiological outputs between male and female ERα neurons. Thus, while it is clear in murine models that estrogen signaling impacts energy expenditure in females, connecting the dots between estrogen and neuronal output remains obscure. This dependency on steroid signaling is perhaps the largest and most important difference between the male and female VMHvl. As mentioned above, to fully understand how these sex-dependent endpoints are established within different VMHvl modules requires the application of newer methods that allow a more granular view and finer genetic manipulation of the VMHvl. This task could be made more challenging if key factors in estrogen-responsive VMHvl neurons needed for neuronal output are not themselves direct downstream targets of ERα. Lastly, we have yet to define how well new findings in rodent models translate to humans, which is especially important if we are to appreciate the full metabolic benefits of estrogen in women’s health.
References
- Brailoiu E, Dun SL, Brailoiu GC, Mizou K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. The Journal of Endocrinology. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15148–15153. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brobeck JR, Wheatland M, Strominger JL. Variations in regulation of energy exchange associated with estrus, diestrus and pseudopregnancy in rats. Endocrinology. 1947;40:65–72. doi: 10.1210/endo-40-2-65. [DOI] [PubMed] [Google Scholar]
- Brock O, De Mees C, Bakker J. Hypothalamic expression of oestrogen receptor alpha and androgen receptor is sex-, age- and region-dependent in mice. Journal of Neuroendocrinology. 2015;27:264–276. doi: 10.1111/jne.12258. [DOI] [PubMed] [Google Scholar]
- Budefeld T, Tobet SA, Majdic G. Altered position of cell bodies and fibers in the ventromedial region in SF-1 knockout mice. Experimental Neurology. 2011;232:176–184. doi: 10.1016/j.expneurol.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Krause WC, Edwards RH, Yang CF, Shah NM, Hnasko TS, Ingraham HA. Sex-dependent changes in metabolism and behavior, as well as reduced anxiety after eliminating ventromedial hypothalamus excitatory output. Molecular Metabolism. 2015;4:857–866. doi: 10.1016/j.molmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Kurrasch DM, Liang JK, Ingraham HA. Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. The Journal of Comparative Neurology. 2012;521:1268–1288. doi: 10.1002/cne.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Kurrasch DM, Liang JK, Ingraham HA. Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. The Journal of Comparative Neurology. 2013;521:1268–1288. doi: 10.1002/cne.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento A, Sirianni R, Casaburi I, Pezzi V. Role of estrogen receptors and g protein-coupled estrogen receptor in regulation of hypothalamus-pituitary-testis axis and spermatogenesis. Frontiers in Endocrinology (Lausanne) 2014;5:1. doi: 10.3389/fendo.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiology & Behavior. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Correa SM, Newstorm DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Reports. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Stallings NR, Zhao L, Parker KL, Tobet SA. Loss of steroidogenic factor 1 alters cellular topography in the mouse ventromedial nucleus of the hypothalamus. Journal of Neurobiology. 2004;60:424–436. doi: 10.1002/neu.20030. [DOI] [PubMed] [Google Scholar]
- Della Torre S, Maggi A. Sex differences: A resultant of an evolutionary pressure? Cell Metabolism. 2017;25:499–505. doi: 10.1016/j.cmet.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthazar N, Cowley MA, Chua S, Jr, Elmquist JK, LOWELL BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Dornan WA, Akesson TR, Micevych PE. A substance P projection from the VMH to the dorsal midbrain central gray: Implication for lordosis. Brain Research Bulletin. 1990;25:791–796. doi: 10.1016/0361-9230(90)90061-4. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiology & Behavior. 1985;35:985–992. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellochio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fu LY, Van Den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. The Journal of Neuroscience. 2008;28:5433–5449. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara K, Hirata S, Osada T, Hirai M, Kato J. Distribution of cells containing progesterone receptor mRNA in the female rat di- and telencephalon: An in situ hybridization study. Brain Research Molecular Brain Research. 1992;14:239–249. doi: 10.1016/0169-328x(92)90179-f. [DOI] [PubMed] [Google Scholar]
- Hathout GM, Bhidayasiri R. Midbrain ataxia: An introduction to the mesencephalic locomotor region and the pedunculopontine nucleus. AJR American Journal of Roentgenology. 2005;184:953–956. doi: 10.2214/ajr.184.3.01840953. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Molecular Endocrinology. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Molecular Endocrinology. 1995;9:478–486. doi: 10.1210/mend.9.4.7659091. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI. Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Developmental Dynamics. 2001;220:363–376. doi: 10.1002/dvdy.1116. [DOI] [PubMed] [Google Scholar]
- Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes & Development. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, Bakirtzi K, Kokkotou E, Stavrakis D, Margolis KG, Thomou T, Giorgadze N, Kirkland JL, Pothoulakis C. Role of substance P in the regulation of glucose metabolism via insulin signaling-associated pathways. Endocrinology. 2011a;152:4571–4580. doi: 10.1210/en.2011-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannides I, Stavrakis D, Bakirtzi K, Kokkotou E, Pirtskhalava T, Nayeb-Hashemi H, Bowe C, Bugni JM, Nuno M, Lu B, Gerard NP, Leeman SE, Kirkland JL, Pothoulakis C. Substance P (SP)-neurokinin-1 receptor (NK-1R) alters adipose tissue responses to high-fat diet and insulin action. Endocrinology. 2011b;152:2197–2205. doi: 10.1210/en.2010-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzales FJ. The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes & Development. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Koch M. Effects of treatment with estradiol and parental experience on the number and distribution of estrogen-binding neurons in the ovariectomized mouse brain. Neuroendocrinology. 1990;51:505–514. doi: 10.1159/000125384. [DOI] [PubMed] [Google Scholar]
- Kopp C, Ressel V, Wigger E, Tobler I. Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains. Behavioural Brain Research. 2006;167:165–174. doi: 10.1016/j.bbr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Iingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. The Journal of Neuroscience. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim DW, Remedios R, Anthoby TE, Chang A, Madisen L, Zeng H, Anderson DJ. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Baker J, Puelles L, Rubenstein JL. Patterning of the basal telencephalon and hypothalamus is essential for guidance of cortical projections. Development. 2002;129:761–773. doi: 10.1242/dev.129.3.761. [DOI] [PubMed] [Google Scholar]
- Mastronardi C, Smiley GG, Raber J, Kusakabe T, Kawaguchi A, Matagne V, Dietzel A, Heger S, Mungenast AE, Cabrera R, Kimura S, Ojeda SR. Deletion of the Ttf1 gene in differentiated neurons disrupts female reproduction without impairing basal ganglia function. The Journal of Neuroscience. 2006;26:13167–13179. doi: 10.1523/JNEUROSCI.4238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccall K. Eggs over easy: Cell death in the drosophila ovary. Developmental Biology. 2004;274:3–14. doi: 10.1016/j.ydbio.2004.07.017. [DOI] [PubMed] [Google Scholar]
- McClallan KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Frontiers in Neuroendocrinology. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, Hallahan NL, Brown SH, Liu M, Mitchell TW, Cooney GJ, Turner N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia. 2013;56:1129–1139. doi: 10.1007/s00125-013-2846-8. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Yokawa T, Nidhihara M, Takahashi M. Interaction between excitatory and inhibitory amino acids in the ventromedial nucleus of the hypothalamus in inducing hyper-running. Brain Research. 1993;603:243–247. doi: 10.1016/0006-8993(93)91243-l. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annual Review of Physiology. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Salvatierra J, Lee DA, Zibetti C, Duran-Moreno M, Yoo S, Newman EA, Wang H, Bedont JL, De Melo J, Miranda-Angulo AL, Gil-Perotin S, Garcia-Verdugo JM, Blackshaw S. The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. The Journal of Neuroscience. 2014;34:16809–16820. doi: 10.1523/JNEUROSCI.1711-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the mullerian inhibiting substance gene: A link to the sex determination cascade. Cell. 1994;77:651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, Grinevich V, Canteras NS, Ragozzino D, Gross CT. Independent hypothalamic circuits for social and predator fear. Nature Neuroscience. 2013;16:1731–1733. doi: 10.1038/nn.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. The Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Garcia-rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Research. 1984;323:385–389. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- Spiteri T, Ogawa S, Musatov S, Pfaff DW, Agmo A. The role of the estrogen receptor alpha in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behavioural Brain Research. 2012;230:11–20. doi: 10.1016/j.bbr.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Stanic D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, Boon WC. Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors alpha and beta, and androgen receptors. PLoS One. 2014;9:e90451. doi: 10.1371/journal.pone.0090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH –> arcuate nucleus microcircuits and their reorganization by fasting. Nature Neuroscience. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O’Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: Evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christainsen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metabolism. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Lee MB, Marin O, Xu B, Jones KR, Reichardt LF, Rubenstein JR, Ingraham HA. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Molecular and Cellular Neurosciences. 2003;22:441–453. doi: 10.1016/S1044-7431(03)00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedugadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metabolism. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Shah NM. Representing sex in the brain, one module at a time. Neuron. 2014;82:261–278. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JL. Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y(+), proopiomelanocortin(+), and tyrosine hydroxylase(+) neurons in neonatal and adult mice. The Journal of Comparative Neurology. 2009;517:37–50. doi: 10.1002/cne.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. The Journal of Comparative Neurology. 2002;448:217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. Estrogen receptor beta expression in the mouse forebrain: Age and sex differences. The Journal of Comparative Neurology. 2014;522:358–371. doi: 10.1002/cne.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]