Abstract
Tetrabromobisphenol A (TBBPA) is a widely used brominated flame retardant that is persistent in the environment and detected in human serum and breast milk. TBBPA is microbiologically transformed in anaerobic environments to bisphenol A (BPA) and in aerobic environments to TBBPA dimethyl ether (TBBPA DME). Despite the detection of TBBPA DME in the environment, the resulting toxicity is not known. The relative toxicity of TBBPA, BPA and TBBPA DME was determined using embryonic exposure of zebrafish, with BPA and TBBPA DME exhibiting lower potency than TBBPA. TBBPA exposure resulted in 100% mortality at 3 (1.6 mg/L) and 1.5 µM (0.8 mg/L), whereas BPA and TBBPA DME did not result in significant embryonic mortality in comparison to controls. While all three caused edema and hemorrhage, only TBBPA specifically caused decreased heart rate, edema of the trunk, and tail malformations. Matrix metalloproteinase (MMP) expression was measured due to the role of these enzymes in the remodeling of the extracellular matrix during tissue morphogenesis, wound healing and cell migration. MMP-2, -9 and -13 expression increased (2–8 fold) after TBBPA exposure followed by an increase in the degradation of collagen I and gelatin. TBBPA DME exposure resulted in only a slight increase (less than 2 fold) in MMP expression and did not significantly increase enzymatic activity. These data suggest that TBBPA is more potent than BPA or TBBPA DME and indicate that the trunk and tail phenotypes seen after TBBPA exposure could be due in part to alteration of proper MMP expression and activity.
Keywords: Tetrabromobisphenol A, zebrafish, brominated flame retardant, tetrabromobisphenol A dimethyl ether, bisphenol A, matrix metalloproteinases
Introduction
Tetrabromobisphenol A (TBBPA) is a widely used brominated flame retardant that is a persistent and prevalent contaminant in the aquatic environment (Fernandes et al., 2008; Kuiper et al., 2007a; Kuiper et al., 2007b; Labadie et al., 2010; Nyholm et al., 2008; Verslycke et al., 2005; Zhang et al., 2009). The widespread production and use of brominated flame-retardants has raised concerns regarding their fate and effects on aquatic species. Tetrabromobisphenol A [TBBPA, 4,4’-isopropylidenebis(2,6-dibromophenol)] is the most commonly used brominated flame retardant produced globally at 120,000 metric tons in 2001 (Hakk and Letcher, 2003). TBBPA is used primarily in the production of epoxy and polycarbonate resins. The environmental persistence of TBBPA is due to its high lipophilicity (log Kow=5.9), low volatility (7.0 × 10−11 atm-m3 /mol), low water solubility (4.16 mg/l at 25°C in H2O) and recalcitrance (Hakk and Letcher, 2003). The widespread use of TBBPA, and its environmental persistence in dust, sediments and accumulation in biota has led to increased concerns regarding its effects on wildlife and humans (Johnson-Restrepo et al., 2008).
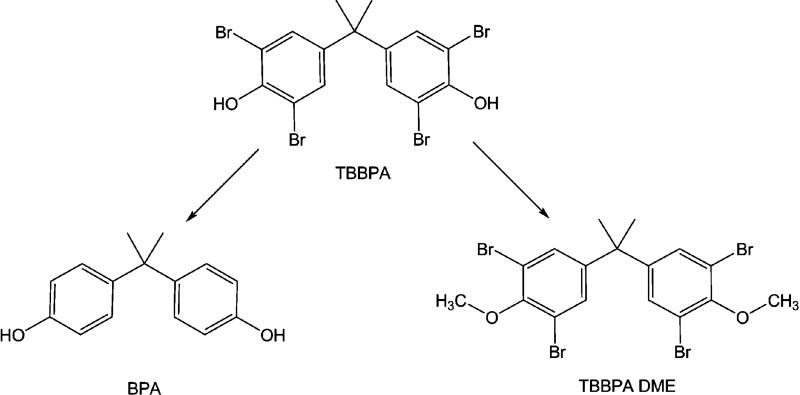
TBBPA undergoes two different types of transformations (Fig. 1) mediated by microorganisms in the environment, reductive debromination to bisphenol A (BPA, 4,4’-isopropylidenediphenol) or O-methylation to TBBPA monomethyl ether and TBBPA dimethyl ether [(TBBPA DME), 4,4’-isopropylidenebis(2,6-dibromo-1-methoxybenzene)] (Arbeli et al., 2006; George and Häggblom, 2008; Voordeckers et al., 2002). Notably, TBBPA DME has been detected in aquatic sediments near a plastic manufacturing plant in Sweden at concentrations of 36 and 2400 ng/g as well as in mussels in Osaka Bay, Japan (Sellström and Jansson, 1995; Watanabe et al., 1983). Toxicological data on this new metabolite of TBBPA are lacking, but are crucial to understanding the impact of accumulation of TBBPA DME in the environment.
Fig. 1.
Microbial induced transformations of tetrabromobisphenol A. The microbial mediated transformations of TBBPA through anaerobic debromination to BPA or aerobic O-methylation to TBBPA
In contaminated sites, TBBPA is typically detected at parts per million (ppm) concentrations in sediments and sewage sludge near brominate flame retardant production facilities (BFR), and parts per billion (ppb) at other sites (Hakk and Letcher, 2003; Hale et al., 2006; Kang et al., 2007). TBBPA DME has been detected in sediments at a higher level than TBBPA, but the levels are still in the ppb range (Hakk and Letcher, 2003). BPA levels in sediments and surface waters are also typically in the ppb range, but can be higher near the manufacturing plants and decrease with distance from the plant (Kang, et al., 2007).
It is known that TBBPA and BPA are estrogen mimics that interfere with normal reproduction and development in mammals and finfish (Hamers et al., 2006; Jakobsson et al., 2002; Kitamura et al., 2005; Kuiper, et al., 2007a; Law, 2006; Meerts et al., 2000; Thomsen et al., 2001). TBBPA (≥ 0.047 µM) exposure effects include a reduction of egg production, survival and overall reproductive success in zebrafish (Kuiper, et al., 2007a). Furthermore, it has been demonstrated that maternal transfer of brominated flame-retardants from adult to embryos occurs in zebrafish (Nyholm, et al., 2008). The estrogenic effects in adult fish involve a number of endocrine regulated pathways that are critical for coordinated signaling in gonadal development and normal reproduction (Thomas 2008). Estrogen and its cytosolic and membrane associated receptors have, been shown to be involved in the regulation of MMPs which have been implicated in vascular permeability, angiogenesis, gonadal development and developmental effects during early embryogenesis (Levin 2008; Marin-Catano et al. 2003; Razndi et al. 2003). Therefore, estrogen mimics such as TBBPA and BPA could also lead to inappropriate expression of MMPs that could explain some of the effects described above and effects observed in this paper.
Previous studies in our laboratory have reported the importance of MMPs in normal development and the effects of chemicals on MMP expression and activity with associated developmental lesions (Hillegass, et al., 2007; Hillegass, et al., 2008). This is not to say that other pathways do not play a role in lesion occurrence, but our studies concentrated on the role of MMP 2, 9 and 13, which are major endopeptidases involved in development (Crawford and Pilgrim, 2005; Ding et al. 2008). MMPs are Zn2+ dependent endopeptidases that are responsible for the cleavage of different components of the extracellular matrix, such as collagen and gelatin. MMPs are involved in regulating cell adhesion, migration, proliferation, differentiation and morphogenesis (Chakraborti et al., 2003; Zagris, 2001). Our study focused on the gelatinases, MMP-2 and -9, and the collagenase MMP-13. These particular MMPs were selected because they have been shown to be critical in mammalian and zebrafish development (Crawford and Pilgrim, 2005; Murphy and Nagase, 2008). The goal of this study was to determine the effect of developmental exposure to TBBPA and determine whether the microbial transformation products of TBBPA (BPA and TBBPA DME) differ in their developmental toxicity. The zebrafish model was used for these studies for comparison to other finfish, and the ability to monitor the individual compounds effects during embryonic stages (Hill et al., 2005; Kuiper, et al., 2007a). Therefore, the effects of TBBPA and its metabolites (BPA and TBBPA DME) are compared during exposure to embryonic stages of the zebrafish. There are currently no toxicity reports for TBBPA DME, which is a primary metabolite of TBBPA and has been detected in environmental samples.
In our studies, TBBPA is more toxic than its metabolites, BPA and TBBPA DME based on embryonic mortality and lesion occurrence. Embryonic exposure to TBBPA resulted in truncated bodies/tails suggesting impairment in the remodeling of tissues during development of the zebrafish embryo caudal region. Our studies concentrated on the role of matrix metalloproteinase (MMP) expression and activity in response to TBBPA exposure in the developing zebrafish embryo. Alterations in MMP expression and activity correlated with the severity of the chemical’s effects. All three compounds following embryonic exposure resulted in decreased juvenile survival (28 dph). This is the first study to characterize and compare the developmental effects of TBBPA, BPA and TBBPA DME in the developing zebrafish embryo.
Materials and Methods
Chemicals
Tetrabromobisphenol A (TBBPA) and bisphenol A (BPA) were obtained from Sigma Aldrich (>98% purity). Tetrabromobisphenol A dimethyl ether (TBBPA DME, purity >99% by GC-MS) was synthesized from TBBPA as previously described (George and Häggblom, 2008). Stock solutions were prepared in dimethyl sulfoxide (DMSO, Sigma-Aldrich), which was also used as the vehicle control.
Zebrafish strains and husbandry
The AB strain of zebrafish (Danio rerio) was used for all experiments, and was obtained from the Zebrafish International Resource Center (ZIRC). Zebrafish were bred and maintained in a recirculating Aquatic Habitat System utilizing a light: dark cycle of 14:10 hours, respectively. All experiments in this study were conducted according to a protocol approved by the Rutgers University Animal Care and Facilities Committee.
Embryonic Dose Response Studies
A static, non-renewal approach was used in a series of studies where one embryo was placed into each vial and observed every 24 hours. The concentrations of TBBPA and BPA used in this study were similar to those used in previous zebrafish studies (Kuiper, et al., 2007b; Muncke et al., 2007). The TBBPA DME doses were selected because they are in the same range as the TBBPA and BPA concentrations. Zebrafish embryos were collected at approximately 3 hours post fertilization (hpf) and were individually exposed for 5 days in capped glass vials containing 1 mL of TBBPA (0.5, 0.75, 1, 1.5, or 3 µM)/(0.27, 0.4, 0.54, 0.8, 1.6 mg/L), BPA (5, 10, 15, 20 or 25 µM)/(1.14, 2.3, 3.42, 4.62, 5.77 mg/L), DME (5,10, 15, 20, 25 µM)/ (2.9, 5.7, 8.58, 11.44, 14.3 mg/L) or dimethyl sulfoxide (0.15% for controls) in sterile zebrafish system water. Studies were conducted with 20 embryos per dose. Developmental lesions, including edema, hemorrhage, death and date of hatching, were recorded.
Embryonic Exposure and 28 Day Survival
Zebrafish embryos were exposed and evaluated for lesions as described above to the following treatments: TBBPA (0.75, 1.5, or 3 µM)/(0.4, 0.8, 1.6 mg/L), BPA (5, 10, or 15 µM)/(1.14, 2.3, 3.42 mg/L), DME (1, 5, or 10 µM)/ (0.57, 2.9, 5.7 mg/L) and dimethyl sulfoxide (0.15% for controls). The embryonic studies were repeated at least 3 times. To examine juvenile survival, larvae were removed from vials after 5 days and placed into a beaker containing system water and boiled wheat seeds. They were fed paramecium culture and AP100 for the next 21 days. At 28 days post fertilization (dpf) all remaining live larvae were counted and euthanized using an overdose of MS-222 (Tricaine methanesulfonate).
Quantitation of Heart Beat in Exposed Zebrafish Embryos
Zebrafish embryos were collected at approximately 3 hpf and exposed to various levels of TBBPA (0.75, 1.5, or 3 µM) or vehicle control, DMSO (0.05% for controls) under the same conditions as in the vial studies above, and were observed at 48 hpf. Observations were made starting at 48 hpf, because the heart is not yet fully formed at 24 hpf (Kimmel et al., 1995). The embryos were observed through a dissecting microscope mounted with a Scion video recording device to determine heart beat rate. Video was recorded at 7 frames per second for a total of 7 seconds. The videos were observed and heart beats were counted. The number of heart beats per specified time interval was converted to heart beats per minute.
Alcian Blue Staining and Visualization
Zebrafish embryos were collected at approximately 3 hpf and exposed in glass plates to either TBBPA (0.75 µM), BPA (15 or 10 µM), TBBPA DME (10 or 5 µM) or DMSO (vehicle) containing 0.003% phenylthiourea (total volume 20 mL). Phenylthiourea was used to prevent pigmentation allowing for better measurement after Alcian blue staining. Embryos were euthanized at 7 dpf with an overdose of MS-222, and fixed with 4% paraformaldehyde. The staining and visualization procedure was performed as previously described (Hillegass et al., 2008).
RNA Isolation from Zebrafish Embryos
Zebrafish embryos were collected at approximately 3 hpf and exposed in glass plates to either TBBPA at 0.75 µM or TBBPA DME at 5 µM or vehicle (DMSO, 0.1 %) in sterile zebrafish system water (total volume 20 mL). These concentrations were selected in order to examine expression at sublethal doses. Embryos were snap frozen in liquid nitrogen at 24 and 48 hpf, and stored at −80°C. RNA was isolated using Trizol (Invitrogen Carlsbad, CA). RNA was treated with Dnase (DNA-free kit Ambion, Austin, TX) and complimentary DNA (cDNA) was generated using iScript cDNA synthesis kit (BioRad, Hercules, CA). Quantitative Real Time RT-PCR was performed on a BioRad iCycler equipped with an iCycler iQ Detection System using the BioRad iQ SYBR Green Supermix (BioRad Hercules, CA). Samples were analyzed in triplicate, and normalized to the zebrafish 28S rRNA gene. Primers previously designed for zebrafish MMP-2, -9 and -13 were used (Hillegass et al., 2007; Hillegass, et al., 2008). Standard curves for the MMP-2, -9 and -13 primers were prepared and used for quantitation (Hillegass, et al., 2007; Hillegass, et al., 2008). All experiments were repeated three times.
In Vitro Zymography
Zebrafish embryos were collected and exposed to TBBPA (0.75 µM), TBBPA DME (5 µM), or vehicle (DMSO, 0.1 %) as described in the RNA isolation section. Embryos were harvested in Lysis buffer containing, 150 mM NaCl, 10 mM HEPES, 2 mM DTT, and 0.1% Triton X-100, at 48 and 72 hpf. Protein concentration was determined and normalized to ensure equal protein loading. The embryos were analyzed according to the in vitro zymography assay as previously described (Crawford and Pilgrim, 2005; Hillegass, et al., 2007). Fluoresceinated gelatin and collagen I were used in these assays to analyze MMP-2 and -9 or MMP-13 activity, respectively. All data were normalized to a no lysate control to take into account background fluorescence.
Statistical Analyses
Sigma Stat Version 1.01 was used to determine the normality of the data, the power and the appropriate statistical test. The Mann-Whitney Rank Sum test was used to examine the hatching data and lesion data. Chi square analysis was used to compare the mortality data. The Students T-test was used to analyze the data from the qRT-Real Time PCR and In Vitro Zymography. All data referred to as significant are p ≤ 0.05.
Results
Embryonic Dose Response Studies
Zebrafish embryos were exposed to TBBPA, BPA or TBBPA DME in order to determine at which concentrations death occurred, what types of lesions occurred, and in what sequence of appearance did they occur in the embryos following exposure to each of the chemicals. There were no deaths or lesion occurrence in the control group. In the embryonic exposure studies the primary lesions observed following TBBPA exposure were pericardial edema (>1.5 µM), yolk-sac edema (>1.0 µM), hemorrhage (>0.75µM) and trunk malformations (>1.0 µM). Hemorrhage preceded the edema and the caudal malformations appeared as the distal somites of the tail were formed by 48 hpf. At 3.0 µM 85% of the embryos were dead at 48 hpf and 100% by 72 hpf. The LC50 was 1.6 + 0.4 µM at 72 hpf.
BPA at 122 hpf resulted in lesions involving pericardial (20%), yolk-sac edema (80%) and hemorrhage (25%) in eleuthroembryos at 25 µM. Fifteen percent of the eleuthroembryos died. The LC50 was calculated from a separate series of studies to be 17.5 + 0.37 µM (McCormick 2010). At the BPA concentrations tested the eleuthroembryos would appear to be more sensitive than the developing embryo. Similar results are shown in the studies discussed below.
TBBPA DME (5–25 µM) resulted in no deaths or significant lesions at any of the concentrations tested in this range finding dose response study. Additional studies carried out did show lesions within this concentration range, but no death following exposure (Table 1).
Table 1.
Developmental lesions (%) observed in zebrafish (N=24 per treatment dose) exposed to TBBPA, BPA or TBBPA DME
| Lesion | Control | TBBPA 0.75µM |
TBBPA 1.5µM |
TBBPA 3µM |
BPA 5 µM |
BPA 10 µM |
BPA 15 µM |
DME 1 µM |
DME 5 µM |
DME 10 µM |
|---|---|---|---|---|---|---|---|---|---|---|
| Death | 0 | 17* | 100* | 100* | 8 | 8 | 4 | 0 | 0 | 0 |
| PC edema | 8 | 63* | 100* | 50*# | 33* | 38* | 46* | 33* | 50* | 46* |
| YS edema | 4 | 67* | 100* | 29* | 29* | 17 | 38* | 50* | 46* | 46* |
| Trunk edema | 0 | 0 | 54* | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemorrhage | 4 | 75* | 63* | 0 | 38* | 54* | 50* | 50* | 58* | 79* |
| Slow heart beat | 0 | 25* | 100* | 50*# | 0 | 0 | 0 | 0 | 0 | 0 |
| Tail malformation | 0 | 21* | 100* | 50*# | 0 | 4 | 0 | 0 | 0 | 0 |
| Curved tail | 0 | 17* | 46* | 0 | 0 | 4 | 25* | 21 | 29* | 21 |
| Time to hatch | 3±0 | 4±0.4* | ------ | ----- | 4±0.4* | 4±0* | 4±0* | 3±0.5 | 4±0.5 | 3±0.5 |
Zebrafish embryos were exposed to TBBPA, BPA, TBBPA DME or vehicle (DMSO) in 4 mL vials for 5 days. Lesions were recorded and presented as lesion percentage occurring at any point during the five day experiment. All data are shown as percentages and data are representative of three replicate experiments.
denote significance as compared to control with a p ≤ 0.05.
denotes complete mortality in three days.
Mortality Following Exposure to TBBPA, BPA or TBBPA DME
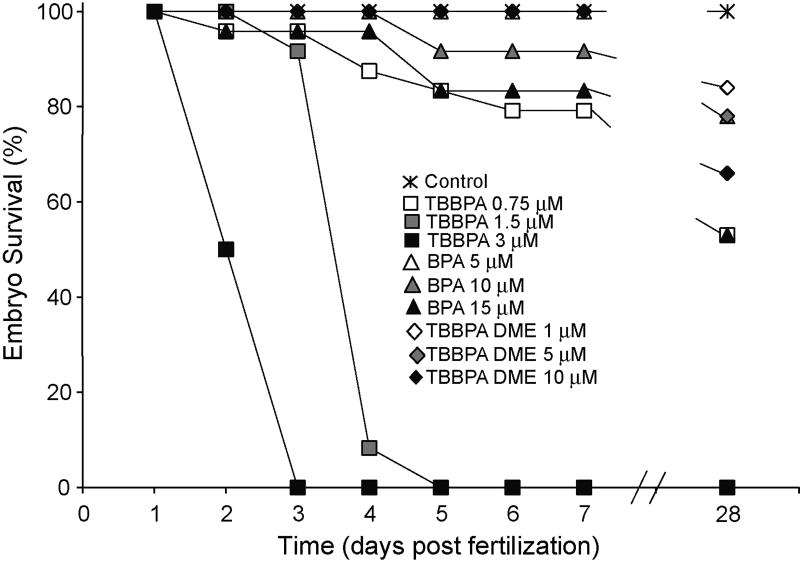
Zebrafish exposed to TBBPA, BPA or TBBPA DME were examined for dose related embryonic mortality and gross developmental lesions (Table 1). TBBPA was more acutely toxic than either BPA or TBBPA DME (Fig. 2) causing 100% mortality by 3 and 5 dpf at 3 µM (1.6 mg/L) and 1.5 µM (0.8 mg/L) respectively. TBBPA DME exposure did not increase mortality in comparison to control at any concentration tested. BPA exposure (5–15 µM) resulted in 4–8% death, however this was not statistically different from mortality in controls (Table 1, Fig. 2).
Fig 2.
Survival curve after exposure to TBBPA, BPA, or TBBPA DME. Zebrafish embryos were exposed to TBBPA (0.75, 1.5, or 3 µM), BPA (5, 10, 15 µM), TBBPA DME (1, 5, 10 µM) or vehicle (DMSO) and observed for 28 days. The 28 day embryo survival (%) were the following: Control 100%, TBBPA 0.75µM – 53%; BPA 5µM –53%, 10µM –78%, 15µM –53%; TBBPA DME 1µM – 84%, 5µM –78%, 10µM –66% Data are representative of three replicate experiments. (n=25 for each experiment)
In order to assess whether exposure to TBBPA, BPA or TBBPA DME alters juvenile survival we assessed the number of animals surviving to 28 dpf. The mortality of exposed embryos at all doses of TBBPA (0.75 µM–47%), BPA (5 µM –47%, 10 µM –22%, and 15 µM–47%) and TBBPA DME (1 µM–16%, 5 µM –22% and 10 µM–34%) was increased as compared to the control embryos (Fig. 2). It is not clear why BPA at 10 µM had lower mortality at 28 days compared to the 5 or 10 µM deaths since the embryonic lesion occurrence was similar at all three concentrations.. The differences in mortality seen in the embryonic, larval and juvenile stages after exposure to TBBPA, BPA or TBBPA DME demonstrate that TBBPA exposure (0.75 µM) was lethal at doses lower than BPA 5 µM or TBBPA DME 5 µM (Table 1, Fig. 2).
Hatching Success Following Exposure to TBBPA, BPA and TBBPA DME
The time to hatch was measured after zebrafish embryos exposure to TBBPA, BPA or TBBPA DME. TBBPA exposure (0.75 µM) displayed a significant delay in time to hatch as compared to control embryos (Table 1). No difference in the time to hatch was observed with TBBPA DME exposed embryos at 1, 5, and 10 µM as compared to control embryos (Table 1). Hatching was significantly delayed at all concentrations of BPA exposure (Table 1). Therefore, both TBBPA and BPA exposure delayed hatching of embryos, whereas TBBPA DME at the tested doses had no effect.
Lesion Occurrence in Exposed Zebrafish Embryos
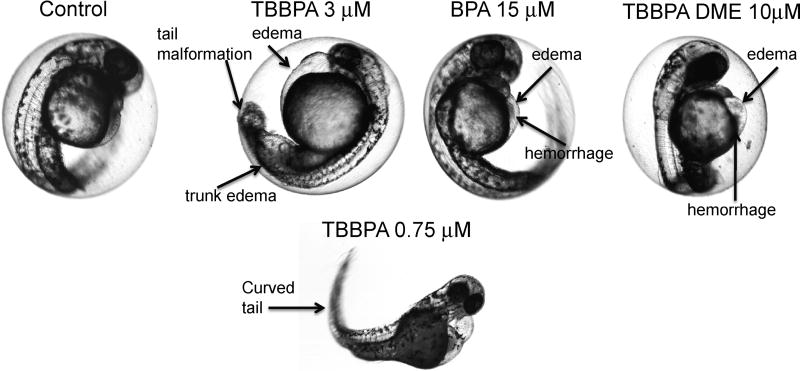
Pericardial (PC) edema, yolk sac (YS) edema, and hemorrhage were observed at all concentrations of TBBPA, BPA and TBBPA DME, as shown in Table 1. Representative photographs of the developing embryo and lesions resulting from exposure are shown in Fig. 3.
Fig. 3.
Representative lesions due to exposure to TBBPA and its metabolites. Representative photographs are shown illustrating lesions due to exposure to TBBPA (3 µM and 0.75 µM), BPA, TBBPA DME and vehicle (DMSO). The photographs of TBBPA at 3µM, BPA 15 µM, and TBBPA DME 10µM are of exposed embryos at 48 hours post fertilization. The photograph of the embryo exposed to TBBPA at 0.75 µM illustrates the tail malformation at a lower dose of exposure resulting in decreased severity. Arrows point to the representative lesions for each compound, including pericardial (PC) edema, hemorrhage, trunk edema and tail malformation.
Rank sum analysis was performed on data comparing control to individual chemical doses for each lesion. TBBPA (0.75, 1.5 and 3 µM) and TBBPA DME (5, 10 and 15 µM) exposure resulted in a significant occurrence of PC and YS edema compared to control (Table 1). For BPA exposed embryos, the incidence of PC edema was significant at all doses, whereas the incidence of YS edema was significant for the 5 and 15 µM doses. Hemorrhaging observed in the developing zebrafish was significant at all doses of BPA and TBBPA DME (Table 1). TBBPA DME resulted in significant YS edema at all concentrations and PC edema was observed at 5 and 10 µM (Table 1). These data indicate that the potency of TBBPA as compared to BPA or TBBPA DME is greater due to the numbers of lesions seen at doses of TBBPA approximately 10 fold lower than doses used for BPA or TBBPA DME exposure. However, TBBPA DME did result in significantly more vascular lesions than BPA at comparable molar concentrations.
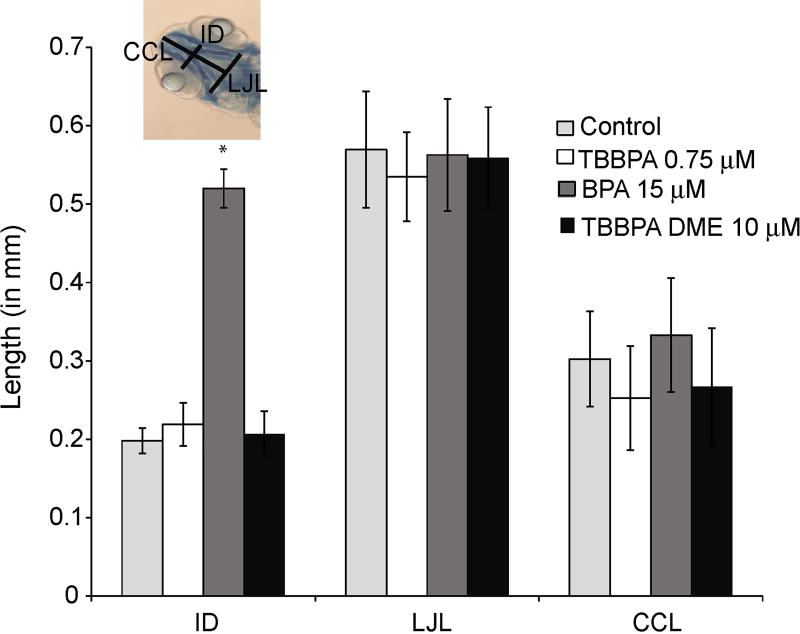
One of the most common developmental lesions following chemical exposure to embryos is disruption of craniofacial development. To determine if there were effects of TBBPA, BPA, or TBBPA DME exposure on the craniofacial features in developing zebrafish embryos, embryos were exposed to TBBPA (0.75 µM), BPA (10, and 15 µM) and TBBPA DME (5 and 10 µM) for 7 days, and then stained with alcian blue. Intraocular distance (ID), lower jaw length (LJL) and ceratohyal cartilage length (CCL) were measured (Fig. 4). Embryos exposed to BPA at 15 µM exhibited a significant increase in intraocular distance (Fig. 4), whereas embryos exposed to TBBPA, BPA at 10 µM (data not shown) or TBBPA DME at 10 or 5 µM showed no significant difference in intraocular distance compared to control. There were no significant craniofacial differences compared to control for any of the compounds and doses tested in LJL and CCL (Fig. 4).
Fig. 4.
Staining of cartilage in TBBPA, BPA, or TBBPA DME exposed embryos. Embryos were exposed in TBBPA (0.75 µM), BPA (10, and 15 µM), TBBPA DME (5 and 10 µM) or vehicle (DMSO) for 7 days, when they were euthanized and stained accordingly. Intraocular distance (ID), lower jaw length (LJL) and certohyal cartilage length (CCL) were measured. A representative photograph is shown depicting the location of each measurement. Error bars represent standard deviation and the asterisk denotes significance relative to control with a p ≤ 0.05.
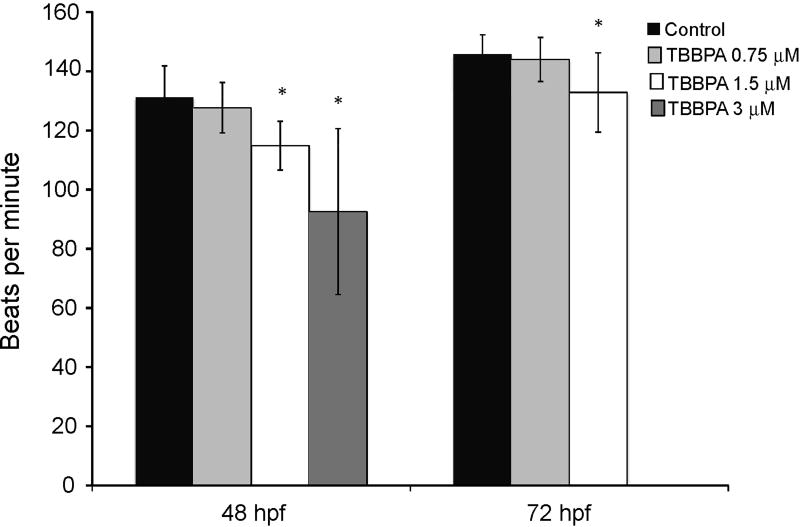
Exposure of zebrafish embryos to TBBPA resulted in trunk edema, tail malformation, and impaired circulation that were not observed following exposure to BPA or TBBPA DME (Table 1 and Fig. 3). These lesions were most severe following exposure to TBBPA at 3 µM, with embryos demonstrating a lack of tail formation, and edema in the caudal region of the animal (Fig. 3). Exposure to 1.5 µM TBBPA resulted in similar lesions but with reduced severity, with the tail partially formed but was shorter and with a bent tip (Fig. 3). At 0.75 µM TBBPA exposure, the tail was fully formed, but was curved (Fig.3). The heart beat was significantly slower following TBBPA (1.5 and 3 µM) exposure at 48 hpf, and 72 hpf as compared to control embryos (Fig. 5). However, embryos exposed to TBBPA at 0.75 µM did not show any significant difference in heart beats compared to the control embryos (Fig.5). Normal sinuous rhythm was observed in all exposed embryos, even those with reduced heart rate. Thus, TBBPA exposure at the two highest doses resulted in decreased heart rate, and stunted growth of the tail that were not seen with exposure to BPA or TBBPA DME.
Fig. 5.
Effect of TBBPA exposure on zebrafish heart beats. Embryos were exposed to TBBPA at 0.75, 1.5 and 3 µM. Data are the mean heart beats at 48 and 72 hpf. Error bars denote standard deviation and asterisks denote significance with a p ≤ 0.05 as compared to control.
TBBPA or TBBPA DME Exposure Leads to Alteration in MMP Gene Expression
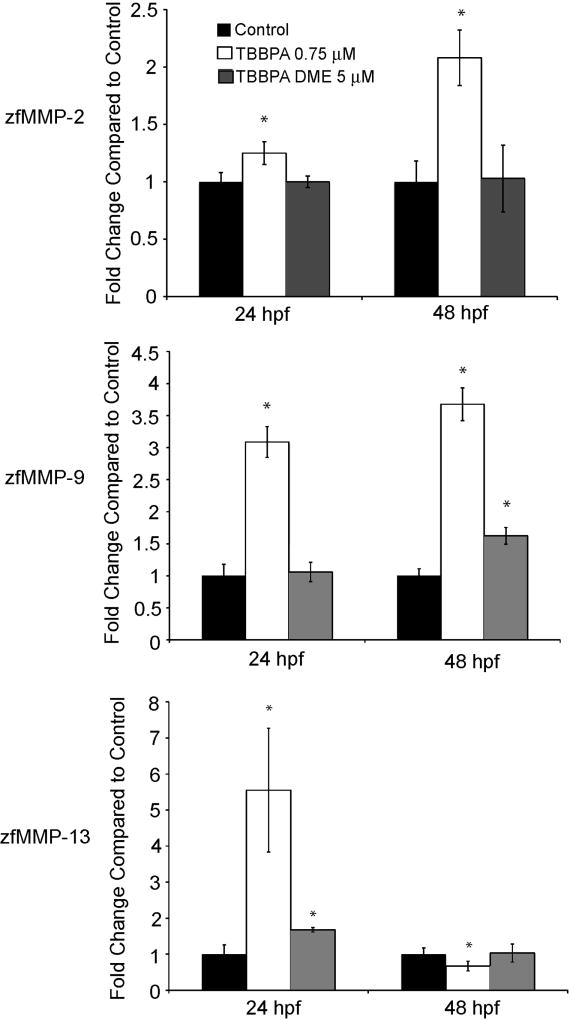
The TBBPA induced lesions seen in the caudal regions of these embryos suggesting a role for MMPs in the deformity. MMPs are known to be involved in tissue morphogenesis, wound repair and are crucial for proper embryo development (Murphy and Nagase, 2008; Zagris, 2001). To ascertain whether MMPs play a role in the appearance of TBBPA induced developmental lesions, expression of the gelatinases MMP-2 and -9, and the collagenase MMP-13, after embryonic exposure to either TBBPA (0.75 µM) or TBBPA DME (5 µM) was examined. Exposure of developing zebrafish embryos to TBBPA resulted in a significant increase in MMP-2 by 1.25 fold at 24 hpf and slightly more than 2 fold at 48 hpf as compared to control (Fig. 6A). TBBPA exposure induced MMP-9 expression at 24 hpf by 3 fold at 24 hpf and by 3.5 fold at 48 hpf as compared to control levels (Fig. 6B). MMP-13 expression in response to TBBPA exposure increased at 24 hpf by 5.5 fold but reduced to slightly below normal at 48 hpf (Fig. 6C). Conversely, TBBPA DME exposure at 24 hpf and 48 hpf did not affect the expression of MMP-2 as compared to control (Fig 6A). MMP-9 expression was unchanged at 24 hpf after TBBPA DME exposure but was increased at 48 hpf by 1.5 fold relative to control (Fig. 6B). MMP-13 expression increased at 24 hpf by 1.5 fold with TBBPA DME exposure but was unchanged compared to that in control embryos at 48 hpf (Fig 6C). TBBPA exposure results in significant alteration in MMP expression at a much lower dose as compared to control in contrast to TBBPA DME exposure which did not appear to have a large impact on MMP expression, despite the dose being over 5 fold higher.
Fig. 6.
Expression of MMP-2, -9, and -13 after exposure to TBBPA or TBBPA DME. Expression levels were measured by Quantitative Real Time PCR on total RNA isolated from embryos exposed to either vehicle, TBBPA at 0.75 µM or TBBPA DME at 5µM at 24 and 48 hpf. Data are represented as fold change relative to control. The top panel is MMP-2 expression, middle panel is MMP-9 expression and the lower panel is MMP-13 expression. Data were normalized to the 28S rRNA gene and were performed in triplicate. Data shown are representative of three separate experiments. Error bars denote standard deviation and asterisks denote significant with a p ≤ 0.05 as compared to control.
TBBPA or TBBPA DME Exposure Leads to Alteration in the Activity of MMPs in the Developing Zebrafish Embryo
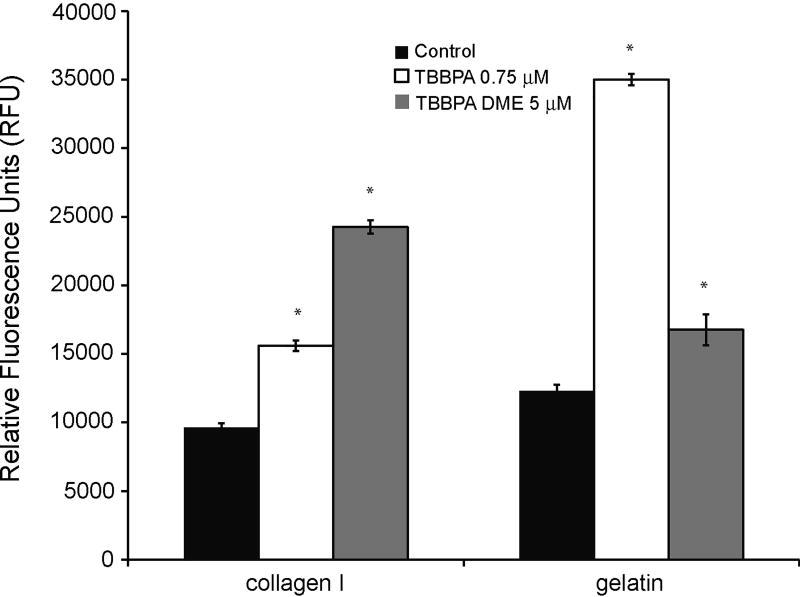
It is important to examine MMP activity to confirm that the changes in gene expression are leading to changes in enzymatic activity. MMP activity was examined using an in vitro zymography assay in which native fluoresceinated substrates (gelatin, degraded by MMPs-2 and -9 and collagen I, degraded by MMP-13) were incubated with lysates from zebrafish embryos exposed to vehicle, TBBPA or TBBPA DME. Extracts from 48 hpf TBBPA-exposed embryos demonstrated a significant increase (1.5 fold), in gelatin degradation (Fig. 7), a substrate for MMP-2 and -9. The 3 fold increase in degradation of gelatin correlates with the increase seen in MMP-2 and -9 mRNA expression after TBBPA exposure as does the expression of MMP-13 and the collagenase activity. Collagen I degradation was also increased (3.5 fold) in 48 hpf embryos in response to TBBPA exposure. TBBPA DME exposure resulted in significant increase in degradation of collagen I (2.5 fold) and gelatin (1.5 fold) as compared to control at 48 hpf (Fig. 7).
Fig. 7.
Activity of gelatinases and collagenase after TBBPA or TBBPA DME exposure. Lysates from zebrafish embryos were obtained at 48 hours post fertilization. In vitro zymography was used to examine the degradation of gelatin and collagen I after TBBPA at 0.75 µM or TBBPA DME at 5 µM embryonic exposure. Data are normalized to a no enzyme control. Data are representative of three separate experiments. Error bars denote standard deviation and asterisks denote significance with a p ≤ 0.05 relative to control.
Discussion
TBBPA is transformed in the environment (Fig.1) by indigenous microbes, through anaerobic reductive dehalogenation leading to BPA (Arbeli and Ronen, 2003; Arbeli, et al., 2006; Ronen and Abeliovich, 2000; Voordeckers, et al., 2002) or by aerobic O-methylation pathways forming TBBPA MME and TBBPA DME (George and Häggblom, 2008). These compounds persist and accumulate in the environment with the potential to cause toxicity to wildlife and humans. Although TBBPA DME has been detected in the environment (Sellström and Jansson, 1995; Watanabe, et al., 1983), it is a newly recognized metabolite of TBBPA for which little toxicological data is available. This study presents the first investigation comparing the toxicological effects of TBBPA and its metabolites in the developing zebrafish embryo and suggests that the microbially mediated transformations result in compounds with lower acute toxicity. In this study, TBBPA concentrations used for exposure were in the range of environmentally detected levels which are reported to be as high as mg/kg in sediments near production plants (Hale, et al., 2006). The doses used for BPA and TBBPA DME exposure are approximately tenfold greater than that of TBBPA. Our data show an increase in embryo or larval mortality following developmental exposure to TBBPA or BPA. TBBPA DME exposure, however, did not result in death as compared to control embryos after one-week post fertilization. TBBPA proved to be 10 times more potent than BPA or TBBPA DME exposure, resulting in 100% mortality at 1.5 and 3 µM. This potency at low doses is important considering the levels of TBBPA detected in the environment.
In contrast to the embryonic and sac-fry mortality measured in this study, all three compounds resulted in lower survival at 28 dph. In this study, long-term survival post-hatch in exposed embryos supported the findings from previous work examining post-hatch survival of embryos whose parents were exposed to 1.5 µM TBBPA (Kuiper, et al., 2007b). After 28 dpf, survival of TBBPA DME exposed embryos, as well as those exposed to TBBPA and BPA, was significantly reduced (Fig. 2). Our data show that TBBPA DME and BPA were less acutely toxic than TBBPA. BPA did have 4 to 8% death by day five and 22–47% death by day 28 dpf., and based on the lesion occurrence the delayed death is likely a result of the these lesions (Table 1). TBBPA DME exposure did not cause embryonic mortality, but the lesions involving the vasculature during embryonic development likely contributed to the delayed mortality (Table 1).
TBBPA, BPA and TBBPA DME exposure resulted in a variety of developmental lesions in the embryos, such as a delay in time to hatch, and vascular lesions (edema and hemorrhage). A delay in the time to hatch is an indication of the chemicals effects on critical biochemical and developmental pathways necessary for the embryo’s ability to free itself from the chorion (Nechaev and Pavlov, 2004; Sano et al., 2008), and is a crucial developmental benchmark to overall larval survival of aquatic species in the environment. Our data show that exposure to TBBPA and BPA resulted in a delay in time to hatch (Table 1), however this was not observed in embryos exposed to TBBPA DME. Exposure to TBBPA or its metabolites caused dose dependent lesions, including PC edema, YS edema and hemorrhage in zebrafish embryos (Table 1). The significant decrease in heart beat at 1.5 and 3.0 µM TBBPA is likely due to vascular lesions (Fig. 5). TBBPA exposure exhibited the highest potency with the highest presence of significant lesions at concentrations an order of magnitude lower than that of its metabolites. A number of common chemically induced lesions were observed (Fig. 3), and included edema, hemorrhage and curved tail. These lesions were previously reported for embryos exposed to similar concentrations of TBBPA and BPA (Hu et al., 2009; Kishida et al., 2001). These data are in keeping with studies from other laboratories showing malformed tails in zebrafish in response to exposure to BPA at higher concentrations (68 µM) then tested in our studies (Duan et al., 2008). The appearance of truncated tails at higher doses of BPA exposure, resembling the tail malformations seen with TBBPA in this study suggests a similar mechanism of action of BPA and TBBPA with BPA being less potent.
Alcian blue staining of cartilage was used to measure the craniofacial features of the exposed larvae after 7 days of exposure. Our data show that there are no significant alterations in the lower jaw length or ceratohyal cartilage in the exposed embryos as compared to control (Fig. 4). For the intraocular distance, the only significant alteration occurred in embryos exposed to BPA at 15 µM (Fig. 4). The reason for this effect on the intraocular is not known. The staining, however, of the cartilage and spine was similar to that of the controls. This observation supports the idea that exposure to TBBPA, BPA or TBBPA DME does not alter cartilaginous staining or cellular appearance of the cartilaginous components in the developing zebrafish embryos.
The tail and trunk lesions seen in previous BPA exposure studies (Duan, et al., 2008), and in this study with TBBPA exposure, suggest an alteration in proper caudal formation that could be related to altered MMP expression. Previous studies illustrate a role of MMPs in the proper formation of the caudal axis (Zhang et al., 2003a; Zhang et al., 2003b) and it is known that the Wnt pathway, which regulates caudal development, also regulates MMP expression (Harrington et al., 2007; Karow et al., 2008). Furthermore, TBBPA exposure is also known to cause the production of reactive oxygen species, which can play a role in the regulation of MMP expression (Reistad et al., 2005; Reistad, et al., 2007; Svineng et al., 2008). Studies in our laboratory showed morpholino knock down of MMP-13 expression in the developing zebrafish embryo resulted in body axis curvature, kinked tail and other malformations (Hillegass, et al., 2007), which demonstrated the role for this enzyme in proper tail formation. In our study, expression of MMP-9 and -13 increased after TBBPA exposure to a greater degree than was seen with TBBPA DME exposure (Fig. 6). MMP-2 expression in TBBPA DME exposed embryos was similar to that of control embryos. MMP activity as measured by in vitro zymography using gelatin and collagen I as substrates was significantly increased at 48 hpf as compared to control (Fig.7). Additionally, the mRNA expression increase in TBBPA exposed embryos was followed by an increase in the degradation of collagen I (1.5 fold) and gelatin (3.5 fold) (Fig. 7). One possible explanation is that the tail malformations and trunk edema seen during development are a result of increased MMP expression and activity. Although we also observed an increase in MMP-9 following TBBPA DME exposure, no truncation in tail structure was observed. MMP-2 and -9 expression patterns may explain the difference in caudal development after TBBPA and TBBPA DME exposure since both enzymes are known for playing a role in promoting cellular migration and both may be required to cause the truncated tail phenotype (Murphy and Nagase, 2008). Altered expression and activity of MMPs could explain in part the lesions observed here and those reported by Kuiper et. al. (2007).
Morphogenesis is a complex process relying on the critical timing of cell proliferation, cell migration and ECM remodeling, which involve a number of growth factors and enzymes. It is likely that compounds which alter the temporal and spatial expression of these factors will alter vascular formation, and normal body axis morphology. Studies examining TBBPA exposure on the effect of tadpole (Pacific Tree Frog, Pseudacris regilla) metamorphosis demonstrate an up regulation of MMP-9 mRNA and also abnormal timing of tail resorption during metamorphosis (Veldhoen et al., 2006). Glucocortocoid exposure of developing zebrafish has been shown to increase MMP-2, -9 and -13 expression and activity and result in similar lesions such as impaired vascularization and tail malformations similar to that seen with TBBPA exposure (Hillegass, et al., 2007; Hillegass, et al., 2008) Taken together, these data support our hypothesis that the increase in MMP expression due to TBBPA exposure is related to the observed tail malformations, but may not be the only pathway affected.
The data presented here demonstrate that developmental exposure to TBBPA, BPA or TBBPA DME result in a reduction in embryo survival. However, there are differences in their potency, with BPA and TBBPA DME being less acutely toxic than the parent compound. However, TBBPA DME does appear to be slightly more toxic than BPA in causing embryonic vascular lesions. Further, the alteration of the expression and activity of MMPs is likely playing a role in these developmental lesions due to their involvement in vascularization and caudal development. The tail malformations in TBBPA exposed embryos, may also be influencing the time to hatch as the movement of the embryos is limited with improper tail formation. These data also demonstrate that developmental exposure to these compounds results in reduced survival one-month post exposure. Ongoing use of TBBPA and increasing environmental contamination of both TBBPA due to production, and TBBPA DME due to microbial metabolism, warrants further study. Specifically, the mechanisms underlying the developmental lesions, notably the trunk and tail lesions in TBBPA exposed embryos, and potential longer-term chronic effects must be examined in order to fully understand the overall risk and toxicity of these compounds.
Acknowledgments
This work was supported by the Hudson River Foundation, the New Jersey Agricultural Experimental Station, the National Institute for Environmental Health and Safety Center Grant (ES05022), and the Department of Defense / SERDP (ER-1492).
We thank Yolanda Pennesi for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arbeli Z, Ronen Z. Enrichment of a microbial culture capable of reductive debromination of the flame retardant tetrabromobisphenol-A, and identification of the intermediate metabolites produced in the process. Biodegradation. 2003;14:385–95. doi: 10.1023/a:1027304222436. [DOI] [PubMed] [Google Scholar]
- Arbeli Z, Ronen Z, Diaz-Baez MC. Reductive dehalogenation of tetrabromobisphenol-A by sediment from a contaminated ephemeral streambed and an enrichment culture. Chemosphere. 2006;64:1472–8. doi: 10.1016/j.chemosphere.2005.12.069. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–85. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- Crawford BD, Pilgrim DB. Ontogeny and regulation of matrix metalloproteinase activity in the zebrafish embryo by in vitro and in vivo zymography. Dev Biol. 2005;286:405–14. doi: 10.1016/j.ydbio.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Ding Yu, Xi Ying, Chen Ting, Wang Ji-yong, Tao Dong-lei, Wu Zhi-Li, Li Yi-ping, Li Chen, Zeng Rong, Li Lin. Caprin-2 enhances canonical Wnt signaling through regulating LRP5/6 phosphorylation. The Journal of Cell Biology. 2008;182(5):865–872. doi: 10.1083/jcb.200803147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Zhu L, Kun Y, Zhu X. Individual and joint toxic effects of pentachlorophenol and bisphenol A on the development of zebrafish (Danio rerio) embryo. Ecotoxicol Environ Saf. 2008 doi: 10.1016/j.ecoenv.2008.01.021. (Epub ahead of print Mar20.) [DOI] [PubMed] [Google Scholar]
- Fernandes A, Dicks P, Mortimer D, Gem M, Smith F, Driffield M, White S, Rose M. Brominated and chlorinated dioxins, PCBs and brominated flame retardants in Scottish shellfish: methodology, occurrence and human dietary exposure. Mol Nutr Food Res. 2008;52:238–49. doi: 10.1002/mnfr.200700135. [DOI] [PubMed] [Google Scholar]
- George KW, Häggblom MM. Microbial O-methylation of the flame retardant tetrabromobisphenol-A. Environ Sci Technol. 2008;42:5555–61. doi: 10.1021/es800038q. [DOI] [PubMed] [Google Scholar]
- Hakk H, Letcher RJ. Metabolism in the toxicokinetics and fate of brominated flame retardants--a review. Environ Int. 2003;29:801–28. doi: 10.1016/S0160-4120(03)00109-0. [DOI] [PubMed] [Google Scholar]
- Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM. Brominated flame retardant concentrations and trends in abiotic media. Chemosphere. 2006;64:181–6. doi: 10.1016/j.chemosphere.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–73. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Harrington MJ, Hong E, Fasanmi O, Brewster R. Cadherin-mediated adhesion regulates posterior body formation. BMC Dev Biol. 2007;7:130. doi: 10.1186/1471-213X-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hillegass JM, Villano CM, Cooper KR, White LA. Matrix Metalloproteinase-13 Is Required for Zebra fish (Danio rerio) Development and Is a Target for Glucocorticoids. Toxicol Sci. 2007;100:168–79. doi: 10.1093/toxsci/kfm192. [DOI] [PubMed] [Google Scholar]
- Hillegass JM, Villano CM, Cooper KR, White LA. Glucocorticoids alter craniofacial development and increase expression and activity of matrix metalloproteinases in developing zebrafish (Danio rerio) Toxicol Sci. 2008;102:413–24. doi: 10.1093/toxsci/kfn010. [DOI] [PubMed] [Google Scholar]
- Hu J, Liang Y, Chen M, Wang X. Assessing the toxicity of TBBPA and HBCD by zebrafish embryo toxicity assay and biomarker analysis. Environ Toxicol. 2009;24:334–42. doi: 10.1002/tox.20436. [DOI] [PubMed] [Google Scholar]
- Jakobsson K, Thuresson K, Rylander L, Sjödin A, Hagmar L, Bergman Å. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46:709–16. doi: 10.1016/s0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B, Adams DH, Kannan K. Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere. 2008;70:1935–44. doi: 10.1016/j.chemosphere.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kang JH, Asai D, Katayama Y. Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms. Crit Rev Toxicol. 2007;37:607–25. doi: 10.1080/10408440701493103. [DOI] [PubMed] [Google Scholar]
- Karow M, Popp T, Egea V, Ries C, Jochum M, Neth P. Wnt Signaling in Mouse Mesenchymal Stem Cells: Impact on Proliferation, Invasion, and MMP Expression. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00619.x. (Epub ahead of print Dec24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kishida M, McLellan M, Miranda JA, Callard GV. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio) Comp Biochem Physiol B Biochem Mol Biol. 2001;129:261–8. doi: 10.1016/s1096-4959(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci. 2005;84:249–59. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Kuiper RV, Canton RF, Leonards PE, Jenssen BM, Dubbeldam M, Wester PW, van den Berg M, Vos JG, Vethaak AD. Long-term exposure of European flounder (Platichthys flesus) to the flame-retardants tetrabromobisphenol A (TBBPA) and hexabromocyclododecane (HBCD) Ecotoxicol Environ Saf. 2007a;67:349–60. doi: 10.1016/j.ecoenv.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kuiper RV, van den Brandhof EJ, Leonards PE, van der Ven LT, Wester PW, Vos JG. Toxicity of tetrabromobisphenol A (TBBPA) in zebrafish (Danio rerio) in a partial life-cycle test. Arch Toxicol. 2007b;81:1–9. doi: 10.1007/s00204-006-0117-x. [DOI] [PubMed] [Google Scholar]
- Labadie P, Tlili K, Alliot F, Bourges C, Desportes A, Chevreuil M. Development of analytical procedures for trace-level determination of polybrominated diphenyl ethers and tetrabromobisphenol A in river water and sediment. Anal Bioanal Chem. 2010;396:865–75. doi: 10.1007/s00216-009-3267-x. [DOI] [PubMed] [Google Scholar]
- Law RJ, Allchin CR, de Boer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Levin ER. Rapid signaling by steroid receptors. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1425–R1430. doi: 10.1152/ajpregu.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Castaño ME, Elliot SJ, Potier M, Karl M, Striker LJ, Striker GE, Csaky CG, Cousins SW. Regulation of Estrogen Receptors and MMP-2 Expression by Estrogens in Human Retinal Pigment Epithelium. Visual Sci. 2003;44:50–59. doi: 10.1167/iovs.01-1276. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem Int. 2003;43:533–42. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- McCormick JM. Dissertation. Rutgers University; New Brunswick, NJ: 2010. Microbial transformations of tetrabromobisphenol A and its metabolites, and their impact on toxicity to the developing zebrafish (Danio rerio) embryos; p. 225. [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman Å, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Muncke J, Junghans M, Eggen RI. Testing estrogenicity of known and novel (xeno-)estrogens in the MolDarT using developing zebrafish (Danio rerio) Environ Toxicol. 2007;22:185–93. doi: 10.1002/tox.20255. [DOI] [PubMed] [Google Scholar]
- Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Saigusa D, Tetsu N, Yamakuni T, Tomioka Y, Hishinuma T. Neurobehavioral effects of tetrabromobisphenol A, a brominated flame retardant, in mice. Toxicol Lett. 2009;189:78–83. doi: 10.1016/j.toxlet.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Nechaev IV, Pavlov DS. The species specificity of hatching enzyme and its effect on the duration of embryogenesis in the fish Cichlasoma nigrofasciatum (Cichlidae) Dokl Biol Sci. 2004;394:78–81. doi: 10.1023/b:dobs.0000017136.04664.66. [DOI] [PubMed] [Google Scholar]
- Nyholm JR, Norma nA, Norrgren L, Haglund P, Andersson PL. Maternal transfer of brominated flame retardants in zebrafish (Danio rerio) Chemosphere. 2008;73:203–8. doi: 10.1016/j.chemosphere.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Razandi M, Ali Pedram A, Park S, Levin ER. Proximal Events in Signaling by Plasma Membrane Estrogen Receptors. J. Biol. Chem. 2003;278(4):2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicol Sci. 2005;83:89–100. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E, Ring A, Fonnum F. In vitro toxicity of tetrabromobisphenol-a on cerebellar granule cells: cell death, free radical formation, calcium influx and extracellular glutamate. Toxicol Sci. 2007;96:268–78. doi: 10.1093/toxsci/kfl198. [DOI] [PubMed] [Google Scholar]
- Ronen Z, Abeliovich A. Anaerobic-aerobic process for microbial degradation of tetrabromobisphenol A. Appl Environ Microbiol. 2000;66:2372–7. doi: 10.1128/aem.66.6.2372-2377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K, Inohaya K, Kawaguchi M, Yoshizaki N, Iuchi I, Yasumasu S. Purification and characterization of zebrafish hatching enzyme - an evolutionary aspect of the mechanism of egg envelope digestion. FEBS J. 2008;275:5934–46. doi: 10.1111/j.1742-4658.2008.06722.x. [DOI] [PubMed] [Google Scholar]
- Sellström U, Jansson B. Analysis of Tetrabromobisphenol A in a product and environmental samples. Chemosphere. 1995;31:3085–3092. [Google Scholar]
- Strack S, Detzel T, Wahl M, Kuch B, Krug HF. Cytotoxicity of TBBPA and effects on proliferation, cell cycle and MAPK pathways in mammalian cells. Chemosphere. 2007;67:S405–11. doi: 10.1016/j.chemosphere.2006.05.136. [DOI] [PubMed] [Google Scholar]
- Svineng G, Ravuri C, Rikardsen O, Huseby NE, Winberg JO. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res. 2008;49:197–202. doi: 10.1080/03008200802143166. [DOI] [PubMed] [Google Scholar]
- Thomas P. The Endocrine System. In: diGiulio RT, Hinton DE, editors. The Toxicology of Fishes. CRC Press; Boca Raton: 2008. pp. 457–488. [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J Environ Monit. 2001;3:366–70. doi: 10.1039/b104304h. [DOI] [PubMed] [Google Scholar]
- Veldhoen N, Boggs A, Walzak K, Helbing CC. Exposure to tetrabromobisphenol-A alters TH-associated gene expression and tadpole metamorphosis in the Pacific tree frog Pseudacris regilla. Aquat Toxicol. 2006;78:292–302. doi: 10.1016/j.aquatox.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Verslycke TA, Vethaak AD, Arijs K, Janssen CR. Flame retardants, surfactants and organotins in sediment and mysid shrimp of the Scheldt estuary (The Netherlands) Environ Pollut. 2005;136:19–31. doi: 10.1016/j.envpol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Voordeckers JW, Fennell DE, Jones K, Häggblom MM. Anaerobic biotransformation of tetrabromobisphenol A, tetrachlorobisphenol A, and bisphenol A in estuarine sediments. Environ Sci Technol. 2002;36:696–701. doi: 10.1021/es011081h. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Kashimoto T, Tatsukawa R. Identification of the flame retardant tetrabromobisphenol-A in the river sediment and the mussel collected in Osaka. Bull Environ Contam Toxicol. 1983;31:48–52. doi: 10.1007/BF01608765. [DOI] [PubMed] [Google Scholar]
- Zagris N. Extracellular matrix in development of the early embryo. Micron. 2001;32:427–38. doi: 10.1016/s0968-4328(00)00011-1. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bai S, Zhang X, Nagase H, Sarras MP., Jr The expression of gelatinase A (MMP-2) is required for normal development of zebrafish embryos. Dev Genes Evol. 2003a;213:456–63. doi: 10.1007/s00427-003-0346-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bai S, Zhang X, Nagase H, Sarras MP., Jr The expression of novel membrane-type matrix metalloproteinase isoforms is required for normal development of zebrafish embryos. Matrix Biol. 2003b;22:279–93. doi: 10.1016/s0945-053x(03)00020-9. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Luo XJ, Chen SJ, Wu JP, Mai BX. Spatial distribution and vertical profile of polybrominated diphenyl ethers, tetrabromobisphenol A, and decabromodiphenylethane in river sediment from an industrialized region of South China. Environ Pollut. 2009;157:1917–23. doi: 10.1016/j.envpol.2009.01.016. [DOI] [PubMed] [Google Scholar]