Abstract
Perfluoroalkylated substances (PFAS) are a class of persistent anthropogenic chemicals that have been detected worldwide. PFASs consist of fluorinated carbon chains of varying length, terminal groups, and have a number of industrial uses. A previous zebrafish study from our laboratory showed that acute (3–120 h post fertilization, 0.02–2.0μM), waterborne embryonic exposure to these chemicals resulted in chemical specific alterations at 5 days post fertilization (dpf), and some effects persisted up to 14 dpf. Using a gene battery consisting of 100 transcripts identified several genes that were up or down regulated. This current study looks at the long-term impacts of PFASs in adult zebrafish using the same exposure regimen. It was hypothesized that sub-lethal exposure of perfluorooctane sulfonate (PFOS), perfluorononanoic acid (PFNA), or perfluorooctane sulfonate (PFOA) in embryonic zebrafish (3–120 hpf) would result in permanent morphometric, gene expression, and behavioral changes in adult fish similar to those observed at 5 and 14 dpf. Zebrafish were exposed to PFOS, PFOA, and PFNA (Control 0μM, 2.0μM) for the first five days post fertilization. At six months post fertilization, no PFAS treatment resulted in a significant change in total body length or weight. In terms of behavior, PFNA males showed a reduction in total distance traveled and time of immobility, and an increase in thigmotaxis behavior, aggressive attacks, and preference for the bright section of the tank. PFOS treated males had a reduced aggression behavior, and PFOA females preferred the dark section of the tank. Gene expression of slco2b1, slco1d1, and tgfb1a were analyzed because these transcripts were previously found to be affected by PFAS exposure in 5dpf and 14 dpf zebrafish and resulted in: significant decrease in expression of slco2b1 for both sexes in PFNA and PFOS treated groups, significant decrease of slco1d1 in all treatment groups for females and PFOS and PFOA exposed males, significant increase of tgfb1a in males treated with PFOS and PFNA, and a significant increase of bdnf in all PFAS male groups. This study demonstrates that acute, embryonic exposure (5 days) to individual PFASs result in significant biochemical and behavioral changes in young adult zebrafish 6 months after exposure. These three PFASs have long term and persistent impacts following short term embryonic exposure that persists into adulthood.
Keywords: PFOS, PFNA, PFOA, PFAS, Zebrafish behavior, Zebrafish locomotion, Danio rerio
1. Introduction
Perfluoroalkylated substances (PFASs) are anthropogenic compounds composed of a long carbon backbone that is fully fluorinated with either a carboxyl, alcohol, or sulfonate terminal group (Conder et al., 2008). Long chain PFASs (greater than 8 carbons) were produced from the 1950s until 2000 (Lehmler, 2005) because they are extremely stable which allows them to be used in a number of manufacturing applications (Renner, 2001). The three most prevalent long chain PFASs that are most commonly found at elevated levels in the environment are perfluorooctanoic acid (PFOA; C8), perfluorooctane sulfonate (PFOS; C8) and perfluorononanoic acid (PFNA; C9).
PFASs are of particular concern because they are persistent in the environment (Houde et al., 2011), have been detected in animal tissue samples world-wide (Lindstrom et al., 2011), and have been detected in both ground and surface waters (Hu et al., 2016; Post et al., 2013). PFNA in particular, since a voluntary ban of long chain PFASs in 2000, has seen increased concentrations in human serum in U.S populations (Kato et al., 2011). Although all three compounds belong to the same chemical class, the subtle structural differences affect the toxicity, toxicokinetics, and toxicodynamics within organisms. The occurrence in both environmental and animal samples as well as the fate and transport of these compounds has been extensively reviewed (Houde et al., 2011; Kannan et al., 2005; Lau 2012; Lindstrom et al., 2011).
Our previous study results are summarized in Table 4 and formed the basis for the endpoints examined in this paper. The effects on morphometrics, behavior analysis, and gene expression in zebrafish after the acute, embryonic exposure (3–120 h post fertilization, 0.02–2.0μM) of yolk sac larvae (5 dpf) and free swimming larvae (14 dpf) time points were reported. The current paper analyzes the long term effects of an acute embryonic exposure (3–120 h post fertilization, 2.0μM) in adult zebrafish, 6 months after exposure has stopped.
Table 4.
Summary of total body length, locomotion, light/dark sensitivity, aggression, and gene expression after exposure to PFNA, PFOS, and PFOA at 5, 14, and 180 days post fertilization.
| PFNA
|
PFOS
|
PFOA
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dpf: | 5* | 14* | 180 Males |
180 Females |
5 | 14* | 180 Males |
180 Females |
5 | 14* | 180 Males |
180 Females |
| Morphometric | ||||||||||||
| Total Body Length | ↓ | ↑ | NS | NS | ↓ | NS | NS | NS | ↓ | ↓ | NS | NS |
| Locomotion | ||||||||||||
| Distance | — | ↑ | ↓ | NS | — | ↑ | NS | NS | — | ↑ | NS | NS |
| Middle | — | ↑ | ↓ | NS | — | NS | NS | NS | — | NS | NS | NS |
| Immobile | — | NS | ↓ | NS | — | NS | NS | NS | — | NS | NS | NS |
| Velocity | — | ↓ | ↑ | NS | — | ↑ | NS | NS | — | NS | NS | NS |
| Light/Dark Assay | ||||||||||||
| Time in Light | — | — | ↑ | NS | — | — | NS | NS | — | — | NS | ↓ |
| Aggression Assay | ||||||||||||
| Number of hits | — | — | ↑ | NS | — | — | ↓ | NS | — | — | NS | NS |
| Gene Expression | ||||||||||||
| slco2b1 | ↑ | ↑ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↑ | ↑ | ↓ | NS |
| slco1d1 | — | — | NS | ↓ | — | — | ↓ | ↓ | — | — | ↓ | ↓ |
| tgfb1a | ↓ | — | ↑ | NS | ↓ | — | ↑ | NS | NS | — | ↓ | NS |
| bdnf | — | — | ↑ | NS | — | — | ↑ | NS | — | — | ↑ | NS |
“NS” indicates no significant. “—” indicates endpoint not assessed for this time/compound. Arrows represent a significantly increased (↑) or significantly decreased (↓) endpoint (p ≤ 0.05).
Indicate data from (Jantzen et al., 2016).
At 5 dpf, exposure to all three PFASs (2.0μM) resulted in a significantly decreased total body size (Jantzen et al., 2016). However, when the fish reached 14 dpf, only PFOA exposed fish continued to be significantly smaller. PFOS exposed fish had recovered and were not significantly different from controls, while PFNA exposed fish were significantly larger. Behavior analysis at 14 dpf (2.0μM) resulted in an increased swimming distance for all PFAS treatments. Additional behavior effects included changes in swimming velocity (PFNA, PFOS) and thigmotaxis behaviors (PFNA, PFOS). Similar observations have been made in mammalian studies, in which exposure to PFOS in mice (0.3 mg/kg/day) induced spontaneous hyperactivity (Spulber et al., 2014) and mice exposed to 0.3 mg/kg PFOA showed changes in exploratory behavior (Onishchenko et al., 2011). Analyzing similar behavior endpoints in adult zebrafish will determine if the growth and behavior affects seen in larval zebrafish persist through adulthood as well as better understand the long term effects of PFAS exposure in our teleost model system.
In the Jantzen et al., 2016 study, a battery of 100 genes relevant to critical development pathways were analyzed in 5 dpf zebrafish exposed to 2.0 μM PFASs. From this analysis, 4 specific pathways were identified to be affected by two or more PFASs. All three PFASs had a significantly decreased expression of adaptor related protein complex 1, sigma subunit 1 (ap1s1) and a significant increase transcription factor 3a (tcf3a). Transforming growth factor beta 1a (tgfb1a) was significantly decreased after PFOS and PFNA exposure, and solute carrier organic anion transporting polypeptide 2b1 (slco2b1) was significantly increased in PFOA and PFOS and decreased in PFNA. The current study aims to determine if these gene expression changes observed in 5 dpf larvae zebrafish after PFAS exposure persists to the adult life stage. Both ap1s1 and tcf3a are transcripts that are only expressed in the developing zebrafish, and therefore could not be analyzed in adult zebrafish. Tgfb1a and slco2b1 are both expressed throughout the lifetime of the zebrafish and were assessed in this study.
Slco1d1 is a solute carrier organic anion transporting polypeptide that is expressed in all zebrafish life stages. This transcript was chosen for this study based on results shown in (Popovic et al., 2014) which determined in vivo that PFASs could be both a substrate and an inhibitor to this transporter. Due to the number of behavior endpoints analyzed in this study, brain derived neurotrophic factor (bdnf) was also assessed. This is a transcription growth factor involved in a number of neurological processes including regulation of neuron differentiation (Reference Genome Group of the Gene Ontology, 2009), serotonin transporter function (Mossner et al., 2000) and has a possible relation to stress responses (Pavlidis et al., 2015)
It is hypothesized that the toxicities induced by these compounds at 5 and 14 dpf in terms of morphometric measurements, behavior, and gene expression will be persistent to adult life stage (6 months). Exposure to PFASs during embryonic development appears to result in altered gene expression of transporters and behavior into adulthood, particularly in PFNA exposed male fish. In the case of finfish, these biochemical alterations could have detrimental effects on endogenous substrate pharmacokinetics thereby altering normal homeostatic pathways. The behavioral alterations could have detrimental effects on prey survival from predators or other behavioral related cues. Concordance between lower and higher vertebrate studies indicate that embryonic developmental stages are the most sensitive to PFASs and that those alterations can be manifested later in life (Lau et al., 2006; Yang et al., 2002).
2. Methods
2.1. Animal handling
The AB strain zebrafish (Zebrafish International Resource Center, Eugene, OR) were used for all experiments. Breeding stocks were bred and housed in Aquatic Habitats (Apopka, FL) recirculating systems under a 14:10 h light:dark cycle. System water was obtained by carbon/sand filtration of municipal tap water and water quality was maintained at <0.05 ppm nitrite, <0.2 ppm ammonia, pH between 7.2 and 7.7, and water temperature between 26 and 28 °C. All experiments were conducted in accordance with the zebrafish husbandry protocol and embryonic exposure protocol (#08-025) approved by the Rutgers University Animal Care and Facilities Committee.
2.2. Exposure
Shown in Fig. 1 is the exposure and data collection timeline. Zebrafish embryos were exposed to PFOS, PFOA or PFNA (Sigma-Aldrich, St. Louis, MO) from 3 hpf to 120 hpf hours in a static non-renewal protocol. All compounds were dissolved in water. The exposure followed a modified OECD 212 protocol (OECD, 2011), where the endpoints of lesion presence, length, weight, and mortality were recorded. Modification to the OECD protocol was to extend the study beyond the exposure time-point which allowed for the analysis of adult zebrafish. After the exposure was terminated (120 hpf), fish were transferred to non-treated system water and fed 2 times daily with Zeigler Larval AP50 (Aquatic Habitats, Apopka, Florida) and brine shrimp. Therefore, the only exposure was through the water from 3 hpf to 120 hpf (5 days), which corresponds to embryonic to yolk sac larval exposure. Morphometric measurements, gene expression, and swim activity endpoints were collected at 6 months post fertilization, which is during the adult life stage. One biological replicate from each control and treatment group consisted of 10 males and 10 females, for a total of 20 fish per treatment group. Two biological replicates were performed. No experiment had mortality greater than 20% of the starting sample size. All treatment water was collected and disposed of through the Rutgers Environmental Health and Safety for proper disposal.
Fig. 1.

Timeline of zebrafish exposure to PFOA, PFOS and PFNA. Water-borne exposure occurred between 3 and 120 hpf. Dashed lines represent exposure periods.
2.3. Animal rearing after exposure
After 120 hpf, fish larvae from both control and treated groups were transferred into system water as described above in 600 mL beakers and fed Zeigler Larval AP50 (Aquatic Habitats, Apopka, Florida) until 30 dpf. This food consists of marine, animal and vegetable proteins, test, vegetable starches, fish and vegetable oils, and vitamin and mineral premixes with a minimum protein content of 50%. When fish reached 30 dpf they were transferred onto the aquatic habitats system (described above) and fed a regimen of brine shrimp (1 mL/tank) in the morning feeding and a Tetramin/Aquatox flake food combination (0.04 g/tank) in the evening. Tetramin/Aquatox combination consists of 43% protein, 13% crude fat, 1.5% crude fiber, 10% moisture and 10.5% ash. Each treatment group was housed together (N = 10–12) in a 3L tank. Two weeks before the study was performed, the sex of each fish was recorded and the fish were individually housed in a divided 1.5 L tank. The sex ratio of control and all treatment groups was approximately 50%. Feeding amounts for individual fish were adjusted to brine shrimp 0.25 mL/tank and Tetramin/Aquatox 0.01 g/tank.
2.4. Light/dark assay
One behavior that is classified as anxiety type behavior is light-avoidance; in which zebrafish prefer the dark, opaque compartment of a tank rather than the light, clear compartment (Champagne et al., 2010). The proportion of time the zebrafish prefer light versus dark is dependent upon the ambient light level (Stephenson et al., 2011).
A modified version of the light/dark box test assay described by (Champagne et al., 2010) was performed. Fish were habituated in tanks in our behavior room for 30 min. After this habituation period, each fish was placed in the assay tank and video recorded for a total of ten minutes. Four Ikegami ICD-49 CCD cameras (Noldus Information Technology, Leesburg, VA) were mounted to the ceiling above each tank. The videos were analyzed with Noldus Ethovision Software (Leesburg, VA) for endpoints of time spent in light or dark part of tank and number of crossings between compartments.
2.5. Open field test and aggression assay
The open field apparatus was modified from (Champagne et al., 2010), and consisted of 3L aquatic habitats tank filed with 1.5L clean system water to minimize vertical swimming. Each camera was able to capture two tanks in one frame. Illumination via fluorescent lights was consistent with housing conditions (300–400 lx).
Adult zebrafish were individually placed into a novel empty tank, and video recording using Noldus MPEG recorder 2.1 (Noldus Information Technology, Leesburg, VA) began immediately after transfer. The total trial length was 30 min, after which the fish were removed and individually housed for the remainder of the experiment. The tanks were rinsed and water renewed between trials to remove waterborne pheromones. Testing occurred between 12:00 and 16:00 h each day to limit circadian rhythm effects. Each fish was subject to this procedure once a day for four days. On the fourth day, the video was analyzed using Noldus Ethovision Software (Leesburg, VA) for endpoints of total distance traveled, mobility (a spatially independent measure of body movement) and thigmotaxis behaviors.
Thigmotaxis is the tendency of an animal to avoid the center of their tank (Sharma et al., 2009; Treit and Fundytus, 1988). This behavior has been identified in teleosts(Ahmad and Richardson, 2013), rodents(Simon et al., 1994), and in humans (Walz et al., 2015). It is thought that thigmotaxis is a way of finding shelter, protection or a way of escape from a predator or stressor (Sharma et al., 2009).
After four consecutive days in the open field assay, on the fifth day the zebrafish were subject to a mirror-induced stimulation assay for aggression adapted from (Norton et al., 2011). A mirror (7.5 × 7.5 cm) was slotted into the trapezoidal end of the tank, and video was recorded for 10 min. Videos were viewed by two independent blind reviewers and the number of attacks against the mirror was counted.
2.6. Morphometric analysis
Prior to dissection for gene expression analysis, morphometric measurements of total body length and wet weight were recorded for each fish. Fish were anesthetized with tricane MS222 (Sigma Aldrich, St Louis MO) and measurements were taken. Each fish was placed into a weigh boat, and measured on an analytical balance. Then, fish were placed into the dissection tray, and total body length was measured from the tip of the mouth to the end of the spinal cord. Immediately after measurements, fish were dissected for liver isolation used in gene expression analysis.
2.7. Gene expression analysis
Livers isolated from adult fish (N = 5–8 per treatment group per sex) were snap frozen in liquid nitrogen and RNA extracted using RNAzol reagent (Sigma-Aldrich, St. Louis, MO). Reverse transcription was performed with the High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA) and real-time qPCR was performed using iQ™ SYBR® Green Supermix (Bio-Rad, Hercules CA). The following qPCR protocol was used: 35 cycles of: 95 °C for 15 s and 60 °C for 1 min. The housekeeping gene used was b-actin. It was ascertained that b-actin expression was not effected by any PFAS treatment. Analysis was performed using a standard curve method for all of the P0 transcripts examined. The genes examined and primer sequences are listed in Table 1. Each independent experiment was replicated 2 times.
Table 1.
List of transcripts and primer sequences for gene expression analysis.
| Gene | Primer sequence | Amplicon Size (Da) |
Primary Function |
|---|---|---|---|
| actb1 | Forward: 5′-CGAGCAGGAGATGGGAACC-3′ Reverse: 5′-CAACGGAAACGCTCATTGC-3′ |
63067.66 | Cell motility; Housekeeping gene |
| slco2b1 | Forward: 5′-TTGCCCTGCCTCACTTCATT-3′ Reverse: 5′-AGGCTGGAGTTGAGTCTGGT-3′ |
23296.14 | Organic anion transporting polypeptide (sulfate conjugates) |
| tgfb1a | Forward: 5′-CCGCATCCAAAGCCAACTTC-3′ Reverse: 5′-CGCCCGAAAACATTCCCAAG-3′ |
25756.68 | Transcription growth factor lateral line development |
| slco1d1 | Forward: 5′-GCCGCATTTCTTCCAAGGAC-3′ Reverse: 5′-TGTAAGGCACGGCAGAACAT-3′ |
29834.33 | Organic anion transporting polypeptide (estron-3-sulfonate, DHEAS) |
| bdnf | Forward: 5′-AGGTCCCCGTGACTAATGGT-3′ Reverse: 5′-CGCTTGTCTATTCCTCGGCA-3′ |
61825.98 | Brain-derived neurotrophic factor |
2.8. Statistical analysis
Using SigmaPlot® 11, one-way ANOVA was used for analysis of all endpoints (Gene expression, morphometric measurements, and behavioral assays) to determine significance. Statistical significance was assigned at a p-value ≤ 0.05. The SigmaPlot software tests for normality and power of the test prior to statistical analysis.
3. Results
3.1. Morphometric measurements
The total body weight and length of the adult zebrafish were measured at 6 months post fertilization.
There were no significant differences between males and females in any control or treatment group. Additionally, between treatment groups of combined sexes, there was no significant difference in either weight (Fig. 2A) or length (Fig. 2B). However, the PFNA treated group was trending towards a decrease in body weight (p = 0.061). This is dissimilar to what we observed at 5 (all PFASs significantly smaller) and 14 days (PFOA significantly smaller; PFNA significantly larger) in our previous study (Jantzen et al., 2016).
Fig. 2.
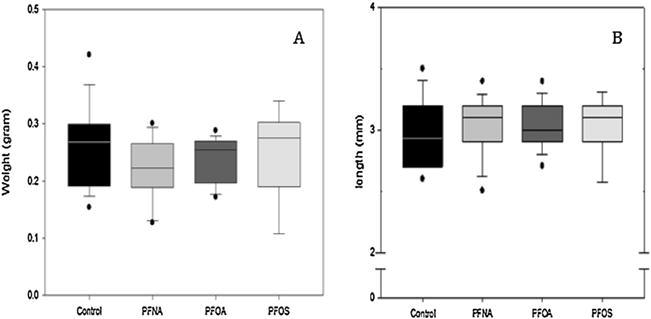
Morphometric measurements of adult zebrafish following acute, embryonic exposure (2.0μM). Box plots represent median ± 25th/75th percentile. Dots indicate the 5th/9th percentile. No significant difference in weight (A) or length (B) were observed for any treatment group. N = 10 fish per treatment group. Statistical significance was tested using a one-way ANOVA, p < 0.05.
3.2. Locomotion activity
The locomotion activity was assessed in six month zebrafish. The fish were separated by sex and males (Fig. 3) and females (Table 2) were analyzed separately. The total distance traveled is a measure of distance swum throughout the duration of the assay. Males exposed to PFNA (Fig. 3A) were the only group affected, in which a significant decrease in total distance was observed.
Fig. 3.
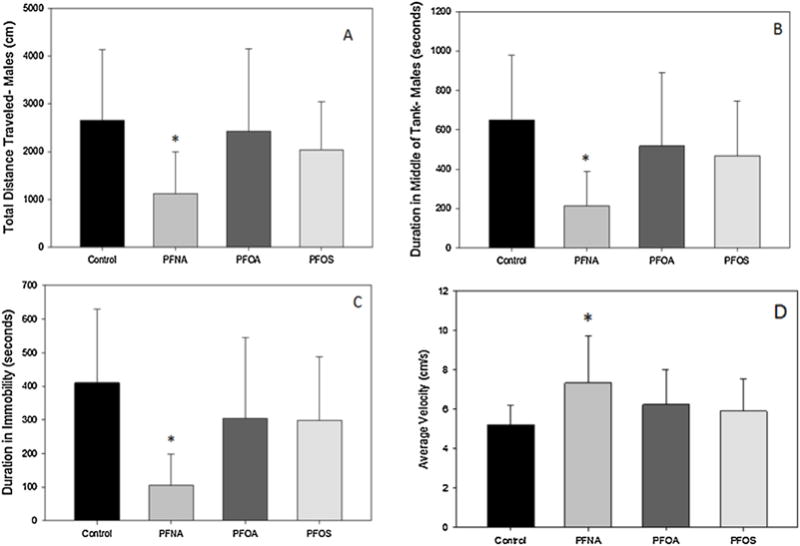
Locomotive behavioral endpoints of total distance traveled (A), middle duration (B), immobile duration (C) and velocity (D) in adult male zebrafish embryonically exposed to PFNA, PFOA, or PFOS (2.0μM). Bars represent mean value and standard deviation. N = 10 animals. An asterisk (*) indicates a statistical significant value, p < 0.05, one-way ANOVA compared to control.
Table 2.
Locomotive behavioral endpoints of total distance traveled, middle duration, immobile duration, and velocity in adult female zebrafish embryonically exposed to PFNA, PFOA, or PFOS (2.0μM). N = 10 animals. Data presented as average ± standard deviation. No statistically significant differences were observed, p < 0.05, one-way ANOVA compared to control.
| Total Distance Traveled (cm) | Duration in middle of tank (s) | Time of immobility (s) | Average velocity (cm/s) | |
|---|---|---|---|---|
| Control | 1997.7 ± 1672.5 | 438.8 ± 382.2 | 263.8 ± 343.9 | 6.3 ± 1.7 |
| PFNA | 2399.2 ± 1058.7 | 544.2 ± 272.4 | 294.7 ± 161.8 | 6.1 ± 1.0 |
| PFOA | 1994.3 ± 882.8 | 445.1 ± 232.4 | 268.9 ± 162.6 | 5.7 ± 1.2 |
| PFOS | 2087.7 ± 1320.6 | 440.9 ± 263.5 | 270.1 ± 153.5 | 6.1 ± 1.4 |
Swimming velocity is a measurement of the average speed traveled per 1 min time bins for the duration of assay. No significant effects were observed in any treatment with the exception of PFNA-exposed males, which exhibited significantly faster swimming velocity (Fig. 3D).
The duration in the middle of the tank is a measure of thigmotaxis, an anxiety type behavior in zebrafish in which they will tend to stay along the walls of the assay arena. PFNA treated males (Fig. 3B) spent a significantly less amount of time in the middle, meaning that there were exhibiting an increase in thigmotaxis.
Immobility duration is an endpoint to assess the amount of movement of a fish independent of swimming. This can include tail and fin movements and body angle changes. Male fish treated with PFNA showed a decrease in the amount of time immobile (Fig. 3C), indicating more body movement independent of swim activity.
3.3. Light/Dark assay
In the light/dark activity assay, the amount of time spent in each area of the assay arena as well as the number of times crossed between the areas was recorded. This assay was performed with a light intensity of 375 Lux. Based on (Stephenson et al., 2011), we would expect control fish at this light intensity to spend approximately 49 ± 15 percent of the time in the dark, which is similar to our results. PFOA exposed females spent a significantly less amount of time in the light compared to controls, while PFNA males (Table 3) spent a significantly larger amount of time in light. There were no significant differences for any treatment in either sex in terms of crossings between the two areas (Table 3).
Table 3.
Average number of crossings between light and dark compartments, and duration of time in the light compartment of the tank during the 10 min assay period. Values represent the average ± standard deviation.
| Number of Crossings between Light and Dark Areas
|
Time Spent in Light Compartment (seconds)
|
|||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Control | 26 ± 20 | 25 ± 14 | 266 ± 75 | 365 ± 112 |
| PFNA | 25 ± 14 | 22 ± 16 | 430 ± 130* | 275 ± 137 |
| PFOA | 29 ± 19 | 23 ± 10 | 361 ± 134 | 153 ± 56* |
| PFOS | 37 ± 26 | 27 ± 12 | 198 ± 1390 | 334 ± 118 |
Asterisk (*) represents statistical significance, one-way ANOVA between treatments for each sex (p < 0.05).
3.4. Aggression assay
The level of aggression is based on the number of times the fish attacked their reflection in the mirror. Fish were analyzed by sex and treatment group. PFNA exposed males had an increased level of aggression, and PFOS treated males showed a decrease in aggressive behavior (Fig. 4B). Females exposed to any PFAS showed no significant difference in aggression compared to controls (Fig. 4A).
Fig. 4.
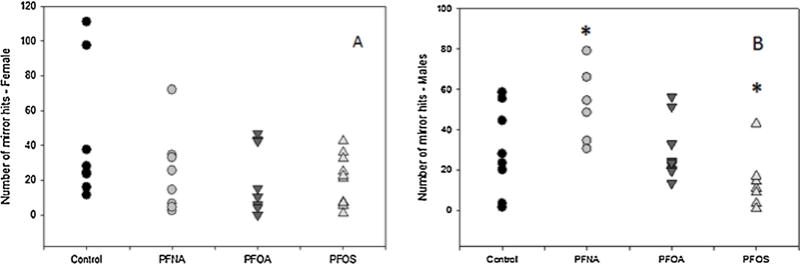
The number of mirror attacks in adult zebrafish in (A) females and (B) males. Each data point represents an individual fish (N = 8–10 fish per treatment per sex). Asterisks represent statistical significance was determined using a one-way ANOVA compared to control (p < 0.05).
3.5. Gene expression
Gene expression of two organic anion transporting polypeptides slco1d1 and slco2b1 were analyzed as well as growth factor tgfb1a. In male zebrafish (Fig. 5A), PFNA and PFOS treatment resulted in a significant decrease in slco2b1 and significant increase tgfb1a transcript expression. Male zebrafish from all PFASs had an increased expression of bdnf. Slco1d1 was increased in males exposed to PFOA and PFOS. In female zebrafish (Fig. 5B), there was no change in tgfb1a expression in any treatment group. All three PFASs examined significantly decreased slco1d1 expression, and PFNA and PFOS exposed fish had a significantly reduced slco2b1 expression (Fig. 5).
Fig. 5.
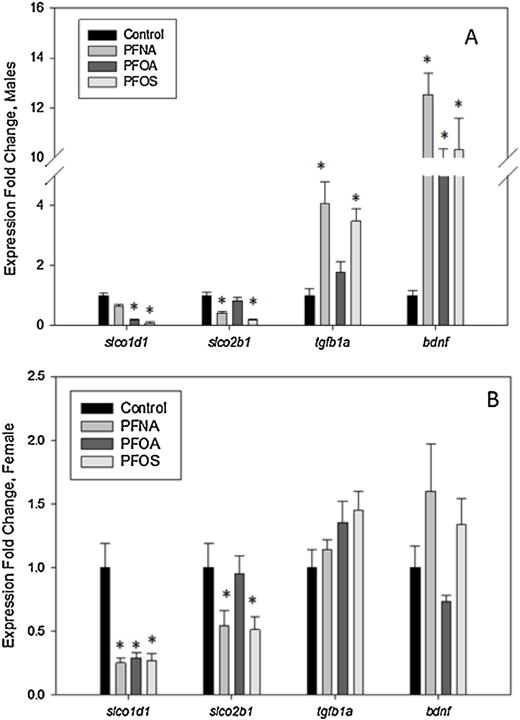
Gene expression of adult (A) male and (B) female zebrafish embryonically exposed to PFOS, PFOA, or PFNA. Bars represent mean fold change and standard mean error. N = 8–10 fish per treatment per sex. An asterisk (*) indicates a statistical significant value, p < 0.05, one-way ANOVA.
4. Discussion
A summary of the morphometric, behavior, and gene expression endpoints after PFAS exposure at 5, 14, and 180 dpf is listed in Table 4. In adult zebrafish, no PFAS treatment at any dose resulted in significant changes in total body weight or length compared to the control group (Fig. 2). This is in contrast to the results found at 5 dpf (all PFAS decreased total body length) and at 14 dpf (PFOA significantly decreased, PFNA significantly increased total body length) at the same exposure concentration and similar study design previously published. Based on these results 6 months after exposure, all PFAS exposed fish were able to recover from changes in size during development and that the behavioral changes reported in the current study are likely not due to physical changes.
A summary of adult behavior and gene expression endpoints is presented in Table 4. Of the three PFASs tested, PFNA exposure appears to have the greatest persistent effects on behavior but only in male zebrafish (Fig. 3, Table 2). PFNA exposed males exhibited a significant decrease in total distance traveled, an increased speed while swimming, a smaller time of immobility, and a higher tendency for thigmotaxis. Additionally, these fish also had a preference for light rather than dark (Table 3), and had a higher level of aggression (Fig. 4B). Males exposed to PFOA showed no significant behavioral deficits in any assay, and PFOS exposed males only showed one significantly altered endpoint which was a decrease of aggression (Fig. 4B). Both PFNA and PFOS exposed females exhibited no behavioral changes, while PFOA exposed females had a preference for the dark compartment of the light/dark assay.
Tgfb1a is one possible pathway that can play a role in locomotive effects observed (Table 4). This gene is a transcription growth factor that can be involved in cell migration, proliferation, apoptosis, and tissue homeostasis (Xing et al., 2015). It was found that when this gene is knocked down, the lateral line of the zebrafish does not form correctly (Xing et al., 2015). The lateral line development is important because it allows the zebrafish to sense water movement, find prey, and avoid predators (Coombs, 2005). Therefore, the tgfb1a transcript was examined to determine if altered gene expression could be correlated to changes in swimming behavior endpoints observed in Fig. 3.
In the present study, the adult fish embryonically exposed to PFASs show a significant increase in tgfb1a expression in males exposed to PFNA and PFOS (Fig. 5). This correlates to a previous acute embryonic exposure to PFOS and PFNA that also resulted in increased tgfb1a expression, indicating that this change is persistent from juvenile until adult zebrafish (Jantzen et al., 2016). However, while both PFOS and PFNA had an increased expression of this transcript, the majority of significantly altered behavioral changes were exhibited by PFNA exposed males(Fig. 3). No female groups from any treatment showed a significant change in tgfb1a expression (Table 4). While the change in tgfb1a expression could be affecting the development of lateral line formation and in turn swimming behavior, this pathway alone is unlikely to account for all of the behavioral changes observed.
Brain derived neurotrophic factor (bdnf) is a transcription growth factor that is expressed throughout all life stages of the zebrafish, and has also been found to affect lateral line formation (Gasanov et al., 2015). Bdnf regulates the migration of the lateral line primordium, and enhances the differentiation of sensory and sympatic neurons (Diekmann et al., 2009). Therefore, an increase in this gene would appear to be beneficial for neurological and central nervous system development. In this study, males from each PFAS had a significantly increased level of bdnf, but PFNA exposed males still expressed many altered behavior endpoints. There are a number of possible scenarios relating bdnf to the endpoints observed in this study. One scenario is that due to the decreased expression of tgfb1a as embryos, bdnf is increased in order enhance neuron development, repair any possible damage of the lateral line, and compensate for this developmental loss. Other possibilities relating bdnf to behavior effect could be the large number of downstream pathways that interact with this transcript. For example, bdnf has been shown to affect the glucocorticoid receptor, which is directly involved in stress response (Lambert et al., 2013). Many of the behavior endpoints tested, such as thigmotaxis, the light/dark box or the aggression assay are indicators of stress (Champagne et al., 2010). However, this increase in expression due to a stress stimulus was transient and was reduced after the stimulus was removed so it would seem unlikely that bdnf expression alone accounts for the anxiety type behaviors observed. Bdnf has also been shown to be involved in synaptic plasticity, longer term potentiation, and memory (Yamada and Nabeshima, 2003). It appears likely that bdnf and its downstream pathways may be directly and indirectly affected by PFAS exposure, the exact role and mechanism of each one are currently not known.
In behavioral endpoints as well as gene expression, there is a sex-specific difference between the compounds. A factor that could be contributing to the different outcomes observed between sexes and compounds is differences in body clearance rates of either the PFASs or endogenous bioactive compounds through competing with transporters. Difference in body clearance rates were observed in PFOA exposure in fat head minnows (Lee and Schultz, 2010) and tilapia (Han et al., 2011), as well as in PFOA and PFNA exposure to rats (Kudo et al., 2001). The elimination of PFASs from tissues in various organisms has been associated with the organic anion transporting polypeptides, oatps (slco). The expression of these transporters is likely one of the reasons for the variable half-lives observed (Klaassen and Aleksunes, 2010). Gene expression analysis of sclo2b1 and slco1d1 gives insight to both differences between compounds and between sexes due to elimination rates.
Previous studies have found that PFASs can be either a substrate or inhibitor of oatps, in particular slco1d1 (Popovic et al., 2014). In our previous manuscript, we reported expression of slco2b1 was significantly changed at both 5 dpf (PFOS significantly decreased; PFNA and PFOA significantly increased) and at 14 dpf (PFOS, PFOA, PFNA significantly increased) (Table 4). In the current study, expression of organic anion transporting polypeptide slco2b1 was significantly decreased in both sexes treated with PFNA and PFOS. Changes in expression of slco2b1 have persisted from larval through adult life stages for PFNA and PFOS treated fish, however there was a change from increased expression at 14 dpf to decreased expression in adults. The mechanism for the altered expression at these different time points will need to be further studied to understand these observations. What is striking is the inhibition at 180 days from exposure during the first five days following fertilization.
Slco1d1 and slco2b1 are only two of the numerous organic ion transporting polypeptides, many of which have overlapping functions and substrates (Klaassen and Aleksunes, 2010). Slco1d1 was significantly decreased in expression in all sex and treatment groups except for males exposed to PFNA. Another role of oatps is to transport steroid conjugate and hormone precursor compounds (Klaassen and Aleksunes, 2010). Changes in these transporters expressions could result in variations of normal hormone production and cycling (Popovic et al., 2014), which in turn could lead to many of the behavior and anxiety-type changes observed in the zebrafish. Our results show that these transporters are dramatically altered both during exposure and long after termination of exposure (180 days). However, at this time the direct relationship between transporter expression and specific hormone functioning resulting in behavioral changes cannot be made. Therefore, while it appears that after PFAS exposure both behavior and transporter expression are affected, more in depth studies will need to be performed to determine the exact role these transporters play in PFAS toxicity.
This study was designed to examine the long term effect on adult zebrafish from a sub-lethal exposure to PFOS, PFNA, or PFOA in embryonic zebrafish (5 days) and will this exposure result in ongoing morphometric, gene expression, and behavioral defects in adult zebrafish (180 days) similar to those observed at 5 and 14 dpf (Table 4). At six months post exposure the morphologic changes observed at 14 dpf did not persist in either sex (Fig. 2). Therefore, a short-term exposure resulted in initial growth affects that are mitigated by six months. In terms of locomotive behavior, light/dark anxiety, and aggression, PFNA exposed fish exhibited the greatest number of significantly altered endpoints. These endpoints were sex specific in that only the male zebrafish were significantly affected (Figs. 3 & 4). Gene expression of slco2b1 and tgfb1a remained altered. Both tgb1a and bdnf were altered in a sex dependent manner in that males exposed to all three PFASs and had higher expression of these transcripts (Fig. 5). Behavioral measurements are the result of a complex set of pathways that can be manifested in teleosts and further studies are needed to determine the mechanism by which these compounds are modifying these behaviors. The results from our study supports the argument that PFAS exposure, particularly to PFNA, at the embryonic life stage is sensitive to persistent effects into adulthood. These behavior changes could have impacts at the population level, which can be extrapolated to other teleost species in the ability to find mates, food, and avoid predators.
Acknowledgments
This work was carried out at the NJ Agricultural Experiment Station with funding support through Cooperative State Research, Education, and Extension Services [01201]; the NJ Water Resources Research Institute [NJWRRI2015]; Department of Biochemistry and Microbiology, Rutgers University.
Footnotes
Disclosure of potential conflicts of interest
The authors have no potential conflicts of interest to declare.
References
- Ahmad F, Richardson MK. Exploratory behaviour in the open field test adapted for larval zebrafish: impact of environmental complexity. Behav Process. 2013;92:88–98. doi: 10.1016/j.beproc.2012.10.014. http://dx.doi.org/10.1016/j.beproc.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Hoefnagels CC, de Kloet RE, Richardson MK. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res. 2010;214(2):332–342. doi: 10.1016/j.bbr.2010.06.001. http://dx.doi.org/10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol. 2008;42(4):995–1003. doi: 10.1021/es070895g. [DOI] [PubMed] [Google Scholar]
- Coombs VN. The hydrodyanmics and structural mechanics of the lateral line system. Fish Physiol. 2005;23:103–139. [Google Scholar]
- Diekmann H, Anichtchik O, Fleming A, et al. Decreased BDNF levels are a major contributor to the embryonic phenotype of huntingtin knockdown zebrafish. J Neurosci. 2009;29(5):1343–1349. doi: 10.1523/JNEUROSCI.6039-08.2009. http://dx.doi.org/10.1523/JNEUROSCI.6039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasanov EV, Rafieva LM, Korzh VP. BDNF-TrkB axis regulates migration of the lateral line primordium and modulates the maintenance of mechanoreceptor progenitors. PLoS One. 2015;10(3):e0119711. doi: 10.1371/journal.pone.0119711. http://dx.doi.org/10.1371/journal.pone.0119711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZX, Zhang M, Lv CX. Toxicokinetic behaviors and modes of perfluorooctane sulfonate (PFOS) and perfluorooctane acid (PFOA) on tilapia (Oreochromis niloticus) Afr J Biotechnol. 2011;10(60):12943–12950. [Google Scholar]
- Houde M, De Silva AO, Muir DC, Letcher RJ. Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ Sci Technol. 2011;45(19):7962–7973. doi: 10.1021/es104326w. http://dx.doi.org/10.1021/es104326w. [DOI] [PubMed] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U. S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett. 2016 doi: 10.1021/acs.estlett.6b00260. http://dx.doi.org/10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed]
- Jantzen CE, Annunziato KM, Bugel SM, Cooper KR. PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat Toxicol. 2016;175:160–170. doi: 10.1016/j.aquatox.2016.03.026. http://dx.doi.org/10.1016/j.aquatox.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP. Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch Environ Contam Toxicol. 2005;48(4):559–566. doi: 10.1007/s00244-004-0133-x. http://dx.doi.org/10.1007/s00244-004-0133-x. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U. S. population 1999–2008. Environ Sci Technol. 2011;45(19):8037–8045. doi: 10.1021/es1043613. http://dx.doi.org/10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. http://dx.doi.org/10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R, Kawashima Y. Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem Biol Interact. 2001;134(2):203–216. doi: 10.1016/s0009-2797(01)00155-7. [DOI] [PubMed] [Google Scholar]
- Lambert WM, Xu CF, Neubert TA, Chao MV, Garabedian MJ, Jeanneteau FD. Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol Cell Biol. 2013;33(18):3700–3714. doi: 10.1128/MCB.00150-13. http://dx.doi.org/10.1128/MCB.00150-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, et al. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol Sci. 2006;90(2):510–518. doi: 10.1093/toxsci/kfj105. http://dx.doi.org/10.1093/toxsci/kfj105. [DOI] [PubMed] [Google Scholar]
- Lau C. Perfluorinated compounds. EXS. 2012;101:47–86. doi: 10.1007/978-3-7643-8340-4_3. http://dx.doi.org/10.1007/978-3-7643-8340-4_3. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Schultz IR. Sex differences in the uptake and disposition of perfluorooctanoic acid in fathead minnows after oral dosing. Environ Sci Technol. 2010;44(1):491–496. doi: 10.1021/es901838y. http://dx.doi.org/10.1021/es901838y. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ. Synthesis of environmentally relevant fluorinated surfactants–a review. Chemosphere. 2005;58(11):1471–1496. doi: 10.1016/j.chemosphere.2004.11.078. http://dx.doi.org/10.1016/j.chemosphere.2004.11.078. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45(19):7954–7961. doi: 10.1021/es2011622. http://dx.doi.org/10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Mossner R, Daniel S, Albert D, et al. Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF) Neurochem Int. 2000;36(3):197–202. doi: 10.1016/s0197-0186(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Norton WH, Stumpenhorst K, Faus-Kessler T, et al. Modulation of Fgfr1a signaling in zebrafish reveals a genetic basis for the aggression-boldness syndrome. J Neurosci. 2011;31(39):13796–13807. doi: 10.1523/JNEUROSCI.2892-11.2011. http://dx.doi.org/10.1523/JNEUROSCI.2892-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. Test No 212: Fish, Short-term Toxicity Test on Embryo and Sac-Fry Stages. OECD Publishing; 2011. [Google Scholar]
- Onishchenko N, Fischer C, Wan Ibrahim WN, et al. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotox Res. 2011;19(3):452–461. doi: 10.1007/s12640-010-9200-4. http://dx.doi.org/10.1007/s12640-010-9200-4. [DOI] [PubMed] [Google Scholar]
- Pavlidis M, Theodoridi A, Tsalafouta A. Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;60:121–131. doi: 10.1016/j.pnpbp.2015.02.014. http://dx.doi.org/10.1016/j.pnpbp.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Popovic M, Zaja R, Fent K, Smital T. Interaction of environmental contaminants with zebrafish organic anion transporting polypeptide, Oatp1d1 (Slco1d1) Toxicol Appl Pharmacol. 2014;280(1):149–158. doi: 10.1016/j.taap.2014.07.015. http://dx.doi.org/10.1016/j.taap.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Post GB, Louis JB, Lippincott RL, Procopio NA. Occurrence of perfluorinated compounds in raw water from New Jersey public drinking water systems. Environ Sci Technol. 2013;47(23):13266–13275. doi: 10.1021/es402884x. http://dx.doi.org/10.1021/es402884x. [DOI] [PubMed] [Google Scholar]
- Reference Genome Group of the Gene Ontology C. The Gene Ontology’s Reference Genome Project: a unified framework for functional annotation across species. PLoS Comput Biol. 2009;5(7):e1000431. doi: 10.1371/journal.pcbi.1000431. http://dx.doi.org/10.1371/journal.pcbi.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner R. Growing concern over perfluorinated chemicals. Environ Sci Technol. 2001;35(7):154A–160A. doi: 10.1021/es012317k. [DOI] [PubMed] [Google Scholar]
- Sharma S, Coombs S, Patton P, Burt de Perera T. The function of wall-following behaviors in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195(3):225–240. doi: 10.1007/s00359-008-0400-9. http://dx.doi.org/10.1007/s00359-008-0400-9. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61(1):59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Spulber S, Kilian P, Wan Ibrahim WN, et al. PFOS induces behavioral alterations, including spontaneous hyperactivity that is corrected by dexamfetamine in zebrafish larvae. PLoS One. 2014;9(4):e94227. doi: 10.1371/journal.pone.0094227. http://dx.doi.org/10.1371/journal.pone.0094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson JF, Whitlock KE, Partridge JC. Zebrafish preference for light or dark is dependent on ambient light levels and olfactory stimulation. Zebrafish. 2011;8(1):17–22. doi: 10.1089/zeb.2010.0671. http://dx.doi.org/10.1089/zeb.2010.0671. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31(4):959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Walz N, Muhlberger A, Pauli P. A human open field test reveals thigmotaxis related to agoraphobic fear. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.12.016. http://dx.doi.org/10.1016/j.biopsych.2015.12.016. [DOI] [PubMed]
- Xing C, Gong B, Xue Y, et al. TGFbeta1a regulates zebrafish posterior lateral line formation via Smad5 mediated pathway. J Mol Cell Biol. 2015;7(1):48–61. doi: 10.1093/jmcb/mjv004. http://dx.doi.org/10.1093/jmcb/mjv004. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91(4):267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- Yang Q, Abedi-Valugerdi M, Xie Y, et al. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int Immunopharmacol. 2002;2(2–3):389–397. doi: 10.1016/s1567-5769(01)00164-3. [DOI] [PubMed] [Google Scholar]


