Abstract
Rotavirus vaccines have demonstrated significant impact in reducing the burden of morbidity and mortality from childhood diarrhoea in countries that have implemented routine vaccination to date. Despite this success, in many countries, rotavirus vaccine coverage remains lower than that of other routine childhood vaccines. Several issues may potentially affect vaccine uptake, namely safety concerns related to intussusception with consequent age restrictions on rotavirus vaccination, contamination with porcine circovirus, vaccine-derived reassortant strains and hospitalization in newborn nurseries at time of administration of live oral rotavirus vaccine. In addition to these safety concerns, other factors may also affect uptake, including lower vaccine efficacy in the developing world, potential emergence of strains escaping from vaccine protection resulting in lower overall impact of a vaccination programme and sustainable vaccine financing. Although further work is needed to address some of these concerns, global policy bodies have reaffirmed that the benefits of rotavirus vaccination outweigh the risks, and vaccine use is recommended globally.
Keywords: Diarrheal disease, Immunization safety, Rotavirus vaccine, Vaccine implementation, Vaccine preventable diseases
Introduction
Rotavirus infection is a major contributor to severe childhood diarrhoea, causing significant morbidity and mortality, with nearly 200 000 deaths among children younger than 5 years of age attributable to rotavirus in 2011 [1–4]. The majority of rotavirus deaths occur in the developing world, with several countries in sub-Saharan Africa reporting rotavirus-specific death rates of over 100 per 100 000 children [4]. While mortality from rotavirus is uncommon in developed settings, the burden of severe morbidity is substantial. In the United States, before vaccine introduction, an estimated 55 000–70 000 hospital admissions for severe rotavirus gastroenteritis occurred each year in children under 5 years of age, with 20 to 60 deaths [5].
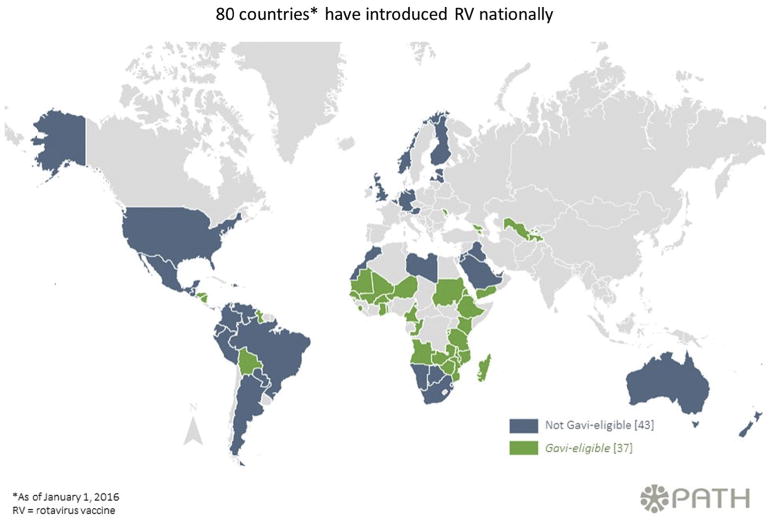
In 2006, two live, attenuated rotavirus (RV) vaccines, a pentavalent (RV5; RotaTeq; Merck and Co.) and a monovalent (RV1 Rotarix; GSK Biologicals) formulation, demonstrated 85–98% efficacy against severe rotavirus gastroenteritis in large clinical trials conducted in the Americas and Europe [6,7]. These vaccines were subsequently recommended and licensed for routine use in the United States [5] and worldwide, with an emphasis on countries where mortality from diarrhoeal deaths was ≥10% among children under 5 years of age [8]. As of January 2016, these vaccines are being used in the national immunization programs of 80 countries [9](http://sites.path.org/rotavirusvaccine/files/2015/12/PATH-Worldwide-Rotavirus-Vaccine-Introduction-Map-EN-2016.01.01_WHO.jpg) (Fig. 1).
Fig. 1.
Rotavirus introduction worldwide, PATH, as of January 2016.
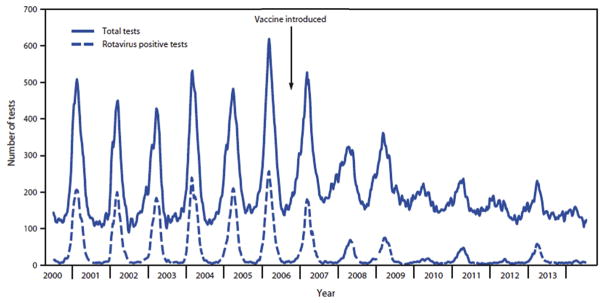
The burden of rotavirus has significantly decreased in many countries that have adopted routine rotavirus vaccination. Notably, reductions in diarrhoea deaths have been reported after rotavirus vaccine introduction in Brazil, Panama and Mexico [9]. In Brazil, rotavirus vaccine coverage reached 90% for the first dose of RV1 and 77% for the second dose among infants by 2008. That same year, the gastroenteritis mortality rate among children less than 1 year of age decreased from 57 per 100 000 in 2004–2005 to 35 per 100 000, representing a relative reduction of 39% (95% confidence interval (CI), 29–49) [10]. In Mexico vaccine coverage reached 74% for the first dose of RV1 and 51% for the second dose among infants before the 2008 rotavirus season; during 2008, diarrhoea-associated mortality among infants 11 months of age or younger declined by 41% (95% CI, 36–47) to 36 per 100 000 compared to 62 per 100 000 during the prevaccine years from 2003 to 2006 [11]; this reduction was sustained for 4 years [12,13]. Similarly, in Panama, where vaccine coverage reached 71% in 2008, gastroenteritis-related mortality rate for children under 1 year of age decreased from 73 deaths per 100 000 in the 2000–2005 prevaccine period to 40 per 100 000 in 2008, representing a 45% decrease [14]. In addition to these remarkable mortality benefits, reductions of 17–55% in hospitalizations for all-cause diarrhoea and of 49–92% in hospitalizations for rotavirus-specific diarrhoea have been reported in the United States, Europe, Australia, Latin America and Africa [9,15–17]. Since vaccine introduction in the United States in 2006, rotavirus seasons have been delayed in onset, of shorter duration and of diminished magnitude (Fig. 2) [18,19].
Fig. 2.
Total and positive rotavirus tests, National Respiratory and Enteric Virus Surveillance System data, United States, 2000–2014 [19].
Despite the remarkable overall impact of rotavirus vaccination, vaccines have not been universally adopted around the world; notably, no country in Asia has adopted a routine nationwide rotavirus vaccination programme to date. In addition, in many countries that have adopted vaccination, coverage of rotavirus vaccines remains lower than that of other established childhood vaccines (http://www.who.int/mediacentre/factsheets/fs378/en/) [20]. Here we discuss some of the potential barriers that might be affecting uptake and use of rotavirus vaccines.
Safety Concerns
Intussusception
Intussusception is a condition where one part of the intestine telescopes into an adjacent section, creating blockage and decreased circulation. If not corrected, it can lead to necrosis and perforation of the bowel and death. Mortality among young children hospitalized for intussusception is primarily related to suboptimal or delayed access to health care, and it ranges from as little as 0.1% in developed settings such as the United States and Europe to as high as 10–35% in some countries of Africa [21].
The first commercially available rotavirus vaccine, the rhesus rotavirus reassortant tetravalent vaccine (RRV-TV; Rotashield; Wyeth Vaccines), was introduced in 1998 in the US market and was recommended by the US Advisory Committee on Immunization Practices (ACIP) to be provided to all US infants as a three-dose schedule given at 2, 4 and 6 months of age. Shortly after vaccine introduction, an increase in reports of intussusception among infants given RRV-TV was noted through the national Vaccine Adverse Event Reporting System [22]. This led to a temporary suspension of vaccine administration, and formal studies were launched to examine the association between RRV-TV and intussusception. Data from these studies confirmed an increased risk of intussusception with RRV-TV, with an almost 30-fold elevated risk of developing intussusception during the first 3 to 7 days after the first dose of RRV-TV [23]. An expert group estimated that the population attributable risk of intussusception was ~1 excess case per 10 000 recipients of RRV-TV [24]. This level of risk was deemed unacceptable for further vaccine use; the ACIP withdrew its recommendation [25], and the manufacturer withdrew RRV-TV from the US market in 1999.
Both RV5 and RV1 have undergone close scrutiny for intussusception risk as a result of the RRV-TV experience. In large pre-licensure clinical trials which included over 60 000 participants each, neither vaccine demonstrated an increased risk of intussusception [6,7]. The RV5 trial reported a relative risk of intussusception within 42 days after any of the three vaccine doses of 1.6 (95% CI, 0.4–6.4) [6] while the RV1 trial reported a relative risk of 0.85 (95% CI, 0.3–2.42) for the period within 30 days of any of the two doses [7]. Given these reassuring data, the World Health Organization (WHO) recommended rotavirus vaccines for global use, noting that further postlicensure monitoring should continue to further examine any possible risk of intussusception.
Postlicensure studies have been conducted in Australia, Brazil, Mexico and the United States to specifically evaluate intussusception risk with use of either rotavirus vaccine [26]. Case series analyses from Australia, where both RV1 and RV5 are available, detected a low level risk in the first 21 days after dose 1 and the first 7 days after dose 2 for both vaccines, with an estimated excess of about 4.3 intussusception cases per 100 000 RV1-vaccinated infants and about 7 intussusception cases per 100 000 RV5-vaccinated recipients [27]. Data using both case series and case–control analyses showed an incidence ratio of 5.3 (95% CI, 3.0, 9.3) and an odds ratio of 5.8 (95% CI, 2.6, 13.0), respectively, during 1 to 7 days after the first dose of RV1 in Mexico and an incidence ratio of 2.6 (95% CI, 1.3, 5.2) and an odds ratio of 1.9 (95% CI, 1.1, 3.4), respectively, in Brazil after the second RV1 dose [28]. These risk figures translated into an excess of one to two cases of intussusception per 100 000 vaccinated children. In the United States, one analysis of administrative data found that RV5 was associated with 1.1 excess cases of intussusception per 100 000 recipients in the 7 days after the first dose and 1.5 cases of intussusception per 100 000 recipients in the 21 days after the first dose [29]. The same study noted an elevated risk after the second dose of RV1, but this analysis was underpowered. Another administrative database analysis of cohort data from six health care organizations found an increased risk of 5.3 cases of intussusception per 100 000 vaccinated with RV1 [30].
Taken together, these data from various settings show an increased risk of intussusception with both RV5 and RV1, with about one to six excess cases per 100 000 vaccinated infants. The WHO and other policy bodies have reviewed these data in the context of the large health benefits of vaccination that have been seen in many countries, and they have reaffirmed the decision to recommend rotavirus vaccination, in view of the benefits greatly exceeding the risks.
Age restriction of rotavirus vaccine schedule
Naturally occurring intussusception is uncommon in the first 2 months of life, after which incidence increases rapidly to peak at 6 months of age and thereafter declines steadily [31]. With the withdrawn RRV-TV vaccine, for which catch-up vaccination was allowed up to 6 months of age with the first dose, some data suggested that the risk of intussusception occurring after receipt of the first dose of vaccine was associated with older age at vaccination, with infants aged 90 days or older accounting for 80% of reported cases of intussusception although they only received 20% of vaccine doses [32]. Furthermore, no cases of intussusception were noted among infants vaccinated at less than 60 days of age. Although it is still debated whether the relative risk of intussusception differed according to the age at which the first vaccine dose was administered [33–35], the absolute risk would undoubtedly be greater at older ages, given the higher baseline rate of intussusception. Given these considerations, an upper age limit of 15 weeks for the first dose of RV5 and RV1 was recommended when they were introduced into national immunization programs [36].
In developed countries, the strict age restriction of 15 weeks for receipt of the first dose of rotavirus vaccination is estimated to reduce the coverage of rotavirus vaccine by 5–10% compared to other childhood vaccines based on data on timing of routine childhood vaccinations [37,38]. However, in developing countries, where delays in the timing of childhood vaccination are far more common, these age restrictions could substantially affect vaccine coverage by as much as 30–40% in some areas. A scenario analysis examined the benefits of rotavirus vaccination on mortality reduction from gastroenteritis versus the risk of fatal intussusception in 158 low- and middle-income countries when vaccine was provided in an age-restricted compared to an unrestricted schedule. This analysis found that lifting age restrictions would prevent 47 200 (18 700–63 700) more gastroenteritis deaths than an age-restricted schedule, while resulting in 294 (161–471) extra intussusception deaths [39]. Given these favorable benefit–risk data, in 2012 the WHO recommended that age restrictions on rotavirus vaccination in developing countries be relaxed, noting that vaccination should be as timely as possible to ensure maximum benefit [36]. Specifically, while WHO still recommends that the first dose of rotavirus vaccine be given as soon as possible after 6 weeks of age, in settings where the mortality burden from rotavirus acute gastroenteritis is high, the 15-week age limit has been removed, and rotavirus vaccine is recommended to be administered orally alongside doses of diphtheria, pertussis and tetanus (DTP: DTP1 and DTP2 for RV1 and DTP1, DTP2 and DTP3 for RV5) with at least 4 weeks between doses, up to 24 months of age [40]. While several low-income countries have recommended rotavirus vaccination without age restriction per the 2012 WHO recommendations, others continue to adhere to the age restrictions. In addition, most high-income countries still recommend vaccination with age restrictions [5,41].
Vulnerable populations
In a study using data from an active surveillance system, rotavirus vaccine coverage was compared to rotavirus prevalence rates in hospitalized children in an urban setting in the United States [42]. Researchers found that physician practices with lowest vaccine coverage (defined as <40%) had the highest prevalence of rotavirus. Of note, one of the low coverage locations was a neonatal intensive care unit (NICU). NICU patients may miss opportunities to receive rotavirus vaccine in the usual outpatient setting if they are hospitalized for prolonged periods to an age beyond 15 weeks, as they may become age ineligible to start the rotavirus vaccine series. Vaccination of these infants while they are still in the NICU has raised concerns about shedding and transmission of vaccine-derived virus to other infants in the NICU. Studies from the United States and Canada examining hospital administration of rotavirus vaccines for infants admitted in the NICU have shown that vaccine does not cause definite rotavirus-attributable symptomatology after vaccination; nor does it lead to increased rates of symptomatic nosocomial rotavirus after vaccination [43,44]. However, practices regarding rotavirus vaccination of infants while they are still in the NICU show substantial variation.
Porcine circovirus contamination
In 2010, contamination with porcine circovirus (PCV) was identified in both RV5 and RV1. PCV is a single-stranded DNA virus that infects pigs but has not been reported to cause disease in humans. RV1 was first found to contain full-length PCV1 genomes [45], and RV5 was later found to contain PCV1 and PCV2 genome fragments [46]. The US Food and Drug Administration (FDA) temporarily suspended the use of RV1 in 2010 (http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm205540). Data suggested that porcine trypsin, a reagent commonly used in the manufacture of biologicals (http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500162147.pdf), was likely the source of the detected PCV1 DNA [47]. After further review of data, an expert FDA advisory committee deemed it safe to resume use of RV1 and continue using RV5 (http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm212140, http://www.who.int/immunization/newsroom/news_rotavirus_vaccine_use/en/index.html), a position that was endorsed by the European regulatory agencies and the WHO.
Despite the recommendation to resume use of rotavirus vaccine, porcine circovirus contamination impacted vaccine integration into national immunization schedules. In France and Spain introduction of vaccine was hindered because of these safety concerns (http://venice.cineca.org/Venice2_WP3_Report_December2010.pdf) [48]. In Spain, although rotavirus vaccine had not been integrated, its use had been supported by the paediatric vaccine advisory committee since 2008, and coverage had reached 40% by 2010. Because of PCV1 contamination, the use of the vaccine was suspended for several months in 2010 in Spain, and the effects of this suspension on disease burden and healthcare-associated costs were examined [49]. It was estimated that in the 5 months of vaccine distribution suspension, assuming coverage had fallen to 0, 85 450 children were not vaccinated, resulting in an estimated 2904 episodes of gastroenteritis due to rotavirus, 497 hospitalizations, 1444 emergency room visits, 1674 paediatrician visits and a total of €2.2 million in avoidable costs.
Vaccine-derived strains associated with diarrhoea
Rotavirus vaccines contain live attenuated virus strains which replicate in the gastrointestinal tract after administration and have the potential to both shed via stool as well as being able to undergo reassortment with other vaccine strains or wild-type virus. Active acute gastrointestinal surveillance in the United States has identified both vaccine-derived strains and reassortant strains circulating in the community. One case report of horizontal transmission of vaccine virus documented the isolation of RV5-derived strains from a child evaluated in the emergency department whose only exposure to vaccine had been via a sibling who had recently been immunized with RV5 [50]. Another case series found that in 106 rotavirus-positive stool specimens from patients with severe gastroenteritis, five were vaccine-reassortant or vaccine-derived strains [51]. Four of these were related to RV5 vaccine, including three patients who had been recently vaccinated and were shedding either the RV5 vaccine strain or a double reassortant derived from two of the five rotavirus vaccine reassortant strains in RV5. The contribution of these identified strains to the patients' illness was not clear cut, as two were coinfected with other pathogens and two had prominent respiratory symptoms. An Australian study examined 61 children who had recently received RV5 and subsequently developed gastroenteritis [52]. Thirteen of these infants had vaccine-derived rotavirus strains, and four of these demonstrated reassortment. These researchers also tested 460 faecal samples from the Australian severe gastroenteritis surveillance programs and found three vaccine-derived strains, two of which demonstrated reassortment. Finally, a case series from Finland also reported the presence of double-reassortant rotavirus in three symptomatic infants shortly after receiving RV5, as well as detection of vaccine strain in one infant [53]. Although these cases of severe gastroenteritis potentially related to vaccine-derived rotavirus strains are of concern, the overall incidence of such events appears to be small (estimated at 1 in 140 000 vaccinees in one study), and the risk is outweighed by the overwhelming positive impact of rotavirus vaccinations.
Other Factors
Lower vaccine efficacy in the developing world
Compared to the 85–98% vaccine efficacy against severe rotavirus gastroenteritis noted in key clinical trials in Latin America, the United States and Europe [6,7], studies from Africa and Asia have reported lower efficacy. In Africa a clinical trial of RV5 conducted in Ghana, Kenya and Mali found an efficacy of 64% in the first year of life [54], while a trial of RV1 in South Africa and Malawi found an overall efficacy of 62% against severe rotavirus gastroenteritis in the first year of life [55]. In Asia RV5 was studied in Vietnam and Bangladesh and was found to be 51% efficacious [56]. Studies of RV1 in routine use in African settings have shown similar figures. When evaluated in routine programmatic use after licensure, RV1 was found to be 40–64% effective in South Africa and Malawi [57,58].
Reasons for lower effectiveness and efficacy compared to more developed settings are multifactorial, involving issues related to vaccine uptake and infants' ability to mount an immunoresponse. The presence of transplacental preexisting circulating antibody in the infant has been shown to decrease the infants' immunoresponse to vaccine [59,60]. Breast-feeding was postulated to be one of the factors that may diminish immunoresponse to the vaccine based on in vitro studies [61], but a series of randomized clinical trials from South Africa, Pakistan and India did not show a difference in vaccine seroconversion among infants with unlimited breast-feeding compared to those whose breast-feeding was withheld around the time of vaccination [62–64]. Additionally, unsanitary living conditions, chronic malnutrition and the presence of coinfections also likely play a role in infants' immunoresponse to rotavirus vaccine, with a recent study from Bangladesh reporting rotavirus vaccine failing in 69% of infants who had received it as a result of a combination of these factors [65]. Finally, coadministration of rotavirus vaccine alongside oral poliovirus, while not having effect on the immunogenicity to oral poliovirus, has been shown to reduce the antibody response to rotavirus vaccine [66].
Despite the lower efficacy, however, the public health impact of vaccination will be substantial in low-income setting because of the tremendous burden of diarrhoeal disease. This is well illustrated by data from the rotavirus vaccine trial in South Africa and Malawi. In this trial, while vaccine efficacy was poorer in Malawi (49%) than in South Africa (77%), vaccination of 100 infants prevented 6.7 cases of severe rotavirus gastroenteritis in Malawi versus 4.2 in South Africa, the result of the higher baseline incidence in Malawi compared to South Africa. Indeed, it was this consideration of the large public health impact of vaccination that drove the WHO decision in 2009 to recommend rotavirus vaccination for all countries globally.
As these vaccines are increasingly in use in high-burden, developing countries, current research efforts are also underway to improve vaccine performance in these settings. Studies to determine the benefit of adding a third doses of RV1 are underway and have shown mixed results. A randomized controlled study in Ghana demonstrated that infants receiving a third dose of RV1 had increased seroconversion and mean titres of antirotavirus IgA compared to infants receiving the standard 2 doses [67], whereas a randomized study in Pakistan showed no such increase using the same dosing schedule [68]. In Bangladesh a 9-month booster dose of rotavirus vaccine, provided alongside the routine dose of measles–rubella vaccine, was shown to increase antirotavirus antibodies compared to infants who received measles–rubella vaccine alone [69]. Further studies will help determine the utility of booster dosing. In addition to these studies, identifying more suitable markers as correlates of protection for rotavirus vaccines is also underway [70].
Potential emergence of rotavirus strains escaping vaccine immunity
The rotavirus genome contains 11 RNA segments, two of which encode the outer capsid proteins VP7 and VP4 (G and P protein, respectively) and form the basis of binary classification (G and P type) of rotavirus [71]. Though reassortment among segments of the genome is common, before vaccine introduction, the majority of strains contained five combinations of G and P proteins: G1P [8], G2P [4], G3P [8], G4P [8] and G9P [8] [72,73]. The two rotavirus vaccine differ in composition; RV5 contains five bovine rotavirus strains that each express a human surface antigen of G1–4 and P1A [8] specificity, while RV1 contains a single human-derived G1P [8] strain. Concern for selective pressure arose in some countries after vaccine implementation, particularly with observed dominance of G2P [4] strains in some settings with RV1 use—a strain with both different G and P type than the vaccine strain [74–77]. However, many of the strain changes observed in the initial years after vaccine implementation were not sustained over time [78] and thus may be related to natural secular variation rather than vaccine-induced selection pressure [79,80]. Furthermore, a recent meta-analysis that examined published genotype data from high- and middle-income countries using either or both RV1 and RV5 [81] found that both vaccines exerted similar effectiveness against a range of rotavirus strains, whether heterotypic or homotypic to the vaccine strain or strains, and no sustained dominance of a particular strain was seen. Although these data are reassuring, further surveillance is warranted, particularly in low-income settings, to further assess any emergence of strains not protected by vaccine.
Sustainable vaccine financing
Financing remains problematic in both developed and developing settings. In Europe, where rotavirus vaccination has not been adopted by all countries, a member-state survey in 2010 identified at least two countries that had decided not to incorporate rotavirus vaccine into their immunization schedules, citing high vaccine cost as a factor (http://venice.cineca.org/Venice2_WP3_Report_December2010.pdf). As an alternative, some groups have advocated for vaccinating high-risk infants only in these settings, to offset costs associated with universally immunizing all infants [82]. Government resources were used for acquiring vaccine in almost all of the early adopting Latin American countries, though insufficient funds were cited as posing constraints in some of these countries [83]. The Global Alliance for Vaccines and Immunization (GAVI) provides financial support for the introduction of rotavirus vaccine, providing subsidies for countries with average gross national income per capital over the last 3 years equal to or less than US$1580 and with WHO/UNICEF vaccine coverage estimates ≥70% (http://www.gavi.org/support/apply/). This has greatly facilitated vaccine introduction in eligible countries, with 37 countries having introduced rotavirus vaccine with support through GAVI as of January 2016 (Fig. 1) (http://sites.path.org/rotavirusvaccine/files/2015/12/PATH-Worldwide-Rotavirus-Vaccine-Introduction-Map-EN-2016.01.01_WHO.jpg). Several of these countries (e.g. Angola, Kenya) have entered GAVI's accelerated transition phase by 2016, where they will receive support to transition to fully financing their own immunization programme over the course of several years (http://www.gavi.org/support/apply/graduating-countries/).
Conclusions
The issues noted in this review highlight the need for ongoing surveillance and targeted research as rotavirus vaccines are increasingly introduced into national immunization programs and as GAVI-assisted countries transition to fully financing their own immunization programs (Table 1). Overall, the many examples of the positive impact of vaccination on morbidity and mortality from rotavirus diarrhoeal disease warrants continued use of these vaccines as these issues are addressed.
Table 1.
Factors influencing uptake of rotavirus vaccines
| Concern | Issue | Major findings | Reference |
|---|---|---|---|
| Safety | Intussusception |
|
[21–30] |
| Age restriction of 15 weeks (dose 1) in vaccine schedule |
|
[31–41] | |
| NICU patients |
|
[42–44] | |
| Porcine circovirus |
|
[45–49], http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm205540, http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/02/WC500162147.pdf, http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm212140, http://www.who.int/immunization/newsroom/news_rotavirus_vaccine_use/en/index.html, http://venice.cineca.org/Venice2_WP3_Report_December2010.pdf | |
| Vaccine-derived strains |
|
[50–52] | |
| Other | Lower efficacy in developing countries |
|
[54–70] |
| Emergence of strains escaping vaccine immunity |
|
[74–81] | |
| Financing |
|
[84–87], http://venice.cineca.org/Venice2_WP3_Report_December2010.pdf |
AGE, acute gastroenteritis; FDA, US Food and Drug Administration; GAVI, Global Alliance for Vaccines and Immunization; NICU, neonatal intensive care unit; PCV, porcine circovirus; RV, rotavirus; WHO, World Health Organization
Footnotes
Transparency Declaration
All authors report no conflicts of interest relevant to this article. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 2.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–72. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD World Health Organization–Coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–105. doi: 10.1093/cid/civ1013. [DOI] [PubMed] [Google Scholar]
- 5.Cortese MM, Parashar UD Centers for Disease Control and Prevention. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58(RR-2):1–25. [PubMed] [Google Scholar]
- 6.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 8.Rotavirus vaccines: an update. Wkly Epidemiol Rec. 2009;84:533–40. [PubMed] [Google Scholar]
- 9.Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clin Infect Dis. 2014;59:1291–301. doi: 10.1093/cid/ciu564. [DOI] [PubMed] [Google Scholar]
- 10.Lanzieri TM, Linhares AC, Costa I, Kolhe DA, Cunha MH, Ortega-Barria E, et al. Impact of rotavirus vaccination on childhood deaths from diarrhea in Brazil. Int J Infect Dis. 2011;15:e206–10. doi: 10.1016/j.ijid.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362:299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 12.Richardson V, Parashar U, Patel M. Childhood diarrhea deaths after rotavirus vaccination in Mexico. N Engl J Med. 2011;365:772–3. doi: 10.1056/NEJMc1100062. [DOI] [PubMed] [Google Scholar]
- 13.Gastanaduy PA, Sanchez-Uribe E, Esparza-Aguilar M, Desai R, Parashar UD, Patel M, et al. Effect of rotavirus vaccine on diarrhea mortality in different socioeconomic regions of Mexico. Pediatrics. 2013;131:e1115–20. doi: 10.1542/peds.2012-2797. [DOI] [PubMed] [Google Scholar]
- 14.Bayard V, DeAntonio R, Contreras R, Tinajero O, Castrejon MM, Ortega-Barria E, et al. Impact of rotavirus vaccination on childhood gastroenteritis-related mortality and hospital discharges in Panama. Int J Infect Dis. 2012;16:e94–8. doi: 10.1016/j.ijid.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011;30(1 Suppl):S1–5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- 16.Rha B, Tate JE, Payne DC, Cortese MM, Lopman BA, Curns AT, et al. Effectiveness and impact of rotavirus vaccines in the United States–2006–2012. Expert Rev Vaccines. 2014;13:365–76. doi: 10.1586/14760584.2014.877846. [DOI] [PubMed] [Google Scholar]
- 17.Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis. 2010;201:1617–24. doi: 10.1086/652403. [DOI] [PubMed] [Google Scholar]
- 18.Tate JE, Haynes A, Payne DC, Cortese MM, Lopman BA, Patel MM, et al. Trends in national rotavirus activity before and after introduction of rotavirus vaccine into the national immunization program in the United States, 2000 to 2012. Pediatr Infect Dis J. 2013;32:741–4. doi: 10.1097/INF.0b013e31828d639c. [DOI] [PubMed] [Google Scholar]
- 19.Aliabadi N, Tate JE, Haynes AK, Parashar UD Centers for Disease Control and Prevention. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination-United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2015;64:337–42. [PMC free article] [PubMed] [Google Scholar]
- 20.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, state, and selected local area vaccination coverage among children aged 19–35 months–United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:889–96. doi: 10.15585/mmwr.mm6433a1. [DOI] [PubMed] [Google Scholar]
- 21.Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS One. 2013;8:e68482. doi: 10.1371/journal.pone.0068482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine–United States, 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48:577–81. [PubMed] [Google Scholar]
- 23.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–72. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 24.Peter G, Myers MG National Vaccine Advisory Committee, National Vaccine Program Office. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics. 2002;110:e67. doi: 10.1542/peds.110.6.e67. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48:1007. [PubMed] [Google Scholar]
- 26.Buttery JP, Standish J, Bines JE. Intussusception and rotavirus vaccines: consensus on benefits outweighing recognized risk. Pediatr Infect Dis J. 2014;33:772–3. doi: 10.1097/INF.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 27.Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s National Immunization Program. Clin Infect Dis. 2013;57:1427–34. doi: 10.1093/cid/cit520. [DOI] [PubMed] [Google Scholar]
- 28.Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–92. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 29.Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in US infants. N Engl J Med. 2014;370:503–12. doi: 10.1056/NEJMoa1303164. [DOI] [PubMed] [Google Scholar]
- 30.Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014;370:513–9. doi: 10.1056/NEJMoa1311738. [DOI] [PubMed] [Google Scholar]
- 31.Tai JH, Curns AT, Parashar UD, Bresee JS, Glass RI. Rotavirus vaccination and intussusception: can we decrease temporally associated background cases of intussusception by restricting the vaccination schedule? Pediatrics. 2006;118:e258–64. doi: 10.1542/peds.2005-2874. [DOI] [PubMed] [Google Scholar]
- 32.Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on Rota-Shield and intussusception: the role of age at the time of vaccination. J Infect Dis. 2005;192(Suppl 1):S36–43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- 33.Rothman KJ, Young-Xu Y, Arellano F. Age dependence of the relation between reassortant rotavirus vaccine (RotaShield) and intussusception. J Infect Dis. 2006;193:898. doi: 10.1086/500217. [DOI] [PubMed] [Google Scholar]
- 34.Gargiullo PM, Murphy TV, Davis RL. Is there a safe age for vaccinating infants with tetravalent rhesus–human reassortant rotavirus vaccine? J Infect Dis. 2006;194:1793–4. doi: 10.1086/509264. [DOI] [PubMed] [Google Scholar]
- 35.Gargiullo PM, Murphy TV, Davis RL. Reply to Gargiullo et al. Is there a safe age for vaccinating infants with tetravalent rhesus–human reassortant rotavirus vaccine? J Infect Dis. 2006;194:1794–5. doi: 10.1086/509264. [DOI] [PubMed] [Google Scholar]
- 36.Meeting of the Strategic Advisory Group of Experts on immunization, April 2012–conclusions and recommendations. Wkly Epidemiol Rec. 2012;87:201–16. [PubMed] [Google Scholar]
- 37.Hull BP, Menzies R, Macartney K, McIntyre PB. Impact of the introduction of rotavirus vaccine on the timeliness of other scheduled vaccines: the Australian experience. Vaccine. 2013;31:1964–9. doi: 10.1016/j.vaccine.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Parashar UD, Alexander JP, Glass RI Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC) Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-12):1–13. [PubMed] [Google Scholar]
- 39.Patel MM, Clark AD, Sanderson CF, Tate J, Parashar UD. Removing the age restrictions for rotavirus vaccination: a benefit–risk modeling analysis. PLoS Med. 2012;9:e1001330. doi: 10.1371/journal.pmed.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rotavirus vaccines. WHO position paper–January 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- 41.Vesikari T, Van Damme P, Giaquinto C, Dagan R, Guarino A, Szajewska H, et al. European Society for Paediatric Infectious Diseases consensus recommendations for rotavirus vaccination in Europe: update 2014. Pediatr Infect Dis J. 2015;34:635–43. doi: 10.1097/INF.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 42.Sahni LC, Tate JE, Payne DC, Parashar UD, Boom JA. Variation in rotavirus vaccine coverage by provider location and subsequent disease burden. Pediatrics. 2015;135:e432–9. doi: 10.1542/peds.2014-0208. [DOI] [PubMed] [Google Scholar]
- 43.Monk HM, Motsney AJ, Wade KC. Safety of rotavirus vaccine in the NICU. Pediatrics. 2014;133:e1555–60. doi: 10.1542/peds.2013-3504. [DOI] [PubMed] [Google Scholar]
- 44.Thrall S, Doll MK, Nhan C, Gonzales M, Perreault T, Lamer P, et al. Evaluation of pentavalent rotavirus vaccination in neonatal intensive care units. Vaccine. 2015;33:5095–102. doi: 10.1016/j.vaccine.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Victoria JG, Wang C, Jones MS, Jaing C, McLoughlin K, Gardner S, et al. Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J Virol. 2010;84:6033–40. doi: 10.1128/JVI.02690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClenahan SD, Krause PR, Uhlenhaut C. Molecular and infectivity studies of porcine circovirus in vaccines. Vaccine. 2011;29:4745–53. doi: 10.1016/j.vaccine.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 47.Dubin G, Toussaint JF, Cassart JP, Howe B, Boyce D, Friedland L, et al. Investigation of a regulatory agency enquiry into potential porcine circovirus type 1 contamination of the human rotavirus vaccine, Rotarix: approach and outcome. Hum Vaccin Immunother. 2013;9:2398–408. doi: 10.4161/hv.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parez N, Giaquinto C, Du Roure C, Martinon-Torres F, Spoulou V, Van Damme P, et al. Rotavirus vaccination in Europe: drivers and barriers. Lancet Infect Dis. 2014;14:416–25. doi: 10.1016/S1473-3099(14)70035-0. [DOI] [PubMed] [Google Scholar]
- 49.Bouzon Alejandro M, Diez Domingo J, Martinon-Torres F. Circovirus and impact of temporary withdrawal of rotavirus vaccines in Spain. Hum Vaccin. 2011;7:798–9. doi: 10.4161/hv.7.7.15683. [DOI] [PubMed] [Google Scholar]
- 50.Payne DC, Edwards KM, Bowen MD, Keckley E, Peters J, Esona MD, et al. Sibling transmission of vaccine-derived rotavirus (RotaTeq) associated with rotavirus gastroenteritis. Pediatrics. 2010;125:e438–41. doi: 10.1542/peds.2009-1901. [DOI] [PubMed] [Google Scholar]
- 51.Boom JA, Sahni LC, Payne DC, Gautam R, Lyde F, Mijatovic-Rustempasic S, et al. Symptomatic infection and detection of vaccine and vaccine-reassortant rotavirus strains in 5 children: a case series. J Infect Dis. 2012;206:1275–9. doi: 10.1093/infdis/jis490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donato CM, Ch’ng LS, Boniface KF, Crawford NW, Buttery JP, Lyon M, et al. Identification of strains of RotaTeq rotavirus vaccine in infants with gastro-enteritis following routine vaccination. J Infect Dis. 2012;206:377–83. doi: 10.1093/infdis/jis361. [DOI] [PubMed] [Google Scholar]
- 53.Hemming M, Vesikari T. Vaccine-derived human-bovine double reassortant rotavirus in infants with acute gastroenteritis. Pediatr Infect Dis J. 2012;31:992–4. doi: 10.1097/INF.0b013e31825d611e. [DOI] [PubMed] [Google Scholar]
- 54.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 55.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 56.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 57.Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, Kapongo CN, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case–control study. Lancet Infect Dis. 2014;14:1096–104. doi: 10.1016/S1473-3099(14)70940-5. [DOI] [PubMed] [Google Scholar]
- 58.Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, Nakagomi O, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case–control study. Lancet Infect Dis. 2015;15:422–8. doi: 10.1016/S1473-3099(14)71060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan J, Nirwati H, Triasih R, Bogdanovic-Sakran N, Soenarto Y, Hakimi M, et al. Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine. 2011;29:1242–7. doi: 10.1016/j.vaccine.2010.11.087. [DOI] [PubMed] [Google Scholar]
- 60.Appaiahgari MB, Glass R, Singh S, Taneja S, Rongsen-Chandola T, Bhandari N, et al. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine. 2014;32:651–6. doi: 10.1016/j.vaccine.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 61.Moon SS, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, et al. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. 2010;29:919–23. doi: 10.1097/INF.0b013e3181e232ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groome MJ, Moon SS, Velasquez D, Jones S, Koen A, van Niekerk N, et al. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Organ. 2014;92:238–45. doi: 10.2471/BLT.13.128066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine. 2014;32(Suppl 1):A134–9. doi: 10.1016/j.vaccine.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 64.Ali A, Kazi AM, Cortese MM, Fleming JA, Moon S, Parashar UD, et al. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine–a randomized trial. PLoS One. 2015;10:e0127622. doi: 10.1371/journal.pone.0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2:1759–66. doi: 10.1016/j.ebiom.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine. 2012;30(Suppl 1):A30–5. doi: 10.1016/j.vaccine.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 67.Armah G, Lewis KD, Cortese MM, Parashar UD, Ansah A, Gazley L, et al. A randomized controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in rural Ghanaian infants. J Infect Dis. 2016 Jun 1;213(11):1678–85. doi: 10.1093/infdis/jiw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ali SA, Kazi AM, Cortese MM, Fleming JA, Parashar UD, Jiang B, et al. Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. J Infect Dis. 2014;210:1772–9. doi: 10.1093/infdis/jiu335. [DOI] [PubMed] [Google Scholar]
- 69.Zaman K, Fleming JA, Victor JC, Yunus M, Bari TI, Azim T, et al. Non-interference of rotavirus vaccine with measles–rubella vaccine at 9 months and improvements in anti-rotavirus immunity: a randomized trial. J Infect Dis. 2016 Jun 1;213(11):1686–93. doi: 10.1093/infdis/jiw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angel J, Steele AD, Franco MA. Correlates of protection for rotavirus vaccines: possible alternative trial endpoints, opportunities, and challenges. Hum Vaccin Immunother. 2014;10:3659–71. doi: 10.4161/hv.34361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Estes MK, Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989;53:410–49. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192(Suppl 1):S146–59. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- 73.Banyai K, Laszlo B, Duque J, Steele AD, Nelson EA, Gentsch JR, et al. Systematic review of regional and temporal trends in global rotavirus strain diversity in the pre rotavirus vaccine era: insights for understanding the impact of rota-virus vaccination programs. Vaccine. 2012;30(Suppl 1):A122–30. doi: 10.1016/j.vaccine.2011.09.111. [DOI] [PubMed] [Google Scholar]
- 74.Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Gentsch JR, et al. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J. 2011;30(1 Suppl):S42–7. doi: 10.1097/INF.0b013e3181fefd78. [DOI] [PubMed] [Google Scholar]
- 75.Carvalho-Costa FA, Volotao Ede M, de Assis RM, Fialho AM, da de Andrade JS, Rocha LN, et al. Laboratory-based rotavirus surveillance during the introduction of a vaccination program, Brazil, 2005–2009. Pediatr Infect Dis J. 2011;30(1 Suppl):S35–41. doi: 10.1097/INF.0b013e3181fefd5f. [DOI] [PubMed] [Google Scholar]
- 76.Kirkwood CD, Boniface K, Barnes GL, Bishop RF. Distribution of rotavirus genotypes after introduction of rotavirus vaccines, Rotarix(R) and RotaTeq(R), into the National Immunization Program of Australia. Pediatr Infect Dis J. 2011;30(1 Suppl):S48–53. doi: 10.1097/INF.0b013e3181fefd90. [DOI] [PubMed] [Google Scholar]
- 77.Nakagomi T, Cuevas LE, Gurgel RG, Elrokhsi SH, Belkhir YA, Abugalia M, et al. Apparent extinction of non-G2 rotavirus strains from circulation in Recife, Brazil, after the introduction of rotavirus vaccine. Arch Virol. 2008;153:591–3. doi: 10.1007/s00705-007-0028-z. [DOI] [PubMed] [Google Scholar]
- 78.Gurgel RQ, de Alvarez AJ, Rodrigues A, Ribeiro RR, Dolabella SS, Da Mota NL, et al. Incidence of rotavirus and circulating genotypes in Northeast Brazil during 7 years of national rotavirus vaccination. PLoS One. 2014;9:e110217. doi: 10.1371/journal.pone.0110217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guerra SF, Linhares AC, Mascarenhas JD, Oliveira A, Justino MC, Soares LS, et al. Rotavirus strain surveillance for three years following the introduction of rotavirus vaccine into Belem, Brazil. J Med Virol. 2015;87:1303–10. doi: 10.1002/jmv.24183. [DOI] [PubMed] [Google Scholar]
- 80.Patel MM, de Oliveira LH, Bispo AM, Gentsch J, Parashar UD. Rotavirus P[4]G2 in a vaccinated population, Brazil. Emerg Infect Dis. 2008;14:863–5. doi: 10.3201/eid1405.071440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leshem E, Lopman B, Glass R, Gentsch J, Banyai K, Parashar U, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:847–56. doi: 10.1016/S1473-3099(14)70832-1. [DOI] [PubMed] [Google Scholar]
- 82.Bruijning-Verhagen P, Mangen MJ, Felderhof M, Hartwig NG, van Houten M, Winkel L, et al. Targeted rotavirus vaccination of high-risk infants; a low cost and highly cost-effective alternative to universal vaccination. BMC Med. 2013;11:112. doi: 10.1186/1741-7015-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7:345–53. doi: 10.1586/14760584.7.3.345. [DOI] [PubMed] [Google Scholar]
- 84.Bruijning-Verhagen P, Mangen MJ, Felderhof M, Hartwig NG, van Houten M, Winkel L, et al. Targeted rotavirus vaccination of high-risk infants; a low cost and highly cost-effective alternative to universal vaccination. BMC Med. 2013;11:112. doi: 10.1186/1741-7015-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7:345–53. doi: 10.1586/14760584.7.3.345. [DOI] [PubMed] [Google Scholar]
- 86.GAVI. [accessed February 2016]; http://www.gavi.org/support/apply/
- 87.GAVI. [accessed February 2016]; http://www.gavi.org/support/apply/graduating-countries/