Abstract
Background
Left atrium (LA) dilatation has been associated with adverse cardiovascular outcomes in patients with sinus rhythm and atrial fibrillation (AF).
Aim of the study
We aimed to evaluate the accuracy of left atrial (LA) size to predict transesophageal echocardiographic (TEE) markers of increased thromboembolic risk left atrial appendage (LAA) thrombus, low LAA velocities and dense spontaneous echocardiographic contrast (SEC), and also to assess the best method to evaluate LA size.
Patients and methods
Cross-sectional study included 64 patients with nonvalvular AF undergoing transthoracic and transesophageal echocardiographic (TTE and TEE) evaluation. LA size was measured on TTE by several methods including the following: anteroposterior diameter (AP), LA area in four and two apical chamber views and volumes by ellipsoid, single plane (1P) and biplane area-length (2P) formulas. All these measures were indexed to the body surface area (BSA). Thromboembolic markers including LAA thrombus, low LAA velocities, dense SEC and LA abnormality (LA ABN) which means the presence of one or more of the previous three parameters were evaluated by TEE.
Results
There was statistically significant increase in indexed and non-indexed LA parameters in patients with LA ABN compared to patients without LA ABN. According to ROC curve, the study found that all indexed LA parameters were predictive for LAA thrombus with the highest AUC was indexed LA 1P area length volume (AUC 0.91, CI 95% 0.81–1.01, p < 0.000), for LAA low flow velocity were indexed and non-indexed LA AP diameters with the highest AUC was indexed LA AP diameter (AUC 0.89, CI 95% 0.80–0.98, p < 0.000), for LA dense SEC were indexed LA ellipsoid volume (AUC 0.78, CI 95% 0.66–0.96, p = 0.002) and indexed LA 1P area length volume (AUC 0.78, CI 95% 0.66–0.90, p = 0.002) and for LA ABN were all LA parameters with the highest AUC was indexed LA 1P area length volume (AUC 0.87, CI 95% 0.79–0.96, p < 0.000). On multivariate logistic regression analysis of TEE parameters, the study found that the most predictive LA measurement for LAA thrombus was indexed LA AP diameter with cutoff 3 cm/m2 (OR 7.5, 95% CI 1.24–45.2, p = 0.02), for LAA low flow velocity was LA AP diameter with cutoff 6 cm (OR 17.6, 95% CI 3.23–95.84, p = 0.001), for LA dense SEC was indexed LA ellipsoid volume with cutoff 42 cm3/m2 (OR 6.5, 95% CI 1.32–32.07, p = 0.02), and for LA ABN was indexed LA ellipsoid volume with cutoff 42 cm3/m2 (OR 10.45, 95% CI 2.18–51.9, p = 0.008).
Conclusion
LA enlargement is suitable to predict thromboembolic markers in patients with non-valvular AF. The indexed and non-indexed LA AP diameter and indexed LA ellipsoid volume were the most accurate parameters for predicting thromboembolic markers.
Abbreviations: ABN, abnormality; AP, anteroposterior; AF, atrial fibrillation; 2P, biplane; BMI, body mass index; BSA, body surface area; DM, diabetes mellitus; EF, ejection fraction; GFR, glomerular filtration rate; HTN, hypertension; ICD, implantable cardioverter defibrillator; INR, international normalized ratio; LA, left atrium; LAA, left atrial appendage; LV, left ventricle; 1P, single plane; SEC, spontaneous echocardiographic contrast; TEE, transesophageal echocardiography; TIA, transient ischemic attack; TTE, transthoracic echocardiography
Keywords: Nonvalvular atrial fibrillation, Left atrial size, Thromboembolic markers, Transthoracic echocardiography, Transesophageal echocardiography
1. Introduction
Enlargement of left atrium (LA) has been established as a prognostic marker for adverse CV outcomes such as atrial fibrillation,1, 2 stroke,3 congestive heart failure,4 and cardiovascular death.5 Different methods exist for the assessment of LA size. The American Society of Echocardiography recommended LA volume and its indexed value assessed by 2-dimensional echocardiography to measure LA size.6
The pathogenesis of LAA thrombus has not been fully elucidated, but the prediction for its formation in the LAA is likely to result from stagnation within the long, blind ended trabeculated pouch.7 Diminished contractility of the appendage understandably leads to reduction in blood flow as well.8
LAA thrombus is associated with a large LAA area.9 The prevalence of LA/LAA thrombi gradually increases with the number of clinical risk factors.10 The LAA is the site most commonly associated with thrombus formation, particularly in patients with nonvalvular AF.5 Larger LA and LAA sizes are associated with lower LAA flow velocity and risk of ischemic stroke.11, 8
Transesophageal echocardiography (TEE) is sensitive in the assessment of parameters associated with thromboembolism including thrombus in the LA appendage (LAA thrombus),12 dense spontaneous echocardiographic contrast (SEC), low LA appendage flow velocities (low LAA velocities).13, 14 The presence of at least one of the three previous TEE changes has been designated by left atrial abnormality (LA ABN) and is associated with a risk of stroke of 7.8% a year.15
TEE is the most sensitive and specific technique to detect LAA thrombus in patients with AF prior to cardioversion and radiofrequency ablation procedures.16 However, several studies demonstrated that LAA is free of thrombi in ∼86% of AF patients who underwent a TEE prior to cardioversion.17 The cost implications of this practice are particularly important because TEE is an increasingly utilized procedure,18 in addition to the associated risk of complications such as oral and esophageal trauma and the risks of conscious sedation.19 Therefore, there may be a role for risk stratification in patients with AF to determine the need for a TEE to exclude the presence of LAA thrombus prior to cardioversion and radiofrequency ablation procedures.
2. Aim of the study
This study aims to evaluate the accuracy of LA size to predict transesophageal echocardiographic (TEE) markers of increased thromboembolic risk left atrial appendage (LAA) thrombus, low LAA velocities and dense spontaneous echocardiographic contrast (SEC), and also to assess the best method to evaluate LA size.
3. Patients and methods
3.1. Patients
This is a cross-sectional study included 65 patients with nonvalvular atrial fibrillation (AF) admitted to cardiology department or referred to transthoracic and transesophageal echocardiography (TTE and TEE) from outpatient cardiology clinic in Zagazig University Hospitals from November 2014 to April 2015. Exclusion criteria were patients with mitral stenosis, mitral regurgitation (moderate or severe), aortic stenosis (moderate or severe), prosthetic mitral or aortic valves, patients with unsuitable images for accurate assessment of transthoracic echocardiography (TTE) measurements or transesophageal echocardiography (TEE) markers of thromboembolic risk and any contraindication to TEE.
3.2. Methods
3.2.1.
All patients in the study were subjected to the following: complete medical history and physical examination including calculated body surface area (BSA), body mass index (BMI),20 CHADS2 and CHA2DS2-VASc scores.21, 22 Electrocardiogram (ECG) and laboratory examination includes prothrombin time, international normalized ratio (INR) and glomerular filtration rate (GFR).
3.2.1. Transthoracic echocardiography
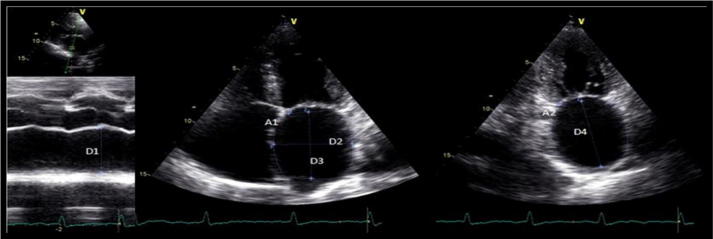
All patients underwent Doppler echocardiographic examination using a commercially available system (GE). Examinations were performed by three trained echo cardiographers according to American society of echocardiography. M-mode and two-dimensional transthoracic images were acquired using a M4S probe (1.5–4.0 MHz), and were used to obtain the following LA measurements: LA anteroposterior diameter (LA AP), left atrium (LA) area, and LA volumes by the ellipsoid, single plane (1P) area length and biplane (2P) area length methods. These measurements were obtained at end-ventricular systole, from the frame immediately preceding mitral valve opening. LA anteroposterior (LA AP) diameter (D1) was measured by M-mode from the parasternal long axis view. LA area was measured using planimetry in (TTE) apical four chamber (A1) and two chamber views (A2) as shown in Fig. 1.
Figure 1.
Echocardiographic parameters used to calculate the left atrial volumes. A1 – LA area in 4C view; A2 – LA area in 2C view D1 – LA AP diameter; D2 – medial–lateral diameter; D3 – superior-inferior diameter in 4C view; D4 – superior-inferior diameter in 2C view. Lang et al.6
LA ellipsoid volume (LAEV) was calculated using AP (D1), medial–lateral (D2) and superior–inferior (D3) LA diameters so LA EV = 4/3π∗(D1/2) ∗ (D2/2) ∗ (D3/2). LA single plane area length volume (LA 1P) was obtained using A1 representing the area and D3 the superior–inferior LA diameter measured from apical four chamber (4C) view so LA 1P = 8/3π∗/D3. Left atrium biplane area-length volume (LA 2P) was obtained using A2 representing the LA area in two chamber view, and L the shortest superior inferior diameter measured in apical four chamber (D3) and two chamber (D4) views using this formula LA 2P = 8/3π∗[(A1) ∗ (A2)/L].6 All these measurements were indexed to body surface area.
3.2.2. Transesophageal echocardiography
TEE images were acquired with a 6 T phased array multiplane TEE probe (2.9–7.0 MHz). The LA and LA appendage (LAA) were imaged in different tomographic planes to detect the presence of LAA thrombus, SEC and LAA flow velocities. LA thrombus was diagnosed by the presence of an echo dense mass in the left atrium or the LAA.23 Spontaneous echocardiographic contrast was diagnosed by the presence of characteristic dynamic smoke-like swirling echoes in the LA or LAA,24 and was classified according to the classification (1 to 4+). Dense SEC was defined as grade 3+ or 4+.25 LAA flow velocities were assessed with a pulsed Doppler sample placed 1 cm from LAA into the body of the LA. Maximum emptying and filling velocities were estimated from an average of five well-defined emptying and filling waves. Patients with maximum emptying and filling velocity ⩽20 cm/s were classified as having low flow velocities.26
4. Study endpoints
The study endpoints were the TEE surrogate markers of thromboembolism as LAA thrombus, LAA low flow velocities and dense SEC. The composite endpoint of LA abnormality was defined by the presence of at least one of the previous markers.
5. Ethics
Informed parental consent was obtained to be eligible for enrollment into the study. The study was done according to the rules of the Local Ethics Committee of Faculty of Medicine, Zagazig University, Egypt.
6. Statistical analysis
All analyses were made using the “SPSS 17 for Windows” software package. Continuous variables were expressed as mean ± standard deviation; categorical variables were expressed as percentages. Independent T-test was used to compare means. Chi-square test was used to compare percentages. ROC curve was plotted to get cutoff value. We used multiple linear regression analysis of TTE parameters to predict thromboembolic risk. A p value of <0.05 means significant and p < 0.001 means highly significant.
7. Results
Basic characteristics of the study population are summarized in Table 1 which shows that the mean age of the studied group was 67.03 ± 5.9 years. There was higher prevalence of males (62.5%). The average CHA2DS2-VASc scores was 3.66 ± 1.2. The INR ranged from 1 to 2.7 with mean 1.69 ± 0.57. We found that 43.7% of the studied group had AF ⩽ one week (paroxysmal AF), 26.6% had AF for more than 1 week to one year (persistent AF), 29.7% had AF for more than one year (long standing persistent AF), 46.9% of patients were on antiplatelet, 40.6% were on oral anticoagulants and 31.1% were on enoxaparine.
Table 1.
Basic characteristics of the study population.
| Demographic data | N (64) |
|---|---|
| Age:(years) mean ± SD | |
| Sex: no (%) | 67.03 ± 5.9 |
| Female | 24 (37.5%) |
| Male | 40 (62.5%) |
| Body mass index (kg/m2): mean ± SD | 29.83 ± 4.41 |
| Body surface area (m2): mean ± SD | 1.88 ± 0.18 |
| Clinical data: no (%) | |
| Smoking | 26 (40.6%) |
| Hypertension | 54 (84.3%) |
| Diabetes | 18 (28.1%) |
| Previous stroke/TIA | 10 (15.6%) |
| Congestive heart failure | 34 (53.1%) |
| Vascular disease | 34 (53.1%) |
| Pacemaker or ICD | 8 (12.5%) |
| CHA2DS2-VASc (mean ± SD) | 3.66 ± 1.2 |
| Laboratory data (mean ± SD) | |
| Hemoglobin (g/dl) | 13.05 ± 1.46 |
| Platelet (103/μL) | 232.56 ± 51.85 |
| INR | 1.69 ± 0.57 |
| GFR (ml/min/1.73 m2) | 66.77 ± 17.51 |
| AF duration | |
| ⩽48 h | 15 (23.4%) |
| >48 h–1 week | 13 (20.3%) |
| >1 week–1 year | 17 (26.6%) |
| >1 year | 19 (29.7%) |
| Antithrombotic treatment | |
| Oral anticoagulation | 26 (40.6%) |
| Antiplatelet agents | 30 (46.9%) |
| Enoxaparine | 20 (31.1%) |
GFR: glomerular filtration rate, ICD: implantable cardioverter defibrillator, INR: international normalized ratio. TIA: transient ischemic attack. Vascular disease is defined as having at least one of the following: myocardial infarctions, peripheral artery disease, and complex aortic plaque.
Echocardiographic findings of the study population are shown in Table 2: TEE examinations identified 8 patients (12.5%) had LAA thrombus, 10 patients (15.6%) had LAA low flow velocities, 14 patients (21.9%) had dense SEC, and 26 patients (40.6%) had LA ABN.
Table 2.
Echocardiographic findings of the study population.
| Echocardiographic finding | (n = 64) |
|---|---|
| TTE (mean ± SD) | |
| LA AP diameter (cm) | 5.51 ± 1.1 |
| LA area (cm2) | 26.3 ± 6.47 |
| LA ellipsoid volume (cm3) | 78.53 ± 16.15 |
| LA 1P area-length volume (cm3) | 131.26 ± 27.04 |
| LA 2P area-length volume (cm3) | 111.64 ± 25.89 |
| LV ejection fraction (%) | 47.63 ± 11.66 |
| TEE (no & %) | |
| LAA thrombus | 8 (12.5%) |
| LAA low flow velocities | 10 (15.6%) |
| Dense SEC | 14 (21.9%) |
| LA ABN | 26 (40.6%) |
Comparison of baseline characteristics of the study population according to the presence of left atrium abnormalities (LA ABN) is summarized in Table 3: patients with LA ABN had statistically significantly higher body surface area (1.93 ± 0.16 vs 1.81 ± 0.19, p = 0.008), CHADS2 score (2.16 ± 1.1 vs 1.77 ± 0.82 p = 0.003), CHADS–VASC score (3.79 ± 1.34 vs 3.46 ± 0.95, p = 0.002) and significantly lower INR (1.49 ± 0.42 vs 1.83 ± 0.62, p = 0.02) than patients without LA ABN respectively. Also Patients with LA ABN had longer AF duration more than one week, significantly lower use of oral anticoagulants than patients without LA ABN (23.1 vs 52.6%, p = 0.02).
Table 3.
Comparison of baseline characteristics of the study population according to the presence of left abnormality.
| Demographic data | Without LA ABN (n = 38) | With LA ABN (n = 26) | P |
|---|---|---|---|
| Age (years) mean ± SD | 66.05 ± 6.2 | 68.46 ± 5.21 | 0.11 |
| Body mass index (kg/m2) | 29.26 ± 4.76 | 30.67 ± 3.77 | 0.21 |
| Body surface area (m2) | 1.81 ± 0.19 | 1.93 ± 0.16 | 0.008 |
| Sex: | |||
| Female | 14 (36.8%) | 10 (38.5%) | 0.9 |
| Clinical data: (n & % ) | |||
| Smoking | 18 (47.4%) | 8 (30.8%) | 0.18 |
| Hypertension | 34 (89.5%) | 20 (76.9%) | 0.17 |
| Diabetes | 6 (23.1%) | 12 (31.6%) | 0.46 |
| Previous stroke/TIA | 2 (7.7%) | 8 (21.1%) | 0.15 |
| Congestive heart failure | 20 (52.6%) | 14 (53.8%) | 0.92 |
| Vascular diseases | 12 (46.2%) | 22 (57.9%) | 0.36 |
| Pacemaker or ICD | 4 (10.5%) | 4 (15.4%) | 0.56 |
| CHADS2 | 1.77 ± 0.82 | 2.16 ± 1.1 | 0.003 |
| CHA2DS2-VASc | 3.46 ± 0.95 | 3.79 ± 1.34 | 0.002 |
| Laboratory data: mean ± SD | |||
| Hemoglobin (g/d) | 12.91 ± 1.54 | 13.25 ± 1.33 | 0.36 |
| Platelet (103/μL) | 241.79 ± 49.06 | 219.08 ± 53.8 | 0.09 |
| INR | 1.83 ± 0.62 | 1.49 ± 0.42 | 0.02 |
| GFR (ml/min/1.73 m2) | 64.37 ± 19.32 | 69.75 ± 14.29 | 0.26 |
| AF duration: (n & % ) | |||
| ⩽48 h | 12 (31.6%) | 3 (11.5%) | 1 |
| >48 h–1 week | 11 (28.9%) | 2 (7.7%) | 0.11 |
| >1 week–1 year | 9 (23.7%) | 8 (30.8%) | 0.01 |
| >1 year | 6 (15.8%) | 13 (50.0%) | 0.004 |
| Antithrombotic treatment: | |||
| Oral anticoagulation | 20 (52.6%) | 6 (23.1%) | 0.02 |
| Antiplatelet agent | 12 (31.6%) | 18 (69.2%) | 0.003 |
| Enoxaparine | 16 (42.1%) | 4 (15.4%) | 0.02 |
There were statistically significant increases in all indexed and non-indexed LA measurements and decrease in LV EF in patients with LA ABN compared to patients without LA ABN (Table 4).
Table 4.
Comparison of echocardiographic findings according to the presence of left atrium abnormalities.
| Characteristics (mean ± SD) | Without ABN (n = 38) | With ABN (n = 26) | T | P |
|---|---|---|---|---|
| LA AP diameter (cm) | 5.12 ± 0.83 | 6.07 ± 1.21 | 3.73 | 0.000 |
| LA area (cm2) | 23.51 ± 6.34 | 30.38 ± 4.09 | 4.88 | 0.000 |
| LA ellipsoid volume (cm3) | 73.31 ± 16.97 | 86.16 ± 11.37 | 3.38 | 0.001 |
| LA 1P area-length volume (cm3) | 119.98 ± 28.21 | 147.75 ± 13.71 | 4.65 | 0.000 |
| LA 2P area-length volume (cm3) | 108.05 ± 27.04 | 128.62 ± 18.6 | 3.37 | 0.001 |
| LV ejection fraction (%) | 52.77 ± 10.43 | 44.11 ± 11.26 | 3.11 | 0.003 |
| Indexed LA AP (cm/m2) | 2.65 ± 0.35 | 3.39 ± 0.78 | 5.16 | 0.000 |
| Indexed LA area (cm2/m2) | 12.16 ± 3 | 17.01 ± 3.29 | 6.1 | 0.000 |
| Indexed LA ellipsoid volume (cm3/m2) | 37.92 ± 7.77 | 47.85 ± 6.76 | 5.29 | 0.001 |
| Indexed LA 1p volume (cm3/m2) | 62.25 ± 14.12 | 82.29 ± 10.90 | 6.09 | 0.000 |
| Indexed LA 2p volume (cm3/m2) | 56.01 ± 13.5 | 71.52 ± 12.26 | 4.68 | 0.001 |
P value <0.001 indicates highly significant difference.
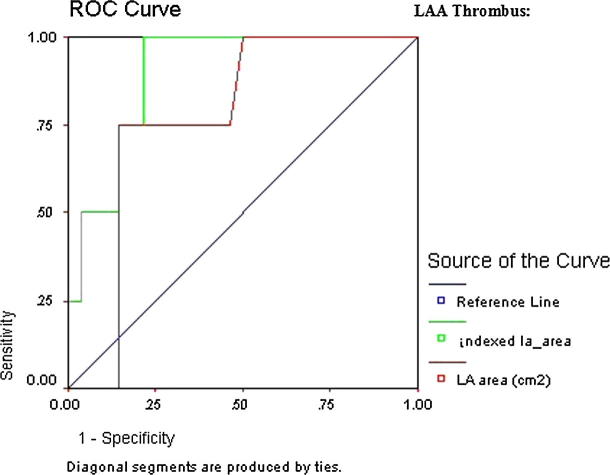
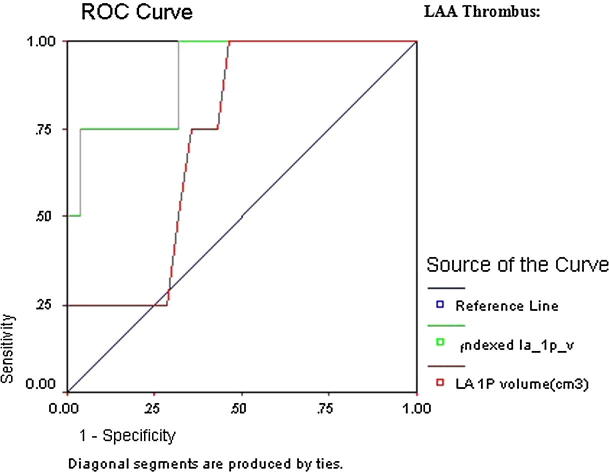
ROC curve revealed LAA thrombus showing that are all indexed and most non-indexed LA parameters that were significant predictors of thromboembolic risk; however, the highest AUC was indexed LA 1P area length volume (AUC 0.91, CI 95% 0.81–1.01, p = 0.000) followed by indexed LA area (AUC 0.90, CI 95% 0.82–0.98, p = 0.000) (Table 5 and Figure 2a, Figure 2b).
Table 5.
Validity of LA measurements in predicting LAA thrombus as a marker for thromboembolic risk.
| AUC | 95% CI | P | |
|---|---|---|---|
| LA AP | 0.75 | 0.62–0.87 | 0.02 |
| Indexed LAAP | 0.88 | 0.75–0.97 | 0.001 |
| LA area | 0.77 | 0.64–0.91 | 0.01 |
| Indexed LA area | 0.90 | 0.82–0.98 | 0.000 |
| LA ellipsoid volume | 0.63 | 0.46–0.79 | 0.26 |
| Indexed LA ellipsoid volume | 0.81 | 0.66–0.96 | 0.004 |
| LA 1P volume | 0.73 | 0.58–0.87 | 0.04 |
| Indexed LA 1P volume | 0.91 | 0.81–1.01 | 0.000 |
| LA 2P volume | 0.76 | 0.61–0.92 | 0.02 |
| Indexed LA 2P volume | 0.88 | 0.72–1.03 | 0.001 |
Figure 2a.
ROC curve of LAA thrombus.
Figure 2b.
Detailed ROC curves of LAA thrombus.
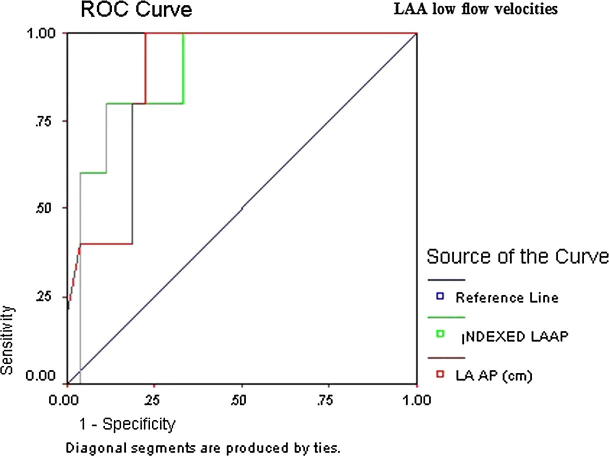
ROC curve of LAA low flow velocity revealed that indexed LA AP diameter (AUC 0.89, CI 95% 0.80–0.98, p = 0.000) followed by non-indexed LA AP diameter (AUC 0.88, CI 95% 0.79–0.97, p = 0.000) was the highest predictor of thromboembolic risk (Table 6 and Fig. 3).
Table 6.
Validity of LA measurements in predicting LAA low flow velocity.
| AUC | 95% CI | P | |
|---|---|---|---|
| LA AP | 0.88 | 0.79–0.97 | 0.000 |
| Indexed LAAP | 0.89 | 0.80–0.98 | 0.000 |
| LA area | 0.65 | 0.50–0.80 | 0.14 |
| Indexed LA area | 0.66 | 0.49–0.83 | 0.11 |
| LA ellipsoid volume | 0.59 | 0.41–0.77 | 0.38 |
| Indexed LA ellipsoid volume | 0.62 | 0.42–0.81 | 0.25 |
| LA 1P volume | 0.71 | 0.54–0.87 | 0.04 |
| Indexed LA 1P Volume | 0.60 | 0.43–0.77 | 0.32 |
| LA 2P volume | 0.74 | 0.59–0.88 | 0.02 |
| Indexed LA 2P Volume | 0.65 | 0.50–0.81 | 0.13 |
Figure 3.
Detailed ROC curves of LAA low flow velocity.
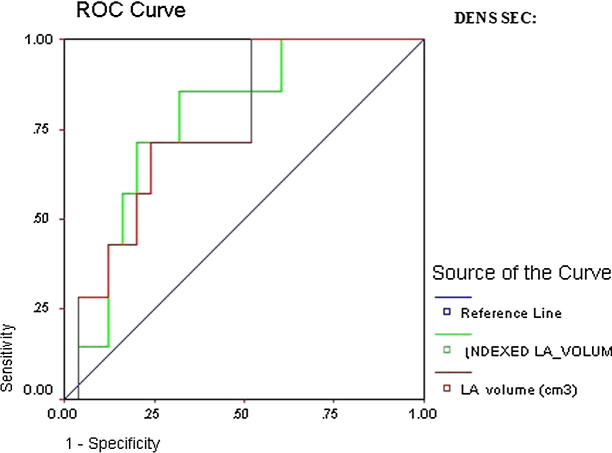
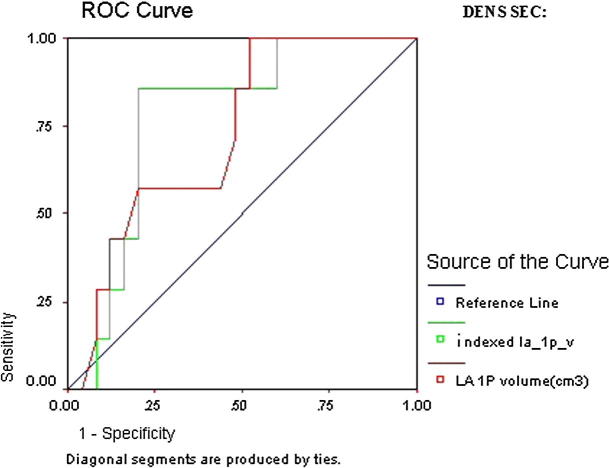
ROC curve of LA dense SEC revealed that all parameters except anteroposterior diameter were significant predictors of thromboembolic risk. However, the highest AUC was indexed LA ellipsoid volume (AUC 0.78, CI 95% 0.66–0.96, p = 0.002) and indexed LA 1P area length volume (AUC 0.78, CI 95% 0.66–0.90, p = 0.002) (Table 7 and Figure 4a, Figure 4b).
Table 7.
Validity of LA measurements in predicting dense SEC as a marker for thromboembolic risk.
| AUC | 95% CI | P | |
|---|---|---|---|
| LA AP | 0.59 | 0.41–0.77 | 0.31 |
| Indexed LAAP | 0.59 | 0.42–0.77 | 0.28 |
| LA area | 0.76 | 0.64–0.89 | 0.003 |
| Indexed LA area | 0.75 | 0.62–0.88 | 0.005 |
| LA ellipsoid volume | 0.76 | 0.65–0.90 | 0.003 |
| Indexed LA ellipsoid volume | 0.78 | 0.66–0.96 | 0.002 |
| LA 1P volume | 0.73 | 0.58–0.87 | 0.009 |
| Indexed LA 1P volume | 0.78 | 0.65–0.90 | 0.002 |
| LA 2P volume | 0.66 | 0.50–0.81 | 0.07 |
| Indexed LA 2P volume | 0.65 | 0.50–0.79 | 0.09 |
Figure 4a.
ROC curve of LA dense SEC.
Figure 4b.
Detailed ROC curves of LA dense SEC.
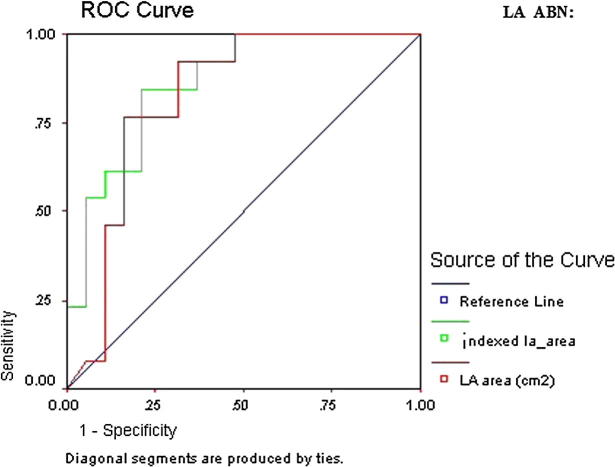
ROC curve of LA ABN showed that all LA parameters were statistically significant in predicting LA ABN. However the highest AUC was indexed LA 1P area length volume (AUC 0.87, CI 95% 0.79–0.96, p = 0.000) followed by indexed LA area (AUC 0.86, CI 95% 0.78–0.95, p = 0.000) (Table 8 and Figure 5a, Figure 5b).
Table 8.
Validity of LA measurements in predicting LA ABN as a marker for thromboembolic risk.
| AUC | 95% CI | P | |
|---|---|---|---|
| LA AP | 0.76 | 0.64–0.89 | 0.000 |
| Indexed LAAP | 0.80 | 0.68–0.91 | 0.000 |
| LA area | 0.82 | 0.72–0.93 | 0.000 |
| Indexed LA area | 0.86 | 0.78–0.95 | 0.000 |
| LA ellipsoid volume | 0.76 | 0.64–0.87 | 0.001 |
| Indexed LA ellipsoid volume | 0.83 | 0.72–0.93 | 0.000 |
| LA 1P volume | 0.81 | 0.71–0.92 | 0.000 |
| Indexed LA 1P volume | 0.87 | 0.79–0.96 | 0.000 |
| LA 2P volume | 0.77 | 0.65–0.88 | 0.000 |
| Indexed LA 2P volume | 0.78 | 0.67–0.89 | 0.000 |
Figure 5a.
ROC curve of LA ABN.
Figure 5b.
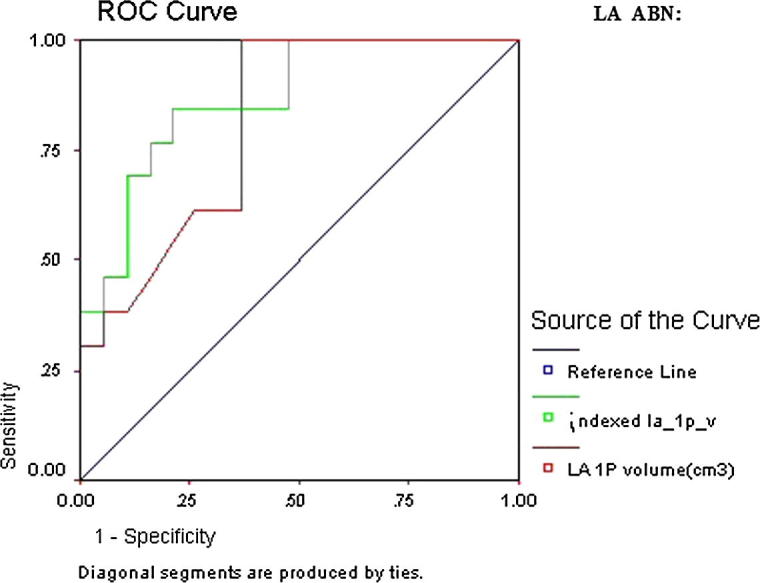
Detailed ROC curves of LA ABN.
Multivariate logistic regression analysis found that the most predictive LA measurement for LAA thrombus was indexed LA AP with cutoff 3 cm/m2 (OR 7.5, 95% CI 1.24–45.2, p = 0.02), for LAA low flow velocity was LA AP with cutoff 6 cm (OR 17.6, 95% CI 3.23–95.84, p = 0.001), for LA dense SEC was indexed LA ellipsoid volume with cutoff 42 cm3/m2 (OR 6.5, 95% CI 1.32–32.07, p = 0.02), and for LA ABN was indexed LA ellipsoid volume with cutoff 42 cm3/m2 (OR 10.45, 95% CI 2.18–51.9, p = .008) followed by indexed LA AP with cutoff 3 cm/m2 (OR 8.2, 95% CI 1.44–44.7, p = 0.01) and LA 1P area length volume with cutoff 125 cm3 (OR 6, 95% CI 1.03–34, p = 0.04) (Table 9).
Table 9.
Multivariate logistic regression analysis of transesophageal parameters of predicting thromboembolic risk.
| OR | 95%CI | P | ||
|---|---|---|---|---|
| LA ABN | Indexed LA ellipsoid volume ⩾ 42 cm3/m2 | 10.45 | 2.18–51.9 | 0.008 |
| Indexed LA AP ⩾ 3 cm/m2 | 8.2 | 1.44–44.7 | 0.01 | |
| LA 1p area length volume ⩾ 125 cm3 | 6 | 1.03–34 | 0.04 | |
| LAA thrombus | Indexed LA AP ⩾ 3 cm/m2 | 7.5 | 1.24–45.2 | 0.02 |
| LA dense SEC | Indexed LA ellipsoid volume ⩾ 42 cm3/m2 | 6.5 | 1.32–32.07 | 0.02 |
| LAA low flow velocity | LA AP ⩾ 6 cm | 17.6 | 3.23–95.84 | 0.001 |
8. Discussion
This study was conducted to evaluate the accuracy of LA size to predict TEE markers of increased thromboembolic risk in patients with nonvalvular AF prior to cardioversion or electrophysiology procedure.
We found that the proportion of patients with LA ABN was 40.6% of the study population (Table 2) which is higher than reported by other previous studies. They found that the proportion of patients with LA ABN was 26.3%, 15.6%, 8.8%, and 29.6%, of their study population respectively.14, 27, 28, 29 This high percentage of patients with LA ABN can be at least partially explained by underutilization of oral anticoagulants that were found in our study.
Providência et al.14 evaluated and compared the accuracy of CHADS2 and CHA2DS2-VASc in the prediction of TEE markers of thromboembolic risk and test the additive value of transthoracic echocardiogram (TTE)-derived parameters (LA area and left ventricle global systolic function) as a possible refinement for prediction of the TEE endpoints.
Ayirala et al.27 and Doukky et al.28 tested the hypothesis that higher LA volume and/or lower left ventricular ejection fraction (LVEF) and the ratio of LVEF to LA volume index (LAVI) might prove valuable as markers of increased risk for LA appendage thrombus formation in patients with nonvalvular AF.
Faustino et al.29 aimed to evaluate the accuracy of LA size to identify TEE markers of thromboembolic risk in patients with AF.
We found that use of anti-coagulants was significantly lower among patients with LA ABN compared to patients without LA ABN (23.1 vs 52.6%), p = 0.002 (Table 3). These results are different from those reported by previous studies.14, 27, 28, 29 They all found that use of anticoagulants was significantly higher among patients with LA ABN compared to patients without LA ABN (42.4 vs 37.5%), (56.5 vs. 21%), (83.3 vs. 44%) and (50 vs 36.6%) respectively. This may be explained by less strict application of guidelines regarding use of oral anticoagulants in our patients.
Our study found that patients with LA ABN had high CHADS2 and CHA2DS2-VASc scores, and this can be explained by high prevalence of thromboembolic risk factors including older age, diabetes, previous stroke or TIA, vascular diseases and heart failure. (Table 3) These results were concordant with those reported by previous studies.14, 28, 29, 30 Also Ayirala et al.27 found that high CHADS(2) score was significant predictors of LA appendage thrombus formation.
Our data showed significant increase in percentage of patients with AF duration more than 1 week (persistent AF) in patients with LA ABN compared to patients without LA ABN (30.8 vs 23.7%), p < 0.01. However the study found no significant difference between both groups regarding the prevalence of paroxysmal AF (duration < 1 week). These results were in agreement with Fatkin et al.25 and Faustino et al.29, who found that patients with paroxysmal AF had no LA ABN, while patients with longer AF duration (persistent AF) had LA ABN.
We found that dilated LA size irrespective of the used method, and lower LV EF were more prevalent in patients with left atrial abnormalities (LA ABN). These results confirm the results of previous studies.12, 27, 28, 29
Zabalgoitia and colleagues12 found statistically significant increase in LA measurements in patients with LA ABN compared to patients without LA ABN.
Ayirala et al.27 and Doukky et al.28 found that the ratio of LVEF to LA volume index ⩽1.5 produced 100% sensitivity for the presence of LA appendage thrombus. They used two different methods to calculate LA volume either three linear dimensions (ellipsoid volume) or the area-length method. Both of these methods are endorsed by the American Society of Echocardiography guidelines.6 They concluded that the excellent diagnostic performance of the prediction rule of this ratio not affected by the method was used to calculate the LA volume.
Faustino et al.29 reported that LA dilation is associated with an increase in the prevalence of LA ABN (TEE markers of increased thromboembolic risk) in patients with AF, independently from recognized clinical risk factors. A stronger association was found for measurements indexed to body surface area.
These results can be explained by decreased LVEF and elevated LV filling pressure lead to LA dilatation and LAA thrombus formation,31 chronic elevation in LA pressure leads to LA volume and pressure overload and deterioration of LAA contractility, leading to blood stasis and thrombus formation,32 and also LA enlargement usually associated with permanent AF, which is known independent predictor of LAA thrombus formation.33
Our results suggest that a larger LA size was associated with LA ABN. Also, most non-indexed and all indexed measurements showed high accuracy in the prediction of all thromboembolic risk markers assessed by TEE without significant differences between different parameters.
We found the highest AUC for the prediction of study endpoints was achieved with indexed LA 1P volume for LAA thrombus (AUC 0.91, p < 0.000), dense SEC (AUC 0.87, p < 0.000) and LA ABN (AUC 0.87, p < 0.000). Regarding LAA low flow velocities, the highest AUC was obtained with indexed LA AP (AUC 0.89, p < 0.000).
Faustino et al.29 found that indexed measurements of LA area 4C, LA 1P and 2P area-length volumes had moderate to high discriminatory power in the prediction of LAA thrombus, LAA low flow velocities, dense SEC and LA ABN, without significant differences between them. Indexed LA area 4C was an independent predictor of all TEE endpoints. For LAA thrombus, indexed 2P area-length volume was a predictor of TEE surrogate markers of stroke.
Russo et al.34 evaluated single-plane and biplane methods for the assessment of LA volume against three-dimensional echocardiography in 527 participants of a community-based Cohort. They found strong correlations between single- and biplane LA volume measurements (r = 0.95, p < 0.01), and single- (r = 0.93, p < 0.01) and biplane (r = 0.93, p < 0.01) area-length with three-dimensional volumes.
On multivariate analysis, the present study found that the most predictive LA parameter for LAA thrombus was indexed LA AP with cutoff 3 cm/m2, for LA dense SEC was indexed LA ellipsoid volume with cutoff 42 cm3/m2, for LAA low flow velocity was LA AP with cutoff 6 cm and for LA ABN was LA 1P area length volume with cutoff 125 cm3 (Table 9).
Faustino et al.29 found that the most predictive LA parameter for LAA thrombus was indexed LA 2P volume, for LA dense SEC, LAA low flow velocity and LA ABN were indexed LA area, and also they found that no significant differences between indexed LA area 4C, LA 2P and 1P area length volume for the discrimination of TEE markers of thromboembolic risk.
9. Limitation
For single-center study with small sample size, we suggest that multicenter approaches may be necessary to attain larger sample sizes. At the time of TEE, 40.6% of the patients were under oral anticoagulation, which may have an impact on the prevalence of thromboembolic risk markers; we thought this is not problem as some of patients on oral anticoagulants had sub therapeutic INR, and also it is known that thrombi may arise even under therapeutic INR. Finally as our study hypothesis is based on surrogate TEE markers endpoints so it needs to be clinically validated in an outcome study looking at systemic thromboembolism, including stroke, as a main endpoint to confirm the accuracy and advantages of TTE-derived LA parameters in prediction of thromboembolic risk.
10. Conclusion
LA enlargement is suitable to predict TEE derived thromboembolic markers (LAA thrombus, LAA low flow velocities, dense SEC and LA ABN) in patients with non-valvular AF. The indexed LA ellipsoid volume, LA 1P area length volume, and indexed and non-indexed LA AP diameter were the most accurate methods for predicting thromboembolic surrogate markers in these patients.
Conflict of interest
The author declares that she has no conflict of interest.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgment
The author thanks the staff of cardiovascular department of Zagazig University Hospital for their expert input and detailed evaluations as well as our patients who participated in the study.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.Tani T., Tanabe K., Ono M. Left atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2004;17(6):644–648. doi: 10.1016/j.echo.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Fatema K., Barnes M.E., Bailey K.R. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10(2):282–286. doi: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin E.J., D’Agostino R.B., Belanger A.J. Left atrial size and the risk of stroke and death. The framing ham heart study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 4.Tsang T.S., Barnes M.E., Gersh B.J. Risks for atrial fibrillation and congestive heart failure in patients ⩾65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol. 2004;93:54–58. doi: 10.1016/j.amjcard.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Beinert R., Boyko V., Schwammenthal E. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol. 2004;44(2):327–334. doi: 10.1016/j.jacc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 6.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Am J Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Thambidorai S.K., Murray R.D., Parakh K. Utility of transesophageal echocardiography in identification of thrombogenic milieu in patients with atrial fibrillation (an ACUTE ancillary study) Am J Cardiol. 2005;96:935–941. doi: 10.1016/j.amjcard.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L.T., Gay M. Characterizing left atrial appendage functions in sinus rhythm and atrial fibrillation using computational models. J Biomech. 2008;41:2515–2523. doi: 10.1016/j.jbiomech.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Rubin D.N., Katz S.E., Riley M.F. Evaluation of left atrial appendage anatomy and function in recent–onset atrial fibrillation by transesophageal echocardiography. Am J Cardiol. 1996;78:744–778. doi: 10.1016/s0002-9149(96)00419-5. [DOI] [PubMed] [Google Scholar]
- 10.Wazni O.M., Tsao H.M., Chen S.A. Cardiovascular imaging in the management of atrial fibrillation. J Am Coll Cardiol. 2006;48:2077–2084. doi: 10.1016/j.jacc.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 11.Li Y.H., Lai L.P., Shyu K.G. Clinical implications of left atrial appendage flow patterns in non-rheumatic atrial fibrillation. Chest. 1994;105:748–752. doi: 10.1378/chest.105.3.748. [DOI] [PubMed] [Google Scholar]
- 12.Zabalgoitia M., Halperin J.L., Pearce L.A. Stroke prevention in atrial fibrillation III investigators. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1998;31:1622–1626. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 13.Albers G.W., Dalen J.E., Laupacis A. Antithrombotic therapy in atrial fibrillation: the sixth ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2001;119:194S–206S. doi: 10.1378/chest.119.1_suppl.194s. [DOI] [PubMed] [Google Scholar]
- 14.Providência R., Trigo J., Paiva L. The role of echocardiography in thromboembolic risk assessment of patients with non valvular atrial fibrillation. J Am Soc Echocardiogr. 2011;26:801–812. doi: 10.1016/j.echo.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 15.The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med. 1998;128:639–647. doi: 10.7326/0003-4819-128-8-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Klein A.L., Grimm R.A., Murray R.D., Apperson-Hansen C., Asinger R.W., Black I.W. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–1420. doi: 10.1056/NEJM200105103441901. [DOI] [PubMed] [Google Scholar]
- 17.Klein A.L., Grimm R.A., Jasper S.E., Murray R.D., Apperson-Hansen C., Lieber E.A. Efficacy of transesophageal echocardiography-guided cardioversion of patients with atrial fibrillation at 6 months: a randomized controlled trial. Am Heart J. 2006;151:380–389. doi: 10.1016/j.ahj.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Garnock-Jones K.P., Curran M.P. Regadenoson. Am J Cardiovasc Drugs. 2010;10:65–71. doi: 10.2165/10489040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Barrett R.J., Lamson M.J., Johnson J., Smith W.B. Pharmacokinetics and safety of binodenoson after intravenous dose escalation in healthy volunteers. J Nucl Cardiol. 2005;12:166–171. doi: 10.1016/j.nuclcard.2004.12.294. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Moss J., Thisted R. Predictors of body surface area. J Clin Anesth. 1992;4(1):4–10. doi: 10.1016/0952-8180(92)90111-d. [DOI] [PubMed] [Google Scholar]
- 21.Gage B.F., Waterman A.D., Shannon W. Validation of clinical classification schemes for predicting stroke: results from the National Registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 22.Lip G.Y., Nieuwlaat R., Pisters R. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 23.Beppu S., Park Y.D., Sakakibara H. Clinical features of intracardiac thrombosis based on echocardiographic observation. Jpn Cite J. 1984;92:835–841. doi: 10.1253/jcj.48.75. [DOI] [PubMed] [Google Scholar]
- 24.Beppu S., Nimura Y., Sakakihara H. Smoke-like echo in the left atrial cavity in mitral valve disease: its features and significance. J Am Coll Cardiol. 1985;6:744–749. doi: 10.1016/s0735-1097(85)80476-9. [DOI] [PubMed] [Google Scholar]
- 25.Fatkin D., Kelly R.P., Feneley M.P. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23:961–969. doi: 10.1016/0735-1097(94)90644-0. [DOI] [PubMed] [Google Scholar]
- 26.Merino A., Hauptman P., Badimon L. Echocardiographic “smoke” is produced by an interaction of erythrocytes and plasma proteins modulated by shear forces. J Am Coli Cardiol. 1992;20:1661–1668. doi: 10.1016/0735-1097(92)90463-w. [DOI] [PubMed] [Google Scholar]
- 27.Ayirala S., Kumar S., O’ Sulivan D.M., Silverman D.I. Echocardiographic predictors of left atrial appendage thrombus formation. J Am Soc Echocardiogr. 2011;24:499–505. doi: 10.1016/j.echo.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Doukky R., Khandelwal A., Garcia-Sayan E., Gage H. External validation of a novel transthoracic echocardiographic tool in predicting left atrial appendage thrombus formation in patients with nonvalvular atrial fibrillation. Eur Heart J. 2013;14(9):876–881. doi: 10.1093/ehjci/jes313. [DOI] [PubMed] [Google Scholar]
- 29.Faustino A., Providência Rui, Barra Sérgio, Paiva Luís, Trigo Joana, Botelho Ana. Which method of left atrium size quantification is the most accurate to recognize thromboembolic risk in patients with non-valvular atrial fibrillation? Cardiovascu Ultrasound. 2014;12:28. doi: 10.1186/1476-7120-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Floria M., De Roy L., Xhaet O., Blommaert D., Jamart J., Gerard M. Predictive value of thromboembolic risk scores before an atrial fibrillation ablation procedure. J Cardiovascu Electrophysiol. 2013;24(2):139–145. doi: 10.1111/j.1540-8167.2012.02442.x. [DOI] [PubMed] [Google Scholar]
- 31.Iwakura K., Okamura A., Koyama Y., Date M., Higuchi Y., Inoue K. Effect of elevated left ventricular diastolic filling pressure on the frequency of left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011;107:417–422. doi: 10.1016/j.amjcard.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 32.Goldman M.E., Pearce L.A., Hart R.G., Zabalgoitia M., Asinger R.W., Safford R. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study) J Am Soc Echocardiogr. 1999;12:1080–1087. doi: 10.1016/s0894-7317(99)70105-7. [DOI] [PubMed] [Google Scholar]
- 33.Wysokinski W.E., Ammash N., Sobande F., Kalsi H., Hodge D., McBane R.D. Predicting left atrial thrombi in atrial fibrillation. Am Heart J. 2010;159:665–671. doi: 10.1016/j.ahj.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 34.Russo C., Hahn R.T., Jin Z., Homma S., Sacco R.L., Di Tullio M.R. Comparison of echocardiographic single- vs biplane method in the assessment of left atrial volume and validation by real time three-dimensional echocardiography. J Am Soc Echocardiogr. 2010;23(9):954–960. doi: 10.1016/j.echo.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]