Abstract
BACKGROUND:
ATP - binding cassette transporter A1 (ABCA1) plays essential roles in the biogenesis of high -density lipoprotein - cholesterol. Variations in the ABCA1 gene may influence the risk of coronary artery disease (CAD).
AIM:
Present study aimed to investigate the association of rs2230806 (R219K) polymorphism of ABCA1 gene with the development and severity of CAD in an Iranian population.
MATERIALS AND METHODS:
Our study population consisted of 100 patients with angiographically confirmed CAD and 100 controls. The genotyping of R219K mutation of ABCA1 gene was determined by PCR - RFLP method. Lipid profile was determined using routine colourimetric assays. Statistical analysis was done by SPSS - 16.
RESULTS:
The genotypic (P = 0.024) and allelic (P = 0.001) distribution of the ABCA1 R219K polymorphism were significantly different between the two groups. In a univariate analysis (with genotype RR as the reference), the RK genotype (OR = 0.46, 95%CI = 0.25-0.86, P = 0.020) and KK genotype (OR = 0.27, 95%CI = 0.11 – 0.66, P = 0.005) was significantly associated with a decreased risk of CAD. A multiple logistic regression analysis revealed that smoking (0.008), diabetes (P = 0.023), triglyceride (P = 0.001), HDL - cholesterol (P = 0.002) and ABCA1 KK genotype (P = 0.009) were significantly and independently associated with the risk of CAD. The association between different genotypes of R219K polymorphism with lipid profile was not significant in both groups (P > 0.05). The R219K polymorphism was significantly associated with severity of CAD (P < 0.05).
CONCLUSION:
The carriage of K allele of ABCA1 R219K polymorphism has a protective effect on CAD risk and correlates with a decreased severity of CAD. This protective effect seems to be mediated independently of plasma lipid levels.
Keywords: ATP - binding cassette transporter A1, Polymerase chain reaction-restriction fragment length polymorphism, R219K mutation, Coronary artery disease
Introduction
Coronary artery disease (CAD) is considered as one of the main leading causes of morbidity and mortality in Iranian population. Several epidemiological studies have indicated a strong inverse association between high-density lipoprotein cholesterol (HDL - C) levels and CAD occurrence [1][2]. Low levels of HDL-C in plasma are considered as an independent risk factor for CAD development [3]. The circulating levels of HDL - C are determined by the interaction of both environmental and genetic factors. Genetic factors contribute considerably (up to 60%) for plasma HDL - C levels and some genes including ATP - binding cassette transporter A1 (ABCA1) have been associated with its plasma concentration [4][5].
The ABCA1 is a membrane transporter protein that plays an essential role in the efflux of cholesterol from peripheral tissues back to the liver. The ABCA1 transporter functions in the initial step of revers cholesterol transfer and transfers cholesterol and phospholipid from peripheral tissues to lipid-poor apolipoprotein AI, creating nascent high - density lipoprotein particles [6]. Reduced activity of the ABCA1 transporter leads to disturbances in plasma lipid composition and levels which are common findings in patients with CAD [6].
Reduced HDL - C level is the most common lipoprotein abnormality observed among CAD patients and is a major determinant of morbidity and mortality rate in these patients [7][8]. Genetic and molecular biology studies have shown that common polymorphisms in the ABCA1 gene can influence the function of ABCA1 transporter resulting in the altered biosynthesis of HDL - C particles [9][11]. Reduced function of the ABCA1 transporter may be involved in the pathogenesis of CAD. The R219K (rs2230806, 107620867C>T) common polymorphism in the ABCA1 gene is located in the two major extracellular loops of ABCA1 protein, which has a critical role for interaction with apoA - I and for cholesterol efflux [12][13]. Therefore, it is likely that the R219K variant acts as a functional mutation to modulate HDL - C level [12]. The R219K polymorphism may affect the development and severity of CAD via altering the lipid profile [14]. Numerous studies have investigated the association of R219K mutation of ABCA1 gene and the development of CAD in different populations [9][10][15]. However, in-consistent results have been reported [9][10][14][15][16]. The aim of the present study was to investigate the role of ABCA1 rs2230806 genetic polymorphism in the development of CAD from an Iranian population. Also, the effect of the ABCA1 rs2230806 common polymorphism on lipid profile was investigated in CAD patients and control subjects.
Material and Methods
Study Population
Our study population consisted of 200 subjects including 100 patients (50 male and 50 female) with a confirmed diagnosis of CAD and 100 matched controls (50 male and 50 female). The mean age of CAD patients and controls were 58.96 ± 11.54 and 57.53 ± 16.1, respectively. The CAD diagnosis was made by angiography conducted by an expert cardiovascular specialist. The minimum required criteria for including of CAD patients in the study were the presence of more than 50% stenosis in at least one major coronary vessel. The severity of CAD was determined based on the number of the stenotic vessel showing ≥ 50% stenosis. Accordingly, patients were classified as single, double and triple vessel stenosis patients. Patients showing fewer than 50% stenosis or taking lipid-lowering drugs were excluded from the study. Patients suffering from valvular heart disease, cardiomyopathy, autoimmune disorders, inflammatory disease, infectious disease, renal, heart and liver failures and cancer were excluded from the study. Control subjects were selected randomly after careful inspection of a cardiovascular specialist. Control subjects were included in the study if they had no personal or family history of CAD or other reasons to suspect CAD. Also, control subjects with overt concomitant diseases such as malignant diseases, autoimmune disease, infectious disease and organ failure were excluded. For all subjects, complete medical history including questions about smoking habits, history of hypertension and diabetes and family history of heart disease was obtained by questionnaire. Diabetes was defined by fasting blood glucose > 126 mg/dL, and hypertension was defined by systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg. All of the study subjects participated voluntarily in the study and written informed consent was obtained from all participants. The study was approved by ethical committee of Zahedan University of Medical Sciences (Ethical committee code: IR.ZAUMS.REC.1394.383), Zahedan, Iran.
Blood Samples Collection
From each participant, 5 ml fasting blood were collected in EDTA containing tubes and instantly centrifuged. Plasma fraction was separated and stored at -40ºC and the cellular fraction was used for DNA extraction.
ABCA1 R219K Polymorphism Analysis
Genomic DNA was purified from blood leukocytes using a commercially available DNA extraction kit (Viogene, Poland). The genotyping of ABCA1 gene R219K polymorphism was conducted by PCR reaction at an annealing temperature of 62ºC. The sequence of primers were obtained from previous studies [17] and was as follows: forward 5’ – AAAGACTTCAAGGACCCAGCTT - 3’ and reverse 5’ - CCTCACATTCCGAAAGCATTA - 3’. After amplification, a seven microliter aliquot of PCR product was digested with 5u EcoN1 restriction enzyme (Fermentas, Inc, Germany) for at least 8 hours at 37 °C. The obtained fragments after restriction digestion were separated on 2.5% agarose gel and stained with Sybr green dye. After digestion, the 309bp PCR amplicon cleaves into 184 and 125 bp fragments in the presence of K allele whereas the R allele produces a single non - digested 309 bp fragment. Genotyping results from ten percent of samples determined by PCR - RFLP method was randomly confirmed by direct sequencing of PCR products (Figure 1).
Figure 1.
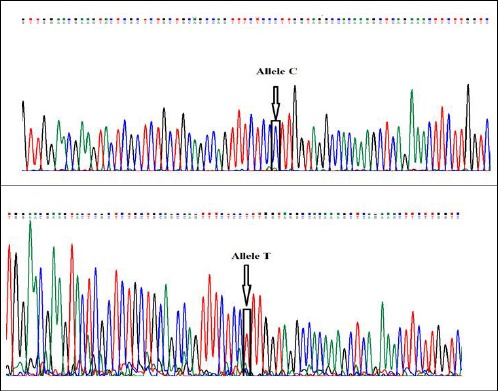
Results of direct sequencing of PCR products showing wild (C) and mutant (T) allele of ABCA1 R219K polymorphism
Biochemical Methods
Plasma total cholesterol (TC), Triglycerides (TG), high-density lipoprotein- cholesterol (HDL - C), low-density lipoprotein cholesterol (LDL - C) and fasting glucose levels were measured with commercially available enzyme assay kits (Pars Azmon Co, Tehran, Iran) using Mindray autoanalyser (BS-200).
Statistical Analysis
In descriptive statistics, numerical variables presented as mean ± SD and were compared using Student t-test. Categorical variables were compared with Chi-square test or Fisher exact test (in case of small sample size). The deviation of genotype distribution from the Hardy - Weinberg equilibrium (HWE) was assessed in both patients and controls by Chi-square test. Multivariate logistic regression analysis was performed to determine the independent association of each covariate with the risk of a CAD. The statistical analysis with a P value < 0.05 was considered significant. All statistical analysis was performed using SPSS 16 software.
Results
The comparison of clinical and biochemical parameters of the CAD patients and control subjects revealed no statistically significant differences in the mean ages and sex distribution between CAD group and control group (P = 0.471; P = 1.000, respectively). However, plasma levels of TC, TG and LDL - C were significantly higher while the plasma levels of HDL - C were significantly lower in CAD group compared with control group. Moreover, the presence of diabetes (P = 0.014), hypertension (P = 0.009) and smoking habit (P = 0.002) were significantly more common in CAD group than control group (Table 1). Also, statistical analysis using Chi-square test indicated that the genotype distribution of ABCA1 R219K polymorphism was significantly different between the two groups (P = 0.024, χ2= 13.6) (Table 1).
Table 1.
Clinical characteristics of the CAD group and control group included in the study
| Variables | CAD group n=100 | Control group n=100 | P value |
|---|---|---|---|
| Age (years) | 58.96 ± 11.54 | 57.53 ± 16.1 | 0.471 |
| Sex (M/F) | 50/50 | 50/50 | 1.000 |
| TG (mg/dl) | 202.21 ± 90.02 | 162.17 ± 69.67 | 0.001 |
| TC (mg/dl) | 200.78 ± 58.13 | 173.57 ± 40.13 | <0.001 |
| HDL-C (mg/dl) | 39.17 ±8.45 | 45.39 ± 12.62 | <0.001 |
| LDL-C (mg/dl) | 114.98± 44.43 | 96.97 ± 34.51 | 0.002 |
| Hypertension (n.%) | 23 (23%) | 8 (8%) | 0.009* |
| Diabetes (n.%) | 24 (24%) | 10 (10%) | 0.014* |
| Smoking (n.%) | 33 (33%) | 12 (12%) | 0.002* |
| R219K genotypes RR:RK:KK | 50:40:10 | 29:50:21 | 0.024 |
CAD: Coronary artery disease, TC: total cholesterol, TG: Triglyceride, HDL: High - density lipoprotein - cholesterol, LDL: Low - density lipoprotein- cholesterol.
P value determined by Fisher exacts test.
The frequency of RR, RK and KK genotypes among the CAD group was 50.0%, 40.0%, and 10.0%, respectively, and among the control group was 29.0%, 50.0% and 21.0%, respectively (Table 2). The χ2 test showed that the genotype distribution of ABCA1 gene R219K polymorphism were in coincidence with the Hardy - Weinberg equilibrium in both CAD patients (P = 0.63) and controls (P = 0.98). In a univariate analysis (with genotype RR as reference) the RK genotype (OR = 0.46, 95% CI: 0.25 - 0.86; P = 0.020) and KK genotype (OR = 0.27, 95% CI: 0.11-0.66; P = 0.005) was significantly correlated with a decreased risk of CAD (Table 2). Also, the frequency of minor K allele of ABCA1 R219K polymorphism was significantly lower in CAD group compared with control group (30% vs. 46%; OR = 0.50; 95% CI: 0.33-0.75; P = 0.001).
Table 2.
Prevalence of ABCA1 R219K allele and genotypes in CAD group and control group
| ABCA1 R219K Polymorphism | CAD group (n=100) | Control group (n=100) | OR (95%CI) | P value* |
|---|---|---|---|---|
| R allele | 140 (70.0%) | 108 (54.0 %) | Ref | - |
| K allele | 60 (30.0%) | 92 (46.0%) | 0.50 (0.33-0.75) | 0.001 |
| RR | 50 (50%) | 29 (29%) | Ref | - |
| RK | 40 (40%) | 50 (50%) | 0.46 (0.25-0.86) | 0.020 |
| KK | 10 (10%) | 21 (21%) | 0.27 (0.11-0.66) | 0.005 |
CAD: Coronary artery disease, RR: Wild-type, RK: Heterozygote; KK: Homozygote;
P values were determined by Fisher’s exact test.
In a multiple binary logistic regression analysis using the study group (CAD group vs. control group) as the dependent variable and using age, sex, TG, TC, HDL - C, LDL - C, smoking, diabetes, hypertension and R219K genotypes as covariates, the KK genotype of ABCA1 R219K polymorphism was independently associated with a decreased risk of CAD (OR = 0.212, 95% CI: 0.069 - 0.653; P = 0.009) but the RK genotype was not (OR = 0.430, 95% CI: 0.194-0.954; P = 0.074). Also, TG levels (P = 0.001), HDL - C levels (P = 0.002), diabetes (P = 0.023) and smoking (P = 0.008) were significant risk factor for CAD development. However, TC levels (P = 0.245), LDL - C levels (P = 0.644) and hypertension (P = 0.279) didn’t show any significant association with CAD risk in regression analysis (Table 3).
Table 3.
Multiple Logistic regression analysis of association between the ABCA1 R219K genotypes and risk of coronary artery disease
| Variable | Wald | Odd ratio | 95% CI | P value |
|---|---|---|---|---|
| Age | 0.075 | 0.997 | 0.973-1.021 | 0.785 |
| Sex | 0.622 | 1.321 | 0.662-2.636 | 0.430 |
| Hypertension | 1.198 | 1.740 | 0.639- 4.741 | 0.279 |
| Diabetes | 5.097 | 2.881 | 1.154-7.175 | 0.023 |
| Smoking | 6.889 | 3.134 | 1.349-7.281 | 0.008 |
| TG | 10.181 | 1.009 | 1.003-1.014 | 0.001 |
| TC | 1.353 | 1.009 | 0.994-1.023 | 0.245 |
| HDL-C | 7.959 | 0.951 | 0.918-0.985 | 0.002 |
| LDL-C | 0.001 | 1.000 | 0.982-1.019 | 0.644 |
| RK vs. RR | 4.313 | 0.430 | 0.194-0.954 | 0.074 |
| KK vs. RR | 7.302 | 0.212 | 0.069-0.653 | 0.009 |
Furthermore, the association of different genotypes of ABCA1 R219K polymorphism with lipid profile was presented in Table 4. As shown, no significant differences were observed in the lipid distribution according to the different genotypes of ABCA1 R219K polymorphism in CAD group and control group.
Table 4.
Lipid profile distribution among different genotypes of ABCA1 R219k polymorphism
| Parameter | Controls* | Cases† | ||||
|---|---|---|---|---|---|---|
| RR | RK | KK | RR | RK | KK | |
| TG mean | 162.2 | 162.4 | 161.6 | 192.1 | 215.1 | 185.9 |
| (SD) | (91.7) | (42.6) | (88.3) | (95.3) | (91.3) | (38.5) |
| TC mean | 181.9 | 173.8 | 160.6 | 196.9 | 197.6 | 232.7 |
| (SD) | (33.6) | (43.4) | (39.0) | (53.1) | (57.5) | (79.3) |
| HDL-C mean | 43.3 | 46.6 | 45.3 | 38.4 | 40.5 | 37.6 |
| (SD) | (12.7) | (13.4) | (10.5) | (8.1) | (9.2) | (6.6) |
| LDL-C mean | 108.4 | 94.7 | 86.5 | 123.0 | 107.3 | 105.4 |
| (SD) | (32.6) | (33.9) | (35.6) | (39.7) | (47.4) | (51.4) |
One way ANOVA test showed insignificant differences in TG (P = 0.99), TC (P = 0.21), HDL-C (P = 0.53) and LDL - C (P = 0.06) levels according to different genotypes of R219K polymorphism.
One way ANOVA test showed insignificant differences in TG (P = 0.31), TC (P = 0.18), HDL - C (P = 0.44) and LDL - C (P = 0.19) levels among different genotypes of R219K polymorphism.
However, investigating the prevalence of different genotypes of ABCA1 R219K polymorphism between patients with one and two or three stenotic vessels revealed significant differences in the genotype distribution between them (P < 0.05) (Table 5).
Table 5.
The association of severity of CAD with the number of stenotic coronary vessels
| ABCA1 R219K genotypes | 1 SV N = 36 | 2 SV N = 42 | 3 SV N = 22 | P value* 2 SV vs. 1SV | P value* 3 SV vs. 1SV |
|---|---|---|---|---|---|
| RR | 12 (33.33%) | 24 (57.14%) | 14 (63.63%) | Ref | Ref |
| RK | 17 (47.22%) | 17 (40.48%) | 6 (27.27%) | 0.225 | 0.080 |
| KK | 7 (19.44%) | 1 (2.38%) | 02 (9.1%) | 0.013 | 0.135 |
| RK+KK | 24 (66.67%) | 18 (42.86%) | 08 (36.36%) | 0.042 | 0.031* |
SV: Stenotic vessel, ABCA1: ATP - binding cassette transporter A1, RR: Wild-type, RK: Heterozygote; KK: Homozygote.
P values were calculated using Fisher’s exact test,
Discussion
We found a significant association between the K allele of ABCA1 R219K polymorphism and a decreased susceptibility to CAD development in an Iranian population. Based on the present study, the carriers of K allele exhibited lower risk of CAD development than the carriers of R allele (OR = 0.50, 95% CI = 0.33-0.75, P = 0.001) Also, the severity of CAD was significantly lower in K allele carriers of R219K polymorphism relative to homozygous carriers of R allele. However, the association of R219K polymorphism with the lipid profile didn’t show any significant association.
The association of an R219K polymorphism of ABCA1 gene with CAD risk and lipid profile has been investigated in several studies. The study by Abd El-Aziz et al. reported that the K allele of ABCA1gene confers protection against CAD development [10]. In another study by Yin et al., it was shown that carriers of K allele relative to carriers of R allele of R219K polymorphism of ABCA1 gene had a significantly lower risk for progression of atherosclerosis (P < 0.01) [18]. Also, the study by Benton et al. conducted in the United States indicated that the KK genotype of R219K polymorphism was associated with slightly higher HDL - C and thus may protect against subclinical cardiovascular disease [19]. Interestingly, in a recently published study by Au et al., it was shown that ABCA1 R219K K variant allele may associate with decreased risk of cerebrovascular disease, and thus provided further evidence for the protective effects of ABCA1 R219K K variant allele on the development of another vascular disease such as cerebrovascular disease [20].
By these studies, the current study demonstrated a lower risk of CAD development in a carrier of K allele than carriers of R allele of ABCA1 R219K polymorphism. On the contrary, Rejeb et al. didn’t find any significant association between R219K polymorphism and CAD risk in a Tunisian population [9]. Moreover, Li et al concluded that the R219K polymorphism does not seem to influence CAD risk in a China population [13]. Also, the study by Cyrus et al in a Saudi Arabians population identified that the KK genotype of ABCA1 R219K polymorphism acts as a promoting risk factor for CAD development [16]. There are several possible speculations for the inconsistent results of association studies. Some of these controversies may be related to different genetic background and genetic diversity among ethnicities. Ethnic differences may influence the impact of this polymorphism on CAD risk [11]. Indeed, a recently published study by Liu et al indicated interethnic differences in the genotype distribution of R219K genetic polymorphism and demonstrated that this genetic variant acts as a significant protective factor against CAD development in Asian population but not in some Caucasians populations [21]. Also, population-specific linkage disequilibrium between markers loci and disease-susceptibility loci, confounding sampling bias, sample selection criteria, variation in study design, misclassification of phenotypes, gene-gene and gene-environment interactions are other factors that influence genetic association results [22]. Moreover, a great difference in the K allele frequency of R219K polymorphism was seen in various ethnic groups that may influence the consistency of association studies [19][23].
Increased circulating level of HDL - C is a significant protective factor against CAD development. HDL - C deficiency is the most common lipid abnormality observed among patients with premature CAD [10]. At least 50% of HDL - C levels are determined by genetic factors [4]. ATP - binding cassette transporter A1 (ABCA1) is shown to play an essential role in the HDL-C biosynthesis by efflux of cellular cholesterol to apolipoprotein - A1. The effect of R219K polymorphism of ABCA1 gene on lipid profile has been studied extensively with discrepant results [9][14][17][24][25]. Our study didn’t find any significant association between this common polymorphism and TG, TC, HDL - C and LDL - C levels. In accordance with present study, some other studies also reported no significant association between the K allele of ABCA1 R219K polymorphism and serum HDL - C levels and TG levels [17][23][25] However, some other studies reported this common polymorphism as a significant contributing factor for the plasma lipid levels [9][19][24]. Recently, a study by Rejeb et al. reported that the K allele was significantly associated with a higher mean HDL - C concentration [9]. Also, another study by Mokuno et al. reported that the mean HDL - C level was slightly higher in KK genotype than in RR genotype in both men and women [24]. Moreover, the study by Benton et al. demonstrated higher HDL - C level and lower LDL - C and TG levels in carriers of homozygote KK genotype [19].
The causes for the discrepant association of ABCA1 R219K polymorphism with lipid profile in different studies remains to be determined. However, the interaction between genetic and environmental factors plays an essential role in determining the lipid profile. So, the variation in environmental factors such as geographic distribution, climate, diet, lifestyle and socioeconomic status may modify the effect of ABCA1 R219K polymorphism on lipid profile which can explain the inconsistencies among different studies [4][11].
On the other hand, some other studies including the current study have reported a significant association of K allele of ABCA1 R219K polymorphism with a decreased risk of CAD development independent of serum lipid levels [21][26]. According to some studies, cholesterol efflux capacity has been associated with carotid intima-media thickness and the risk of cardiovascular disease independent of HDL-C levels [27, 28]. So, the independent association of K allele of ABCA1 R219K polymorphism with decreased susceptibility to CAD development may be attributed to increased cholesterol efflux capacity seen in K allele carriers of R219K polymorphism [29]. Recently, in a study by Villard et al., it was shown that cholesterol efflux capacity is significantly and inversely associated with an incident of coronary heart events independent of established cardiovascular risk factors including the HDL cholesterol or apoA-I concentrations [30].
The present study bears some limitations as follows (i) The cholesterol efflux capacity and its association with different genotypes of ABCA1 R219K polymorphism were not determined (ii) The plasma concentration of Apo-A and Apo-B were not measured (iii) The other polymorphisms of ABCA1 gene and their interaction with R219K polymorphism were not determined.
In conclusion, the carriage of K allele of the ABCA1 R219K polymorphism has a protective effect on CAD development and correlates with a decreased severity of CAD. This protective effect of the K allele seems to be mediated independently of plasma lipid levels.
Acknowledgment
We would like to thanks, all volunteers for their participation in the study.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Tsompanidi EM, Brinkmeier MS, Fotiadou EH, Giakoumi SM, Kypreos KE. HDL biogenesis and functions: role of HDL quality and quantity in atherosclerosis. Atherosclerosis. 2010;208(1):3–9. doi: 10.1016/j.atherosclerosis.2009.05.034. https://doi.org/10.1016/j.atherosclerosis.2009.05.034 PMid:19595353. [DOI] [PubMed] [Google Scholar]
- 2.Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA. High-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studies. Circ Res. 2014;114(1):205–213. doi: 10.1161/CIRCRESAHA.114.300760. https://doi.org/10.1161/CIRCRESAHA.114.300760 PMid:24385513 PMCid: PMC3918097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmoodi K, Nasehi L, Karami E, Soltanpour MS. Association of Nitric Oxide Levels and Endothelial Nitric Oxide Synthase G894T Polymorphism with Coronary Artery Disease in the Iranian Population. Vasc Specialist Int. 2016;32:105–112. doi: 10.5758/vsi.2016.32.3.105. https://doi.org/10.5758/vsi.2016.32.3.105 PMid:27699157 PMCid: PMC5045252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ZZhang S, Liu X, Necheles J, et al. Genetic and environmental influences on serum lipid tracking: a population-based, longitudinal Chinese twin study. Pediatr Res. 2010;68(4):316–22. doi: 10.1203/PDR.0b013e3181eeded6. https://doi.org/10.1203/PDR.0b013e3181eeded6 PMid:20606601. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Lin J, Zhu X, et al. Effects of R219K polymorphism of ATP-binding cassette transporter 1 gene on serum lipids ratios induced by a high-carbohydrate and low-fat diet in healthy youth. Biol Res. 2014;47:54. doi: 10.1186/0717-6287-47-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye D, Lammers B, Zhao Y, et al. ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis. Curr Drug Targets. 2011;12(5):647–660. doi: 10.2174/138945011795378522. https://doi.org/10.2174/138945011795378522 PMid:21039336. [DOI] [PubMed] [Google Scholar]
- 7.Mahdy Ali K, Wonnerth A, Huber K, Wojta J. Cardiovascular disease risk reduction by raising HDL cholesterol–current therapies and future opportunities. Br J Pharmacol. 2012;167(6):1177–94. doi: 10.1111/j.1476-5381.2012.02081.x. https://doi.org/10.1111/j.1476-5381.2012.02081.x PMid:22725625 PMCid: PMC3504986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams R, Carnethon M, De Simone G, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. https://doi.org/10.1161/CIRCULATIONAHA.108.191259 PMid:19171871. [DOI] [PubMed] [Google Scholar]
- 9.Rejeb J, Omezzine A, Rebhi L, et al. Associations between common polymorphisms of adenosine triphosphate-binding cassette transporter A1 and coronary artery disease in a Tunisian population. Arch Cardiovasc Dis. 2010;103(10):530–537. doi: 10.1016/j.acvd.2010.10.003. https://doi.org/10.1016/j.acvd.2010.10.003 PMid:21130966. [DOI] [PubMed] [Google Scholar]
- 10.Abd El-Aziz TA, Mohamed RH, Hagrass HA. Increased risk of premature coronary artery disease in Egyptians with ABCA1 (R219K), CETP (TaqIB), and LCAT (4886C/T) genes polymorphism. J Clin Lipidol. 2014;8(4):381–389. doi: 10.1016/j.jacl.2014.06.001. https://doi.org/10.1016/j.jacl.2014.06.001 PMid:25110219. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Tang K, Zhou K, et al. Quantitative assessment of the effect of ABCA1 R219K polymorphism on the risk of coronary heart disease. Mol Biol Rep. 2012;39(2):1809–1813. doi: 10.1007/s11033-011-0922-z. https://doi.org/10.1007/s11033-011-0922-z PMid:21643759. [DOI] [PubMed] [Google Scholar]
- 12.Zhao GJ, Yin K, Fu YC, Tang CK. The interaction of ApoA-I and ABCA1 triggers signal transduction pathways to mediate efflux of cellular lipids. Molecular Medicine. 2012;18(2) doi: 10.2119/molmed.2011.00183. https://doi.org/10.2119/molmed.2011.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Wang LF, Li ZQ, Pan W. Effect of R219K polymorphism of the ABCA1 gene on the lipid-lowering effect of pravastatin in Chinese patients with coronary heart disease. Clin Exp Pharmacol Physiol. 2009;36(5-6):567–570. doi: 10.1111/j.1440-1681.2008.05119.x. https://doi.org/10.1111/j.1440-1681.2008.05119.x PMid:19673941. [DOI] [PubMed] [Google Scholar]
- 14.Zargar S, Wakil S, Mobeirek AF, Al-Jafari AA. Involvement of ATP-binding cassette, subfamily A polymorphism with susceptibility to coronary artery disease. Biomed Rep. 2013;1(6):883–888. doi: 10.3892/br.2013.163. https://doi.org/10.3892/br.2013.163 PMid:24649047 PMCid: PMC3916977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coban N, Onat A, Komurcu Bayrak E, et al. Gender specific association of ABCA1 gene R219K variant in coronary disease risk through interactions with serum triglyceride elevation in Turkish adults. Anadolu Kardiyol Derg. 2014;14:18–25. doi: 10.5152/akd.2013.234. PMid:24084154. [DOI] [PubMed] [Google Scholar]
- 16.Cyrus C, Vatte C, Al-Nafie A, et al. The impact of common polymorphisms in CETP and ABCA1 genes with the risk of coronary artery disease in Saudi Arabians. Hum Genomics. 2016;2(10):8. doi: 10.1186/s40246-016-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marvaki A, Kolovou V, Katsiki N, et al. Impact of 3 Common ABCA1 Gene Polymorphisms on Optimal vs Non-Optimal Lipid Profile in Greek Young Nurses. Open Cardiovasc Med J. 2014;8:83–87. doi: 10.2174/1874192401408010083. https://doi.org/10.2174/1874192401408010083 PMid:25279016 PMCid: PMC4181169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin YW, Li JC, Gao D, et al. Influence of ATP-Binding Cassette Transporter 1 R219K and M883I Polymorphisms on Development of Atherosclerosis: A Meta-Analysis of 58 Studies. PLoS One. 2014;9(1):e86480. doi: 10.1371/journal.pone.0086480. https://doi.org/10.1371/journal.pone.0086480 PMid:24466114 PMCid: PMC3900558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benton JL, Ding J, Tsai MY, et al. Associations between two common polymorphisms in the ABCA1 gene and subclinical atherosclerosis: Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;193:352–360. doi: 10.1016/j.atherosclerosis.2006.06.024. https://doi.org/10.1016/j.atherosclerosis.2006.06.024 PMid:16879828. [DOI] [PubMed] [Google Scholar]
- 20.Au A, Griffiths LR, Irene L, Kooi CW, Wei LK. The impact of APOA5, APOB, APOC3 and ABCA1 gene polymorphisms on ischemic stroke: Evidence from a meta-analysis. Atherosclerosis. 2017;265:60–70. doi: 10.1016/j.atherosclerosis.2017.08.003. https://doi.org/10.1016/j.atherosclerosis.2017.08.003 PMid:28865324. [DOI] [PubMed] [Google Scholar]
- 21.Liu N, Hou M, Ren W, et al. The R219K polymorphism on ATP-binding cassette transporter A1 gene is associated with coronary heart disease risk in Asia population: evidence from a meta-analysis. Cell Biochem Biophys. 2015;71:49–55. doi: 10.1007/s12013-014-0161-8. https://doi.org/10.1007/s12013-014-0161-8 PMid:25104170. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg NA, Vanliere JM. Replication of Genetic Associations as Pseudoreplication due to Shared Genealogy. Genet Epidemiol. 2009;33(6):479–87. doi: 10.1002/gepi.20400. https://doi.org/10.1002/gepi.20400 PMid:19191270 PMCid: PMC2766236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan T, Wang Y, Liu X. Relationship between single nucleotide polymorphism of ABCA1 gene and susceptibility of coronary heart disease in Mongolian/Han population. Int J Clin Exp Med. 2017;10(2):3478–3485. [Google Scholar]
- 24.Mokuno J, Hishida A, Morita E, et al. ATP-binding cassette transporter A1 (ABCA1) R219K (G1051A, rs2230806) polymorphism and serum high density lipoprotein cholesterol levels in a large Japanese population: cross-sectional data from the Daiko Study. Endocr J. 2015;62(6):543–549. doi: 10.1507/endocrj.EJ14-0577. https://doi.org/10.1507/endocrj.EJ14-0577 PMid:25877294. [DOI] [PubMed] [Google Scholar]
- 25.Kolovou V, Kolovou G, Marvaki A, et al. ATP-binding cassette transporter A1 gene polymorphisms and serum lipid levels in young Greek nurses. Lipids Health Dis. 2011;10:56. doi: 10.1186/1476-511X-10-56. https://doi.org/10.1186/1476-511X-10-56 PMid:21489276 PMCid: PMC3090361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halalkhor S, Mesbah-Namin SA, Daneshpour MS, Hedayati M, Azizi F. Association of ATP-binding cassette transporter-A1 polymorphism with apolipoprotein AI level in Tehranian population. J Genet. 2011;90(1):129–132. doi: 10.1007/s12041-011-0030-9. https://doi.org/10.1007/s12041-011-0030-9 PMid:21677398. [DOI] [PubMed] [Google Scholar]
- 27.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135. doi: 10.1056/NEJMoa1001689. https://doi.org/10.1056/NEJMoa1001689 PMid:21226578 PMCid: PMC3030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–2393. doi: 10.1056/NEJMoa1409065. https://doi.org/10.1056/NEJMoa1409065 PMid:25404125 PMCid: PMC4308988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villard EF, EI Khoury P, Frisdal E, et al. Genetic determination of plasma cholesterol efflux capacity is gender-specific and independent of HDL-cholesterol levels. Arterioscler Thromb Vasc Biol. 2013;33(4):822–828. doi: 10.1161/ATVBAHA.112.300979. https://doi.org/10.1161/ATVBAHA.112.300979 PMid:23372063. [DOI] [PubMed] [Google Scholar]
- 30.Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet. Diabetes Endocrinol. 2015;3(7):507–13. doi: 10.1016/S2213-8587(15)00126-6. https://doi.org/10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


