Abstract
INTRODUCTION:
Type 1 Diabetes Mellitus (T1D) is an autoimmune disease that results from the destruction of insulin-producing beta cells of the pancreas by autoreactive T cells. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells that can potently suppress T cell responses.
AIM:
To detect the presence of MDSCs in T1D and compare their percentage in T1D versus healthy individuals.
METHOD:
Thirty T1D patients were included in the study. Diabetic patients with nephropathy (n = 18) and diabetic patients without nephropathy (n = 12). A control group of healthy individuals (n = 30) were also included. CD33+ and HLA-DR– markers were used to identify MDSCs by flow cytometry. CD14 positive and negative MDSCs subsets were also identified.
RESULTS:
MDSCs was significantly increased in T1D than the control group and diabetic patient with nephropathy compared to diabetic patients without nephropathy. M-MDSCs (CD14+ CD33+ HLA–DR−) were the most abundant MDSCs subpopulation in all groups, however their percentage decrease in T1D than the control group.
CONCLUSION:
MDSCs are increased in the peripheral blood of T1D with a predominance of the CD14+ MDSCs subset. Future studies are needed to test the immune suppression function of MDSCs in T1D.
Keywords: Myeloid-derived suppressor cells, Type 1 Diabetes, Diabetic nephropathy, CD 14+, MDSCs subsets
Introduction
Autoimmune Diabetes (AID) also known as type 1 diabetes (T1D) usually develops as a result of the triad of environmental factors, genes and immune system interaction [1]. Several genes like cytotoxic T-lymphocyte antigen 4 (CLTA4), major histocompatibility complex class II (MHC II), and others drive an immune response that controls the T1D outcome. The over-reactive immune system also occurs in AID patients [2]. Subsequently, loss of insulin-producing pancreatic β – cells, caused by infiltrating immune cells, results in hypoinsulinemia, hyperglycemia and fatal complications. To date, no means for preventing or curing AID exists [3]. Numerous strategies have been developed, trying to restore physiological insulin production in diabetic patients [4]. Despite some progress, restoring self - tolerance or specifically correcting autoimmunity remains a crucial step toward reversing AID. In this respect, research and clinical interest in regulatory Treg and MDSC have increasingly grown [5][6][7].
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells that are defined by their myeloid origin, immature state and ability to suppress T cell responses potently. Under normal conditions, they differentiate into dendritic cells, macrophages and granulocytes. However, in pathological states such as infection, inflammation or cancer, there is an arrest of differentiation of this population of cells resulting in their accumulation [8][9][10]. The composition and percentage of MDSCs were found to vary according to disease nature. However, MDSC of different origins has potent suppressive activities [11][12].
Human MDSCs were initially defined as HLA-DR-CD33+ or CD14-CD11b+ cells, with both phenotypes identifying cell populations with T cell suppressive activity [13][14]. Recently, other markers have defined MDSC subsets, including CD14- positive monocytic MDSC (Mo-MDSC) and CD15- positive granulocytic MDSC (G-MDSC). The expression of CD14 on MDSCs as a Mo-MDSC marker was initially controversial but has now become accepted. The CD14 expression is difficult to be used as a lineage marker as low levels of this marker are expressed on polymorphonuclear leukocytes (PMNs). Therefore, differentiation between CD14bright versus CD14low in the assessment of MDSCs is crucial [15].
Accumulative evidence shows that T- cells play a pivotal role in AID pathogenesis through the secretion of inflammatory cytokines and destruction of β-cells [16]. Accordingly, the importance of studying MDSCs emerges since several therapeutic strategies targeting MDSCs could inhibit immune responses in the setting of autoimmune disease [17].
This study aimed to determine the frequency of MDSCs and study MDSCs subsets in type1 autoimmune diabetes and to evaluate the effect of diabetic nephropathy on the frequency of MDSCs for later studying of their suppressor activities on T cells and their potential therapeutic use in AID.
Material and Methods
Subjects
This study enrolled thirty type 1 diabetic patients from the diabetes outpatient clinic of National Research Center (NRC) and thirty age - and gender-matched healthy controls. In particular, inclusion criteria for patients were age range 20 - 60 years. The first group of patients included 12 T1D without diabetic nephropathy. Diagnosis of diabetes was based on criteria of the American Diabetes Association [18]
The second group of patients included 18 T1D with diabetic nephropathy. Diagnosis of diabetic nephropathy is based on detection of abnormal levels of urinary albumin in diabetic patients combined with exclusion of other causes of albuminuria. Albumin measurements are defined as follows [19]:
Normal albuminuria: urinary albumin excretion < 30 mg/24h;
Microalbuminuria: urinary albumin excretion in the range of 30 - 299 mg/24h;
Macroalbuminuria: urinary albumin excretion ≥ 300 mg/24h.
Exclusion criteria included Type 2 diabetes, any febrile illness during the last three months, chronic inflammatory/rheumatic disease or other chronic kidney disease.
Clinical data were collected from patients’ records. Informed consent was obtained from each subject. Approval for the study was obtained from the Ethics Committee of National Research Center, Cairo, Egypt (Number of the approval: 15160).
Samples
From each subject, 3 ml venous blood was taken and divided into three sterile tubes. Two tubes were sent to the Clinical Chemistry laboratory in NRC for the determination of fasting blood sugar level; two hours postprandial blood sugar test, glycated haemoglobin level. The third tube was sent to the flow cytometry unit in Kasr El Aini hospital for immunofluorescent staining for the determination of myeloid-derived suppressor cells.
Samples were kept at room temperature and were not shaken. They were analysed within 24 hours of venipuncture.
Microalbuminuria was defined by urinary albumin excretion of at least 30 mg in a 24 - hour period
Flowcytometry
First, isolation of human PBMCs was performed using a Ficoll - Hypaque density gradient before antibody staining. Second, 100 µl of test sample was incubated with 20 µl of specific monoclonal antibodies: CD33 PE (Beckman Coulter, USA), CD14 - FITC (Beckman Coulter, USA) and HLA - DR PC5 (Beckman Coulter, USA) at room temperature in the dark, same species isotypes served as a negative control.
After 20 min of incubation, 2 ml of Phosphate Buffer Saline were added to each tube of monoclonal treated cells. The mixtures were then centrifuged for 5 min at 150 xg at room temperature followed by discarding the supernatant and resuspending the pellet in 3 ml PBS. Cell analysis was performed using CYTOMICS FC 500 Flow Cytometer (Beckman Coulter, FL, USA) and analysed using CXP Software version 2.2.
Statistical analysis
The data were analysed using Microsoft Excel 2010 and statistical package for social science (SPSS version 24.0) for Windows (SPSSIBM, Chicago, IL). Results were expressed as mean ± SD with 95% confidence interval using the range for quantitative variables, and using the frequencies and percentage for qualitative ones; a p-value < 0.05 was considered statistically significant. Spearman’s rank correlation coefficient (r) was done to show the correlation between different parameters in this study. Diagnostic parameters of subjects were compared using the non-parametric Wilcoxon – Mann - Whitney U - test, whereas the parametric parameters were compared using the Paired samples (t) test. Also, Chi-square (χ2) test was used for comparison of categorical data. Whenever the expected values in one or more of the cells in a 2 x 2 tables were less than 5, Fisher exact test was used instead and using linear by linear association in larger than 2 x 2 cross - tables.
Results
Characteristics and clinical data
A total number of 30 T1D patients were included in the study. Diabetic patients with nephropathy represented 60% (n = 18) and diabetic patients without nephropathy represented 40% (n = 12). A control group of healthy individuals (n = 30) were also included. Demographic and laboratory data of Patients and controls are shown in Table 1. Human MDSCs are immature myeloid cells that express the cell surface antigen CD33 and weakly express HLA-DR being a mature myeloid cell marker [20]. Accordingly, we used the surface markers CD33+ within HLA-DR neg cells to identify MDSCs and compared the frequency of MDSCs in the peripheral blood of the two groups of patients - diabetes with nephropathy (Figure 1a) and diabetes without nephropathy (Figure 1b) - and of the control group as well (Figure 1c).
Table 1.
Demographic and laboratory data of the two groups of patients and control
| Descriptive parameters | Control N=30 | Diabetic patients Without nephropathy N=12 | Diabetic patients With nephropathy N=18 | |
|---|---|---|---|---|
| Range | 28 - 55 | 35 - 55 | 28 – 56 | |
| Age | Mean ± SD | 38.3 ± 5.3 | 49.1 ± 5.3 | 40.0 ± 8.7 |
| Female | 21 (70.0%) | 9 (75.0%) | 15(83.3%) | |
| Sex | Male | 9 (30.0%) | 3 (25.0%) | 3(16.7%) |
| Range | 80-120 | 86-243 | 98-272 | |
| FBS mg/dl | Mean ± SD | 95.6 ± 13.8 | (125.4 ± 47.7) | (123.3 ± 40.3) |
| Range | 90-150 | 110-242 | 120-250 | |
| PP.BS mg/dl | Mean ± SD | 119.6 ± 20.6 | (152.9 ± 32.3) | (155.1 ± 27.9) |
| Range | 3-5.5 | 4.5-7.0 | 5.1-7.2 | |
| Hba1C % | Mean ± SD | 4.22 ± 0.79 | (5.7 ± 0.7) | (5.8 ± 0.6) |
| Range | 16.5-29.0 | 185-310 | ||
| Microalbumin mg/24h | Mean ± SD | --------- | (23.97 ± 2.9) | (253.8 ± 33.8) |
FBS: Fasting blood sugar, PP; BS = postprandial blood sugar; SD = standard deviation.
Figure 1.
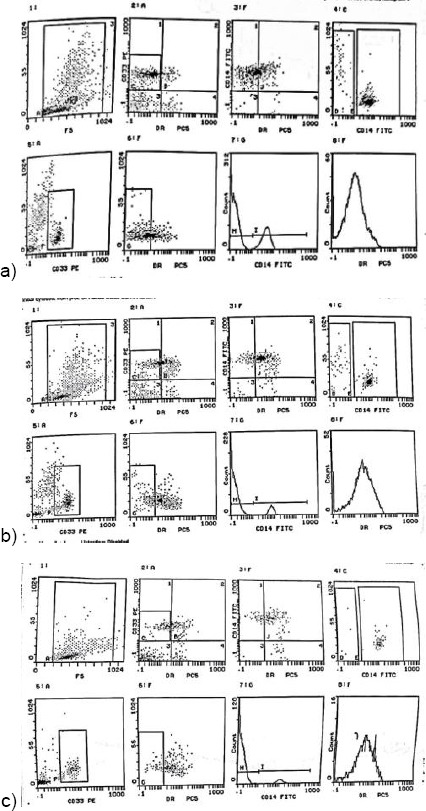
a) Flowcytometry analysis of the percentage of CD33+ HLA-DR- MDSCS in T1D patients with diabetic nephropathy; b) Flowcytometry analysis of the percentage of CD33+ HLA-DR- MDSCS in T1D patients without diabetic nephropathy; c) Flowcytometry analysis of the percentage of CD33+ HLA-DR- MDSCS in healthy control group
To determine the percentage of MDSCs in patients, acquired cells were first gated based on the expression of HLA DR (PC5). The PC5 subset was comprised of HLA DR- cells. Within this population, the fraction of cells expressing CD33 (PE) and CD14 (FITC) was determined.
Increased frequency of CD33+ HLA - DR-MDSCs in the peripheral blood of T1D patients
The frequency of total MDSCs was increased in the peripheral blood of type 1 diabetes patients compared to the control group with a highly significant difference (Figure 2), (Table 2).
Figure 2.
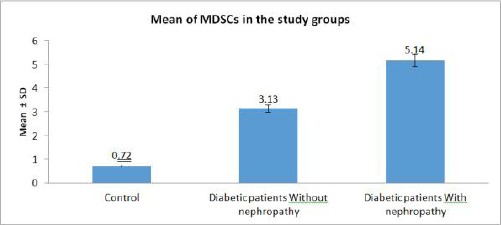
Mean of MDSCs in the study groups
Table 2.
The frequency of MDSCs in both groups of T1D and the control group. Percentage of the two subpopulations of MDSCs CD14+ and CD14- in both patients group compared to the control group
| Descriptive parameters | Control N = 30 | Diabetic patients Without nephropathy N = 12 | Diabetic patients With nephropathy N = 18 | Total Diabetic Patients | |
|---|---|---|---|---|---|
| MDSCs | Range | 0.3-1.01 | 2.4-3.8 | 3.0-10.3 | 2.4-10.3 |
| Mean ± SD | 0.72 ± 0.24 | (3.13 ± 0.5) | (5.14 ± 2.3) | (4.4 ± 2.07) | |
| CD14 | Range | 98.9-99.7 | 96.0-97.5 | 87.5-99.5 | 87.5-99.5 |
| Positive | Mean ± SD | 99.3 ± 0.3 | (96.9 ± 0.6) | (95.4 ± 3.8) | (96.5 ± 3.02) |
| CD14 | Range | 0.1-1.1 | 2.5-4.0 | 0.52-12.5 | 0.52-12.5 |
| Negative | Mean ± SD | 0.62 ± 0.33 | (3.09 ± 0.56) | (4.6 ± 3.8) | (3.98 ± 3.0) |
Then, we subdivided the group of diabetic patients according to nephropathy into two groups: Diabetic patients without nephropathy (n = 12), and Diabetic patients with nephropathy (n = 18), and we compared the frequency of total MDSCs among the two groups and the control group. We found that the frequency of total MDSCs in a diabetic patient with nephropathy was increased than diabetic patients without nephropathy with a highly significant difference (P - value < 0.001) (Figure 2), (Table 2).
The total population of CD33+ HLA–DR- MDSCs obtained from peripheral blood of T1D patients were further divided into two subpopulations of cells expressing CD14+ and CD14-. We compared the two subsets in the two groups of diabetic patients in relation to the control group. Then, we compared the percentage of these 2 MDSCs subsets in the two groups of diabetic patients (Table 2).
Analysis of the previous results of MDSC subsets (CD14+ and CD14-) demonstrated that all healthy individuals in the control group, diabetic patients with nephropathy and diabetic patients without nephropathy had higher numbers of CD14+ MDSC and lower number of CD14- MDSCs.
The percentage of CD14+ subset tended to decrease in the T1D patients compared to the control group even though their absolute number increased due to the increase of total MDSCs. On the contrary, Percentage of CD14- subset has increased in T1D patients than the control group.
Among the diabetes patients, the percentage of CD14+ subset was less in diabetic patients with nephropathy than diabetic without nephropathy. On the contrary, the percentage of CD14- was increased in diabetic patients with nephropathy than diabetic without nephropathy (Figure 3), (Table 2).
Figure 3.
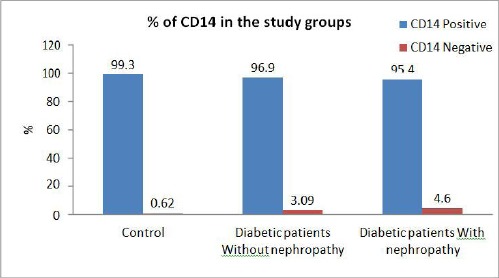
Comparison of CD14+ and CD14- subsets among the three tested groups
In Brief, comparing diabetic patients with and without nephropathy to the control group demonstrated a highly significant difference in the frequency of total MDSCs and a significant difference in the proportion of CD14+ and CD14- subset of MDSCs among the three groups.
Correlation between CD33+ HLA - DR- MDSCs and HbA1C
We examined the possible correlations between CD33+ HLA - DR- MDSCs and HbA1C in T1D patients. As shown in Figure 4, CD33+ HLA - DR- MDSCs were positively correlated with levels of HbA1C (r = 0.702, P < 0.001) (Figure 4).
Figure 4.
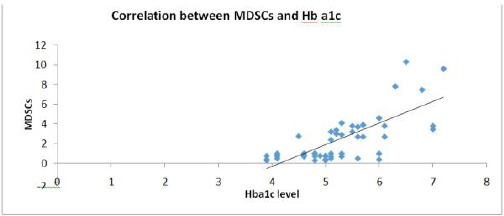
Positive correlation (direct proportion) between MDSCs and Hba1c (r = 0.702; P < 0.001)
Correlation of CD33+ HLA - DR- MDSCs, microalbumin
We examined the possible correlations of CD33+ HLA - DR- MDSCs, levels of microalbumin in T1D patients. As shown in Figure 5, CD33+ HLA-DR- MDSCs were positively correlated with levels of microalbumin (r = 0.814, P < 0.001) (Figure 5).
Figure 5.
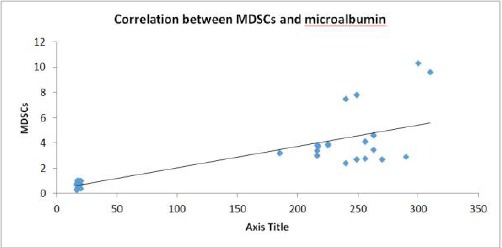
Positive correlation (direct proportion) between MDSCs and microalbumin (r = 0.814; P < 0.001)
Discussion
MDSCs are a heterogeneous population of cells that may inhibit the immune response, which makes them important goals for the treatment of autoimmune diseases [21]. Some studies focused on the importance of MDSCs, and they suggested the possibility of their use in the prevention and treatment of T1D due to their potential suppressor function [22]. On the contrary, MDSCs was found at different stages of differentiation, accumulating in various pathological conditions like inflammation and autoimmune diseases [21]. These findings appear to be ambiguous. In our work, we studied the frequency of MDSCs in T1D patients.
According to Kracht et al. destruction of Beta-islets of the pancreas and development of autoimmune T1D and the antitumour immunity may share some common pathways and may differ in others [23]. Environmental factors have an important role in the aetiology of T1D as well as the antitumor immune response. The subsequent inflammation induces massive destruction of the target cells. Both beta cells and tumour cells respond to inflammatory signals by releasing proinflammatory cytokines such as (IL-1b), chemokines (CCL2, CXCL10) [24][25], and by producing neoantigens that are recognised by a tumour - specific or autoreactive T cells [26][27]. Overexpression of human leukocyte antigen (HLA) is an early indicator of islet distress, seemingly before insulitis in the case of T1D [28]. While tumours can evade immune detection by downregulation of HLA class I or direct inhibition of lymphocytes [29]. The hyperexpression of HLA class I by beta – cells together with the production of chemo-attractants, results in amplification of the immune response [30]. This, in conjunction with signals produced by infiltrating immune cells (CD8 and CD4 T cells), that lead to a microenvironment comparable to antitumor response. The microenvironment of progressive tumours contains numerous cells that promote tumour growth. Among these cells, we can find MDSCs that prevent cytotoxic T cell activity [31]. In case of T1D, Whitfield - Larry et al., reported an increased frequency of MDSCs in T1D patients in contrast to the islet of diabetic Non-Obese Diabetic (NOD) mice that showed fewer MDSCs suggesting an underlying defect in immune suppression [22]. Even though studies of MDSCs in autoimmunity in mice are frequent, only a few data about MDSCs in human autoimmune diseases are available.
Our work reveals a highly significant increase in the frequency of CD33+ HLA-DR- MDSCs in the peripheral blood of T1D patients. The underlying mechanism of this increase, however, is not well known. In a previous study, Haile et al. had described an increased frequency of peripheral MDSCs in Crohn’s disease and ulcerative colitis [32]. A strong association reported in their study was that most of their patients followed an immunosuppression regimen. In our study, none of the T1D patients was on immunosuppressive treatment, yet our results similarly show an increased frequency of peripheral MDSCs. Therefore, we suggest that the high frequency of peripheral MDSCs might be a general finding in autoimmune diseases and not a result of the use of immunosuppressive drugs.
A possible explanation of MDSCs increase in T1D raised when a previous cancer study suggested that some factors produced by cancer cells and inflammatory cells may have a role in the increased frequency of MDSCs [33][34]. These factors included three cytokines known to be important in the pathogenesis of T1D which are IFN - gamma, IL-1beta, and IL-6 [35][36]. So, increased frequency of peripheral MDSCs in T1D patients is likely a result of the elevation of these cytokines. Some recent studies reported a relation between end-stage renal disease (ESRD) and expansion of MDSCs [37], so we investigated the effect of renal complication of T1D known as diabetic nephropathy on the frequency of MDSCs and whether MDSCs would significantly differ in T1D without nephropathy than in T1D with diabetic nephropathy. We found a highly significant increase of MDSCS in diabetic patients with nephropathy than in those without nephropathy.
Human MDSCs can be identified as CD33+ HLA-DR−, and further divided into 2 subsets: granulocytic CD14− and monocytic CD14+ MDSCs [38][39]. In our study, we analysed the peripheral blood of T1D patients with and without diabetic nephropathy for the presence of 2 recently described MDSCs subpopulations (G-MDSCs and M-MDSCs). Our data demonstrate that MDSCs show highly significant increase in the peripheral blood of patients with T1D compared to healthy controls. M-MDSC phenotype (CD14+ CD33+ HLA–DR-) were the most abundant MDSCs subpopulation in the blood of healthy control group, T1D with and without nephropathy. To our knowledge, this is the first study that compares the difference in the percentage of MDSCs subsets (CD14+ and CD14-) in T1D and stating the decrease in the percentage of CD14+ and the increase in CD14- in T1D compared to the control group.
In conclusion, this study reports an increase in MDSCs in the peripheral blood of T1D patients versus healthy control with a predominance of the CD14+ MDSCs subset. Considering the immunosuppressive effect of MDSCs, we realise that continuous monitoring of the composition of these cells in T1D patients may be highly rewarding. Further future studies are needed to test the immune suppression function of MDSCs in T1D and their potential use as targets to inhibit the immune response in autoimmune disease.
Footnotes
Funding: This work was funded by the National Research Center, Egypt. Grant Number: P100508
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Ermann J, Fathman CG. Autoimmune diseases: genes, bugs and failed regulation. Nature Immunology. 2001;2(9):759–761. doi: 10.1038/ni0901-759. [DOI] [PubMed] [Google Scholar]
- 2.Yang WC. Myeloid-derived Suppressor Cells in Autoimmune Diabetes: Their Anti-diabetic Potential and Mechanism. J Diabetes Metab S. 2013;12:2. https://doi.org/10.4172/2155-6156.S12-004. [Google Scholar]
- 3.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi: 10.1016/S0140-6736(13)60591-7. https://doi.org/10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquali L, Giannoukakis N, Trucco M. Induction of immune tolerance to facilitate βcell regeneration in type 1 diabetes. Advanced drug delivery reviews. 2008;60(2):106–13. doi: 10.1016/j.addr.2007.08.032. https://doi.org/10.1016/j.addr.2007.08.032 PMid:18053613. [DOI] [PubMed] [Google Scholar]
- 5.Hu C, Du W, Zhang X, Wong FS, Wen L. The role of Gr1+cells after anti-CD20 treatment in type 1 diabetes in nonobese diabetic mice. The Journal of Immunology. 2012;188(1):294–301. doi: 10.4049/jimmunol.1101590. https://doi.org/10.4049/jimmunol.1101590 PMid:22140261 PMCid: PMC4361178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Medical microbiology and immunology. 2010;199(3):273–81. doi: 10.1007/s00430-010-0151-4. https://doi.org/10.1007/s00430-010-0151-4 PMid:20376485. [DOI] [PubMed] [Google Scholar]
- 7.Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK. Immunopathology of the human pancreas in type-I diabetes. Seminars in immunopathology. 2011;33(1):9–21. doi: 10.1007/s00281-010-0205-0. https://doi.org/10.1007/s00281-010-0205-0 PMid:20424842. [DOI] [PubMed] [Google Scholar]
- 8.Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunology, Immunotherapy. 2012;61(2):255–63. doi: 10.1007/s00262-011-1161-9. https://doi.org/10.1007/s00262-011-1161-9 PMid:22120756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atretkhany KS, Drutskaya MS. Myeloid-derived suppressor cells and proinflammatory cytokines as targets for cancer therapy. Biochemistry (Moscow) 2016;81(11):1274–83. doi: 10.1134/S0006297916110055. https://doi.org/10.1134/S0006297916110055 PMid:27914453. [DOI] [PubMed] [Google Scholar]
- 10.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. European journal of immunology. 2010;40(11):2969–75. doi: 10.1002/eji.201040895. https://doi.org/10.1002/eji.201040895 PMid:21061430 PMCid: PMC3277452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. The Journal of Immunology. 2008;181(8):5791–802. doi: 10.4049/jimmunol.181.8.5791. https://doi.org/10.4049/jimmunol.181.8.5791 PMid:18832739 PMCid: PMC2575748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Current opinion in immunology. 2010;22(2):238–44. doi: 10.1016/j.coi.2010.01.021. https://doi.org/10.1016/j.coi.2010.01.021 PMid:20171075. [DOI] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews immunology. 2009;9(3):162. doi: 10.1038/nri2506. https://doi.org/10.1038/nri2506 PMid:19197294 PMCid: PMC2828349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nature Reviews Cancer. 2013;13(10):739. doi: 10.1038/nrc3581. https://doi.org/10.1038/nrc3581 PMid:24060865 PMCid: PMC4358792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Petersen-Baltussen HM, Ter Laan M, Wesseling P, Adema GJ. Increase in both CD14-positive and CD15-positive myeloid-derived suppressor cell subpopulations in the blood of patients with glioma but predominance of CD15-positive myeloid-derived suppressor cells in glioma tissue. Journal of neuropathology &experimental neurology. 2015;74(5):390–400. doi: 10.1097/NEN.0000000000000183. https://doi.org/10.1097/NEN.0000000000000183 PMid:25853692. [DOI] [PubMed] [Google Scholar]
- 16.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105–15. doi: 10.1111/imm.12036. https://doi.org/10.1111/imm.12036 PMid:23216602 PMCid: PMC3575763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM, Casares S, Chen SH, Yang WC, Pan PY. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. The Journal of Immunology. 2010;185(10):5828–34. doi: 10.4049/jimmunol.0903636. https://doi.org/10.4049/jimmunol.0903636 PMid:20956337 PMCid: PMC4355963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33(Suppl 1):S62. doi: 10.2337/dc10-S062. https://doi.org/10.2337/dc10-S062 PMid:20042775 PMCid: PMC2797383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazar CM. Diabetic nephropathy;principles of diagnosis and treatment of diabetic kidney disease. Journal of nephropharmacology. 2014;3(1):15. [PMC free article] [PubMed] [Google Scholar]
- 20.Luan Y, Mosheir E, Menon MC, Wilson D, Woytovich C, Ochando J, Murphy B. Monocytic Myeloid-Derived Suppressor Cells Accumulate in Renal Transplant Patients and Mediate CD4+Foxp3+Treg Expansion. American journal of transplantation. 2013;13(12):3123–31. doi: 10.1111/ajt.12461. https://doi.org/10.1111/ajt.12461 PMid:24103111. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Fujino M, Xu J, Li XK. The role and potential therapeutic application of myeloid-derived suppressor cells in allo-and autoimmunity. Mediators of inflammation. 2015:1–14. doi: 10.1155/2015/421927. Article ID: 421927. https://doi.org/10.1155/2015/421927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitfield-Larry F, Felton J, Buse J, Su MA. Myeloid-derived suppressor cells are increased in frequency but not maximally suppressive in peripheral blood of Type 1 Diabetes Mellitus patients. Clinical Immunology. 2014;153(1):156–64. doi: 10.1016/j.clim.2014.04.006. https://doi.org/10.1016/j.clim.2014.04.006 PMid:24769355. [DOI] [PubMed] [Google Scholar]
- 23.Kracht MJ, Zaldumbide A, Roep BO. Neoantigens and microenvironment in type 1 diabetes: lessons from antitumor immunity. Trends in Endocrinology &Metabolism. 2016;27(6):353–62. doi: 10.1016/j.tem.2016.03.013. https://doi.org/10.1016/j.tem.2016.03.013 PMid:27094501. [DOI] [PubMed] [Google Scholar]
- 24.Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, Aldrighetti L. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51(1):55–65. doi: 10.2337/diabetes.51.1.55. https://doi.org/10.2337/diabetes.51.1.55 PMid:11756323. [DOI] [PubMed] [Google Scholar]
- 25.Roep BO, Kleijwegt FS, Van Halteren AG, Bonato V, Boggi U, Vendrame F, Marchetti P, Dotta F. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clinical &Experimental Immunology. 2010;159(3):338–43. doi: 10.1111/j.1365-2249.2009.04087.x. https://doi.org/10.1111/j.1365-2249.2009.04087.x PMid:20059481 PMCid: PMC2819499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Büll C, den Brok MH, Adema GJ. Sweet escape: sialic acids in tumor immune evasion. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2014;1846(1):238–46. doi: 10.1016/j.bbcan.2014.07.005. https://doi.org/10.1016/j.bbcan.2014.07.005 PMid:25026312. [DOI] [PubMed] [Google Scholar]
- 27.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature medicine. 2013;19(11):1423. doi: 10.1038/nm.3394. https://doi.org/10.1038/nm.3394 PMid:24202395 PMCid: PMC3954707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, Roep BO, von Herrath MG. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. Journal of Experimental Medicine. 2012:jem-20111187. doi: 10.1084/jem.20111187. https://doi.org/10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nature Reviews Endocrinology. 2009;5(4):219. doi: 10.1038/nrendo.2009.21. https://doi.org/10.1038/nrendo.2009.21 PMid:19352320. [DOI] [PubMed] [Google Scholar]
- 30.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. https://doi.org/10.1016/j.cell.2010.01.025 PMid:20303878 PMCid: PMC2866629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motallebnezhad M, Jadidi-Niaragh F, Qamsari E S, et al. The immunobiology of myeloid-derived suppressor cells in cancer. Tumor Biology. 2015;37(2):1387–1406. doi: 10.1007/s13277-015-4477-9. https://doi.org/10.1007/s13277-015-4477-9 PMid:26611648. [DOI] [PubMed] [Google Scholar]
- 32.Haile LA, Von Wasielewski R, Gamrekelashvili J, Krüger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135(3):871–81. doi: 10.1053/j.gastro.2008.06.032. https://doi.org/10.1053/j.gastro.2008.06.032 PMid:18674538. [DOI] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews immunology. 2009;9(3):162. doi: 10.1038/nri2506. https://doi.org/10.1038/nri2506 PMid:19197294 PMCid: PMC2828349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lechner MG, Liebertz DJ, Epstein AL. Correction: Characterization of Cytokine-Induced Myeloid-Derived Suppressor Cells from Normal Human Peripheral Blood Mononuclear Cells. The Journal of Immunology. 2010;185(9):5668–5668. doi: 10.4049/jimmunol.1000901. https://doi.org/10.4049/jimmunol.1090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced βcell production of IL-1βcontributes to glucotoxicity in human pancreatic islets. The Journal of clinical investigation. 2002;110(6):851–60. doi: 10.1172/JCI15318. https://doi.org/10.1172/JCI200215318 PMid:12235117 PMCid: PMC151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell IL, Kay TW, Oxbrow L, Harrison LC. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. The Journal of clinical investigation. 1991;87(2):739–42. doi: 10.1172/JCI115055. https://doi.org/10.1172/JCI115055 PMid:1899431 PMCid: PMC296368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing YF, Cai RM, Lin Q, Ye QJ, Ren JH, Yin LH, Li X. Expansion of polymorphonuclear myeloid-derived suppressor cells in patients with end-stage renal disease may lead to infectious complications. Kidney international. 2017;91(5):1236–42. doi: 10.1016/j.kint.2016.12.015. https://doi.org/10.1016/j.kint.2016.12.015 PMid:28215666. [DOI] [PubMed] [Google Scholar]
- 38.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. International immunopharmacology. 2011;11(7):802–7. doi: 10.1016/j.intimp.2011.01.003. https://doi.org/10.1016/j.intimp.2011.01.003 PMid:21237299 PMCid: PMC3478130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ning G, She L, Lu L, Liu Y, Zeng Y, Yan Y, Lin C. Analysis of monocytic and granulocytic myeloid-derived suppressor cells subsets in patients with hepatitis C virus infection and their clinical significance. BioMed research international. 2015;2015:1–8. doi: 10.1155/2015/385378. https://doi.org/10.1155/2015/385378. [DOI] [PMC free article] [PubMed] [Google Scholar]


