Abstract
AIM:
This work investigated associations between tissue inhibitor metalloproteinase-1 and diabetic cardiovascular diseases in type 2 diabetic patients; also it investigated the role of osteopontin in the diagnosis of type 2 cardiovascular diabetes complications.
SUBJECTS AND METHODS:
These were examined on eighty subjects, divided into three groups as follows: twenty volunteer healthy control subjects, thirty type 2 diabetes mellitus (DM) patients, and thirty cardiovascular, diabetic patients. Full clinical measurements were carried out, and the expression level of tissue inhibitor metalloproteinase-1 in blood samples was analysed by real-time PCR, using gene-specific primer pairs. Also osteopontin concentrations had been measured by the enzyme-linked immunosorbent assay. Data were tested statistically by parametric tests.
RESULTS:
The concentrations of osteopontin and the expression levels of tissue inhibitor metalloproteinase-1 were significantly increased in diabetic and cardiovascular diabetic groups compared to control group also they were significantly increased in the cardiovascular diabetic group compared to the diabetic group.
CONCLUSION:
Tissue inhibitor metalloproteinase-1 and osteopontin concentrations were significantly increased in diabetic patients with cardiovascular complications than other groups.
Keywords: Molecular markers, expression level, tissue inhibitor metalloproteinase-1, OPN concentrations, diabetic cardiovascular diseases
Introduction
The expanding commonness of type 2 diabetes mellitus (T2DM) calls for creating corresponding screening systems for early recognisable proof of high-risk people for the disease or its complexities [1]. The condition is comorbid with macrovascular disorders, for example, coronary artery disease, peripheral arterial disease, and stroke. Diabetes mellitus is a major risk cause for cardiovascular disease (CVD) which is the main cause of death among the diabetic patients. Diabetes mellitus intensifies atherosclerosis and heart failure mechanisms. Regrettably, these mechanisms are not sufficiently modulated by therapeutic strategies focusing only on the glycemic control with currently available drugs or approaches [2], underscoring the requirement for forceful CVD risk factor management.
The diagnosis and screening of diabetes mellitus have been changed since the earlier scientific statement, with the incorporation of glycated haemoglobin (HbA1c) in the diagnostic gauges of type II diabetes of at least 6.5% [3]. Specifically, HbA1c was used as a preferable standardised assay over glucose, a better indicator for overall glycemic exposure, and as less subject to pre-analytic instability, prandial status, biological variability, and intense stress [4].
While insulin and glucose are the most entrenched biomarkers, progresses in the “omics” technologies have recognised the extent of molecules including genetic variants, small metabolites, RNA transcripts, and proteins that could serve as indicators of diabetes risk [5].
Circulating markers of extracellular matrix turnover, such as matrix metalloproteinases (MMP) and their tissue inhibitors (TIMP), are essential to the vascular alternations of atheromatous vascular disease. TIMP-1 was considered as an endogenous MMP inhibitor that might include in the vascular matrix fibrosis [6]. Also, it has a role in diastolic dysfunction and left ventricular hypertrophy by diminishing cardiac collagen type I turnover and expanding cardiac stiffness and mass [7].
In diabetes, TIMP-1 and MMP-9 levels are significantly increased [8], also in this condition, peripheral and central artery stiffness is increased [9]. Amelioration in the metabolic control leads to decreasing in TIMP-1 levels, and this raises TIMP-1 probability as a marker of vascular composition in diabetes mellitus [10], a potential purpose of mediation.
Phosphoprotein osteopontin (OPN), also called secreted phosphoprotein 1 (SPP1), urinary stone protein, early T lymphocyte activation 1, and bone sialoprotein is found in various cell types [11]. In prevalent patients, a significant association was observed between atherosclerotic plaque development and plasma OPN levels, independently of conventional risk factors [12]. Moreover, high glucose and advanced glycation end product may lead to upregulation of OPN expression in the vascular wall of diabetic animal models and diabetic patients [13].
In this study, the hypothesis that there may be a relationship between elevated both OPN levels and TIMP-1 expression levels and the presence of CVD in type 2 diabetic patients were tested.
Subjects and Methods
Patients’ data
T of 80 subjects was recruited for that study and divided into three groups. Twenty volunteer healthy control subjects (G1), thirty type 2 diabetes mellitus (DM) patients (G2); disease duration 5 ± 3 years, and thirty type 2 diabetic patients with cardiovascular (coronary heart) diseases (G3); disease duration 3 ± 1 years. Type II diabetic patients were those consecutively referred during routine medical care visits from an outpatient diabetes clinic (National Institute of Diabetes in Egypt). If patients had any known systemic diseases other than diabetes that can influence the needed results such as epidemiological diseases, chronic periodontitis, nephropathy, retinopathy, neuropathy, gallbladder, liver, and lung diseases, they were excluded in this study. Participation in this study is completely voluntary. Patients decide to participate, can stop participating at any time and may decide not to answer any specific question. Participants’ decision not to continue participating will not influence the nature of their relationship with a researcher or with a staff of this study either now or in the future. If a patient withdraws from the study, all associated data collected will be immediately destroyed wherever possible.
Laboratory markers of diabetes mellitus
Blood samples were collected from all fasting participants in this study, Lipemic and hemolyzed samples were excluded, samples were analysed for HbA1c by cation - exchange resin method [14]. Total cholesterol (TC) and triglycerides (TG) in serum were measured by colourimetric enzymatic method [15]. Also, high-density lipoprotein cholesterol (HDL cholesterol) was measured [16], and low-density lipoprotein cholesterol (LDL cholesterol) was evaluated by Friedewald formula [17]. Moreover, heart function biomarkers such as creatine phosphokinase-MB (CK-MB), creatine phosphokinase (CK), and lactate dehydrogenase (LDH) were measured by enzymatic method [18].
Tissue inhibitor of metalloproteinases-1 (TIMP-1) analysis TIMP-1 expression levels were determined using Real Time PCR (qPCR) which performed with SYBR Green (5X HOT FIREPol®EvaGreen® qPCR Mix Plus) for tenfold diluted cDNA samples in a triplet set using specific primers pairs which synthesized in HVD Life Sciences co., Vienna, Austria; forward primer sequence (CTGGCTTCTGGCATCCTGTTG) and reverse primer sequence (GTCTGGTTGACTTCTGGTGTCC) (NG_012533.1, NM_003254.2) against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a reference gene; forward primer sequence (CAGCCTCAAGATCATCAGCAATG) and reverse primer sequence (CAGTCTTCTGGGTGGCAGTGA) (NG_007073.2, Variant1: NM_002046.5, Variant 2: NM_001256799.2, Variant 3: NM_001289745.1, Variant 4: NM_001289746.1). Blood RNA was extracted by EZ-10 column blood RNA purification kit (cat.# BS82313) according to the manufacturer’s instructions, and it was eluted from the membrane in the presence of RNase - free water. First cDNA strand was synthesised immediately after RNA extraction. Reaction components were mixed as follows: 5 μg RNA, 0.5 μl (10 μmol) Oligo dT and 0.5 μl (10 μmol) random primer, then the volume was adjusted to 12.5 μl using diethyl pyrocarbonate (DEPC) treated water. The reaction tubes were mixed well and incubated 5 min at 60°C to remove the RNA secondary structure, then chilled on ice. Finally, the following components were added to the reaction mixture: 2 μl (10 mM) dNTPs, 4 μl (5 X) Moloney murine leukaemia virus (MMLV) buffer, 0.5 μl ribolock (40 u/μl) and 1 μl reverse transcriptase enzyme (MMLV-RTase) (20 u/μl). As a termination step, the final reaction mixture (20 μl) was spun down and incubated at room temperature for 10 min, then at 42°C for 1hour followed by 10 min at 70°C. cDNA samples were stored at - 20°C for the amplification steps which performed on Bio-Rad CFX ManagerTM software, version 3.1. The qPCR was performed according to the following program: Initial denaturation and polymerase activation step for 10 min at 95°C, denaturation step at 95°C for 30 sec, annealing step for 30 sec at 57°C for GAPDH and TIMP-1 and finally, the elongation step for 45 sec at 72°C. The denaturation, the annealing and the elongation steps were performed at 50 cycles.
Osteopontin (OPN) analysis
Osteopontin concentrations were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s protocol (BOSTER Biological Technology Co., Inc., USA; cat.# EK0482). All samples were analysed in duplicate. Horseradish peroxidase was conjugated with the secondary antibody, and tetramethyl benzidine (TMB) was used as a substrate. The absorbance was measured at 450 nm. Osteopontin concentration was expressed as pg/ml.
Statistical analysis
SPSS 17.0 software was used to execute the statistical analyses, and the results were represented by the mean ± SD. Student’s t-test was used to make a comparison between the groups and correlations were assessed by Pearson’s correlation test using P < 0.05 as considered to be significant.
Results
Clinical findings in the diabetic patients
Fasting blood sugar (FBS), glycosylated Hb (HbA1c%), Lipid profile; total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C), LDL-C/HDL-C ratio, cardiac biomarkers; creatine phosphokinase (CK), creatine phosphokinase-MB isoenzyme (CK-MB), and lactate dehydrogenase (LDH) activities were significantly higher in the cardiovascular diabetic groups than that in the control and the diabetic group, while high-density lipoprotein cholesterol (HDL-C) concentrations were significantly lower in the cardiovascular, diabetic groups than that in the control and the diabetic group as clarified in Table 1.
Table 1.
Some clinical data in control and different tested groups
| Normal range | Control group | Diabetic Group | Cardiovascular diabetic group | |
|---|---|---|---|---|
| FBS (mg/dl) | 60-110 | 84.0 ± 8.9** | 268.6 ± 94.2** | 347.0 ± 66.9 |
| HbA1c (%) | < 6.5 | 4.9 ± 0.7** | 9.1 ± 2.0** | 10.3 ± 1.1 |
| TC (mg/dl) | < 200 | 105.8 ± 9.1** | 157.9 ± 28.9** | 262.7 ± 19.8 |
| TG (mg/dl) | 35-140 | 75.6 ± 8.7** | 123 ± 22.8** | 387.8 ± 71.9 |
| HDL-C (mg/dl) | >55 | 59.9 ± 4.6** | 52.9 ± 6.8** | 22.9 ± 4.7 |
| LDL-C (mg/dl) | < 150 | 30.6 ± 11.0** | 80.4 ± 30.1** | 162.2 ± 21.6 |
| LDL-C/HDL-C | < 2 | 0.5** | 1.6** | 7.4 |
| CK (U/L) | Up to 120 | 54.5 ± 10.2** | 42.5 ± 7.8** | 128.1 ± 23.7 |
| CK-MB (U/L) | 0-24 | 21.9 ± 3.9* | 18.5 ± 3.8** | 25.2 ± 3.7 |
| LDH (U/L) | 160-320 | 207.3 ± 12.5** | 214.6 ± 23.8** | 620.3 ± 101.9 |
Mean ± SD, vs. cardiovascular diabetic group.
P ≤ 0.05;
P ≤ 0.001.
TIMP-1 expression findings in the diabetic patients
The resulted data of the normalized expression ratio of TIMP-1 to GAPDH as a reference gene was represented in Table 2, and Fig. 1, A. Also individuals’ data were statistically analyzed from the point of view as ± S.D and S.E. Then the t-value results were checked on student’s t-test to detect the significance level (p-value).
Table 2.
Statistical analysis of TIMP-1 expression levels and osteopontin concentrations in control and different tested groups
| Control group | Diabetic group | Cardiovascular diabetic group | |||
|---|---|---|---|---|---|
| Statistical analysis of TIMP-1 | Avg. dCT | 7.03 | 2.27 | 1.39 | |
| ddCT | 0.0 | -4.77 | -5.65 | ||
| FC than control group | 1.0 | 27.20 | 50.07 | ||
| FC than cardiovascular diabetic group | 0.12 | 0.54 | 1.0 | ||
| ±S.D | 0.75 | 0.9 | 0.72 | ||
| S.E | 0.17 | 0.16 | 0.13 | ||
| F-value (ANOVA) | 327.0 | ||||
| T-value | G1 | - | 20.3** | 26.4** | |
| G2 | - | - | 4.2** | ||
| Statistical analysis of osteopontin | OPN conc. range | 6000-8500 | 9000-14700 | 9550-22000 | |
| OPN conc. mean | 7140 | 10765 | 14421 | ||
| ±S.D | 619.8 | 1588.3 | 3411.6 | ||
| S.E | 138.6 | 290.0 | 622.9 | ||
| F-value ANOVA | 59.6 | ||||
| T-value | G1 | - | 9.7** | 9.4** | |
| G2 | - | - | 5.3** | ||
P ≤ 0.001.
Figure 1.
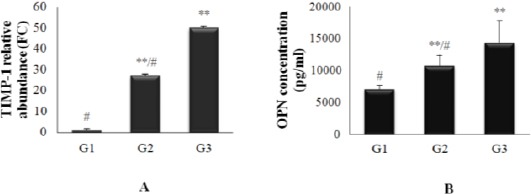
(A) TIMP-1 relative expression level (FC ± S.D). (B) Osteopontin concentrations (Mean ± S.D) in control and different tested groups. **Highly significant different with the control group G1 (p ≤ 0.001), # Highly significant different with cardiovascular, diabetic group G4
The present study showed that; the expression level of TIMP-1 gene significantly increased by 27.2 and 50.07 folds to reference gene; GAPDH, in diabetic and cardiovascular diabetic patients respectively than normal volunteers, so it might be considered as diagnostic marker for DM, and its expression level significantly increased by 1.84 folds in cardiovascular, diabetic patients than the diabetic patients, so it might be considered as diagnostic marker for detection of cardiovascular complications in type 2 diabetes.
Osteopontin (OPN) concentration findings in the diabetic patients
The individuals’ data of osteopontin (OPN) concentration of different tested groups was statistically analysed in Table 2 and evaluated from the point of view as ± S.D and S.E. The result of the t-value is then checked on student’s t-test to find out the significance level (p-value).
From the table, it appeared that highly significant difference (p ≤ 0.001) was found in diabetic group (G2) (10765 ± 1588.3 pg/ml), and cardiovascular, diabetic group (G3) (14421 ± 3411.6 pg/ml) as compared with the control group (G1) (7140 ± 619.8 pg/ml), also highly significant difference was appeared in G3 as compared with G2 (Fig. 1, B).
Table 3 showed highly significant positive correlation observed between osteopontin with some variables; FBS, HbA1c, cholesterol, triglyceride, LDL-C, CK, and LDH, and between TIMP-1 and HDL-C, while there was low significant positive correlation between osteopontin and CK-MB concentrations.
Table 3.
Correlation between osteopontin concentrations and TIMP-1 with other variables
| Osteopontin | TIMP-1 | |||
|---|---|---|---|---|
| Variable | Pearson Correlation (r) | Pearson Correlation (r) | Sig. (2-tailed) | |
| FBS | 0.605 | H.S** | -0.740 | H.S** |
| HbA1c | 0.656 | H.S** | -0.777 | H.S** |
| Cholesterol | 0.736 | H.S** | -0.735 | H.S** |
| Triglyceride | 0.709 | H.S** | -0.640 | H.S** |
| HDL – cholesterol | - 0.666 | H.S** | 0.663 | H.S** |
| LDL- cholesterol | 0.701 | H.S** | -0.734 | H.S** |
| CK | 0.644 | H.S** | -0.407 | H.S** |
| CK-MB | 0.257 | L.S* | -0.074 | N.S |
| LDH | 0.618 | H.S** | -0.549 | H.S** |
N.S. P > 0.05;
P ≤ 0.05;
P ≤ 0.001.
Also there was highly significant negative correlation between osteopontin and HDL-C, also between TIMP-1 and some variables; FBS, HbA1c, cholesterol, triglyceride, LDL-C, CK, and LDH, while there was no correlation between TIMP-1 and CK-MB concentrations. (Fig. 2, A-L).
Figure 2.
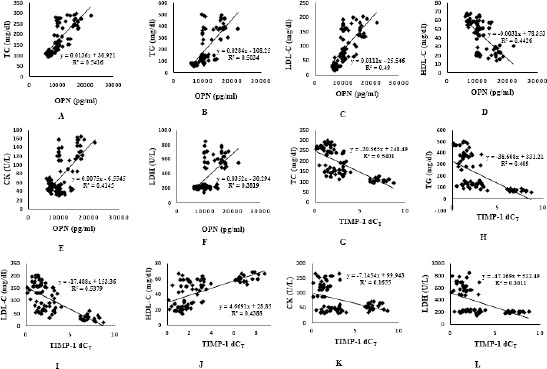
Correlation between osteopontin concentrations and TIMP-1 with some variable
Discussion
Diabetes mellitus is a complex disease affecting nearly all tissues and organs, with 158 metabolic ramifications extending far beyond impaired glucose metabolism. Biomarkers may reflect the presence and/or severity of hyperglycemia and the vascular complications of diabetes [19].
TIMP-1 is a diagnostic marker for diabetes, in this study, the expression level of TIMP-1 was significantly increased in diabetic and cardiovascular diabetic patients than normal volunteers, also increased significantly in cardiovascular, diabetic patients than other diabetic groups, higher levels of circulating TIMP-1 was connected with the higher risk of diabetes and cardiovascular demise. This finding was consistent with Usmanova [20], furthermore, Lee and his colleagues who found that plasma TIMP-1 concentration was significantly raised in type II diabetic patients [21]. Also in this study, the TIMP-1 expression level was significantly higher in diabetic patients with cardiovascular diseases (CVD) than in those without cardiovascular diseases, these were in agreement with Papazafiropoulou and Tentolouris results [22]. TIMP-1 is an endogenous MMP inhibitor that might be involved in vascular matrix fibrosis [6] and has a role in left ventricular hypertrophy and diastolic dysfunction by reducing cardiac collagen type I turnover, thereby increasing cardiac mass and stiffness [6][23]. Increased Central and peripheral artery stiffness [9] occurs with diabetes [24], and higher TIMP-1 circulating levels complete the hypothesis that altered TIMP-1 activities can be related to arterial stiffness.
Another biomarker studied in that work, was osteopontin (OPN) which is a phosphoprotein found in different types of cells [11]. In this study, Levels of serum OPN increased in all diabetic groups significantly in comparing with the control group, the expression of OPN was highly induced by glucose [25], this agreed with Takemoto and his colleagues [26] who reported an increase of serum OPN in diabetic patients, suggesting that it may be involved in the accelerated atherosclerosis-related to diabetes. Also in this study, its concentration increased in a cardiovascular, diabetic group significantly in comparing with the diabetic group. OPN is a pleiotropic cytokine that is a common and relevant component of many acute and chronic vascular or endothelial responses to injury characterised by inflammation and/or fibrosis, including atherosclerosis, arterial neointimal hyperplasia, and aortic stenosis [27], as well as the vascular damage, accompanied diabetes [28]. OPN has been acting as the main factor in the atherosclerosis development [29][ 30]. Plasma OPN concentration is elevated in the essential hypertension, coronary artery disease (CAD), and restenosis [31][32][33]. In the cardiovascular system, OPN is one of the main regulators of the vascular disease and the chronic inflammation [34]. In other aspects of vascular diseases, Yan and his colleagues [35] found that plasma OPN level, parallels with the severity of nephropathy and CAD in diabetes, suggesting that an elevated plasma OPN level can be used as indicator for the diabetic vasculopathy screening.
This study revealed that, the expression levels of TIMP-1 and osteopontin concentrations had highly significant differences between diabetic and cardiovascular diabetic patients in Egypt which appeared in Table 2, so they could be used as molecular biomarkers in the diagnosis of cardiovascular complications in type 2 diabetic patients to predict potential progression and aid in case management.
In the future, we can be hopeful that new blood-based biomarkers will facilitate the discovery, prohibition, and treatment of diabetes and its complications much sooner than overt disease develops.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. https://doi.org/10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133:2459–502. doi: 10.1161/CIRCULATIONAHA.116.022194. https://doi.org/10.1161/CIRCULATIONAHA.116.022194 PMid:27297342 PMCid: PMC4910510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes. Diabet Care. 2015;38(Suppl. 1):S1–S94. PMCid: PMC4582912. [Google Scholar]
- 4.International Expert Committee. International expert committee report on the role of the a1c assay in the diagnosis of diabetes. Diabet Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. https://doi.org/10.2337/dc09-9033 PMid:19502545 PMCid: PMC2699715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindström J, Hellmich M, et al. Development and validation of a risk-score model for subjects with impaired glucose tolerance for the assessment of the risk of type 2 diabetes mellitus—the stop-niddm risk-score. Diabet Res Clin Pract. 2010;87:267–74. doi: 10.1016/j.diabres.2009.11.011. https://doi.org/10.1016/j.diabres.2009.11.011 PMid:20022651. [DOI] [PubMed] [Google Scholar]
- 6.Tayebjee MH, MacFadyen RJ, Lip GYH. Extracellular matrix biology: A new frontier in linking the pathology and therapy of hypertension?J Hypertens. 2003; 21:2211–18. doi: 10.1097/01.hjh.0000098178.36890.81. https://doi.org/10.1097/00004872-200312000-00002 PMid:14654734. [DOI] [PubMed] [Google Scholar]
- 7.Tayebjee MH, Lim HS, Nadar S, MacFadyen RJ, Lip GYH. Tissue inhibitor of metalloproteinse-1 is a marker of diastolic dysfunction using tissue doppler in patients with type 2 diabetes and hypertension. Eur J Clin Invest. 2005;35:8–12. doi: 10.1111/j.1365-2362.2005.01438.x. https://doi.org/10.1111/j.1365-2362.2005.01438.x PMid:15638813. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell P, Timms P, Chandran S, Gordon D. Peripheral blood level alterations of timp-1, mmp-2 and mmp-9 in patients with type 1 diabetes. Diabet Med. 2001;18:777–80. doi: 10.1046/j.1464-5491.2001.00542.x. https://doi.org/10.1046/j.1464-5491.2001.00542.x PMid:11678966. [DOI] [PubMed] [Google Scholar]
- 9.Van Bortel LM, Struijker-Boudier HA, Safar ME. Pulse pressure, arterial stiffness, and drug treatment of hypertension. Hypertension. 2001;38:914–21. doi: 10.1161/hy1001.095773. https://doi.org/10.1161/hy1001.095773 PMid:11641309. [DOI] [PubMed] [Google Scholar]
- 10.Tayebjee MH, Lim HS, MacFadyen RJ, Lip GYH. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 and -2 in type 2 diabetes: Effect of 1 year's cardiovascular risk reduction therapy. Diabet Care. 2004;27:2049–51. doi: 10.2337/diacare.27.8.2049. https://doi.org/10.2337/diacare.27.8.2049. [DOI] [PubMed] [Google Scholar]
- 11.Zhong X-J, Shen X-D, Wen J-B, et al. Osteopontin-induced brown adipogenesis from white preadipocytes through a pi3k-akt dependent signaling. Biochem Biophys Res Commun. 2015;459:553–9. doi: 10.1016/j.bbrc.2015.02.153. https://doi.org/10.1016/j.bbrc.2015.02.153 PMid:25749339. [DOI] [PubMed] [Google Scholar]
- 12.Minoretti P, Falcone C, Calcagnino M, et al. Prognostic significance of plasma osteopontin levels in patients with chronic stable angina. Eur Heart J. 2006;27:802–7. doi: 10.1093/eurheartj/ehi730. https://doi.org/10.1093/eurheartj/ehi730 PMid:16421174. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson-Berglund LM, Zetterqvist AV, Nilsson-Öhman J, et al. Nuclear factor of activated t cells regulates osteopontin expression in arterial smooth muscle in response to diabetes-induced hyperglycemia. Arterioscler Thromb Vasc Bio. 2010;30:218–24. doi: 10.1161/ATVBAHA.109.199299. https://doi.org/10.1161/ATVBAHA.109.199299 PMid:19965778 PMCid: PMC2823568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Eng J Med. 1984;310:341–6. doi: 10.1056/NEJM198402093100602. https://doi.org/10.1056/NEJM198402093100602 PMid:6690962. [DOI] [PubMed] [Google Scholar]
- 15.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–80. PMid:6812986. [PubMed] [Google Scholar]
- 16.Sugiuchi H, Uji Y, Okabe H, et al. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated alpha-cyclodextrin. Clin Chem. 1995;41:717–23. PMid:7729051. [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. PMid:4337382. [PubMed] [Google Scholar]
- 18.Pasupathi P, Rao YY, Farook J, Bakthavathsalam G. Biochemical cardiac markers in clinical cardiology. Medicine. 2009;10:100–8. [Google Scholar]
- 19.Lyonsa TJ, Basub A. Biomarkers in diabetes: Hemoglobin A1c, vascular and tissue markers. Transl Res. 2012;159:303–12. doi: 10.1016/j.trsl.2012.01.009. https://doi.org/10.1016/j.trsl.2012.01.009 PMid:22424433 PMCid: PMC3339236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usmanova ZA. Relationship between the levels of mmp-9, timp-1, and zinc in biological samples of patients with carotid atherosclerosis. Int J BioMedicine. 2015;5:60–4. https://doi.org/10.21103/Article5(2)_CR2. [Google Scholar]
- 21.Lee SW, Song KE, Shin DS, et al. Alterations in peripheral blood levels of timp-1, mmp-2, and mmp-9 in patients with type-2 diabetes. Diabet Res Clin Pract. 2005;69:175–9. doi: 10.1016/j.diabres.2004.12.010. https://doi.org/10.1016/j.diabres.2004.12.010 PMid:16005367. [DOI] [PubMed] [Google Scholar]
- 22.Papazafiropoulou A, Tentolouris N. Matrix metalloproteinases and cardiovascular diseases. Hippokratia. 2009;13(2):76–82. PMid:19561775 PMCid: PMC2683462. [PMC free article] [PubMed] [Google Scholar]
- 23.Lim H, Tayebjee M, MacFadyen R, Lip GY. Tissue inhibitor of matrix metalloproteinase-1 is a marker of left ventricular diastolic dysfunction in diabetic heart disease. Am Coll Cardiol. 2004;43:221A. https://doi.org/10.1016/S0735-1097(04)90938-2. [Google Scholar]
- 24.Schram M, Henry R, Van Dijk R, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176–81. doi: 10.1161/01.HYP.0000111829.46090.92. https://doi.org/10.1161/01.HYP.0000111829.46090.92 PMid:14698999. [DOI] [PubMed] [Google Scholar]
- 25.Bidder M, Shao J-S, Charlton-Kachigian N, et al. Osteopontin transcription in aortic vascular smooth muscle cells is controlled by glucose-regulated upstream stimulatory factor and activator protein-1 activities. J Biol Chem. 2002;277:44485–96. doi: 10.1074/jbc.M206235200. https://doi.org/10.1074/jbc.M206235200 PMid:12200434. [DOI] [PubMed] [Google Scholar]
- 26.Takemoto M, Yokote K, Nishimura M, et al. Enhanced expression of osteopontin in human diabetic artery and analysis of its functional role in accelerated atherogenesis. Arterioscler Thromb Vasc Bio. 2000;20:624–28. doi: 10.1161/01.atv.20.3.624. https://doi.org/10.1161/01.ATV.20.3.624. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzen JM, Neunhoffer H, David S, et al. Angiotensin II receptor blocker and statins lower elevated levels of osteopontin in essential hypertension-Results from the EUTOPIA trial. Atherosclerosis. 2010;209:184–8. doi: 10.1016/j.atherosclerosis.2009.09.009. https://doi.org/10.1016/j.atherosclerosis.2009.09.009 PMid:19801149. [DOI] [PubMed] [Google Scholar]
- 28.Riedl M, Vila G, Maier C, et al. Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab. 2008;93:2307–12. doi: 10.1210/jc.2007-2383. https://doi.org/10.1210/jc.2007-2383 PMid:18334587. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda T, Shirasawa T, Esaki Y, et al. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest. 1993;92:2814–20. doi: 10.1172/JCI116901. https://doi.org/10.1172/JCI116901 PMid:8254036 PMCid: PMC288482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isoda K, Kamezawa Y, Ayaori M, et al. Osteopontin transgenic mice fed a highcholesterol diet develop early fatty-streak lesions. Circulation. 2003;107:679–81. doi: 10.1161/01.cir.0000055739.13639.d7. https://doi.org/10.1161/01.CIR.0000055739.13639.D7 PMid:12578867. [DOI] [PubMed] [Google Scholar]
- 31.Ohmori R, Momiyama Y, Taniguchi H, et al. Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis. 2003;170:333–7. doi: 10.1016/s0021-9150(03)00298-3. https://doi.org/10.1016/S0021-9150(03)00298-3. [DOI] [PubMed] [Google Scholar]
- 32.Kato R, Momiyama Y, Ohmori R, et al. High plasma levels of osteopontin in patients with restenosis after percutaneous coronary intervention. Arterioscler Thromb Vasc Biol. 2006;26:1–2. doi: 10.1161/01.ATV.0000194157.26665.e6. https://doi.org/10.1161/01.ATV.0000194157.26665e6 PMid:16373617. [DOI] [PubMed] [Google Scholar]
- 33.Kurata M, Okura T, Watanabe S, et al. Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin Sci (Lond) 2006;111:319–24. doi: 10.1042/CS20060074. https://doi.org/10.1042/CS20060074 PMid:16776647. [DOI] [PubMed] [Google Scholar]
- 34.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–9. doi: 10.1161/ATVBAHA.107.144824. https://doi.org/10.1161/ATVBAHA.107.144824 PMid:17717292. [DOI] [PubMed] [Google Scholar]
- 35.Yan X, Sano M, Lu L, et al. Plasma concentrations of osteopontin, but not thrombin-cleaved osteopontin, are associated with the presence and severity of nephropathy and coronary artery disease in patients with type 2 diabetes mellitus. Cardiovascular Diabetology. 2010;9:70. doi: 10.1186/1475-2840-9-70. https://doi.org/10.1186/1475-2840-9-70 PMid:21034455 PMCid: PMC2988001. [DOI] [PMC free article] [PubMed] [Google Scholar]


