Abstract
BACKGROUND:
We present to the attention of the medical, dermatological and oncosurgical community data that serves to indicate the indispensability of optimisation of the algorithm and recommendations for diagnosis and surgical treatment of cutaneous melanoma. These recommendations could be referred to different subgroups of patients in different clinical stages as well as to patients with different initial characterisation (histological morphology) of the primary tumours. One step surgery is not a myth, even more, it could prove to be one of the best solutions for some patient collectives with advanced stages of melanoma.
CASE REPORT:
We present a case of a 74 - year old patient with a congenital medium sized melanocytic nevus, located directly above the lateral part of the elbow joint. In one month and a half, an achromatic nodular formation evolves with a diameter of 2.7 x 2.3 cm, prominent over the skin level, painful by palpation and spontaneously bleeding. By the anamnestic, clinical and dermoscopic findings the patient was diagnosed with nodular melanoma associated with a congenital medium sized melanocytic nevus. A primary excision with a field of safety 0.5 cm in all directions was performed. After confirmation of the primary diagnosis (tumour thickness 8 mm with no ultrasonographic detection of enlarged lymph nodes), seven days later are - excision was performed with an additional field of surgical safety of 1.5 cm in all directions.
CONCLUSIONS:
In this case remains unclear the following question: For what reason a preoperative high - frequent ultrasonography (HFUS) is not recommended to be used as it will allow only one surgical excision with the elimination of a tumour with a safety field of 2cm in all directions? The enigma about the obstacles preventing such a rational optimisation of the current diagnostic and therapeutic algorithm in patients with melanomas remains unresolved. One step surgery for cutaneous melanoma is widely used in many countries although it continues to be considered as a matter of dispute for some experts. Once again, by a clinical case and the following analysis, we would like to focus the attention of the dermatosurgical community on this crucial and highly significant problem. Innovations are very often resulting from the simplicity of logic, which unfortunately is not always accepted appropriately.
Keywords: melanoma, congenital nevus, confocal, surgery, survival benefit
Introduction
Congenital melanocytic nevi (CMN) are benign proliferations of cutaneous melanocytic cells with incidence rate around 1% of the newborn infants [1]. They are composed of melanocytes which are grouped in focal nests in the epidermis, dermis or other tissues [2]. The definition “congenital” is expanded to melanocytic nevi that have occurred 6 months to 2 years after birth, according to different authors who explain this late occurrence with the insufficient melanogenesis or the extremely small size of the nevus postpartum [3]. Clinical classification of CMN is based on their size as following: small nevi (greatest diameter less than 1.5 cm); medium nevi (greatest diameter between 1.5 - 19.9cm) and giant nevi (diameter 20 cm or more) [4]. The most important concern related to the CMN is their malignancy potential [5]. There are many investigations that serve to evaluate the risk of malignant transformation, and at the current stage of knowledge, it is proven that the larger size of the lesion is associated with a significantly higher risk of malignant melanoma development [6]. The estimated lifetime risk for evolution in melanoma is a matter of controversies, but conforming to most of the reported medical data it is approximately 5%, depending on the size of the primary lesion (1 - 5% for small CMN, to 5 - 10% for giant GMN) [7].
There are several main problematic points in the management of patients with congenital melanocytic nevi: 1) The lack of organized and well -functioning centers for dermabrasio threatening of children in their first weeks to months after birth (concerning mainly giant congenital nevi) [8]; 2) The lack of well - trained dermatopathologists, who can quickly and accurately distinguish pseudomelanomas in infants from true melanomas (pseudomelanomas are dysplastic nevi, which in most cases are congenital small melanocytic nevi that are clinical, dermoscopically and histologically difficult for differentiating from real melanomas) [9][10]; 3) The lack of determination to more aggressive approach when it refers to medium sized melanocytic nevi, which are showing tendency of enhanced malignancy risk associated with increased age [11].
Last but not least, it should be taken into account the reluctance of some dermatologists to perform a preventive surgical resection of medium-sized congenital nevi, due to their insufficient competency level (national observations).
To establish the widespread so-called confocal laser microscopy, it is appropriate the following important facts be presented: 1,) Diagnosis melanoma is based on a clinical examination in 60% of the cases and up to 25% it is based on dermoscopic findings. In only 15% of the cases, confocal laser microscopy can give some clarity for the genesis of the lesions and whether they have to be surgically eliminated [13][14]. 2) Confocal microscopy has its limitations in certain areas of the human body [15][16][17][18].
Additionally, it has been found that the multifactorial genesis of melanomas, particularly in patients with dysplastic nevi syndrome shows various genetic mutations within a single patient, but also in every single lesion [19][20]. In simple terms, different nevi whether dysplastic or not, show diverse tendency and speed of nevus – to - melanoma evolving within the life of each patient. This means that two congenital or dysplastic nevi which seem to have completely identical clinical, dermoscopic, confocal - microscopic and even histological appearance, show entirely different malignisation tendency within an equal period in the same patient. It is interesting to be noted that the mutation analysis of several nevi in one patient shows significant differences [21][22][23]. This leads us to the conclusion that the personalisation of the medicine, in general, is inevitable even though it is still hard to achieve it by now. This particular reasoning underlay the logical statement that algorithms and high technologies could provide some advantages in the treatment of skin tumours, but they could by no means be equivalent or even a percentile equivalent of human logic. Melanoma guidelines suffer from lack of case – by - case personalisation and this leads to an inability of optimising the ultimate results.
Case report
A 74 – year - old female patient presented to the department of dermatologic surgery because of a nodular lesion with signs of malignancy, evolved within the borders of middle-sized congenital nevus. The lesion is located in the lateral brachial region of the right arm and has occurred one month and a half ago. The patient noticed rapidly increase in size and regular spontaneous bleeding. Local pruritus, pain and paresthesia were reported as additional subjective complaints.
Clinical examination observed brown pigmented macula with a diameter of 6.3 x 4.1cm, irregular borders and uneven distribution of colour. On approximately half of its size, an elevated nodule with diameter 2.7 x 2.3 cm, asymmetrical shape, dark red colour and irregular borders with central bleeding erosion is situated (Figure 1).
Figure 1.
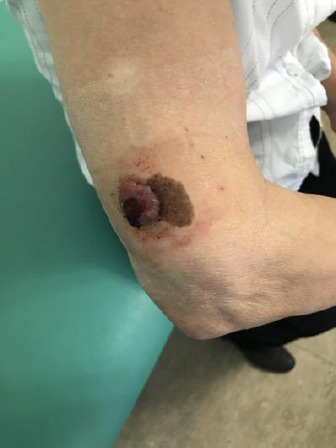
Clinical manifestation of nodular melanoma associated with congenital medium sized melanocytic nevus with Breslow thickness 8 mm, located in the lateral brachial region of the right arm of a 74 - year old patient
No enlarged lymphatic nodes were identified by palpation. Conducted paraclinical examinations revealed elevated ESR – 52 mm/h (< 39 mm/h); WBC – 12.01 /μl (3.5 - 10.5 /μl); Neu – 8.990 μl (1.900 - 7.900μl); GGT – 43 U/l (6.00 – 40 U/l); CRP – 5.70 mg/l (< 5 mg/l). Chest radiography detected poorly expressed emphysematous and fibrous changes.
The right paracardial and basal regions are showing linear non homogenous infiltrative changes probably due to small pleural effusion or adhesions. Normal cardiac silhouette was found.
Ultrasound examination did not detect any axillar, cervical or inguinal enlarged lymphatic nodes. The liver was no focal changes, sharp borders and homogenous structure. The lesion was removed by surgical excision under local anaesthesia, with 0.5 cm field of safety margins in all directions (Figure 2 - 5).
Figure 2.
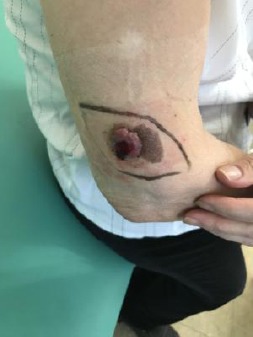
Preoperative surgical skin marking with 0.5cm filed for safety in all directions
Figure 3.
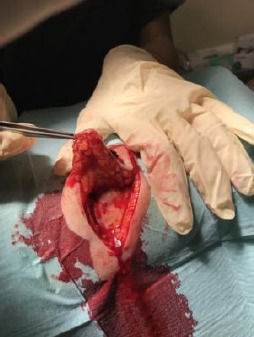
Elliptical surgical excision of the lesion under local anaesthesia
Figure 4.
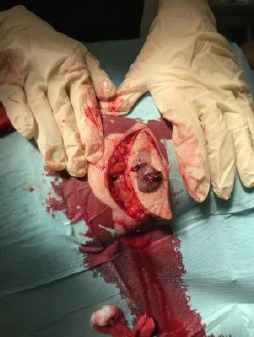
Elliptical surgical excision of the lesion under local anesthesia
Figure 5.
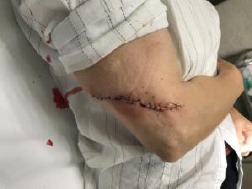
Wound closure with simple interrupted sutures
Histological examination of the cutaneous lesion revealed nodular malignant melanoma with tumour thickness 8mm (Breslow), Clark IV, with no signs of spontaneous regression, high mitotic activity, epidermal erosion, insignificant lymphocytic stromal reaction and clear resection margins.
The patient was diagnosed in stage IIC and underwent reoperation with 1.5 cm field of safety (Figure 6 - 9). Afterwards was referred for registration in oncologic dispensary for regular monitoring.
Figure 6.
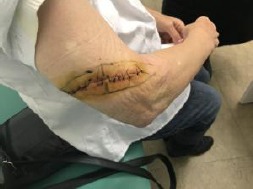
Preoperative surgical skin marking of the re-excision with 1.5 cm field for safety in all directions
Figure 7.
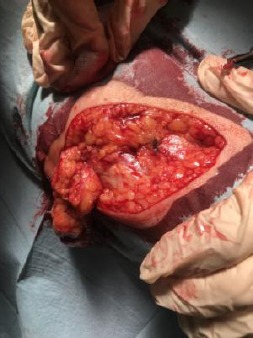
Wide elliptical surgical re-excision under local anaesthesia
Figure 8.
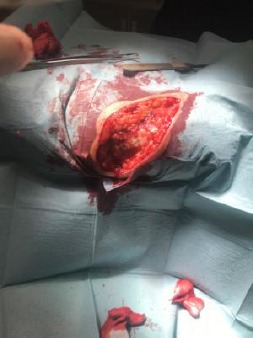
Wide elliptical surgical re-excision under local anaesthesia
Figure 9.
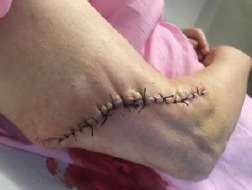
Wound closure with simple interrupted sutures
Discussion
In the era of so-called personalised medicine, the current solutions for diagnosis and treatment of various diseases often are and should be challenged. There are numerous factors that motivate the nation - following of certain guidelines but taking individual decisions for the therapeutic approach of a patient instead. Malignant melanoma should be considered as one of the most illustrative examples of such a non - standard model.
Critical reviews of the standard surgical treatment should not surprise the so-called experts because of four main facts and circumstances, as follows:
1) In the controversy with the great medical progress, we observe that even though pathogenesis of melanoma is multifactorial, the therapy is often (considered 2 years later) identical, regardless of the newly introduced target therapies.
2) It is unclear why melanomas over 8mm or 16mm do not evolve locoregional or distant metastases compared to significantly thinner melanomas, which show high metastatic tendency and extremely aggressive potential [24][25].
3) Rapidly changing therapeutic strategies for treatment of melanoma indicate a serious deficiency of orientation and a kind of helplessness among the medical community towards this never-ending problem.
4) Medical centres have different access possibilities which are reflecting in diverse approaches to patients in general. Why OMICS analyses are available for certain collectives, and not for others? Isn’t this some high - tech personalised medicine which is available for a limited number and types of patients?
All these facts lead our minds to the logical question concerning not the difficulty of the pathogenesis or the target therapy, but the significantly more simplified surgical treatment: Isn’t there any possibility for alleviation of the surgical treatment and reduction in the number of therapeutic interventions as well as the chances of incorrect assessment of the preoperative status? It is a simple question whether these factors can be somehow limited? And we believe that the answer is - definitely YES!
By the presented case, we would like to express our critical view regarding the lack of any individual approach in the recommendations of melanoma treatment in Europe, the US and worldwide at least for some collectives of patients. In the case of our patient two medium - sized surgical interventions were performed with a favourable outcome despite the initial 8 mm tumour thickness. In cases of melanoma, over 4 mm and no locoregional metastases a sentinel lymph node biopsy and lymph denectomy are not recommended. Re - excisions, however, are. An open question remains - why in this initially clear clinical and dermoscopic case, guidelines do not recommend preoperative HFUS for detecting of the tumour thickness? Then, depending on the ultrasonographically measured thickness, only one single surgical excision could be performed?! In less thick melanomas this approach would lead to primary excision of the lesion with or without a sentinel lymph node biopsy at once, in a single surgical session. The surgical field of safety would be 1 cm or 2 cm in all directions, depending on whether the ultimately established thickness of the tumor is under or more than 2 cm [26]. This approach would be limited in cases of achromatic melanomas so they should be excluded from the category of tumors appropriated for this strategy. A possibility for their inclusion in the one - step melanoma surgery would be the use of confocal microscopy and/or cytological analysis in combination with immunohistochemical methods [27][28].
Although this concept would be considered as “frivolous” by many experts, the number of reduced surgical interventions and the optimization of the approach, in general, would lead to 1) Reduction of healthcare costs, 2) Limited possibilities of different mistakes by the therapists and patients (occurring between the two surgical interventions) and as an ultimate and most important outcome - 3) Long-term survival of the affected patient collectives would be expected.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Tannous ZS, Mihm MC, Jr, Sober AJ, Duncan LM. Congenital melanocytic nevi: clinical and histopathologic features, risk of melanoma, and clinical management. J Am Acad Dermatol. 2005;52(2):197–203. doi: 10.1016/j.jaad.2004.07.020. https://doi.org/10.1016/j.jaad.2004.07.020 PMid:15692463. [DOI] [PubMed] [Google Scholar]
- 2.Nikfarjam J, Chambers E. Congenital melanocytic nevi and the risk of malignant melanoma: establishing a guideline for primary-care physicians. Einstein J Biol Med. 2011;27(2):59. https://doi.org/10.23861/EJBM20112745. [Google Scholar]
- 3.Kovalyshyn I, Braun R, Marghoob A. Congenital melanocytic naevi. Australas J Dermatol. 2009;50:231–240. doi: 10.1111/j.1440-0960.2009.00553_1.x. https://doi.org/10.1111/j.1440-0960.2009.00553_1.x PMid:19916964. [DOI] [PubMed] [Google Scholar]
- 4.Alikhan A, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now?Part I. Clinical presentation, epidemiology, pathogenesis, histology, malignant transformation, and neurocutaneous melanosis. J Am Acad Dermatol. 2012;67(4):495e1–17. doi: 10.1016/j.jaad.2012.06.023. quiz 512-4. [DOI] [PubMed] [Google Scholar]
- 5.Shah J, Feintisch AM, Granick MS. Congenital Melanocytic Nevi. Eplasty. 2016;16:ic4. PMid:26904155 PMCid: PMC4740346. [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes AR. Melanocytic precursors of cutaneous melanoma. Estimated risks and guidelines for management. Med Clin North Am. 1986;70(1):3–37. doi: 10.1016/s0025-7125(16)30966-x. https://doi.org/10.1016/S0025-7125(16)30966-X. [DOI] [PubMed] [Google Scholar]
- 7.Viana AC, Gontijo B, Bittencourt FV. Giant congenital melanocytic nevus. An Bras Dermatol. 2013;88(6):863–78. doi: 10.1590/abd1806-4841.20132233. Erratum in: An Bras Dermatol 2014; 89 (1):190. https://doi.org/10.1590/abd1806-4841.20132233 PMid:24474093 PMCid: PMC3900335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Natarajan K, Arunachalam P, Sundar D, Srinivas CR. Congenital Melanocytic Nevi: Catch Them Early! Journal of Cutaneous and Aesthetic Surgery. 2013;6(1):38–40. doi: 10.4103/0974-2077.110097. https://doi.org/10.4103/0974-2077.110097 PMid:23723605 PMCid: PMC3663176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mooi WJ, Krausz T. Biopsy Pathology of Melanocytic Disorders. 1st ed. New York: A Hodder Arnold Publication; 1992. https://doi.org/10.1007/978-1-4899-6908-8. [Google Scholar]
- 10.McCarthy SW, Scolyer RA. Pitfalls and Important Issues in the Pathologic Diagnosis of Melanocytic Tumors. The Ochsner Journal. 2010;10(2):66–74. PMid:21603360 PMCid: PMC3096206. [PMC free article] [PubMed] [Google Scholar]
- 11.Bastian BC, Xiong J, Frieden IJ, Williams ML, Chou P, Busam K, Pinkel D, LeBoit PE. Genetic changes in neoplasms arising in congenital melanocytic nevi: differences between nodular proliferations and melanomas. Am J Pathol. 2002;161(4):1163–9. doi: 10.1016/S0002-9440(10)64393-3. https://doi.org/10.1016/S0002-9440(10)64393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigel DS, Russak J, Friedman R. The Evolution of Melanoma Diagnosis:25 Years Beyond the ABCDs. CA: A Cancer Journal for Clinicians. 2010;60:301–316. doi: 10.3322/caac.20074. https://doi.org/10.3322/caac.20074. [DOI] [PubMed] [Google Scholar]
- 13.Debarbieux S, Depaepe L, Poulalhon N, Balme B, Dalle S, Thomas L. Reflectance confocal microscopy accurately discriminates between benign and malignant melanocytic lesions exhibiting a 'dermoscopic island'. J Eur Acad Dermatol Venereol. 2013;27(2):e159–65. doi: 10.1111/j.1468-3083.2012.04533.x. https://doi.org/10.1111/j.1468-3083.2012.04533.x PMid:22486883. [DOI] [PubMed] [Google Scholar]
- 14.Scope A, Benvenuto-Andrade C, Agero AL, Halpern AC, Gonzalez S, Marghoob AA. Correlation of dermoscopic structures of melanocytic lesions to reflectance confocal microscopy. Arch Dermatol. 2007;143(2):176–85. doi: 10.1001/archderm.143.2.176. https://doi.org/10.1001/archderm.143.2.176 PMid:17309998. [DOI] [PubMed] [Google Scholar]
- 15.Wielowieyska-Szybińska D, Białek-Galas K, Podolec K, Wojas-Pelc A. The use of reflectance confocal microscopy for examination of benign and malignant skin tumors. Advances in Dermatology and Allergology/Postępy Dermatologii i Alergologii. 2014;31(6):380–387. doi: 10.5114/pdia.2014.40961. https://doi.org/10.5114/pdia.2014.40961 PMid:25610353 PMCid: PMC4293386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(Suppl 2):59–69. doi: 10.1016/s0001-7310(09)73380-0. https://doi.org/10.1016/S0001-7310(09)73380-0. [DOI] [PubMed] [Google Scholar]
- 17.Guida S, Longo C, Casari A, Ciardo S, Manfredini M, Reggiani C, Pellacani G, Farnetani F. Update on the use of confocal microscopy in melanoma and non-melanoma skin cancer. G Ital Dermatol Venereol. 2015;150(5):547–63. PMid:26140397. [PubMed] [Google Scholar]
- 18.Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170(4):802–8. doi: 10.1111/bjd.12678. https://doi.org/10.1111/bjd.12678 PMid:24124911 PMCid: PMC3984366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva JH, de Sá BCS, de Ávila ALR, Landman G, Neto JPD. Atypical mole syndrome and dysplastic nevi: identification of populations at risk for developing melanoma - review article. Clinics. 2011;66(3):493–499. doi: 10.1590/S1807-59322011000300023. https://doi.org/10.1590/S1807-59322011000300023 PMid:21552679 PMCid: PMC3072014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein AM, Tucker MA. Dysplastic Nevi and Melanoma. Cancer epidemiology, biomarkers &prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(4):528–532. doi: 10.1158/1055-9965.EPI-12-1346. https://doi.org/10.1158/1055-9965.EPI-12-1346 PMid:23549396 PMCid: PMC3616416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaal LH, Mooi WJ, Sillevis Smitt JH, van der Horst CM. Classification of congenital melanocytic naevi and malignant transformation: a review of the literature. Br J Plast Surg. 2004;57(8):707–19. doi: 10.1016/j.bjps.2004.04.022. https://doi.org/10.1016/j.bjps.2004.04.022 PMid:15544766. [DOI] [PubMed] [Google Scholar]
- 22.Roh MR, Eliades P, Gupta S, Tsao H. Genetics of Melanocytic Nevi. Pigment cell &melanoma research. 2015;28(6):661–672. doi: 10.1111/pcmr.12412. https://doi.org/10.1111/pcmr.12412 PMid:26300491 PMCid: PMC4609613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloethner S, Snellman E, Bermejo JL, Hiripi E, Gast A, Thirumaran RK, Wellenreuther R, Hemminki K, Kumar R. Differential gene expression in melanocytic nevi with the V600E BRAF mutation. Genes, chromosomes &cancer. 2007;46:1019–1027. doi: 10.1002/gcc.20488. https://doi.org/10.1002/gcc.20488 PMid:17696195. [DOI] [PubMed] [Google Scholar]
- 24.Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC, Slominski A. Current concepts of metastasis in melanoma. Expert review of dermatology. 2008;3(5):569–585. doi: 10.1586/17469872.3.5.569. https://doi.org/10.1586/17469872.3.5.569 PMid:19649148 PMCid: PMC2601641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Annals of Surgery. 1970;172(5):902–908. doi: 10.1097/00000658-197011000-00017. https://doi.org/10.1097/00000658-197011000-00017 PMid:5477666 PMCid: PMC1397358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tchernev G. One Step Surgery for Cutaneous Melanoma: “We Cannot Solve Our Problems with the Same Thinking We Used When We Created Them?”. Open Access Maced J Med Sci. 2017;5(6):774–776. doi: 10.3889/oamjms.2017.168. https://doi.org/10.3889/oamjms.2017.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cinotti E, Perrot JL, Labeille B, Adegbidi H, Cambazard F. Reflectance confocal microscopy for the diagnosis of vulvar melanoma and melanosis: preliminary results. Dermatol Surg. 2012;38(12):1962–7. doi: 10.1111/dsu.12009. https://doi.org/10.1111/dsu.12009 PMid:23127153. [DOI] [PubMed] [Google Scholar]
- 28.Lazarou I, Purek L, Duc C, Licker M-J, Spiliopoulos A, Tschopp J-M. Primary malignant achromic melanoma of the lung. Thoracic Cancer. 2014;5(1):85–88. doi: 10.1111/1759-7714.12011. https://doi.org/10.1111/1759-7714.12011 PMid:26766979 PMCid: PMC4704277. [DOI] [PMC free article] [PubMed] [Google Scholar]


