Abstract
IMPORTANCE
Survival from sepsis has improved in recent years, resulting in an increasing number of patients who have survived sepsis treatment. Current sepsis guidelines do not provide guidance on posthospital care or recovery.
OBSERVATIONS
Each year, more than 19 million individuals develop sepsis, defined as a life-threatening acute organ dysfunction secondary to infection. Approximately 14 million survive to hospital discharge and their prognosis varies. Half of patients recover, one-third die during the following year, and one-sixth have severe persistent impairments. Impairments include development of an average of 1 to 2 new functional limitations (eg, inability to bathe or dress independently), a 3-fold increase in prevalence of moderate to severe cognitive impairment (from 6.1% before hospitalization to 16.7% after hospitalization), and a high prevalence of mental health problems, including anxiety (32% of patients who survive), depression (29%), or posttraumatic stress disorder (44%). About 40% of patients are rehospitalized within 90 days of discharge, often for conditions that are potentially treatable in the outpatient setting, such as infection (11.9%) and exacerbation of heart failure (5.5%). Compared with patients hospitalized for other diagnoses, those who survive sepsis (11.9%) are at increased risk of recurrent infection than matched patients (8.0%) matched patients (P < .001), acute renal failure (3.3% vs 1.2%, P < .001), and new cardiovascular events (adjusted hazard ratio [HR] range, 1.1–1.4). Reasons for deterioration of health after sepsis are multifactorial and include accelerated progression of preexisting chronic conditions, residual organ damage, and impaired immune function. Characteristics associated with complications after hospital discharge for sepsis treatment are not fully understood but include both poorer presepsis health status, characteristics of the acute septic episode (eg, severity of infection, host response to infection), and quality of hospital treatment (eg, timeliness of initial sepsis care, avoidance of treatment-related harms). Although there is a paucity of clinical trial evidence to support specific postdischarge rehabilitation treatment, experts recommend referral to physical therapy to improve exercise capacity, strength, and independent completion of activities of daily living. This recommendation is supported by an observational study involving 30 000 sepsis survivors that found that referral to rehabilitation within 90 days was associated with lower risk of 10-year mortality compared with propensity-matched controls (adjusted HR, 0.94; 95% CI, 0.92–0.97, P < .001).
CONCLUSIONS AND RELEVANCE
In the months after hospital discharge for sepsis, management should focus on (1) identifying new physical, mental, and cognitive problems and referring for appropriate treatment, (2) reviewing and adjusting long-term medications, and (3) evaluating for treatable conditions that commonly result in hospitalization, such as infection, heart failure, renal failure, and aspiration. For patients with poor or declining health prior to sepsis who experience further deterioration after sepsis, it may be appropriate to focus on palliation of symptoms.
Sepsis is defined as life-threatening acute organ dysfunction secondary to infection1 and affects more than 19 million people each year.2 In-hospital mortality has declined,3,4 from 35% in 2000 to 18% in 2012, resulting in a large number of sepsis survivors. Emerging data suggest that patients who survive sepsis frequently experience new symptoms,5 long-term disability,6 and worsening of chronic health conditions7,8 for which they will seek care from many types of clinicians.
An international survey suggests a need for improved management after hospital discharge. Of 1475 patients who survived hospitalization for sepsis, there was only low to moderate satisfaction with support services after they were discharged.5 In addition, re-hospitalization after sepsis accounts for 12.2% of all US hospital readmissions and 14.5% of readmission costs.9 Therefore, improving medical care after sepsis hospitalizations may reduce health care utilization and costs. Although physical disability, cognitive impairment, and hospital readmission are common after sepsis, sepsis treatment guidelines provide no recommendations on posthospital management.10 This article reviews the epidemiology, pathophysiology, and clinical sequelae in the months following hospital discharge of patients treated for sepsis. Management strategies and directions for future research are also reviewed.
Methods
A literature search of MEDLINE was conducted in PubMED through April 26, 2017, using search terms and synonyms for sepsis and survivors. Non-English language articles or those published before January 1, 2000, were excluded. Bibliographies of retrieved studies were searched for other relevant studies. Articles were reviewed for their contribution to current understanding of sepsis survivorship, with priority given to clinical trials, large longitudinal observational studies, and more recently published articles.
Observations
Epidemiology
Throughout the world, an estimated 19.4 million patients develop sepsis each year, of whom 14.1 million survive to hospital discharge.2 In 2014, 1.3 million US adults survived a hospitalization for sepsis11(Figure 1), of whom 56% were aged 65 years or older.11 Approximately half of patients who survive hospitalization for sepsis have a complete or near complete recovery. Overall, one-sixth experience severe persistent physical disability or cognitive impairment, and one-third die during the following year.6,12 Half of deaths in the year after hospitalization for sepsis are related to complications of sepsis, while half are explained by age or preexisting comorbidities.13
Figure 1. US Hospitalizations and Live Discharges for a Diagnosis of Sepsis, 2005–2014.
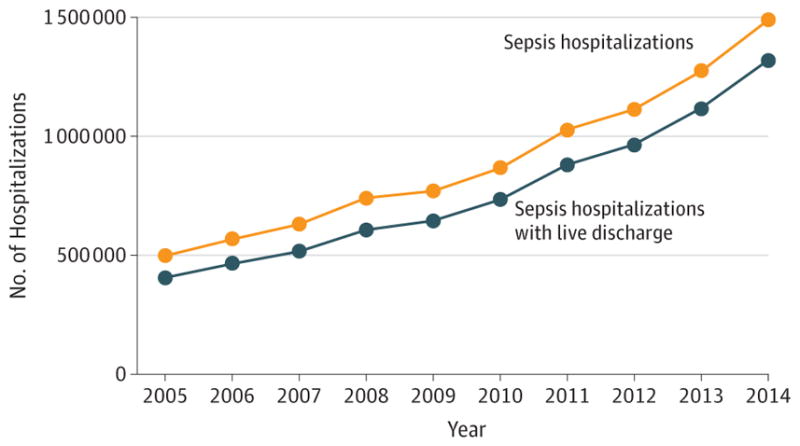
This figure depicts the number of adult hospitalizations and live discharges in the United States with a principal diagnosis of sepsis, severe sepsis, or septic shock over time. The data were abstracted from the National Inpatient Sample using HCUPnet.11
Pathophysiology
Sepsis can occur due to either community-acquired or nosocomial infection. Among 307 491 US hospitalizations for sepsis, 63% of underlying infections were community acquired, 11% were hospital acquired, and 26% were health care associated (acquired outside a hospital by patients with recent exposure to health care facilities, such as nursing home residents, hemodialysis recipients, or recently hospitalized patients).14 The most common underlying infection is pneumonia (40%), followed by abdominal, genitourinary, primary bacteremia, and skin or soft tissue infections.15,16
Based on experimental and human volunteer models, sepsis was initially presumed to be an extreme, body-wide inflammatory response that led to alterations in microvascular flow, endothelial leak, and compromised parenchymal cell function, manifesting clinically as inadequate tissue perfusion and multisystem organ dysfunction. However, more recent evidence demonstrates that the pathophysiological response is more complex and variable17 (Figure 2). First, the initial host response includes activation of pro-inflammatory pathways and anti-inflammatory innate immune pathways, as well as alterations in adaptive immune pathways. Second, the characteristics of immune system changes vary and depend on both host and pathogen characteristics, as well as recent medical events (eg, surgery, other infection) and treatment (eg, timing of antibiotics).17 Third, the resolution of immune system changes in response to sepsis is complex and frequently prolonged. Many patients continue to have inflammatory changes, immune suppression, or both after sepsis.19
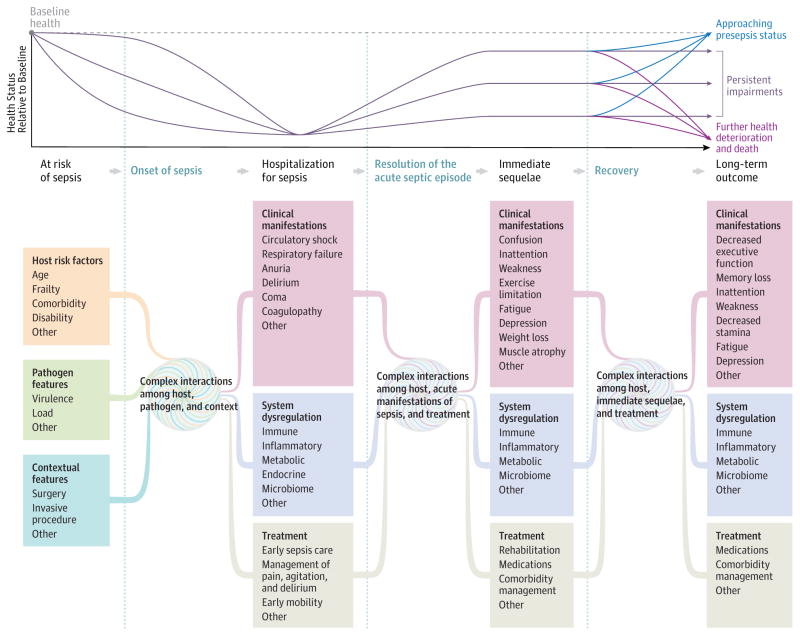
Figure 2. A Conceptual Model of the Potential Network of Factors and Interactions Important to Determining a Patient’s Clinical Course and Long-term Outcome After Sepsis.
There are many potential clinical courses that a patient may experience after a hospitalization for sepsis, from rapid complete recovery to recurrent complications and death. This figure depicts examples of common clinical trajectories and presents a conceptual model of factors important to shaping a patient’s clinical course and long-term outcome. This figure draws from the Wilson-Cleary model,18 which links underlying biological factors to physical function and quality of life, but extends the representation of the biological factors to demonstrate their complex and unmeasurable interactions.
The reasons for these immune system changes are complex and include epigenetic20 and metabolic21 reprogramming of immune cells induced by the original septic insult and by on-going changes in the host environment, such as neuroendocrine22 or microbiome23 alterations. These processes continue despite successful eradication of the initial pathogen and increase a patient’s risk of secondary episodes of infection or sepsis. The combination of the initial septic insult and ongoing abnormalities in host control systems contributes to persistent organ dysfunction. The severity of immune suppression and organ dysfunction after sepsis treatment is influenced by a patient’s presepsis health and by characteristics of the infection (pathogen load, virulence), host response, and the quality of early sepsis treatment. Patients may also experience sequelae from iatrogenic complications24,25 and medication errors26–28 during and after hospitalization.
Recovery from sepsis also varies (Figure 2). There are no validated tools to estimate a patient’s likelihood of complete recovery. However, several prognostic factors have been identified. Patients with preexisting disability, frailty, or nursing home use are less likely to regain functional independence,30–35 while previously healthy patients have a higher chance of recovery. Importantly, the severity of cognitive impairments shortly after hospitalization do not predict well subsequent impairment.29
Clinical Sequelae
Physical Limitations
After hospitalization for sepsis, a patient’s ability to function independently frequently declines. Patients treated for sepsis typically develop 1 to 2 new limitations of activities of daily living (ADLs), such as inability to manage money, bathe, or toilet independently6 after hospital discharge (Table 1). The causes of functional decline are multifactorial. Patients often develop physical weakness following critical illness, which may be due to myopathy, neuropathy,40 cardio-respiratory impairments, cognitive impairment, or a combination of these conditions.
Table 1.
Matched Cohort Studies Examining Risk for Physical Disability, Cognitive Impairment, and Common Medical Conditions in Patients Surviving a Hospitalization for Sepsisa
| Source | Study Population | Comparison | Findings |
|---|---|---|---|
| Physical Disability | |||
| Iwashyna et al,6 2010 | 516 HRS participants who survived hospitalization for sepsis, ≥65 y with linked Medicare claims | 4517 HRS participants, ≥65 y, who survived a nonsepsis hospitalization | Patients with no functional limitations prior to sepsis developed a mean 1.57 new limitations (95% CI, 0.99–2.15) after sepsis vs 0.48 new limitations among comparison patients (P < .001 for difference) Patients with mild to moderate limitations before sepsis developed a mean 1.50 new limitations (95% CI, 0.87–2.12) after sepsis hospitalization; 59.3% (95% CI, 55.5%–63.2%) of patients who completed follow-up assessment (median, 1 y postsepsis) had worse cognitive or physical function than their own presepsis baseline assessment |
| Cognitive Impairment | |||
| Iwashyna et al,6 2010 | 516 HRS participants who survived hospitalization for sepsis, ≥65 y, with linked Medicare claims | 4517 HRS participants, ≥65 y, who survived a nonsepsis hospitalization | Moderate to severe cognitive impairment increased 10.6% after sepsis, from 6.1% presepsis to 16.7% postsepsis In a multivariable model, sepsis was associated with an adjusted OR of 3.34 (95% CI, 1.53–7.25) for developing moderate to severe cognitive impairment (P < .001), while nonsepsis hospitalization was not associated with increased odds (adjusted OR, 1.15; 95% CI, 0.80–1.67) |
| Shah et al,7 2013 | 198 CHS participants who survived a hospitalization for sepsis, 320 pneumonia, or 1049 infection | 2556 CHS participants not hospitalized with infection | Adjusted HRs for progression to dementia were 2.28 (95% CI, 1.38–3.77) postsepsis, 2.24 (95% CI, 1.62–3.11) after pneumonia, and 1.98 (95% CI, 1.61–2.43) after infection (P < .001 for each) |
| Common Medical Conditions | |||
| Yende et al,8 2014 | 4179 Medicare beneficiaries who survived an ICU hospitalization for sepsis | 4179 Medicare beneficiaries hospitalized in ICU without sepsis; 4179 hospitalized with infection; 4179 hospitalized without infection, and 4179 nonhospitalized. All controls were matched on age, sex, prior cardiovascular disease, and propensity for sepsis | Cardiovascular events occurred 29.5% of patients in the year after sepsis (498.2 events/1000 person-y) Rate of cardiovascular events was higher after sepsis vs matched population controls (incidence RR, 1.9; P < .01) and matched hospitalized controls (incidence RR, 1.1; P = .002). Rates were indistinguishable from matched ICU controls (P = .28) |
| Zielske et al,36 2014 | 30 Adult survivors of ICU stay with sepsis | 30 Adult survivors of ICU stay without sepsis | After 14 d in the ICU, aspiration was present on fiberoptic endoscopic evaluation of swallowing in 63% (19/30) of patients with sepsis vs 23% (7/30) of patients without sepsis (P = .002) |
| Prescott et al,37 2015 | 2617 HRS participants who survived a sepsis hospitalization, ≥65 y with linked Medicare claims | 2617 Age, sex, and health status–matched HRS participants who survived a hospitalization for a nonsepsis acute medical condition | 90-d Hospital readmission for a principal diagnosis of infection occurred in 11.9% (95% CI, 11.9%–13.1%) after vs in 8.0% (95% CI, 7.0%–9.1%) of controls (P < .001 P Readmission for sepsis occurred in 167 patients (6.4%) of 2617 vs 73 (2.8%; P < .001) Readmission for acute renal failure occurred in 87 (3.3%) vs 30 (1.2%; P < .001) Readmission for acute respiratory failure occurred in 65 (2.5%) vs 38 (1.5%; P = .007) Readmission for aspiration pneumonitis occurred in 47 (1.8%) vs 31 (1.2%; P = .06) |
| Ou et al,38 2016 | All 93 862 adult patients hospitalized with sepsis in Taiwan during a 3-y period, of whom, 67 926 were matched to population controls and 42 855 were matched to nonsepsis hospitalizations | 67 926 Population controls and 42 855 survivors of nonsepsis hospitalization, matched by propensity for sepsis hospitalization | During long-term follow-up (average 6.7 y), patients postsepsis had increased risk of cardiovascular events vs matched population controls (adjusted HR, 1.37; 95% CI, 1.34–1.41) and matched survivors on nonsepsis hospitalization (adjusted HR, 1.27; 95% CI, 1.22–1.32) |
| Shen, et al,39 2016 | All 10 818 adult patients hospitalized with sepsis in Taiwan during a 3-y period, who had no prior sepsis and survived to 90 d without recurrent sepsis | 10 818 Age- and sex-matched population controls with no prior history of sepsis | During 8-y follow-up, 35.0% of patients after sepsis vs 4.3% of matched controls had a recurrent hospitalization for sepsis Adjusted HR for subsequent sepsis among sepsis survivors, 8.89 (95% CI, 8.04–9.83) |
Abbreviations: CHS, Cardiovascular Health Study, an observational cohort study; HR, hazard ratio; HRS, US Health and Retirement Study, an observational cohort study; ICU, intensive care unit; OR, odds ratio; RR, rate ratio.
Ou et al38 defined sepsis by principal diagnosis of sepsis (ICD-9-CM 038.x). All other studies identified sepsis by evidence of acute infection plus acute organ dysfunction.
Swallowing difficulty is common and may be due to muscular weakness or neurological damage. Among patients discharged from the intensive care unit(ICU), those with sepsis are more likely to have aspiration on fiberoptic endoscopic evaluation of swallowing (63% vs 23%, P < .01) than those without sepsis.36 Among older US residents who survive hospitalization for sepsis, risk of 90-day readmission for aspiration is 1.8% vs 1.2% after hospitalizations for other diagnoses (P = .06).37
Physical function typically improves after hospital discharge. In a prospective study of functional recovery after sepsis, patients experienced clinically important improvements in 6-minute walk distance (from a mean of 45.9% of predicted distance for age at hospital discharge to a mean of 69% 3 months after discharge, P < .05); quadriceps strength (68.5% vs 50.9%, P < .05); and handgrip strength (68.5% vs 54.6%, P < .05).41 By 3 months after discharge, 60% of 51 patients could walk for 30 minutes per day.41 However, physical function typically remained below population norms and often does not return to presepsis levels.6,41
Cognitive Impairment
Patients may acquire neurological damage during hospitalization for sepsis through a variety of mechanisms, including cerebral ischemia, metabolic derangements, and neuroinflammation.42 Patients frequently experience delirium and impaired consciousness. After hospitalization, patients may have long-term impairments in memory, attention, verbal fluency, and executive functioning.42
In an observational study of 516 US Health and Retirement Study participants who survived a hospitalization with sepsis, the prevalence of moderate to severe cognitive impairment increased from 6.1% before hospitalization to 16.7% after hospitalization.6 By contrast, patients surviving hospitalization without sepsis did not have an increase in the incidence of moderate to severe impairment.6 The prevalence of milder cognitive impairments after sepsis is unknown. However, even patients with normal neurocognitive testing after sepsis may report new difficulties with memory and executive functioning that limit return to work or school.43
Mental Health Impairment
Patients discharged from an ICU report a high prevalence of anxiety (32%) within 2 to 3 months44; depression (29%) within 2 to 3 months)45; and posttraumatic stress disorder (PTSD) symptoms (44%) within 1 to 6 months46 (eTable in the Supplement). Among 2 studies of patients after sepsis, rates of mental health impairments were high.47,48 Sepsis was an independent risk factor of stress disorders after critical illness in observational studies.48,49 Somatic symptoms of depression, such as weakness, appetite change, and fatigue, were common.50
The extent to which anxiety, depression, or PTSD are exacerbated by sepsis is unclear. In a study involving 439 Health and Retirement Study participants, the prevalence of clinically significant depressive symptoms was 28% before sepsis and 28% after sepsis. However, in a population-based Danish cohort, 9912 critically ill patients without prior psychological history were more likely than hospitalized controls to receive new psychoactive prescriptions (12.7% vs 5.0%; adjusted HR, 2.5; 95% CI, 2.19–2.74; P<.001) or new psychiatric diagnoses(0.5% vs 0.2%; adjusted HR, 3.4;95% CI, 1.96–5.99; P<.001) in the 3 months after hospitalization.51 It is unclear whether depression, anxiety, or PTSD are exacerbated by sepsis, or merely more common among patients who develop sepsis. However, it is important to recognize and treat mental health impairments because they are associated with a more complicated clinical course.52,53
Recurrent Infection and Sepsis
Patients are susceptible to health deterioration after sepsis recovery (Table 1). In a study involving 2617 Medicare beneficiaries who survived hospitalization for sepsis, 40% were readmitted within 90 days.37 The most common readmission diagnosis was infection; 11.9% were readmitted for sepsis, pneumonia, urinary tract, or skin or soft tissue infection compared with 8.0% of age-and comorbidity-matched patients surviving hospitalizations for other acute medical diagnoses (P < .001).37 In a nationwide study involving 10 818 patients who survived hospitalization for sepsis in Taiwan, risk of subsequent sepsis was elevated 9-fold (from 4.3% to 35.0%) relative to matched population controls.39
Exacerbation of Chronic Medical Conditions
Patients discharged after treatment for sepsis have high rates of hospital readmission for conditions that are potentially treatable in the outpatient setting, including exacerbation of congestive heart failure, acute renal failure, and exacerbation of chronic obstructive pulmonary disease37 (Table 1). These diagnoses reflect common comorbidities of patients who develop sepsis and conditions that may be exacerbated by sepsis-induced organ dysfunction (eg, reduced glomerular filtration rate) or impaired homeostatic mechanisms (eg, labile blood pressure or fluid imbalance).
Risk of cardiovascular events and acute renal failure are increased relative to matched controls, suggesting that sepsis may directly contribute to the development or progression of these conditions. In 2 observational studies involving 4179 and 67 926 patients who survived hospitalization for sepsis, the incidence of new cardiovascular events (myocardial infarction, stroke, sudden cardiac death, and ventricular arrhythmias) was increased 1.4- to 1.9-fold relative to population controls, and 1.1- to 1.3-fold relative to hospitalized controls.8,38 Among 2617 Medicare beneficiaries discharged after sepsis, risk of 90-day readmission for acute renal failure was increased 2.7-fold for patients with sepsis (3.3%) vs matched patients (1.2%; P < .001).
Other Symptoms and Sequelae
Patients may report other symptoms, such as numbness, pain, visual disturbance, hair loss, and problems with dentition and nails.54 Amputation due to limb gangrene is a rare but extreme sequela of sepsis, which may occur from cardiovascular shock, microcirculatory dysfunction, or high vasopressor dosages.55
Impact on Quality of Life, Return to Work, and Social Relationships
Patients who survive sepsis report lower quality of life compared with population averages and often cannot resume prior roles or activities.56 For example, in one study, 35% of elderly patients were discharged to a postacute care facility.12 Only 43% of previously employed patients returned to work within a year of contracting septic shock,57 and only 33% of patients living at home prior to contracting sepsis returned to independent living by 6 months after discharge.58 Spouses and family members must often serve as informal caregivers. A study47 of spouses caring for partners who had survived sepsis found that the spouses were at increased risk of depression with 20% having depressive symptoms before sepsis vs 34% after sepsis.
Hospital and ICU-Based Strategies to Prevent Adverse Sequelae After Sepsis
Current treatment guidelines emphasize interventions that reduce short-term mortality, with little information on strategies to minimize physical disability, cognitive impairment, or health deterioration after sepsis. It is uncertain whether improvement in 30-day outcomes are associated with lasting benefit; there may be instances when that is not the case. For example, conservative fluid administration during sepsis-induced acute respiratory distress syndrome was shown to reduce ICU length of stay and increase ventilator-free days but was subsequently associated with worse late cognitive function, possibly due to compromised cerebral perfusion.59 Although data are limited, Our strategy for preventing long-term sequelae after sepsis focuses on 3 strategies: high-quality early sepsis care10; management of pain, agitation, and delirium60; and early mobilization to prevent or minimize muscle atrophy60 (Table 2).
Table 2.
Recommended Practices for Reducing Long-term Morbidity in Septic and Critically Ill Patients
| Element of Care | Guideline Recommendation | Guideline | Level of Evidencea |
|---|---|---|---|
| Early Sepsis Care | |||
| Antibiotics | Recommendation: empirical broad-spectrum therapy with ≥1 antimicrobials for patients presenting with sepsis or septic shock to cover all likely pathogens | SSC | Moderate |
| Administration of intravenous antimicrobials should be initiated as soon as possible after recognition and within 1 h for both sepsis and septic shock | SSC | Moderate | |
| Fluid resuscitation | In the resuscitation from sepsis-induced hypoperfusion, at least 30 mL/kg of intravenous crystalloid fluid should be given within the first 3 h | SSC | Low |
| Vasopressors | Apply vasopressors (for hypotension that does not respond to initial fluid resuscitation) to maintain a mean arterial pressure ≥65 mm Hg within the first 6 h | SSC | Low |
| Source control | A specific anatomic diagnosis of infection requiring emergent source control should be identified or excluded as rapidly as possible. Any required source control intervention should be implemented as soon as medically and logistically practical after the diagnosis is made | SSC | Ungraded |
| Recommendation: prompt removal of intravascular access devices that are a possible source of sepsis or septic shock after other vascular access has been established | SCC | Ungraded | |
| Pain, Agitation, and Delirium Management | |||
| Pain assessment | Pain should be routinely monitored for adult ICU patients using validated scales such as Behavioral Pain Scale or Critical-Care Pain Observation Tool | PAD | Moderate |
| Pain treatment | Intravenous opioids should be considered the first-line drug class of choice to treat nonneuropathic pain in critically ill patients | PAD | Low |
| Sedative choice | Recommendation: analgesia-first sedation for mechanically ventilated adult ICU patients | PAD | Moderate |
| Sedation strategies using nonbenzodiazepine sedatives (either propofol or dexmedetomidine) may be preferred over sedation with benzodiazepines (either midazolam or lorazepam) to improve clinical outcomes in mechanically ventilated adult ICU patients | PAD | Moderate | |
| Sedative monitoring | Richmond Agitation-Sedation Scale and Sedation-Agitation Scale are the most valid and reliable sedation assessment tools for measuring quality and depth of sedation in adult ICU patients | PAD | Moderate |
| Depth of sedation | Sedative medications should be titrated to maintain a light rather than a deep level of sedation in adult ICU patients, unless clinically contraindicated | PAD | Moderate |
| Recommendation: either daily sedation interruption or a light target level of sedation be routinely used in mechanically ventilated adult ICU patients | PAD | Moderate | |
| Recommendation: that continuous or intermittent sedation be minimized for mechanically ventilated sepsis patients, targeting specific titration end points | SSC | Ungraded | |
| Delirium monitoring | Recommendation: routine monitoring of delirium in adult ICU patients | PAD | Moderate |
| The Confusion Assessment Method for the ICU and the Intensive Care Delirium Screening Checklist are the most valid and reliable delirium-monitoring tools in adult ICU patients | PAD | High | |
| Early Mobility | |||
| Mobilization | Recommendation: performing early mobilization of adult ICU patients whenever feasible to reduce the incidence and duration of delirium | PAD | Moderate |
| During the patient’s critical care stay and as early as clinically possible, perform a short clinical assessment to determine the patient’s risk of developing physical and nonphysical morbidity. | NICE | Ungraded | |
| For patients at risk of physical and nonphysical morbidity, perform a comprehensive clinical assessment to identify their current rehabilitation needs. This should include assessments by health care professionals experienced in critical care and rehabilitation. | NICE | Ungraded | |
| For patients at risk, start rehabilitation as early as clinically possible, based on the comprehensive clinical assessment and the rehabilitation goals. | NICE | Ungraded | |
Abbreviations: ICU, intensive care unit; PAD, clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit61; NICE, National Institute for Health Care and Excellence Clinical Guideline on Rehabilitation after Critical Illness62; SSC, Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock: 2016.10
For SSC and PAD, level of evidence is graded as high (high-quality randomized clinical trial [RCT]), moderate (downgraded RCTs or upgraded observational studies), or low (observational study).
Early Hospital Treatment for Sepsis
Early hospital care for sepsis focuses on prompt recognition, treatment with broad-spectrum antibiotics, elimination of infectious sources (eg, removing infected indwelling catheters), and resuscitation with intravenous fluids and vasopressors for patients with low blood pressure or elevated lactate.10 In a recent observational study involving 49 331 patients, prompt delivery of these treatments was associated with improved survival (odds ratio for in-hospital mortality, 1.04 per hour delay to antibiotic administration, P < .001).63 Earlier treatment may also result in fewer long-term sequelae by minimizing duration of pathogen invasion, host response, host-pathogen interaction, and limiting the opportunity for adverse sequelae.
Pain, Agitation, and Delirium Management During Hospitalization for Sepsis
In critically ill patients, pain, agitation, and delirium are common complications that are associated with increased risk of mortality, cognitive impairment, and PTSD.64 Clinical practice guidelines recommend (1) completing regular assessments of pain using a validated scale (eg, Behavioral Pain Scale or Critical Care Pain Observation Tool); (2) prescribing intravenous narcotic analgesics as the first-line pharmacological treatment for pain; (3) using short-acting sedative medications (eg, propofol or dexmedetomidine) over benzodiazepines; (4) monitoring depth of sedation using a validated scale (eg, Richmond Agitation Sedation Scale or Sedation Analgesia Scale); (5) maintaining light levels of sedation (ie, a patient should be arousable and able to respond to simple commands); (6) stopping continuous sedative medications at least once daily to allow patients to awaken and be reoriented; and (7) monitoring for delirium regularly using a validated scale (eg, Confusion Assessment Method for the ICU or Intensive Care Delirium Checklist). Lighter sedation is associated with lower 1-year mortality (eg, 44% in patients randomized to less sedation combined with a ventilation weaning protocol vs 58% with usual care, P = .01),65 without increased risk of PTSD.66
Early Mobility
In a randomized clinical trial (RCT),67 early mobilization, which promotes early and progressive activity (bed-based exercises, to sitting, standing, and ultimately walking), resulted in shorter time to physical therapy (median, 1.5 vs 7.4 days; P < .001), time to ambulation (median, 3.8 vs 7.6 days; P < .001), and duration of delirium (median 2.0 vs 4.0 days, P = .03) during hospitalization. Randomization to early mobility interventions has also been associated with improved physical function at hospital discharge67–69 and increased likelihood of being discharged directly home(53 of 104[51%] vs 26 of 96 [27%], P < .001).68 Although early mobility has not been proven to improve late physical function, it is possible that short-term improvement in function also results in improvement in long-term (eg, 6-month) function.
Postdischarge Assessment and Treatment of Sepsis Survivors
There is limited clinical trial evidence to guide management of patients after hospitalization for sepsis70,71(Table 3). Randomized clinical trials to promote recovery after critical illness have examined specialized nurse-led ICU follow-up clinics,79,80 in-person exercise rehabilitation programs,73–75 provision of self-guided exercise rehabilitation manuals,72 and case management interventions.76,77 However, these interventions have yielded only small and inconsistent benefits in short- and moderate-term physical function by patient report (eg, 36-Item Short Form Physical Function Component) or physical assessment (eg, anaerobic threshold).
Table 3.
Randomized Clinical Trials of Postdischarge Rehabilitation Interventions for Survivors of an Intensive Care Unit Stay
| Source | Location | Patients | Intervention | Control | Trial Length | Primary Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|
| No. | Characteristics | No. (%) With Sepsis | Mean Age, y | ||||||
| Rehabilitation Programs | |||||||||
| Jones et al,72 2003 | United Kingdom | 126 | Adult ICU survivors who received mechanical ventilation | Not reported | 58 | Routine ICU follow-up, plus a 6-wk self-help rehabilitation manual | Routine ICU follow-up, consisting of 2 general ward visits, 3 telephone calls at home, and follow-up in a dedicated ICU follow-up clinic at 8 wk and 6 mo | 6 mo | “A repeated-measures ANOVA (group by time interaction effect) of the SF-36 physical function scores at the 3 time points (premorbid, 8 wk, and 6 mo), when controlled for length of ICU stay, was significant, P=.006.” Anxiety (HADS-A) was present in 33% (19/58) of intervention vs 34% (15/44) of control patients at 6 mo (P > .05) Depression (HADS-D) was present in 12% (8/63) of intervention vs 25% (13/51) of control patients at 8 wk (P = .07) and 10% vs 12% at 6 mo (P > .05) IES scores were “lower in the intervention patients at 8 wk” (absolute numbers not reported; P = .03) PTSD (IES>19) was present in 53% (31/58) vs 48% (21/44) at 6 mo (P = .57) |
| Elliot, et al,73 2011 | Australia and New Zealand | 195 | Adult ICU survivors who received mechanical ventilation ≤24 h, mean APACHE II score, 19; median ICU LOS, 6 d | Not reported (8) | 57 | 8-wk Individualized home-based physical rehabilitation program, including 3 physical trainer home visits, 4 follow-up telephone calls, and a printed exercise manual | Community-based care (eg, visits to primary care physician). 3 visits for study assessment only | 6 mo | Estimated treatment effects (change in intervention minus change in control patients) at 8 wk were 0.7 (95% CI, −2.5 to 3.8) for SF-36 physical function subscale, 8.4 m (95% CI, −29.6 to 46.4 m) for 6-minute walk distance, −1.3 (95% CI, −4.3 to 1.7) for SF-36 Physical Component Summary, and 1.8 (95% CI, −2.6 to 6.2) for SF-36 Mental Component Summary. Differences at 26 wk were 0.9 (95% CI, −2.7 to 4.6) for SF-36 physical function subscale, 9.6 m (95% CI, −31.4 to 50.5 m) for 6-minute walk distance, 0.3 (95% CI, −3.2 to 3.7) for SF-36 Physical Component Summary, and 1.5 (95% CI, −3.1 to 6.2) for SF-36 Mental Component Summary |
| Jackson et al,74 2012 | United States | 21 | Adult survivors of an ICU hospitalization with shock or respiratory failure, mean APACHE II score, 23; mean ICU LOS, 3 d | 6 (30.0) | 47 | 12-wk Home-based cognitive, physical, and functional rehabilitation program; 12 in-person visits, alternating between cognitive and physical rehabilitation; 12 televisits focusing on cognitive and physical rehabilitation on off-wk | Usual care: referral to physical, occupational or nursing care as determined by medical providers | 3 mo | At 3 mo, median Tower score (overall executive functioning), 13.0 (IQR, 11.5–14.0) in intervention vs 7.5 (IQR, 4.0–8.5) control (P < .01); Functional Activities Questionnaire score, 1.0 (IQR, 0.0–2.5) vs 8.0 (IQR, 6.0–11.0; P = .04); median Timed Up and Go test, 9.0 s (IQR, 8.5–11.8 s) vs 10.2 s (IQR, 9.2–11.7 s; P = .51); rates of moderate to severe ADL limitations, 0% (0/7) vs 25% (2/8; P = .78); Activities Balance and Confidence Scale, 82 (IQR, 38–91) vs 83 (IQR, 78–89; P = .35); Dysexecutive Questionnaire, 8.0 (IQR, 6.0–13.5) vs 16.0 (P = .74); MMSE, 30.0 (IQR, 29.0–30.0) vs 26.5 (IQR, 24.8–28.5; P = .25) |
| Batterham et al,75 2014 | United Kingdom | 59 | Adult ICU survivors who received mechanical ventilation ≤72 h, mean APACHE II score, 16; median ICU LOS, 15 d | 31 (52.5) | 42 | 8-wk Physiotherapist-supervised exercise program, consisting of 2 physiotherapist-led ergometer exercise sessions plus 1 unsupervised exercise session per wk | Usual care | 6 mo | 9-wk Difference in anaerobic threshold (in mL O2 kg-1 min-1), 1.8 (95% CI, 0.4 to 3.2); difference in SF-36 Physical Function sub-scale, 3.4 (95% CI, −1.4 to 8.2); and difference in SF-36 Mental Health subscale, 1.9 (95% CI, −3.9 to 7.7); 26-wk difference in anaerobic threshold, 0.6 (95% CI, −1.6 to 2.8); difference in SF-36 Physical Function subscale, 0.1 (95% CI, −6.0 to 6.2); and difference in SF-36 Mental Health subscale, 4.4 (95% CI, −2.4 to 11.2); differences in secondary outcomes (EQ-5D, HADS-A, HADS-D) were not significant at either time point |
| Case Management and/or Enhanced Primary Care | |||||||||
| Daly et al,76 2005 Douglas et al,77 2007 | United States | 334 | Adult ICU survivors who received mechanical ventilation for at least 72 h, mean APACHE III, 76; mean ICU LOS, 16 d | Not reported; but 399 (38.3) had pneumonia | 62 | 2-mo Disease management program, including care coordination, family support, education, and monitoring of treatment by advanced-practice nurse with geriatrician and pulmonologist consultation | Usual care | 2 mo | Hospital readmission occurred in 40.4% (93/231) of intervention vs 41.9% (39/103) of control patients, P = .65. Mean time to readmission was 15.9 d (95% CI, 12.8–18.9 d) vs 13.9 d (95% CI, 9.4–18.5 d; P = .64); mean No. of days spent readmitted was 11.4 (95% CI, 9.3–12.6) vs 16.7 (95% CI, 12.5–21.0; P = .03) |
| Schmidt et al,78 2016 | Germany | 291 | Adult survivors of an ICU hospitalization for severe sepsis or septic shock, 84% received mechanical ventilation, mean ICU LOS, 34 d | 291 (100) | 61 | 12-mo Primary Care Management Program, including patient and clinician education, case management, and consultant decision support | Usual care | 1 y | Estimated treatment effect (change in intervention minus change in control patients) for primary outcome SF-36 Mental Component Summary at 6 mo was 2.2 (95% CI, −1.8 to 6.1; P = .28); treatment effect was significant for 5 of 63 secondary outcome measures: independent ADL completion at 6 mo, 1.0 (95% CI, 0.2 to 1.8), and at 12 mo, 0.9 (95% CI, 90.0 to 1.7; P = .3 and P = .05); XSMFA-F at 6 mo, −8.9 (95% CI, −17.0 to 0.7; P = .04); XSMFA-B at 6 mo, −9.9 (95% CI, −18.5 to −1.2; P = .03); Regensburg Insomnia Scale at 12 mo, −1.8 (95% CI, −3.5 to −0.1; P = .03) |
| ICU Follow-up Clinics | |||||||||
| Cuthbertson et al,79 2009 | Scotland | 286 | >97% of adult ICU survivors received mechanical ventilation, median, APACHE II score, 19; median, ICU LOS, 3 d | Not reported | 59 | 3-mo Self-directed physical rehabilitation and nurse-led intensive care follow-up clinic visits at 3 and 9 mo | Usual care | 1 y | Estimated treatment effects (change in intervention minus change in control patients) were SF-36 Physical Component score at 12 mo, 1.1 (95% CI, −1.9 to 4.2; P = .46; SF-36 Mental Component score at 12 mo, 0.4 (95% CI, −3.0 to 3.7), P = .83. There was no significant treatment effect for any secondary outcome measures, including SF-36 at 6 mo; quality of life (EQ-5D) at 6 or 12 mo; anxiety (HADS-A) at 6 or 12 mo; or depression (HADS-D) at 6 or 12 mo |
| Jensen et al,80 2016 | Denmark | 386 | Adult ICU survivors who received mechanical ventilation for at least 48 h, median, APACHE II score, 25; median, ICU LOS, 10 d | 112 (29.0) | 66 | Nurse-led ICU Recovery Program, including an informational pamphlet, 1 clinic visit at 1–3 mo, and 2 telephone visits, at 5- and 10-mo post-ICU | Usual care | 1 y | Estimated treatment effects (change in intervention minus change in control patients) were SF-36 Physical Component Score at 12 mo, 1.4 (95% CI, −1.5 to 4.4; P = .35); SF-36 Mental Component Score at 12 mo, 1.9 (95% CI, −1.1 to 4.9; P = .11); there was no significant treatment effects for any secondary outcomes at 3 or 12 mo, including anxiety (HADS-A), depression (HADS-D), PTSD (HTQ-IV), or sense of coherence (orientation to life scale) |
Abbreviations and Definitions: Activities Balance and Confidence scale (range, 0%–100%) rates self-confidence in balance with higher scores reflecting greater confidence in balance; ADL, activities of daily living; ANOVA, analysis of variance; APACHE, acute physiology and chronic health evaluation; Dysexecutive Questionnaire is a self-reported measure of behavioral markers of executive function (score range, 0–80) with higher scores reflecting worse functioning; EQ-5D, EuroQol Five Dimensions Questionnaire; HADS-A, Hospital Anxiety and Depression Scale–Anxiety Subscale (range, 0–21) with higher scores representing greater anxiety; HADS-D, Depression Subscale (range, 0–21), with higher scores representing greater depression; Functional Activities Questionnaire is a 10-item self-report measure of complex instrumental activities of daily living (range, 0–30) with higher scores reflecting worse performance; HTQ-IV, Harvard Trauma Questionnaire; ICU, intensive care unit; IES, Impact of Event Scale (range, 0–88) with higher scores representing greater posttraumatic stress symptoms (PTSD) symptoms; IQR, interquartile range; LOS, length of stay; MMSE, Mini-Mental Status Examination, which assesses overall cognitive function (range, 0–30) with higher scores reflecting better function; SF-36, 36-Item Short Form Health Survey (range, 0–100) with higher scores represent greater quality of life; Timed Up and Go test assesses ambulation ability, longer times reflect worse performance; Tower test assesses overall executive functioning ability on a test of planning and strategy (range, 1–19) with higher scores reflecting better performance; XSFMA-B, Extra Short Musculoskeletal Function Assessment regarding disability (range, 0–100) with higher scores represent greater impairment; XSFMA-F, function (range, 0–100) with higher scores represent greater impairment.
The only RCT78 that studied recovery after sepsis hospitalization randomized 291 patients to a multicomponent primary care management intervention vs usual care. The intervention included education for patients and clinicians about sepsis and its common sequelae; case management by nurses with ICU experience, focusing on proactive symptom monitoring; and decision support by physicians trained in both primary and critical care. The primary outcome was mental health—related quality of life at 6 months. However, 32 outcomes were measured, each at 6 and 12 months. The intervention group performed better on 5 of 64 outcomes (Short Musculoskeletal Function Assessment, Physical Function (XSMF-A [Extra Short Musculoskeletal Function Assessment regarding physical function] ) and Disability (XSMF-B) scales at 6 months, ADL limitations at 6 and 12 months, and Regensburg Insomnia Scale at 12 months), suggesting a potential effect on functional outcomes.78 However, given the number of outcomes measured, these positive findings must be considered exploratory.78
Despite the lack of high-quality evidence, several expert panels suggest that rehabilitation with physical, occupational, and speech therapy benefits patients who develop new weakness following sepsis.69,81 The National Institute for Health and Care Excellence’s Guidelines on Rehabilitation after Critical Illness recommend multiprofessional rehabilitation after critical illness,62 starting in the ICU and continuing in the ward and after hospital discharge. Indirect evidence also comes from the benefits of physical rehabilitation in related populations, such as older patients with cognitive impairment,82 patients surviving stroke or traumatic brain injury,83,84 and residents in long-term care.85 In addition, an observational study of 30 000 sepsis survivors showed that referral to rehabilitation within 90 days of hospital discharge was associated with lower risk of 10-year mortality compared with propensity-matched controls (adjusted HR, 0.94; 95% CI, 0.92–0.97; P < .001).86 Furthermore, a small pilot RCT74 of a multicomponent post-ICU rehabilitation program incorporating cognitive, functional, and physical rehabilitation showed improved cognitive and functional outcomes at 3 months using the Tower test for planning and strategic thinking (median, 13.0; interquartile range, 11.5–14.0 vs 7.5; interquartile range, 4.0–8.5; adjusted P <.01), suggesting that neurocognitive deficits may be also be amenable to treatment.
Patients with new impairments that are not rapidly improving should be referred for assessment by a physical, occupational, or speech therapist, given the strong theoretical rationale for benefit. The goals of rehabilitation, as in analogous clinical situations, include improving exercise capacity, strengthening skeletal and respiratory muscles, and promoting independent completion of ADLs.81 Potential tools to screen for new functional disability warranting referral include the Katz Index of ADLs, Timed Up and Go test, and 6-minute walk distance.
Screening for Treatable Medical Conditions
Physicians should assess patients’ risk of common and potentially preventable causes of hospital readmission (infection, congestive heart failure exacerbation, acute renal failure, chronic obstructive pulmonary disease exacerbation, and aspiration pneumonitis) and tailor medical care to anticipate and prevent these problems (Box).
Box. Framework for Evaluating and Treating Patients in the 90 Days After Hospitalization for Sepsisa.
Screen for Common, Treatable Impairments After Sepsis
Functional Disability
Patients aged 65 years or older develop an average of 1 to 2 new functional limitations6
For patients with newly reduced exercise capacity, consider enrollment in a clinical trial of rehabilitation. If a trial is not available, consider referral to physical therapy, referral to pulmonary or cardiac rehabilitation, or prescribe a structured exercise program, depending on the severity of impairments and motivation of the patients
For patients with new limitations of activities or instrumental activities of daily living, consider referral to occupational therapy
If sepsis has occurred in the setting of long-standing comorbidity and declining health, discuss whether transition to palliative focus is appropriate
Swallowing Impairment
Of patients aged 65 years or older, 1.8% (95% CI, 1.3%–2.3%) are readmitted within 90 days for principal diagnosis of aspiration pneumonitis37
For patients with evidence of swallowing impairment (dysphagia, weak voice, or cough), consider referral to speech therapy for further evaluation (eg, fluoroscopic swallow evaluation) and treatment (eg, swallow strengthening exercises, modified diet)
Mental Health Impairments
Point prevalence for clinically significant anxiety is 32% (95% CI, 27%–38%) at 2 to 3 months41; for depression, 29% (range, 22%–36%) at 2 to 3 months42; and for PTSD, 44% (range, 36%–52%) at 1 to 6 months43
Review the details of the hospital course with interested patients because ICU diaries are associated with decreased PTSD
Consider screening for depression and anxiety with validated surveys
Consider referring patients and caregivers to peer support programs or mental health services
Review and Adjust Long-term Medications
Medication Errors
Errors of omission occur in 10% to 25% of patients, depending on medication class.25 Errors of commission occur in 1% to 25%, depending on medication class26,27
Confirm that long-term medications should remain on list
Discontinue hospital medications without ongoing indication (eg, inhalers, atypical antipsychotics, gastric acid suppressants)
Assess whether any doses should be adjusted based on changes in body mass, renal, or cardiac function, focusing on diuretics, antihypertensives, and renally cleared medications
Anticipate and Mitigate Risk for Common and Preventable Causes of Health Deterioration
Infection
Of patients aged 65 years or older, 11.9% (95% CI, 10.6%–13.1%) are readmitted within 90 days for principal diagnosis of infection (sepsis, pneumonia, urinary tract, and skin or soft tissue infection), 6.4% are readmitted for a principal diagnosis of sepsis37
Counsel patients about their risk of infection and recurrent sepsis
Ensure receipt of vaccines appropriate for the patient
Encourage patients to seek medical care for infectious signs and symptoms
Counsel patients on signs and symptoms that infection has progressed to sepsis (eg, decreased urine output, confusion, cyanosis, mottled skin), indicating that immediate evaluation is needed
For patients presenting with signs or symptoms of infection, consider chest x-ray, complete blood cell count, urinalysis, or cultures to confirm or rule out suspected infection
Schedule in-person or telephone follow-up to monitor for symptomatic improvement in patients with suspected infection
Heart Failure Exacerbation
Of patients aged 65 years or older, 5.5% (95% CI, 4.6%–6.4%) are readmitted within 90 days for principal diagnosis of congestive heart failure37
Reassess need and dosage for diuretics, β-blockers, and ACE-inhibitors because dosage requirements may change after sepsis due to changes in body weight, renal function, or cardiac function
Monitor volume status and weight at each visit, recognizing that dry weight may have declined due to lost muscle mass
Acute Renal Failure
Of patients aged 65 years or older, 3.3% (range, 2.6%–4.0%) were readmitted within 90 days for principal diagnosis of acute renal failure37
For patients with acute renal injury during sepsis, consider surveillance laboratory testing to ensure that renal function improves or stabilizes (eg, check chemistry panel once a week for 3 weeks, then monitor less frequently once blood work is stable)
Reassess need and dosages for renally cleared and nephrotoxic agents (eg, ACE-inhibitors, NSAIDS, statins, ranitidine, opiates, benzodiazepines)
COPD Exacerbation
Of patients aged 65 years or older, 1.9% (95% CI, 1.4%–2.4%) are readmitted within 90 days for principal diagnosis of COPD exacerbation37
Confirm and initiate appropriate controller inhalers
Ensure receipt of vaccines appropriate for the patient
Review and consider stopping or reducing dosages of medications that may suppress respiration such as benzodiazepines and opiates
Abbreviations: ACE, angiotensin-converting enzyme inhibitor; COPD, chronic obstructive pulmonary disease; NSAIDS, nonsteroidal anti-inflammatory drugs; PTSD, posttraumatic stress disorder.
a This Box provides a framework to approach medical evaluation and treatment of patients who have recently survived sepsis hospitalization. Posthospital care should focus on screening for new impairments; reviewing and adjusting long-term medications; and screening for common, preventable causes for medical deterioration.
Medications
Physicians should review a patient’s medication list at hospital discharge, resume essential medications that may have been held during the hospital stay, and assess whether any newly added medications can be discontinued. Patients’ glomerular filtration rate, fluid balance, vascular tone, and weight may be labile in the weeks following hospitalization. Doses of antihypertensives, diuretics, and renally cleared medications should be reassessed at each visit until patients have stabilized.
Referrals
Because of the heterogeneity of potential problems after sepsis, clinicians may consider early referrals to multiple subspecialists and ancillary services. However, it is important to consider the experience of sepsis survivors within the cumulative complexity model framework,87 which conceptualizes patient experience as a balance between workload (the work of being a patient, including effort to understand, access, and use medical care) and capacity (the quality and availability of resources to facilitate being a patient). This framework acknowledges the challenges of adhering to medical care, and suggests that overly complex treatment plans have limitations. Patients with a recent sepsis hospitalization may experience several new barriers to carrying out treatment plans, such as new weakness, cognitive impairment, fatigue, lost income, or stressed caregivers. Clinicians should be aware of these challenges and should consider starting with 1 or 2 referrals to address the most significant symptoms, then place additional referrals over time.
Self-management
Patients and caregivers should be educated about sepsis (including common sequelae) and informed of peer support resources. Many patients are unaware of their sepsis diagnosis,88 and even fewer realize its association with long-term disability.89 Intensive care unit diaries—nonmedical accounts of a patient’s hospitalization written by nurses and family members—are shown to reduce PTSD symptoms when provided to patients and caregivers 1 month after an ICU stay.90,91 Although ICU diaries are uncommon outside Europe, providing a narrative of the hospital course in understandable terms to interested patients may provide similar benefit. Peer-to-peer support groups, a common resource for patients with cancer and other chronic diseases, have not existed for patients suffering the sequelae of sepsis until recently.92 Since 2015, Society of Critical Care Medicine has organized a growing number of in-person, online, and telephone-based support groups for patients and families surviving critical illness.92 Patients and families may benefit from sharing their story, receiving empathy, and learning coping mechanisms from others who have overcome or adapted to new impairments.92
Establishing Goals of Care
Given the high rates of death,13 disability,6 and health care use after sepsis, it is important to discuss goals of care and consider whether a palliative focus is appropriate, in particular for patients with declining health prior to sepsis. However, despite reduced quality of life relative to population norms,56 long-term sepsis survivors are often satisfied with their quality of life and would undergo ICU treatment again.93 Patient-specific conversation is needed.
Important Unanswered Questions
Many important questions about postsepsis morbidity remain un answered. Researchers generally consider sepsis from the starting point of hospital admission. Although this may be appropriate for healthy patients, it may be inappropriate for patients whose health was declining prior to sepsis. Future research is needed to better characterize how presepsis health affects long-term outcomes after sepsis.
Is Sepsis Different From Any Other Hospitalization?
Many challenges described above apply to all patients surviving acute illness.24,25 However, certain sequelae (eg, immunesuppression)may be more common after sepsis, while other aspects of care (eg, confirming correct medications) may be particularly important to address after sepsis.
Which Aspects of Illness and Treatment Contribute to Which Postsepsis Sequelae?
Long-term sequelae may be associated with both disease (eg, infection, organ dysfunction) and treatment (eg, sedation). Measuring the individual contributions of characteristics of sepsis and sepsis treatment to outcomes is challenging but necessary for targeting interventions to the most important mediators of long-term adverse sequelae.
Can Adverse Sequelae Be Prevented?
The most common outcome in RCTs of sepsis is mortality. Interventions that reduce mortality are assumed to be uniformly beneficial. However, interventions that reduce short-term mortality may increase long-term mortality or worsen other patient-centered outcomes such as physical disability.94 As survival from sepsis improves, the effect of interventions on long-term physical and cognitive function must be explicitly tested.
Which Clinicians Should Address Postsepsis Morbidity?
With increasing specialization of medical care, it is unclear who is best suited to address postsepsis morbidity and in what setting. Multi-disciplinary clinics for post-ICU care have been established in several countries,95 but their benefit is unknown. A multicenter collaborative was recently established to study and refine best practices for post-ICU clinics.96
Conclusions
As in-hospital sepsis mortality has decreased, an estimated 14 million patients survived hospitalization for sepsis in 2016. These patients often acquire new physical disability and cognitive impairment following sepsis and may experience further health deterioration after hospital discharge. Risk of subsequent infection, cardiovascular events, acute renalfailure, and aspiration are increased after hospitalization for sepsis. Further research is needed to determine the optimal approach to caring for patients who have survived sepsis.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grants K08 GM115859 (Dr Prescott) and R01 GM097471 (Dr Angus) from the National Institute of General Medical Sciences of the National Institutes of Health.
Footnotes
Disclaimer: The views expressed in this article do not necessarily reflect the position or policy of the US government or the Department of Veteran Affairs. Dr Angus, a JAMA associate editor, was not involved in the editorial evaluation of or decision to publish this article.
Additional Contributions: We thank Theodore “Jack” Iwashyna, MD, PhD, of the University of Michigan for thoughtful feedback on this review and Bronwen Connolly, MSc, PhD, MCSP, from St Thomas’ hospital in London for stimulating discussion on rehabilitation after sepsis. Neither were compensated for their contributions. We also thank the Michigan Center for Integrative Research in Critical Care.
Submissions: We encourage authors to submit papers for consideration as a Review. Please contact Edward Livingston, MD, at Edward.livingston@jamanetwork.org or Mary McGrae McDermott, MD, at mdm608@northwestern.edu.
Conflict of Interest Disclosures: Both authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Prescott reported receiving grant support from the National Institutes of Health and serving on the advisory board of a Bristol-Myers Squibb–sponsored study. Dr Angus reported that he has served as a consultant for Ferring Pharmaceuticals.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NKJ, et al. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Daniels R, Lembo A, et al. Sepsis survivors’ satisfaction with support services during and after their hospitalization [abstract] Crit Care Med. 2016;44(12):425. doi: 10.1097/01.ccm.0000510071.45969.89. [DOI] [Google Scholar]
- 6.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah FA, Pike F, Alvarez K, et al. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188(5):586–592. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, Reis S, Angus DC. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189(9):1065–1074. doi: 10.1164/rccm.201307-1321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayr FB, Talisa VB, Balakumar V, Chang CH, Fine M, Yende S. Proportion and cost of unplanned 30-day readmissions after sepsis compared with other medical conditions. JAMA. 2017;317(5):530–531. doi: 10.1001/jama.2016.20468. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 11.Healthcare Cost and Utilization Project. [Accessed November 23, 2016];Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov/
- 12.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190(1):62–69. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375–i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page DB, Donnelly JP, Wang HE. Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the University HealthSystem Consortium. Crit Care Med. 2015;43(9):1945–1951. doi: 10.1097/CCM.0000000000001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A, Kumar A Co-operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189(10):1204–1213. doi: 10.1164/rccm.201310-1875OC. [DOI] [PubMed] [Google Scholar]
- 17.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 18.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carson WF, Cavassani KA, Dou Y, Kunkel SL. Epigenetic regulation of immune cell functions during post-septic immunosuppression. Epigenetics. 2011;6(3):273–283. doi: 10.4161/epi.6.3.14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Xie M, Yang M, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun. 2014;5:4436. doi: 10.1038/ncomms5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio. 2014;5(5):e01361–e14. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 26.Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847. doi: 10.1001/jama.2011.1206. [DOI] [PubMed] [Google Scholar]
- 27.Scales DC, Fischer HD, Li P, et al. Unintentional continuation of medications intended for acute illness after hospital discharge: a population-based cohort study. J Gen Intern Med. 2016;31(2):196–202. doi: 10.1007/s11606-015-3501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morandi A, Vasilevskis E, Pandharipande PP, et al. Inappropriate medication prescriptions in elderly adults surviving an intensive care unit hospitalization. J Am Geriatr Soc. 2013;61(7):1128–1134. doi: 10.1111/jgs.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woon FL, Dunn CB, Hopkins RO. Predicting cognitive sequelae in survivors of critical illness with cognitive screening tests. Am J Respir Crit Care Med. 2012;186(4):333–340. doi: 10.1164/rccm.201112-2261OC. [DOI] [PubMed] [Google Scholar]
- 30.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors associated with functional recovery among older intensive care unit survivors. Am J Respir Crit Care Med. 2016;194(3):299–307. doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brummel NE, Bell SP, Girard TD, et al. Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med. 2017;196(1):64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hopkins RO, Suchyta MR, Kamdar BB, Darowski E, Jackson JC, Needham DM. Instrumental activities of daily living after critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(8):1332–1343. doi: 10.1513/AnnalsATS.201701-059SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heyland DK, Stelfox HT, Garland A, et al. Canadian Critical Care Trials Group and the Canadian Researchers at the End of Life Network. Predicting performance status 1 year after critical illness in patients 80 years or older: development of a multivariable clinical prediction model. Crit Care Med. 2016;44(9):1718–1726. doi: 10.1097/CCM.0000000000001762. [DOI] [PubMed] [Google Scholar]
- 36.Zielske J, Bohne S, Brunkhorst FM, Axer H, Guntinas-Lichius O. Acute and long-term dysphagia in critically ill patients with severe sepsis: results of a prospective controlled observational study. Eur Arch Otorhinolaryngol. 2014;271(11):3085–3093. doi: 10.1007/s00405-014-3148-6. [DOI] [PubMed] [Google Scholar]
- 37.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313(10):1055–1057. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou S-M, Chu H, Chao P-W, et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors: a nationwide population-based study. Am J Respir Crit Care Med. 2016;194(2):209–217. doi: 10.1164/rccm.201510-2023OC. [DOI] [PubMed] [Google Scholar]
- 39.Shen H-N, Lu C-L, Yang H-H. Risk of recurrence after surviving severe sepsis: a matched cohort study. Crit Care Med. 2016;44(10):1833–1841. doi: 10.1097/CCM.0000000000001824. [DOI] [PubMed] [Google Scholar]
- 40.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 41.Borges RC, Carvalho CRF, Colombo AS, da Silva Borges MP, Soriano FG. Physical activity, muscle strength, and exercise capacity 3 months after severe sepsis and septic shock. Intensive Care Med. 2015;41(8):1433–1444. doi: 10.1007/s00134-015-3914-y. [DOI] [PubMed] [Google Scholar]
- 42.Annane D, Sharshar T. Cognitive decline after sepsis. Lancet Respir Med. 2015;3(1):61–69. doi: 10.1016/S2213-2600(14)70246-2. [DOI] [PubMed] [Google Scholar]
- 43.Jackson JC, Hopkins RO, Miller RR, Gordon SM, Wheeler AP, Ely EW. Acute respiratory distress syndrome, sepsis, and cognitive decline: a review and case study. South Med J. 2009;102(11):1150–1157. doi: 10.1097/SMJ.0b013e3181b6a592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744–1753. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a meta-analysis. Crit Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 47.Davydow DS, Hough CL, Langa KM, Iwashyna TJ. Depressive symptoms in spouses of older patients with severe sepsis. Crit Care Med. 2012;40(8):2335–2341. doi: 10.1097/CCM.0b013e3182536a81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wintermann G-B, Brunkhorst FM, Petrowski K, et al. Stress disorders following prolonged critical illness in survivors of severe sepsis. Crit Care Med. 2015;43(6):1213–1222. doi: 10.1097/CCM.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 49.Bienvenu OJ, Gellar J, Althouse BM, et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013;43(12):2657–2671. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wunsch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014;311(11):1133–1142. doi: 10.1001/jama.2014.2137. [DOI] [PubMed] [Google Scholar]
- 52.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 54.Huang C, Daniels R, Lembo A, et al. Mental, physiologic, and functional disabilities in post-sepsis syndrome an international survey [abstract] Crit Care Med. 2016;44(12):429. [Google Scholar]
- 55.Johansen K, Hansen ST., Jr Symmetrical peripheral gangrene (purpura fulminans) complicating pneumococcal sepsis. Am J Surg. 1993;165(5):642–645. doi: 10.1016/s0002-9610(05)80452-0. [DOI] [PubMed] [Google Scholar]
- 56.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 57.Poulsen JB, Møller K, Kehlet H, Perner A. Long-term physical outcome in patients with septic shock. Acta Anaesthesiol Scand. 2009;53(6):724–730. doi: 10.1111/j.1399-6576.2009.01921.x. [DOI] [PubMed] [Google Scholar]
- 58.Yende S, Austin S, Rhodes A, et al. Long-term quality of life among survivors of severe sepsis: analyses of two international trials. Crit Care Med. 2016;44(8):1461–1467. doi: 10.1097/CCM.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF bundle in critical care. Crit Care Clin. 2017;33(2):225–243. doi: 10.1016/j.ccc.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barr J, Fraser GL, Puntillo K, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 62. [Accessed September 22, 2017];Rehabilitation after critical illness in adults. https://www.nice.org.uk/guidance/cg83/chapter/About-this-guideline.
- 63.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370(5):444–454. doi: 10.1056/NEJMra1208705. [DOI] [PubMed] [Google Scholar]
- 65.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 66.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168(12):1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 67.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaller SJ, Anstey M, Blobner M, et al. International Early SOMS-guided Mobilization Research Initiative. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388(10052):1377–1388. doi: 10.1016/S0140-6736(16)31637-3. [DOI] [PubMed] [Google Scholar]
- 69.Connolly B, O’Neill B, Salisbury L, Blackwood B Enhanced Recovery After Critical Illness Programme Group. Physical rehabilitation interventions for adult patients during critical illness: an overview of systematic reviews. Thorax. 2016;71(10):881–890. doi: 10.1136/thoraxjnl-2015-208273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehlhorn J, Freytag A, Schmidt K, et al. Rehabilitation interventions for postintensive care syndrome: a systematic review. Crit Care Med. 2014;42(5):1263–1271. doi: 10.1097/CCM.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 71.Connolly B, Salisbury L, O’Neill B, et al. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database Syst Rev. 2015;(6):CD008632. doi: 10.1002/14651858.CD008632.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones C, Skirrow P, Griffiths RD, et al. Rehabilitation after critical illness: a randomized, controlled trial. Crit Care Med. 2003;31(10):2456–2461. doi: 10.1097/01.CCM.0000089938.56725.33. [DOI] [PubMed] [Google Scholar]
- 73.Elliott D, McKinley S, Alison J, et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15(3):R142. doi: 10.1186/cc10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson JC, Ely EW, Morey MC, et al. Cognitive and physical rehabilitation of intensive care unit survivors: results of the RETURN randomized controlled pilot investigation. Crit Care Med. 2012;40(4):1088–1097. doi: 10.1097/CCM.0b013e3182373115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Batterham AM, Bonner S, Wright J, Howell SJ, Hugill K, Danjoux G. Effect of supervised aerobic exercise rehabilitation on physical fitness and quality-of-life in survivors of critical illness: an exploratory minimized controlled trial (PIX study) Br J Anaesth. 2014;113(1):130–137. doi: 10.1093/bja/aeu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daly BJ, Douglas SL, Kelley CG, O’toole E, Montenegro H. Trial of a disease management program to reduce hospital readmissions of the chronically critically ill. Chest. 2005;128(2):507–517. doi: 10.1378/chest.128.2.507. [DOI] [PubMed] [Google Scholar]
- 77.Douglas SL, Daly BJ, Kelley CG, O’Toole E, Montenegro H. Chronically critically ill patients: health-related quality of life and resource use after a disease management intervention. Am J Crit Care. 2007;16(5):447–457. [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt K, Worrack S, Von Korff M, et al. SMOOTH Study Group. Effect of a primary care management intervention on mental health-related quality of life among survivors of sepsis: a randomized clinical trial. JAMA. 2016;315(24):2703–2711. doi: 10.1001/jama.2016.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cuthbertson BH, Rattray J, Campbell MK, et al. PRaCTICaL study group. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jensen JF, Egerod I, Bestle MH, et al. A recovery program to improve quality of life, sense of coherence and psychological health in ICU survivors: a multicenter randomized controlled trial, the RAPIT study. Intensive Care Med. 2016;42(11):1733–1743. doi: 10.1007/s00134-016-4522-1. [DOI] [PubMed] [Google Scholar]
- 81.Major ME, Kwakman R, Kho ME, et al. Surviving critical illness: what is next? an expert consensus statement on physical rehabilitation after hospital discharge. Crit Care. 2016;20(1):354. doi: 10.1186/s13054-016-1508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis M, Peiris CL, Shields N. Long-term home and community-based exercise programs improve function in community-dwelling older people with cognitive impairment: a systematic review. J Physiother. 2017;63(1):23–29. doi: 10.1016/j.jphys.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 83.Vanderbeken I, Kerckhofs E. A systematic review of the effect of physical exercise on cognition in stroke and traumatic brain injury patients. Neuro Rehabilitation. 2017;40(1):33–48. doi: 10.3233/NRE-161388. [DOI] [PubMed] [Google Scholar]
- 84.Pollock A, Baer G, Campbell P, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev. 2014;(4):CD001920. doi: 10.1002/14651858.CD001920.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forster A, Lambley R, Hardy J, et al. Rehabilitation for older people in long-term care. Cochrane Database Syst Rev. 2009;(1):CD004294. doi: 10.1002/14651858.CD004294.pub2. [DOI] [PubMed] [Google Scholar]
- 86.Chao PW, Shih C-J, Lee Y-J, et al. Association of postdischarge rehabilitation with mortality in intensive care unit survivors of sepsis. Am J Respir Crit Care Med. 2014;190(9):1003–1011. doi: 10.1164/rccm.201406-1170OC. [DOI] [PubMed] [Google Scholar]
- 87.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gallop KH, Kerr CEP, Nixon A, Verdian L, Barney JB, Beale RJ. A qualitative investigation of patients’ and caregivers’ experiences of severe sepsis*. Crit Care Med. 2015;43(2):296–307. doi: 10.1097/CCM.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 89.Govindan S, Iwashyna TJ, Watson SR, Hyzy RC, Miller MA. Issues of survivorship are rarely addressed during intensive care unit stays: baseline results from a statewide quality improvement collaborative. Ann Am Thorac Soc. 2014;11(4):587–591. doi: 10.1513/AnnalsATS.201401-007BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jones C, Bäckman C, Capuzzo M, et al. RACHEL group. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Crit Care. 2010;14(5):R168. doi: 10.1186/cc9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jones C, Bäckman C, Griffiths RD. Intensive care diaries and relatives’ symptoms of posttraumatic stress disorder after critical illness: a pilot study. Am J Crit Care. 2012;21(3):172–176. doi: 10.4037/ajcc2012569. [DOI] [PubMed] [Google Scholar]
- 92.Mikkelsen ME, Jackson JC, Hopkins RO, et al. Peer support as a novel strategy to mitigate post-intensive care syndrome. AACN Adv Crit Care. 2016;27(2):221–229. doi: 10.4037/aacnacc2016667. [DOI] [PubMed] [Google Scholar]
- 93.Cuthbertson BH, Elders A, Hall S, et al. Scottish Critical Care Trials Group; Scottish Intensive Care Society Audit Group. Mortality and quality of life in the five years after severe sepsis. Crit Care. 2013;17(2):R70. doi: 10.1186/cc12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA. 2014;311(13):1295–1297. doi: 10.1001/jama.2014.2639. [DOI] [PubMed] [Google Scholar]
- 95.Huggins EL, Bloom SL, Stollings JL, Camp M, Sevin CM, Jackson JC. A clinic model: post-intensive care syndrome and post-intensive care syndrome-family. AACN Adv Crit Care. 2016;27(2):204–211. doi: 10.4037/aacnacc2016611. [DOI] [PubMed] [Google Scholar]
- 96.Mikkelsen ME, Iwashyna TJ, Thompson CL, Andrews A. THRIVE: accomplishments, challenges, and next steps. Society of Critical Care Medicine Critical Connections; [Accessed December 7, 2017]. http://www.sccm.org/Communications/Critical-Connections/Archives/Pages/THRIVE-Accomplishments-Challenges-And-Next-Steps.aspx. Published December 6, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.