Abstract
Liquiritigenin (LQ) is a flavonoid that can be isolated from Glycyrrhiza radix. It is frequently used as a tranditional oriental medicine herbal treatment for swelling and injury and for detoxification. However, the effects of LQ on cognitive function have not been fully explored. In this study, we evaluated the memory-enhancing effects of LQ and the underlying mechanisms with a focus on the N-methyl-D-aspartic acid receptor (NMDAR) in mice. Learning and memory ability were evaluated with the Y-maze and passive avoidance tests following administration of LQ. In addition, the expression of NMDAR subunits 1, 2A, and 2B; postsynaptic density-95 (PSD-95); phosphorylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII); phosphorylation of extracellular signal-regulated kinase 1/2 (ERK 1/2); and phosphorylation of cAMP response element binding (CREB) proteins were examined by Western blot. In vivo, we found that treatment with LQ significantly improved memory performance in both behavioral tests. In vitro, LQ significantly increased NMDARs in the hippocampus. Furthermore, LQ significantly increased PSD-95 expression as well as CaMKII, ERK, and CREB phosphorylation in the hippocampus. Taken together, our results suggest that LQ has cognition enhancing activities and that these effects are mediated, in part, by activation of the NMDAR and CREB signaling pathways.
Keywords: Liquiritigenin, Cognition, N-methyl-D-aspartic acid receptor, Postsynaptic density-95, cAMP response element binding
INTRODUCTION
Hippocampal N-methyl-D-aspartic acid receptors (NMDARs) have been linked to spatial and long-term learning and memory (Fleischmann et al., 2003; Nakazawa et al., 2004). Functional NMDARs are generally comprised of a heterotetrameric asSEM bly typically containing two NMDA receptor 1 (GluN1) and two GluN2 or mixed GluN2 and GluN3 subunits (Paoletti et al., 2013). The contribution of NMDAR signaling to synaptic plasticity and learning and memory in the central nervous system is well established (Rao and Finkbeiner, 2007). Enhanced NMDAR signaling facilitates synaptic dopamine (DA) plasticity and superior learning and memory in various behavioral tasks (Shimizu et al., 2000; Kwon et al., 2015).
Clinical studies have revealed the potential for cognitive enhancement via plant sources as therapeutic tools in several neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease, and stroke (Rajendran et al., 2001; Lewis and Garcia, 2003; Oskouei et al., 2013; Kumar and Nisha, 2014). These enhancements can partially compensate for the cognitive deficits induced by AD (Griffith et al., 2010; Qin et al., 2012; Tang et al., 2013). In addition to these clinical benefits, cognitive enhancements have been observed in declarative and working memory and other cognitive functions in healthy subjects (Jensen et al., 2016; Veroniki et al., 2016). In this case, potential natural products or their active compounds may promote neurogenesis-associated cell proliferation, migration, differentiation, apoptosis, and synaptogenesis via modulation of NMDARs in the central nervous system (CNS) (Kim and Oh, 2013; Lai et al., 2016).
Liquiritigenin (LQ) is a flavonoid that is extracted from the Glycyrrhiza radix and is found in a variety of plants and foods. LQ has anti-oxidant, anti-inflammatory, and anti-cancer properties. The chemical structure of LQ is shown in Fig. 1. In previous reports, Lie and colleagues demonstrated that LQ could inhibit neurotoxicity-induced production of amyloid beta-peptide in primary hippocampal neurons (Liu et al., 2009) and that it significantly improved learning and memory in transgenic mice in terms of AD-like amyloid beta precursor protein expression (Liu et al., 2011). Pharmacokinetic data showed that LQ is absorbed well in vivo and (Kang et al., 2009) it efficiently penetrates the blood-brain barrier both in cultured rat brain microvascular endothelial cells and astrocyte systems (Liu et al., 2010). The increasing understanding of LQ has gradually suggested a potential medicinal role for AD that deserves further investigation. Therefore, in the present study, we investigated whether LQ improved learning and memory in mice. To assess the cognitive-enhancing effects of LQ in mice, we evaluated the effect of LQ on learning and memory with the Y-maze and passive avoidance tests. We also conducted Western blot analysis to test whether LQ affected the NMDAR or cAMP response element binding (CREB) signaling pathways in mouse hippocampus.
Fig. 1.
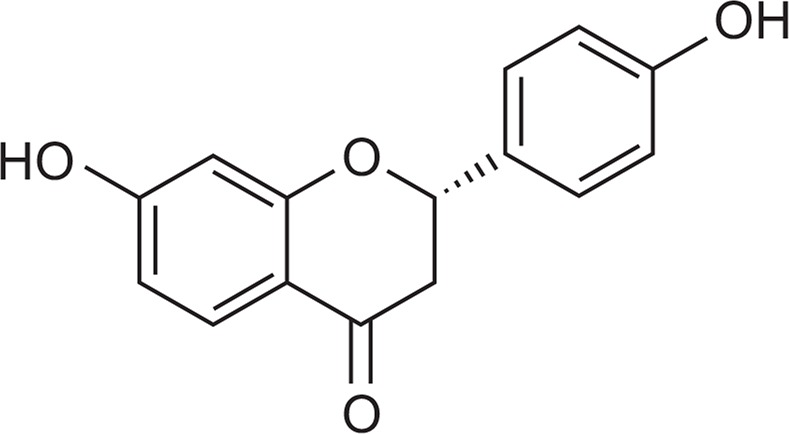
The chemical structure of liquiritigenin.
MATERIALS AND METHODS
Chemicals and reagents
LQ was purchased from Extrasynthese (Genay Cedex, France). Anti-β-actin antibody and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemicals (St. Louis, MO, USA). Rabbit anti-postsynaptic density protein-95 (PSD-95), anti-phospho Ca2+/calmodulin-dependent protein kinase II (CaMKII); anti-CaMKII, anti-phospho extracellular signal-regulated kinase 1/2 (ERK 1/2), anti-ERK 1/2, anti-NMDAR subunit 1 (NR1), anti-NMDAR subunit 2A (NR2A), and anti-NMDAR subunit 2B (NR2B) antibodies were purchased from Cell Signaling Technology Inc (Danvers, MA, USA). Rabbit anti-phospho CREB and anti-CREB antibodies were purchased from Abcam Company (Cambridge, MA, USA). Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories Inc (West Grove, PA, USA). All other chemicals were of analytical grade.
Animals
Male ICR mice (4-weeks-old, 20–23 g) were purchased from Koatech Co., Ltd (Pyongtaek, Korea). Mice were housed 10 per cage (26×42×18 cm), allowed access to water and food ad, libitum and maintained at constant temperature (23 ± 1°C) and humidity (55 ± 5%) conditions under a 12-h light/dark cycle (lights on 07:00 to 19:00 h). All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and with the approval of the Institutional Animal Care and Use Committee of Sungkyunkwan University (Suwon, Korea).
Y-maze test
The spontaneous alternation behavior Y-maze test is a horizontal maze (30 cm long and 5 cm wide, with walls 12 cm high) with three arms (labeled A, B, and C). The maze floor and walls are constructed of dark grey, polyvinyl plastic. Mice were initially placed within one arm, and the number of alternations (i.e., consecutive entry sequences of ABC, CAB, or BCA but not BAB) and the number of arm entries were manually recorded for each mouse over an 8-min period. One hour before each test, the mice were given LQ (5 or 20 mg/kg, p.o.) or vehicle (10% tween 80 with 5% DMSO in distilled water) for the control group. Percentage alternation was calculated according to the following equation: Percentage alternation= [(Number of alternations)/(Total arm entries-2)]×100. The number of arm entries per trial was used to indicate locomotor activity. The Y-maze arms were cleaned with 10% ethanol between tests to remove odors and residues.
Passive avoidance test
The step-through passive avoidance apparatus consisted of one clear and one dark chamber separated by a guillotine door. The floors of both the clear (12×10×12 cm) and dark chambers (12×10×12 cm) were made of 2-mm stainless steel rods spaced 0.5 cm apart. A 50-W lamp positioned 1 m above both chambers illuminated the apparatus. The mice underwent two separate trials, a training trial and a test trial 24 h later. One hour before the training trial, mice were given LQ (5 or 20 mg/kg, p.o.) or vehicle (10% tween 80 with 5% DMSO in distilled water) for the control group. For the training trial, mice were initially placed in the clear chamber. When they entered the dark chamber, the door closed and an electrical foot shock (0.5 mA for 3 seconds) was delivered through the stainless steel rods. Twenty-four hours after the training trial, mice were placed in the illuminated compartment for the test trial. During any trial (training or test trial), the time taken for a mouse to enter the dark compartment after the door opened was defined as the latency. Latency was recorded up to 300 seconds. The step-through passive avoidance apparatus was cleaned with 10% ethanol between tests to remove odors and residues.
Western blot analysis
Western blot analysis was performed as previously described (Kwon et al., 2013). In brief, isolated hippocampal tissues from both hemispheres were promptly excised and homogenized in a rotary homogenizer with 200 μl of ice-cold lysis T-per tissue protein extraction buffer (Thermo Scientific, Rockford, IL, USA) containing protease and phosphatase inhibitor cocktails (Roche Diagnostics, GmbH, Mannheim, Germany) and incubated on ice for 30 min. After centrifugation at 10,000×g for 15 min, the supernatant was separated and stored at −70°C. The protein concentration was determined with a protein assay kit (Thermo Scientific). The protein samples were subjected to 8–12.5 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). These samples were transferred onto polyvinylidene difluoride (PVDF) membranes (Pall Corporation, Pensacola, FL, USA) in transfer buffer [25 mM Tri-HCl buffer (pH 7.4) containing 192 mM glycine and 20% v/v methanol], and blocked with 5% non-fat milk in 0.5 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween 20 for 1h at room temperature. Each membrane was incubated in primary antibodies, anti-NR1 (1:1000), anti-NR2A (1:1000), anti-NR2B (1:1000), anti-PSD-95 (1:1000), anti-phospho CaMKII (1:1000), anti-CaMKII (1:1000), anti-phospho ERK 1/2 (1:2000), anti-ERK 1/2 (1:2000), anti-phospho CREB (1:1000), and anti-CREB (1:1000), overnight at 4°C. After washing the membranes with TBST (Tris-buffered saline with 0.1% Tween 20), blots were incubated in horseradish-peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) for 1 h at room temperature. After the blots were washed again with TBST to determine band density, enhanced chemiluminescence (ECL) was used by immersing the probed membrane in a 1:1 mixture of ECL reagents A and B (Animal Genetics Inc., Suwon, Korea) for 5 min. Membranes were then exposed to photographic film for a few minutes. Protein bands were quantified by densitometric analysis with ImageJ software from NIH (Bethesda, MD, USA).
Statistical analyses
Data are expressed as mean ± SEM and analyzed with Prism 6.0 software (GraphPad Software, Inc., San Diego, CA, USA). Data from the Y-maze and passive avoidance tests were analyzed with one-way analysis of variance (ANOVA) followed by Newman-Keuls test. Western blot data were analyzed with an unpaired t-test. Statistical significance was set at p<0.05.
RESULTS
Effects of LQ on the spontaneous alternation behavior
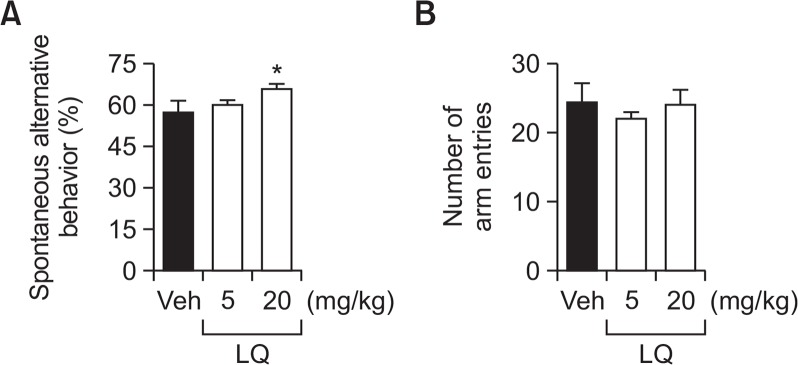
To evaluate the effects of LQ on short-term or working memory, improvement was investigated in the spontaneous alternation behavior. After 20 mg/kg LQ was administered, the percentage of spontaneous alternation behavior increased without a change in the number of arm entries compared to the vehicle group (Fig. 2A, F(2, 21)=3.249, p<0.05 and Fig. 2B, F(2, 33)=0.3208).
Fig. 2.
Effects of LQ on learning and memory as determined by spontaneous alternation behavior in a Y-maze. Mice were treated with LQ (5 and 20 mg/kg, p.o.) or vehicle solution 60 min before the tests (A and B). Data are expressed as the mean ± SEM (n=10). *p<0.05 compared with the vehicle group.
Effects of LQ on the step-through passive avoidance test
We evaluated the effects of LQ on long-term memory in mice by using the step-through passive avoidance test. Mice treated with LQ (20 mg/kg) had a significantly longer step-through latency time in test trials than did the vehicle group (Fig. 3, F(5, 42)=7.227, p<0.05).
Fig. 3.
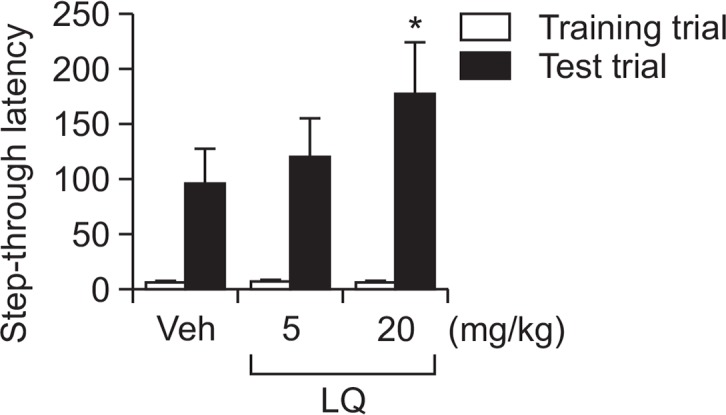
Effects of LQ on learning and memory as determined by using the step-through passive avoidance test. Mice were treated with LQ (5 and 20 mg/kg, p.o.) or vehicle 60 min before the tests. Data are expressed as the mean ± SEM (n=10). *p<0.05 compared with the vehicle group.
Effects of LQ on expression levels of NMDAR subunits 1, 2A, and 2B in the hippocampus
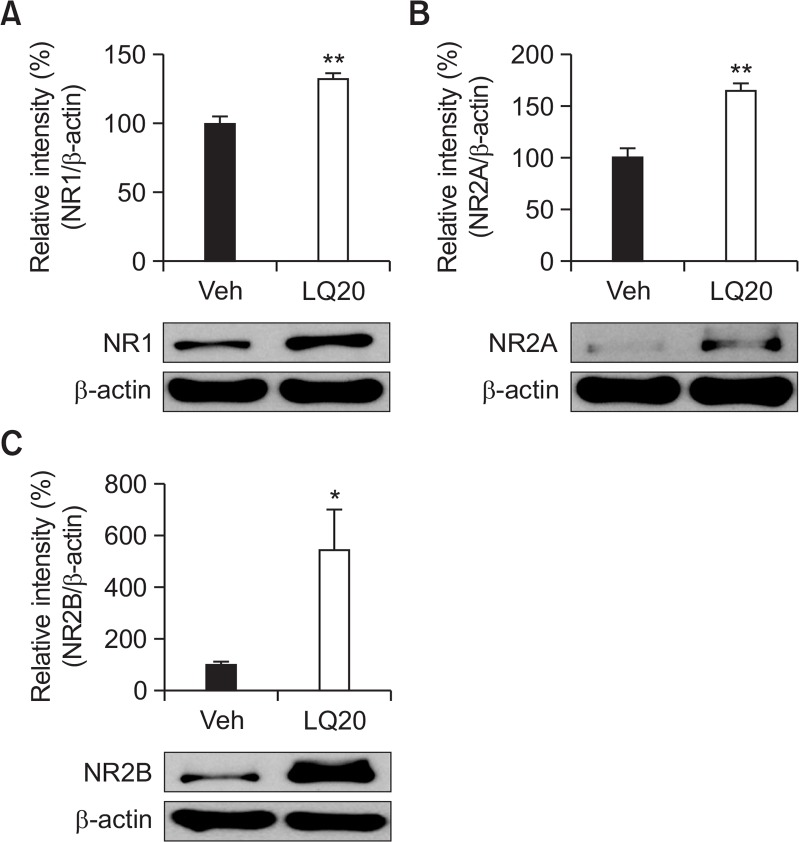
To determine the mechanism that underlies the effects of LQ on cognitive enhancement, we used Western blot analysis to investigate whether administering LQ influenced expression of NMDAR subunits 1, 2A, and 2B in the hippocampus. The LQ-treated group had significantly higher expression levels of subunits 1 (Fig. 4A, t=4.926, p<0.01), 2A (Fig. 4B, t=5.597, p<0.01), and 2B (Fig. 4C, t=2.802, p<0.05) in the hippocampus than did the vehicle group.
Fig. 4.
Effects of LQ on NR1 (A), NR2A (B), and NR2B (C) expression levels in the hippocampus. Mice were decapitated 60 min after test trials of the passive avoidance test. The hippocampus was dissected for Western blot analysis. Data are expressed as the mean ± SEM (n=5). *p<0.05 and **p<0.01 compared with the vehicle group.
Effects of LQ on PSD-95 expression levels and CaMKII, ERK, and CREB phosphorylation in the hippocampus
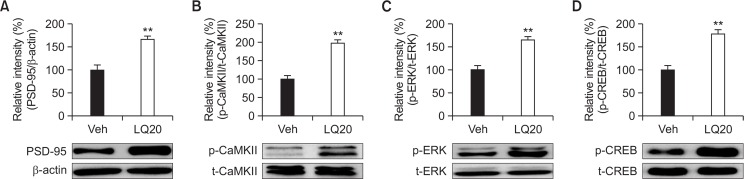
In order to assess the effects of LQ on PSD-95 expression and CaMKII, ERK, and CREB phosphorylation, which are downstream targets of NMDARs, Western blot analysis was performed after the behavioral tests. LQ significantly increased PSD-95 (Fig. 5A, t=5.033, p<0.01) expression levels and CaMKII phosphorylation (Fig. 5B, t=7.188, p<0.01), ERK phosphorylation (Fig. 5C, t=5.599, p<0.01) and CREB phosphorylation (Fig. 5D, t=5.636, p<0.01) in the hippocampus compared with the vehicle group.
Fig. 5.
Effects of LQ on PSD-95 expression levels (A) and CaMKII phosphorylation (B), ERK phosphorylation (C), and CREB phosphorylation (D) in the hippocampus. Mice were decapitated 60 min after test trials of the passive avoidance test. The hippocampus was dissected for Western blot analysis. Data are expressed as the mean ± SEM (n=5). **p<0.01 compared with the vehicle group.
DISCUSSION
The present study was designed to assess the effects of LQ on learning and memory in normal mice by using the Y-maze and passive avoidance tests. We also confirmed the effects of LQ on biochemical markers in the NMDA signaling pathway, expression of NMDARs (subunits 1, 2A, and 2B), PSD-95 and CaMKII, ERK, and CREB phosphorylation in the hippocampus.
To evaluate the effects of LQ on learning and memory, Y-maze and passive avoidance behavioral tests were performed after LQ was administered. Spontaneous alternation behavior in the Y-maze test is a surrogate measure of short-term and working memory (Kwon et al., 2009). LQ (20 mg/kg) significantly improved spontaneous alternation behavior without changing the number of arm entries in normal mice. The one trial passive avoidance task, which is used to measure fear-motivated contextual long-term memory, was also employed. A single dose of LQ (20 mg/kg) significantly enhanced the step-through latency time during the test trial, indicating that LQ-mediated enhancement of the survival and synaptic plasticity of newborn neurons may facilitate learning and memory.
We subsequently questioned how LQ affected cognition, including short-term, working, and long-term memory, in mice. NMDARs were previously reported to be essential regulators of synaptic plasticity, neuronal development, and synaptic transmission (Aamodt and Constantine-Paton, 1999; Armano et al., 2000). NMDARs play an important role in long-term potentiation (LTP), which is regarded as the molecular basis of learning and memory (Morris et al., 1986). Many previous studies have shown that the administration of NMDAR antagonists impairs memory in learning and memory tasks such as the radial maze and Morris water maze, suggesting that hippocampal NMDARs play an important role in learning and memory, especially spatial memory (Yamada et al., 2015; Song et al., 2016). In the present study, to determine whether LQ influenced the NMDA signaling pathway, we measured the expression of NMDAR subunits NR1, NR2A, and NR2B in the hippocampus after LQ administration. Western blot analyses showed that LQ significantly increased NR1, NR2A, and NR2B expression levels in the hippocampus. In addition, we assessed the effects of LQ on PSD-95 expression by Western blot after LQ administration. Patients with AD have decreased levels of post-synaptic intracellular scaffold proteins, including PSD-95, suggesting post-synaptic disruption precedes the loss of pre-synaptic proteins and initiates cognitive deficits (Gong and Lippa, 2010; Stranahan and Mattson, 2010; Danysz and Parsons, 2012). Moreover, NMDAR subunits interact with PSD-95 protein at the C-terminus of the NMDAR, and significant decreases in the PSD-95-NMDAR complex were observed in mice with long-term spatial learning and memory impairments (Barki-Harrington et al., 2009). In a previous report, decreased levels of PSD-95 were induced by decreased expression of NMDAR subunits (NR1, NR2A, and NR2B) in the hippocampus (Stan et al., 2015; Goodfellow et al., 2016). In our Western blots, LQ significantly increased PSD-95 expression in the hippocampus.
We also observed the effects of LQ on CaMKII phosphorylation in the hippocampus. The CaMKII protein plays an essential role in NMDA receptor-dependent hippocampal LTP and is a mediator of synaptic plasticity and learning and memory formation (Wei et al., 2006; Lamsa et al., 2007). Furthermore, CaMKII, a crucial serine/threonine kinase, is activated in a calmodulin (CaM)-dependent manner following calcium influx associated with many neuronal functions through NMDARs (Xu et al., 2008). A previous report demonstrated that the alteration of NMDARs could affect CaMKII activation in the CNS (Zhao et al., 2015). Our data provide the first evidence that the administration of LQ significantly increased the level of CaMKII phosphorylation in the hippocampus.
In addition, ERK is activated by multiple upstream molecular cascades including NMDAR/CaMKII signaling (Easton et al., 2013; Lee et al., 2016). Many previous studies have demonstrated that phosphorylation of ERK is linked to memory function, synaptic plasticity, and induction and maintenance of LTP (Vaynman et al., 2004; Ruscheweyh et al., 2011). Our Western blot results showed LQ administration markedly increased ERK phosphorylation in the hippocampus of mice. This increase might be due to the effect of LQ on NMDAR signaling pathway in the hippocampus.
The transcription factor CREB is implicated in neuronal plasticity and long-term memory. CREB is also related to the survival, proliferation, and maturation of neuronal cells (Alberini, 2009; Ortega-Martinez, 2015). The present study showed that the phosphorylation of hippocampal CREB was significantly higher in the LQ group than in the vehicle group. Taken together, our results suggest that the increase in NMDAR expression may activate CREB phosphorylation, which is critical for synaptic function and increasing NMDAR levels via a positive feedback loop. An increase in post-synaptic proteins may activate CREB phosphorylation resulting in enhanced cognitive consolidation. Thus, to the best of our knowledge, the effects of LQ on learning and memory are directly associated with synaptic function. We found increased expression of NMDAR subunits (NR1, NR2A, and NR2B) in the hippocampus due to LQ. These results provide evidence that the potent memory-enhancing effects of LQ in mice are associated with activation of the NMDA receptor pathway in the hippocampus. Moreover, LQ significantly increased PSD-95 level, phosphorylation of CaMKII, and phosphorylation of ERK, which are upstream molecules of phosphorylated CREB.
In conclusion, our study showed that administering LQ enhanced cognitive performance, which may have resulted from activated signaling by NMDARs and PSD-95 as well as CaMKII, ERK, and CREB phosphorylation in mouse hippocampi. Therefore, our results suggest that cognitive enhancement by LQ may be a candidate for treating cognitive dysfunction in neurological disorders such as AD.
Acknowledgments
This research was supported by grants (NRF-2013R1A-6A3A01027711 and NRF-2016R1D1A1A009919739) from the Basic Science Research Program through the National Research Foundation (NRF) and this research was also supported by a grant (HI12C0035) of the Korean Health Technology R&D project, Ministry of Health & Welfare, Korea.
Footnotes
CONFLICT OF INTEREST
The authors state that they have no conflicts of interest.
REFERENCES
- Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–144. [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armano S, Rossi P, Taglietti V, D’Angelo E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J Neurosci. 2000;20:5208–5216. doi: 10.1523/JNEUROSCI.20-14-05208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barki-Harrington L, Elkobi A, Tzabary T, Rosenblum K. Tyrosine phosphorylation of the 2B subunit of the NMDA receptor is necessary for taste memory formation. J Neurosci. 2009;29:9219–9226. doi: 10.1523/JNEUROSCI.5667-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine-searching for the connections. Br J Pharmacol. 2012;167:324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton AC, Lucchesi W, Mizuno K, Fernandes C, Schumann G, Giese KP, Müller CP. αCaMKII autophosphorylation controls the establishment of alcohol-induced conditioned place preference in mice. Behav Brain Res. 2013;252:72–76. doi: 10.1016/j.bbr.2013.05.045. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Hvalby O, Jensen V, Strekalova T, Zacher C, Layer LE, Kvello A, Reschke M, Spanagel R, Sprengel R, Wagner EF, Gass P. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow MJ, Abdulla KA, Lindquist DH. Neonatal Ethanol Exposure impairs trace fear conditioning and alters NMDA receptor subunit expression in adult male and female rats. Alcohol Clin Exp Res. 2016;40:309–318. doi: 10.1111/acer.12958. [DOI] [PubMed] [Google Scholar]
- Gong Y, Lippa CF. Review: disruption of the postsynaptic density in Alzheimer’s disease and other neurodegenerative dementias. Am J Alzheimers Dis Other Demen. 2010;25:547–555. doi: 10.1177/1533317510382893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TN, Varela-Nallar L, Dinamarca MC, Inestrosa NC. Neurobiological effects of Hyperforin and its potential in Alzheimer’s disease therapy. Curr Med Chem. 2010;17:391–406. doi: 10.2174/092986710790226156. [DOI] [PubMed] [Google Scholar]
- Jensen C, Forlini C, Partridge B, Hall W. Australian university students’ coping strategies and use of pharmaceutical stimulants as cognitive enhancers. Front Psychol. 2016;7:277. doi: 10.3389/fpsyg.2016.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HE, Jung HY, Cho YK, Kim SH, Sohn SI, Baek SR, Lee MG. Pharmacokinetics of liquiritigenin in mice, rats, rabbits, and dogs, and animal scale-up. J Pharm Sci. 2009;98:4327–4342. doi: 10.1002/jps.21702. [DOI] [PubMed] [Google Scholar]
- Kim HG, Oh MS. Memory-enhancing effect of Mori Fructus via induction of nerve growth factor. Br J Nutr. 2013;110:86–94. doi: 10.1017/S0007114512004710. [DOI] [PubMed] [Google Scholar]
- Kumar NS, Nisha N. Phytomedicines as potential inhibitors of beta amyloid aggregation: significance to Alzheimer’s disease. Chin J Nat Med. 2014;12:801–818. doi: 10.1016/S1875-5364(14)60122-9. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Hong SI, Ma SX, Lee SY, Jang CG. 3′,4′,7-Trihydroxyflavone prevents apoptotic cell death in neuronal cells from hydrogen peroxide-induced oxidative stress. Food Chem Toxicol. 2015;80:41–51. doi: 10.1016/j.fct.2015.02.014. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Ma SX, Joo HJ, Lee SY, Jang CG. Inhibitory effects of Eucommia ulmoides Oliv. Bark on scopolamine-induced learning and memory deficits in mice. Biomol. Ther. (Seoul) 2013;21:462–469. doi: 10.4062/biomolther.2013.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Q, Hu P, Li Q, Li X, Yuan R, Tang X, Wang W, Li X, Fan H, Yin X. NMDA receptors promote neurogenesis in the neonatal rat subventricular zone following hypoxicischemic injury. Mol Med Rep. 2016;13:206–212. doi: 10.3892/mmr.2015.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa K, Irvine EE, Giese KP, Kullmann DM. NMDA receptor-dependent long-term potentiation in mouse hippocampal interneurons shows a unique dependence on Ca(2+)/calmodulin-dependent kinases. J Physiol. 2007;584:885–894. doi: 10.1113/jphysiol.2007.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Choi SY, Yang JH, Lee YJ. Preventive effects of imperatorin on perfluorohexanesulfonate-induced neuronal apoptosis via inhibition of intracellular calcium-mediated ERK pathway. Korean J Physiol Pharmacol. 2016;20:399–406. doi: 10.4196/kjpp.2016.20.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RJ, Garcia ML. Therapeutic potential of venom peptides. Nat Rev Drug Discov. 2003;2:790–802. doi: 10.1038/nrd1197. [DOI] [PubMed] [Google Scholar]
- Liu RT, Tang JT, Zou LB, Fu JY, Lu QJ. Liquiritigenin attenuates the learning and memory deficits in an amyloid protein precursor transgenic mouse model and the underlying mechanisms. Eur J Pharmacol. 2011;669:76–83. doi: 10.1016/j.ejphar.2011.07.051. [DOI] [PubMed] [Google Scholar]
- Liu RT, Zou LB, Fu JY, Lu QJ. Effects of liquiritigenin treatment on the learning and memory deficits induced by amyloid beta-peptide (25–35) in rats. Behav Brain Res. 2010;210:24–31. doi: 10.1016/j.bbr.2010.01.041. [DOI] [PubMed] [Google Scholar]
- Liu RT, Zou LB, Lu QJ. Liquiritigenin inhibits Aβ25–35-induced neurotoxicity and secretion of Aβ1–40 in rat hippocampal neurons. Acta Pharmacol Sin. 2009;30:899–906. doi: 10.1038/aps.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Ortega-Martinez S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci. 2015;8:46. doi: 10.3389/fnmol.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouei DS, Rikhtegar R, Hashemilar M, Sadeghi-Bazargani H, Sharifi-Bonab M, Sadeghi-Hokmabadi E, Zarrintan S, Sharifipour E. The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. J Stroke Cerebrovasc Dis. 2013;22:e557–e563. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Qin RA, Yao XX, Huang ZY. Effects of compound danshen tablets on spatial cognition and expression of brain beta-amyloid precursor protein in a rat model of Alzheimer’s disease. J Tradit Chin Med. 2012;32:63–66. doi: 10.1016/S0254-6272(12)60033-8. [DOI] [PubMed] [Google Scholar]
- Rajendran PR, Thompson RE, Reich SG. The use of alternative therapies by patients with Parkinson’s disease. Neurology. 2001;57:790–794. doi: 10.1212/WNL.57.5.790. [DOI] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, SandKühler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Song JC, Seo MK, Park SW, Lee JG, Kim YH. Differential effects of olanzapine and haloperidol on mk-801-induced memory impairment in mice. Clin Psychopharmacol Neurosci. 2016;14:279–285. doi: 10.9758/cpn.2016.14.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan TL, Sousa VC, Zhang X, Ono M, Svenningsson P. Lurasidone and fluoxetine reduce novelty-induced hypophagia and NMDA receptor subunit and PSD-95 expression in mouse brain. Eur Neuropsychopharmacol. 2015;25:1714–1722. doi: 10.1016/j.euroneuro.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s disease. Neural Plast. 2010;2010:108190. doi: 10.1155/2010/108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Wang Z, Zhou Y, Xu W, Li S, Wang L, Wei D, Qiao Z. A novel drug candidate for Alzheimer’s disease treatment: gx-50 derived from Zanthoxylum bungeanum. J Alzheimers Dis. 2013;34:203–213. doi: 10.3233/JAD-121831. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Veroniki AA, Straus SE, Ashoor HM, Hamid JS, Hemmelgarn BR, Holroyd-Leduc J, Majumdar SR, McAuley G, Tricco AC. Comparative safety and effectiveness of cognitive enhancers for Alzheimer’s dementia: protocol for a systematic review and individual patient data network meta-analysis. BMJ Open. 2016;6:e010251. doi: 10.1136/bmjopen-2015-010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Wang GD, Zhang C, Shokat KM, Wang H, Tsien JZ, Liauw J, Zhuo M. Forebrain overexpression of CaMKII abolishes cingulate long term depression and reduces mechanical allodynia and thermal hyperalgesia. Mol Pain. 2006;2:21. doi: 10.1186/1744-8069-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Chandler LJ, Woodward JJ. Ethanol inhibition of recombinant NMDA receptors is not altered by coexpression of CaMKII-alpha or CaMKII-beta. Alcohol. 2008;42:425–432. doi: 10.1016/j.alcohol.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Shimizu M, Kawabe K, Ichitani Y. Hippocampal AP5 treatment impairs both spatial working and reference memory in radial maze performance in rats. Eur J Pharmacol. 2015;758:137–141. doi: 10.1016/j.ejphar.2015.03.080. [DOI] [PubMed] [Google Scholar]
- Zhao B, Wang Y, Li Y, Qiao X, Yan P, Zhu Y, Lai J. Differential phosphorylation of NMDAR1-CaMKII-MAPKs in the rat nucleus accumbens following chronic ethanol exposure. Neurosci Lett. 2015;597:60–65. doi: 10.1016/j.neulet.2015.03.061. [DOI] [PubMed] [Google Scholar]