Abstract
Quercetin and resveratrol are known to have beneficial effects on the diabetes and diabetic complication, however, the effects of combined treatment of these compounds on diabetes are not fully revealed. Therefore, the present study was designed to investigate the combined antidiabetic action of quercetin (QE) and resveratrol (RS) in streptozotocin (STZ)-induced diabetic rats. To test the effects of co-treated with these compounds on diabetes, serum glucose, insulin, lipid profiles, oxidative stress biomarkers, and ions were determined. Additionally, the activities of hepatic glucose metabolic enzymes and histological analyses of pancreatic tissues were evaluated. 50 male Sprague-Dawley rats were divided into five groups; normal control, 50 mg/kg STZ-induced diabetic, and three (30 mg/kg QE, 10 mg/kg RS, and combined) compound-treated diabetic groups. The elevated serum blood glucose levels, insulin levels, and dyslipidemia in diabetic rats were significantly improved by QE, RS, and combined treatments. Oxidative stress and tissue injury biomarkers were dramatically inhibited by these compounds. They also shown to improve the hematological parameters which were shown to the hyperlactatemia and ketoacidosis as main causes of diabetic complications. The compounds treatment maintained the activities of hepatic glucose metabolic enzymes and structure of pancreatic β-cells from the diabetes, and it is noteworthy that cotreatment with QE and RS showed the most preventive effect on the diabetic rats. Therefore, our study suggests that cotreatment with QE and RS has beneficial effects against diabetes. We further suggest that cotreatment with QE and RS has the potential for use as an alternative therapeutic strategy for diabetes.
Keywords: Diabetes, Quercetin, Resveratrol, Streptozotocin, Glucose
INTRODUCTION
Diabetes, the most common serious metabolic disorder and one of the five leading causes of death worldwide, is characterized by persistent hyperglycemia associated with carbohydrate, protein, and lipid metabolism abnormalities (Ramesh and Pugalendi, 2006; Go et al., 2015). Currently, 171 million people are affected with diabetes and the number is expected to rise to 366 million by 2030 (Wild et al., 2004). This disease is caused by a decrease or deficiency in insulin secretion and increased cellular resistance coincident with the induction of oxidative stress (Machha et al., 2007) and alterations in glucose and lipid metabolism-regulating enzymes (Gupta et al., 1999; Ramesh and Pugalendi, 2006). Therefore, controlling of blood glucose level is essential for preventing diabetic complications and improving the health of patients with diabetes (Ceriello, 2005). However, the currently available diabetic drugs have numerous limitations because of unwanted side effects including hypoglycemia, cell death, and high rates of secondary failure. Recently, emphasis has been placed on complementary and alternative treatments for diabetes focused on functional foods and their bioactive compounds (Tahrani et al., 2010).
Quercetin (QE) and resveratrol (RS), two structurally-related flavonoids, are naturally occurring phenolic compounds that are widely distributed in plants and natural foods (such as fruits and vegetables) and have been reported to have many beneficial effects including anti-inflammatory, anti-oxidative, anti-hypertensive, anticancer, antiviral, neuroprotective, hepatoprotective and anti-diabetic activities (ElAttar and Virji, 1999; Szkudelski and Szkudelska, 2011). In addition, both RS and QE have been suggested to extend the lifespan of model organisms and reduce cell senescence of normal cells (Machha et al., 2007; Zamin et al., 2009). QE supplementation has proven beneficial in normalizing blood glucose levels, augmenting liver glycogen content and enzymes, and reducing serum cholesterol. Furthermore, it has shown to improve the antioxidant status and prevent oxidative damage, therby promoting the regeneration of pancreatic islets and increasing insulin release in diabetes-induced rats (Vessal et al., 2003). RS also has antidiabetic effects and decreases blood glucose by increasing the intracellular transport of glucose and improving insulin action. It has also been reported to attenuate oxidative damage of the pancreatic tissue in an animal model of diabetes (Palsamy and Subramanian, 2009, 2011).
Many research studies have reported the combinatory effects of cotreatment with RS and QE on several diseases. In glioma cell lines, the combination of QE and RS cooperatively triggered apoptosis, inhibited cell proliferation and most strikingly induced a senescence-like growth arrest, although supplementation with QE alone had no anti-tumor effects on these cancer cell lines (Vessal et al., 2003). The combination of these drugs has shown antitumor effects on several types of cancers, such as colon cancer, by repressing oncogenic microRNA-27a (Del Follo-Martinez et al., 2013) and inhibiting cell growth and DNA synthesis in oral squamous carcinoma cells (ElAttar and Virji, 1999). They have also exhibited inhibitory effects on angiogenesis in several cancer cells (Igura et al., 2001).
To the best of our knowledge, there are no reports on the combined effect of these two flavonoids on glycemic control in STZ-induced diabetic rats. Therefore, the aim of this study was to evaluate the effect of cotreatment with QE and RS on blood glucose, plasma insulin, glycated hemoglobin (HbA1c), C-peptides, antioxidant activities, carbohydrate and lipid metabolism-regulating enzymes, metabolic acidosis, anionic gap, and osmolality in STZ diabetic rats.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats weighing 200–250 g were purchased from Koatech (Gyeonggi, Korea) and housed at 23 ± 2°C with 50 ± 5% humidity and with a 12 h light/dark cycle. The rats had free access to a standard rat pellet diet and tap water. All experimental protocols were approved by the Committee on the Care of Laboratory Animal Resources, Chonbuk National University (Approval number: CBNU2016-67) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. All efforts were made to minimize suffering.
Induction of experimental diabetes mellitus and experimental design
Diabetes was induced by intraperitoneally injecting the rats with STZ (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 50 mg/kg body weight (BW). The STZ was dissolved in 0.1 mL sodium citrate buffer (pH 4.5). On the day 3 after the STZ injection, blood glucose levels from the non-fasted rats were measured and rats with levels >350 mg/dL were considered to have type 1-diabetes. After establishing the diabetic model, 50 rats were divided into five equal groups: normal control, STZ-induced diabetes, diabetes with QE (Sigma-Aldrich) treatment, diabetes with RS (Enzo Life Sciences Inc., Plymouth Meeting, PA, USA) treatment, and diabetes with QE and RS cotreatment (n=10 in each group). The dosages of QE (Roslan et al., 2017) and RS (Yonamine et al., 2016) were used as previously described. The compounds-treated groups were daily administered intraperitoneal injections at doses of 30, 10, and 30+10 mg·kg−1·day−1 for QE, RS, and the cotreatment, which were dissolved in 0.3% dimethyl sulfoxide (DMSO), respectively for 14 days from the 3rd day after STZ injection for the establishment of diabetes. The normal control and STZ-induced diabetic rats were treated with 0.3% DMSO. During the experimental period, all animals were carefully monitored.
Measurement of blood glucose levels
To measure the glucose levels, fresh blood samples were collected from the rat tail vein, and glucose levels were then determined using a blood glucose meter (ACCU-CHEK® Active, Roche Diagnostics, Mannheim, Germany) that uses test strips to assess a glucose oxidoreductase mediated dye reaction, according to the manufacturer’s instruction. The blood glucose levels were measured on the day of 0, 7, 9, 12 and 14th.
Oral glucose tolerance test (OGGT)
The rats were orally administrated glucose (2 g/kg) after overnight fasting for 12 h at 14th day after compounds treatment. Blood samples were collected from the tail vein at 0, 30, 60, 90, 120 min after the administration of glucose. The glucose levels were then determined using a blood glucose meter (ACCU-CHEK® Active, Roche Diagnostics) according to the manufacturer’s instruction.
Biochemical analysis
Blood was collected from the caudal vena cava after all rats were euthanized. Blood collection, storage, and measurement were performed as previously described (Rahman et al., 2014). A Nova Stat Profiler pHOxr Ultra analyzer (NOVA Biomedical Corp., Waltham, MA, USA) was used to measure the lactate, pH, bicarbonate (HCO3), hemoglobin (Hb), hematocrit (Hct), Na+, and Cl− ion levels in the freshly collected whole blood. The anion gap values were calculated using the formula (Na+-[Cl−+HCO−3]). Serum was collected by centrifuging the whole blood samples at 1,500 g for 15 min. A Hitachi 7020 system (Hitachi, Tokyo, Japan) was used to analyze the serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), lactate dehydrogenase (LDH), creatinine kinase (CK), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low density lipoprotein (LDL), creatinine (CRE), and blood urea nitrogen (BUN) levels. The very low-density lipoprotein (VLDL) was calculated using the following formula: VLDL=TG/5, as previously described (Friedewald et al., 1972). Finally, osmolality (Osm) values were calculated using the following formula: 1.86×Na++(Glucose/18)+ (BUN/2.8)+9, as previously described (Rahman et al., 2014).
Measurements of serum insulin, C-peptide, and HbA1c
Fasting serum insulin levels were measured using a rat insulin enzyme-linked immunosorbent assay (ELISA) kit (ALP-CO Diagnostics, Windham, NH, USA) according to the manufacturer’s protocol. Commercially available C-peptide enzyme immune assay (EIA, Sigma-Aldrich) and HbA1c ELISA (Cusabio Biotech Co., Ltd., Wuhan, China) kits were used to quantify these parameters according to the manufacturers’ protocols.
Measurements of hepatic glucose regulating enzymes
The rat livers were quickly rinsed and lysed in homogenizing buffer containing 0.25 M sucrose, 0.02 M triethanolamine (pH 7.4), and 0.12 mM dithiothreitol. The cytosolic fractions were then prepared by centrifugation at 100,000 g for 1 h, and they were used for further enzyme assays. Glucose-6-phosphate dehydrogenase (G6PD), Glucose-6-phosphatase (G6P), and malic enzyme (ME) levels were measured using a spectrophotometer as previously described (Baquer et al., 1976; Zak et al., 1977). glucokinase (GK) isozymes were assayed as described by (Gumaa and McLean, 1972) using the coupled enzymatic reaction system using nicotinamide adenine dinucleotide phosphate (NADP) at 25°C. Phosphoenolpyruvate carboxykinase (PEPCK) enzyme activity was assayed at 25°C in the reverse direction, carboxylation of phosphoenolpyruvate to form oxaloacetic acid in the presence of NADH (Roslan et al., 2017).
Measurement of oxidative stress-related enzymes
The serum levels of malondialdehyde (MDA) were measured using an OXI-TEK thiobarbituric acid reactive substances (TBARS) kit (Enzo Life Sciences Inc.). The reaction products were determined by measuring the absorbance of the reaction solution at 532 nm using a SpectraMax M2 microplate reader (Molecular Devices, Sunnyvale, CA, USA), according to the manufacturer’s instructions. The serum levels of superoxide dismutase (SOD) were measured using an SOD activity kit (Enzo Life Sciences Inc.), which measures the absorbance of the reaction products at 450 nm. Total glutathione (tGSH) and oxidized GSH (GSSG) were measured using a GSH (total) detection kit (Enzo Life Sciences Inc.). The absorbances of the reaction products were measured at 405 nm. The concentration of reduced GSH was calculated using the following formula: GSH=(tGSH-GSSG). The redox ratio was calculated using the following formula: GSH:GSSG=(tGSH-2 GSSG/GSSG), as previously described (Go et al., 2015).
Histological analysis of Pancreas tissues
The pancreas tissue samples were dissected, rinsed, and fixed in 10% neutral-buffered formalin. After fixation, the samples were embedded in paraffin, 5-μm sections were cut, stained with hematoxylin and eosin (H&E), and then examined using light microscopy.
Statistical analysis
All the data are expressed as the mean ± standard error of the mean (SEM). Statistical significance was evaluated using an analysis of variance (ANOVA) with the Bonferroni post hoc test (Prism 5.03, GraphPad Software Inc., San Diego, CA, USA). A p<0.05 was considered statistically significant.
RESULTS
Effects of QE, RS, and cotreatments on body weight changes and water intake
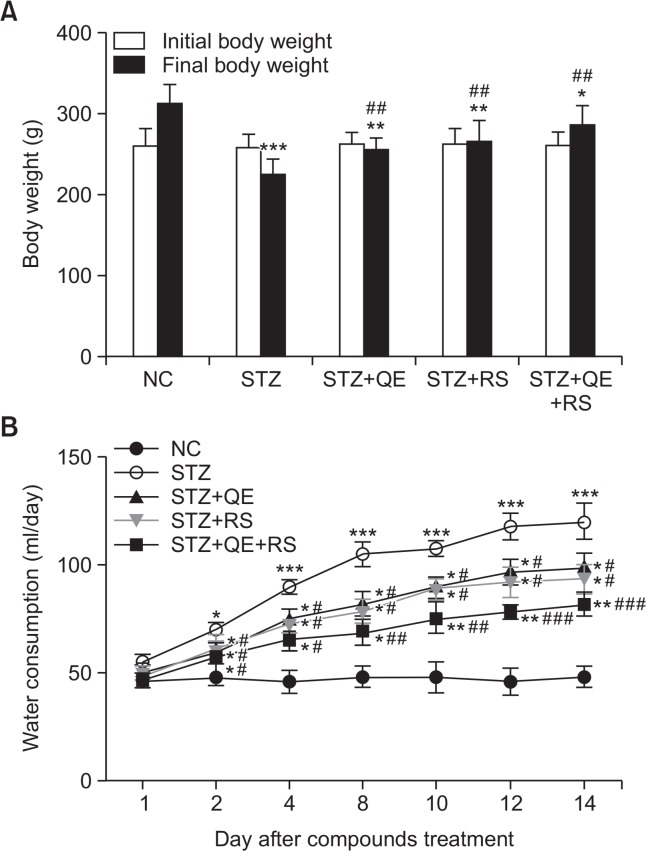
The diabetic rats showed the loss of body weight compared with that in normal control group at the 14th day after starting compounds treatment. Otherwise, it was inhibited in compounds-treated groups, including QE, RS, and cotreated with QE and RS compared with diabetic-induced group (Fig. 1A). Water intake gradually increased, and remained stable from the 8th day in diabetic group compared with that in normal control group, however, the increases of water intake significantly inhibited in compounds-treated groups compared with that in diabetic group (Fig. 1B). Of particular, the cotreated group showed the most effective in these parameters than those in each individual treated group.
Fig. 1.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on body weight change and water consumption. (A) Initial body weight and final body weight (at 14th day after compounds treatment) of rats in all groups. (B) Average daily water intake during the experimental period. Rats were equally divided into each group (n=10 per group). Data are means ± standard error of the mean (SEM). Groups: NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. *p<0.05, **p<0.01, and ***p<0.005 vs. NC group; #p<0.05, ##p<0.01, and ###p<0.001 vs. STZ-treated group.
Effects of QE, RS, and cotreatments on glucose levels
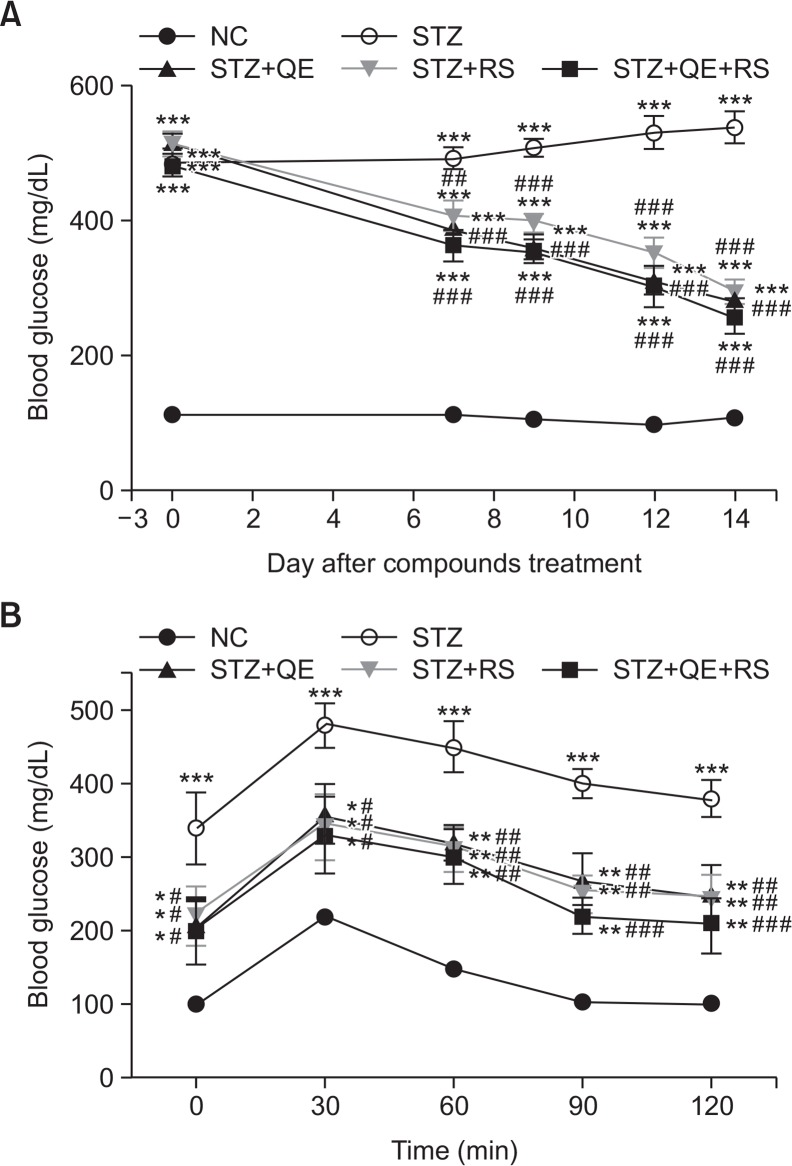
Fig. 2A shows the changes of glucose levels during experimental period. The glucose level steadily increased in the STZ-treated group compared with that in the normal control group while QE, RS, and cotreated groups showed significantly decreased glucose levels, especially compounds treated groups have shown to significant decreases of blood glucose levels from the 7th day compared with that shown by the STZ-treated group. On 14th day after compounds treatment, the glucose level of the STZ-treated group significantly increased compared with that in the normal control group. In contrast, QE or RS treatment significantly decreased the glucose levels compared with that in the STZ-treated group. Notably, the cotreated group showed the lowest glucose level of all the treated groups (Fig. 2A). In addition, the OGTT showed that glucose tolerance was impaired in diabetic rats, while this impairment was improved in compounds-treated diabetic rats (Fig. 2B).
Fig. 2.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on blood glucose levels. The blood glucose levels during the experimental period (A) and oral glucose tolerance test (OGTT) conducted at 14th day after compounds treatment (B). The Rats were equally divided into each group (n=10 per group). Data are means ± standard error of the mean (SEM). Groups: NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. *p<0.05, **p<0.01, and ***p<0.001 vs. NC group; #p<0.05, ##p<0.01, and ###p<0.001 vs. STZ-treated group.
Effects of QE, RS, and cotreatment on serum insulin, C-peptide, HbA1c, Hb, and Hct levels
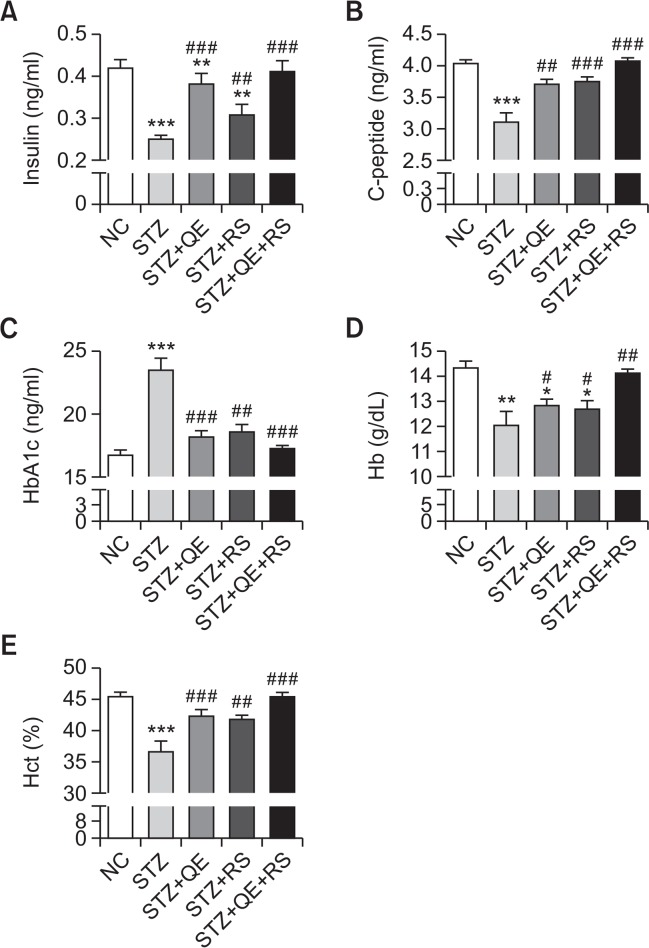
In the STZ-treated group, the serum insulin, C-peptide, blood Hct and Hb levels significantly decreased compared with those in the normal control group. In contrast, QE, RS, or combined treatments restored these hematological parameters in diabetic rats compared with the levels in the STZ-treated group. Conversely, the HbAlc was significantly elevated in the STZ-induced diabetic rats, while the levels were nearly normal in the QE, RS, and cotreated groups (Fig. 3).
Fig. 3.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on serum levels of (A) Insulin, (B) C-peptide, (C) glycated hemoglobin (HbA1c), (D) hemoglobin (Hb) and (E) hematocrit (Hct). Rats were equally divided into each group (n=10 per group). Data are means ± standard error of the mean (SEM). Groups: NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. Significance was measured using one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test. *p<0.05, **p<0.01, and ***p<0.005 vs. NC group; #p<0.05, ##p<0.01, and ###p<0.001 vs. STZ-treated group. HbA1c, glycated hemoglobin; Hb, hemoglobin; Hct, hematocrit.
Effects of QE, RS, and cotreatment on serum lipid profiles
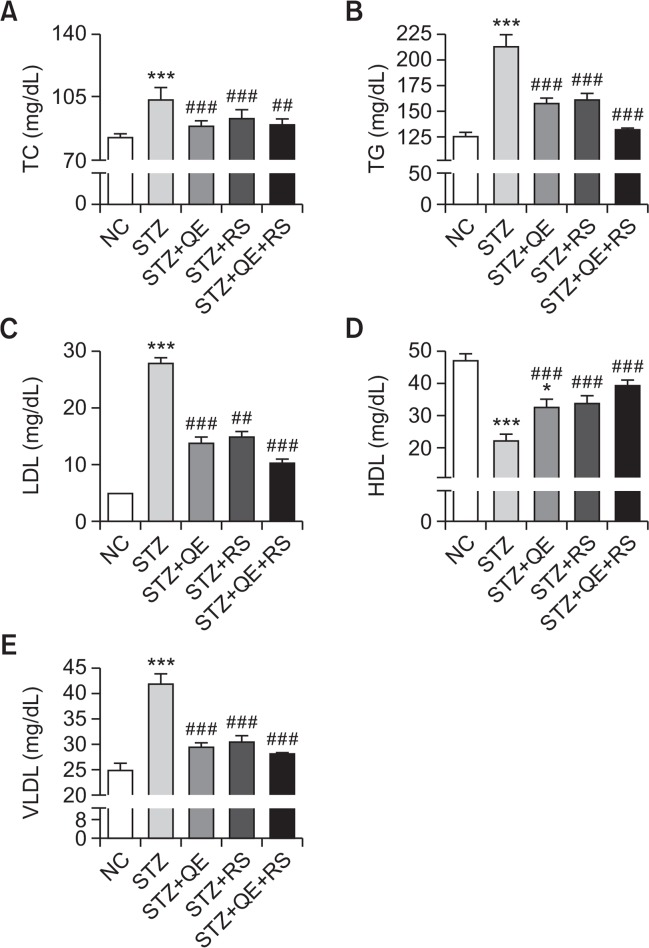
To investigate whether the hypoglycemic effect of the test compounds was mediated through protective actions against dyslipidemia in diabetic rats, we determined the serum lipid profiles. As shown in Fig. 4, TC, TG, LDL, and VLDL levels markedly increased in the STZ-treated group compared with those in normal control group. In contrast, increases in these parameters were significantly inhibited in the QE, RS, and cotreated groups. The HDL level exhibited an opposing increasing trend, which was dramatically decreased in the STZ-induced diabetic rats. Compared to the STZ-treated group, the QE, RS, and cotreated groups showed significant improvements (Fig. 4D). Notably, we observed that the cotreatment was more effective on the lipid profiles than each individual treatment was.
Fig. 4.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on serum lipid profiles. Rats were equally divided into each group (n=10 per group). Data are means ± standard error of the mean (SEM). Groups: NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. Significance was measured using one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test. *p<0.05 and ***p<0.005 vs. NC group; ##p<0.01 and ###p<0.001 vs. STZ-treated group. TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; VLDL, very low density lipoprotein.
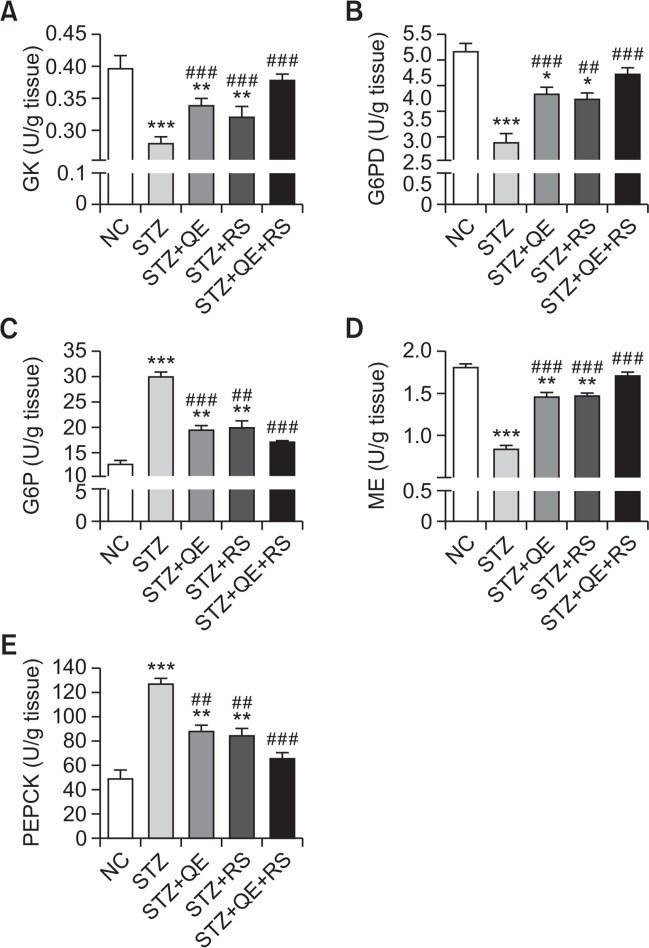
Effects of QE, RS, and cotreatment on hepatic glucose regulating enzymes
To determine the effects of QE, RS, and cotreatment on glucose and lipid metabolism in the liver under diabetic conditions, we investigated the hepatic glucose contents after treating the STZ-induced diabetic rats with the test compounds. The GK, G6PD, and ME levels were decreased in the STZ-treated group. Especially, cotreatment of QE and RS were shown to the most significantly protective effects compared with those in diabetic group. In contrast, the enzyme levels of all the compound-treated groups were conserved their properties. In addition, the levels of G6P and PEPCK enzymes were significantly elevated in the STZ-treated group while increases in the levels of the diabetic QE, RS, and cotreatment groups were dramatically inhibited to levels that were similar to those in normal control group (Fig. 5).
Fig. 5.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on hepatic glucose-regulating enzymes activities. Rats were equally divided into each group (n=10 per group). Data are means ± standard error of the mean (SEM). Groups: NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. Significance was measured using one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test. *p<0.05, **p<0.01, and ***p<0.005 vs. NC group; ##p<0.01 and ###p<0.001 vs. STZ-treated group. GK, glucokinase; G6PD, glucose 6 phosphate dehydrogenase; G6P, glucose 6 phosphatase; ME, malic enzyme; PEPCK, phosphoenolpyruvate carboxykinase.
Effects of QE, RS, and cotreatment on blood ion and metabolite concentrations
The blood pH and HCO3− concentrations were decreased while the blood lactate concentration, anionic gap, and osmolality were increased in the STZ-treated group. Similarly, alterations in Na+ and Cl− ion concentrations were observed in the STZ-treated group. However, the QE, RS, and cotreated groups showed a significant preservation of these blood parameters and ion concentration levels at similar levels to those of the normal control group (Table 1).
Table 1.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on hematological parameters and blood ions in streptozotocin (STZ)-induced diabetic rats
| NC | STZ | STZ+QE | STZ+RS | STZ+QE+RS | |
|---|---|---|---|---|---|
| pH | 7.44 ± 0.01 | 7.32 ± 0.02*** | 7.43 ± 0.01## | 7.41 ± 0.01## | 7.43 ± 0.01## |
| Lactate (mmol/L) | 4.8 ± 0.3 | 6.2 ± 0.4** | 5.2 ± 0.3### | 5.7 ± 0.1## | 4.9 ± 0.3### |
| HCO3− (mmol/L) | 29.0 ± 1.0 | 22.9 ± 0.6* | 26.9 ± 1.4 | 26.5 ± 0.7## | 27.0 ± 0.6*## |
| Osmolality (mOsm/L) | 295 ± 1.0 | 335 ± 3.0*** | 308 ± 2.0## | 312 ± 3.0### | 302 ± 1.0### |
| Anion gap (mmol/L) | 9.6 ± 1.4 | 17.5 ± 2.0 | 12.0 ± 1.5## | 15.1 ± 1.9# | 13.3 ± 1.2*## |
| Na+ (mmol/L) | 147 ± 1.0 | 152 ± 1.0* | 149 ± 1.0 | 150 ± 1.0 | 148 ± 1.2# |
| Cl− (mmol/L) | 109 ± 1.0 | 111 ± 1.0* | 110 ± 1.0 | 109 ± 2.0# | 107 ± 1.0## |
Rats were equally divided into each group (n=10 per group). NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. Data are means ± standard error of the mean (SEM). Significance was measured using one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test.
p<0.05,
p<0.01, and
p<0.005 vs. NC group;
p<0.05,
p<0.01, and
p<0.001 vs. STZ-treated group, respectively.
Effects of different treatments on serum levels of tissue injury markers
As shown in Table 2, levels of ALT, AST, ALP, GGT, BUN, and CRE were elevated while the TP level was reduced in the STZ-treated group compared with those of the normal control group. Notably, changes in these values were significantly inhibited by the QE, RS, and especially, the cotreatment.
Table 2.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on serum liver and kidney injury markers in streptozotocin (STZ)-induced diabetic rats
| NC | STZ | STZ+QE | STZ+RS | STZ+QE+RS | |
|---|---|---|---|---|---|
| AST (IU/L) | 87 ± 3 | 166 ± 7*** | 117 ± 9***### | 126 ± 14*### | 107 ± 10***### |
| ALT (IU/L) | 50 ± 4 | 115 ± 6*** | 68 ± 7*## | 87 ± 9*### | 56 ± 5### |
| ALP (IU/L) | 272 ± 19 | 920 ± 52*** | 520 ± 44***### | 606 ± 80***### | 501 ± 76**### |
| GGT (IU/L) | 10.68 ± 0.84 | 19.25 ± 1.2*** | 14.65 ± 2.2***### | 13.32 ± 1.5***### | 12.36 ± 2.1**### |
| BUN (mg/dL) | 25 ± 1 | 39 ± 2** | 30 ± 2### | 35 ± 2## | 29 ± 2### |
| CRE (mg/dL) | 0.5 ± 0.0 | 0.9 ± 0.0* | 0.7 ± 0.0 | 0.7 ± 0.0## | 0.6 ± 0.0## |
| TP (mg/dL) | 4.7 ± 0.1 | 3.9 ± 0.2** | 4.5 ± 0.1*## | 4.3 ± 0.1### | 4.7 ± 0.1### |
Rats were equally divided into each group (n=10 per group). NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. Data are means ± standard error of the mean (SEM). Significance was measured using one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test.
p<0.05,
p<0.01, and
p<0.005 vs. NC group;
p<0.01 and
p<0.001 vs. STZ-treated group, respectively. AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; BUN, blood urea nitrogen; CRE, creatinine; TP, total protein.
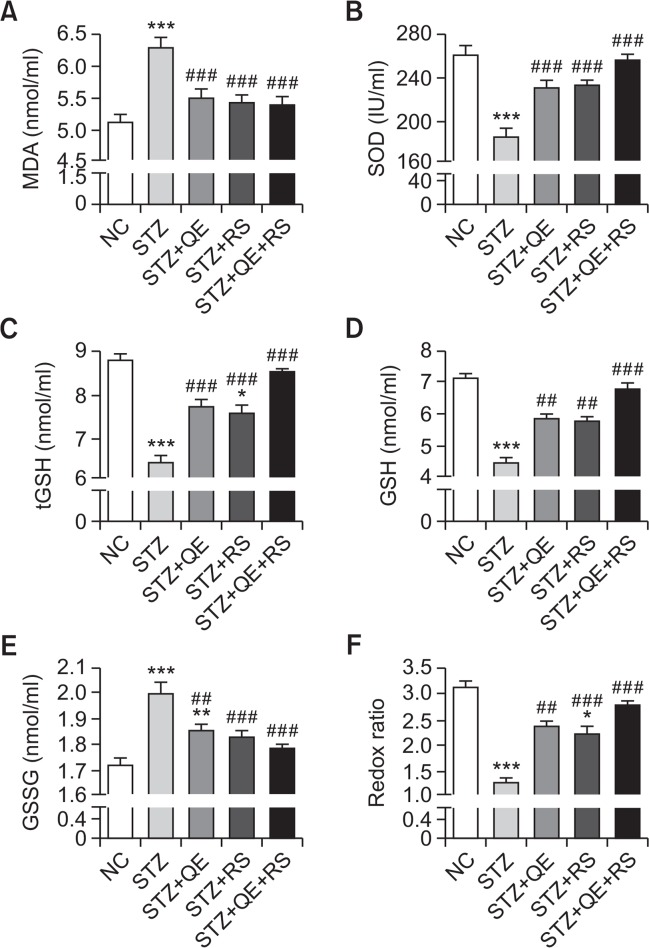
Effects of QE, RS, and cotreatment on serum oxidative stress parameters
To investigate the protective actions of our test compounds against oxidative stress in diabetic conditions, we determined the levels of various serum oxidative stress parameters after treatment of STZ-induced diabetic rats. Serum SOD, tGSH, and GSH levels significantly decreased while MDA and GSSG levels markedly increased in the STZ-treated group compared with those in the normal group. These changes were accompanied by a significant decrease in the redox ratio (GSH:GSSG). However, we found that these effects were reduced by the QE, RS, or cotreatment in diabetic rats (Fig. 6).
Fig. 6.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on serum oxidative stress-related parameters in streptozotocin (STZ)-induced diabetic rats. Rats were equally divided into each group (n=10 per group). Data are means ± standard error of the mean (SEM). Groups: NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. Significance was measured using one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test. *p<0.05, **p<0.01, and ***p<0.005 vs. NC group; ##p<0.01 and ###p<0.001 vs. STZ-treated group. MDA, malondialdehyde; SOD, superoxide dismutase; tGSH, total glutathione; GSH, reduced glutathione; GSSG, oxidized glutathione.
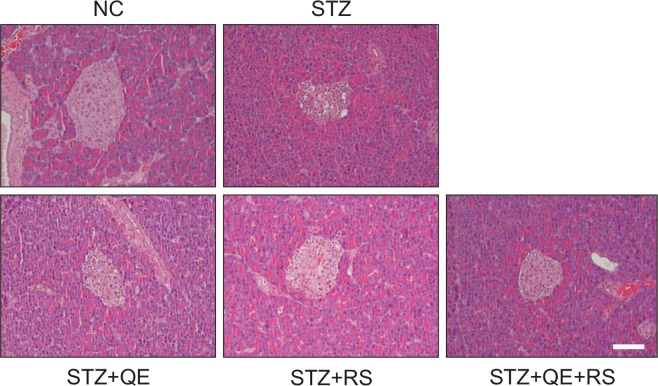
Histological analysis of pancreatic tissues
The STZ-induced diabetic rats exhibited extensive granulation of the β-cells and severe vacuolation of the pancreatic islets, while normal pancreatic-β cells in the islet of Langerhans and the acini were observed in the normal control group. Conversely, histological analysis of tissues from diabetic rats in the QE, RS, and cotreatment groups revealed less β-cell granulation and reduced pancreatic islet vacuolation compared with that shown by the STZ-treated group (Fig. 7).
Fig. 7.
Effects of quercetin (QE), resveratrol (RS), and their cotreatment on pancreatic tissues. Rats were equally divided into each group (n=10 per group). H&E staining were conducted using pancreatic tissues from rats at 14 days after compounds treatments. Groups: NC, normal control; STZ, streptozotocin-treated diabetic; STZ+QE, QE-treated STZ-diabetic rats; STZ+RS, RS-treated STZ diabetic rats; STZ+QE+RS, QE and RS-cotreated STZ-treated diabetic rats. Scale bar, 50 μm.
DISCUSSION
Hyperglycemia occurs in type-1 diabetes because of the toxic conditions destroy the pancreatic β-cells, leading to a deficiency in insulin secretion (McLellan et al., 1994; Rolo and Palmeira, 2006). This effect subsequently causes disturbances in all the biochemical mechanisms of the body, leading to hyperlipidemia as well as cardiac and renal failure (American Diabetes Association, 2010; Zhou et al., 2016).
Recently, natural plant compounds are becoming attractive as therapeutic agents against diabetes because they have fewer side effects than currently used diabetes drugs. Among them, QE and RS are naturally occurring flavonoids that are found in food items such as fruits, vegetables, and red wines. These compounds have been reported to have many beneficial effects, including anti-inflammatory, anti-oxidative, antihypertensive, anticancer, antiviral, neuroprotective, hepatoprotective and antidiabetic activities (Zamin et al., 2009; Szkudelski and Szkudelska, 2011). Notably, previous studies have reported that treatment with QE or RS alone lowered blood glucose levels in diabetic mice and rats (Vessal et al., 2003; Su et al., 2006).
Individual treatment of QE and RS has also shown to have ameliorative effects on type 2 diabetes in both in vitro and in vivo studies. QE has been shown to effectively lower the plasma glucose levels and improve other diabetic-related parameters, such as liver gluconeogenesis and serum lipid- parameters (Chen et al., 2016). RS treatment also reported to exhibit the anti-diabetic activity in type 2 diabetes-induced mice (Oshaghi et al., 2017). Additionally, few studies have investigated the addictive or synergistic effects of combined treatment of QE and RS by in vitro or in vivo approaches. Combination of QE and RS caused to a significant reduction in all fat deposits in rats fed the obesogenic diet (Arias et al., 2016). The combined supplementation of these compounds resulted in the restoration of genes involved in glucose/lipid metabolism, liver function, cardiovascular system, and inflammation/immunity, which were altered by high fat diet (Zhou et al., 2012). However, the actual mechanisms of these compounds are not fully understood. Moreover, their combined effect has not been evaluated in diabetic rats. Thus, in the present study, we investigate the antidiabetic properties and underlying mechanisms of QE and RS alone and in combination using STZ-induced diabetic rats.
We investigated whether treatment with QE and RS alone or in combination could reduce the FBG levels in diabetic rats. The results showed that the FBG levels of QE- or RS-treated diabetic rats were close to normal while the levels were significantly increased in the STZ-induced diabetic rats. Notably, the diabetic rats cotreated with QE and RS showed the most marked decrease in glucose to the lowest levels compared to treatment with QE or RS alone. Similarly, the decreased levels of serum insulin in diabetic rats caused by the destruction of pancreatic β-cells were restored by QE, RS, or QE+RS treatments. Other hematological parameters such as the HbA1c level have been found to be directly proportional to the blood glucose concentration (Go et al., 2015). In addition, C-peptide, a protein that connects the α- and β-chains of insulin in the proinsulin molecule, is important for insulin synthesis (Cersosimo et al., 2014). Therefore, it is thought to have a crucial role in controlling glucose levels (Saisho et al., 2011). As expected, QE, RS, or QE+RS treatment restored the serum HbA1c and C-peptide levels in diabetic rats, especially cotreatment with QE and RS, which was more effective than either QE or RS alone.
Additionally, our histological analysis showed that our test compounds, especially with cotreatment, preserved the pancreatic tissue while that of the STZ-induced diabetic rats exhibited severe damages to both β-cells and pancreatic islets. Indeed, the destruction of the pancreatic β-cells is main symptoms in type 1 diabetes and consequently caused to impairment of insulin production. Collectively, our results suggest that QE, RS, and especially their combination controlled the insulin levels and exerted hypoglycemic activity in diabetic rats. Furthermore, these beneficial effects are thought to be medicated by the improved function of the diabetic pancreatic tissue. Under diabetic conditions, insulin can alter lipid metabolism, and abnormalities in fatty acid metabolism, which cause accumulation of lipids in the blood and liver, which in turn impair pancreatic β-cells function (Kulkarni et al., 2012).
Thus, diabetic dyslipidemia is an important risk factor for many complications including atherosclerosis, myocardial infarction, coronary diseases, and nephrotoxicity (Yim et al., 2007). Our study reveals that QE and RS treatments reduced the serum lipids levels (TC, TG, LDL, and VLDL). Especially, cotreatment with QE and RS more effectively improved these levels than treatment with either compound did alone. In addition, these compounds also showed similar positive effects on the lipid metabolism-related hepatic enzymes. Therefore, our results indicate that these compounds have beneficial effects in controlling dyslipidemia associated with diabetic complications.
Liver has a major role of endogenous glucose production by glycogenolysis and gluconeogenesis (Vidal-Puig and O’Rahilly, 2001). Under the diabetic conditions, hepatic glucose overproduction is occurred, and further results in hyperglycemia (Kurukulasuriya et al., 2003). The present study revealed that the activities of hepatic glucose-regulating enzymes were impaired in diabetic rats, otherwise, combined treatment of QE and RS as well as individual treatments of QE and RS effectively reserved the activities of these enzymes against the diabetes. Especially, G6Pase and PEPCK, which plays a role in glucose homeostasis and are key rate-limiting enzymes of gluconeogenesis, were dramatically reduced their activities when cotreated with QE and RS treatment. Therefore, the hypoglycemic effects of these compounds in diabetic rats might be due to the restoration of these hepatic glucose-regulating enzymes.
In the past decades, considerable evidence has established the role of oxidative stress in the pathogenesis of diabetic complications. Indeed, several studies have reported that hyperglycemia and hyperlipidemia contribute to the accumulation of ROS and antioxidants deficiency (e.g., SOD and GSH) in both diabetic experimental animals and patients (West, 2000; Piwkowska et al., 2011). Consistent with these findings, our data have shown that antioxidants were suppressed in diabetic rats while QE and RS treatments restored these antioxidant levels. Furthermore, cotreatment with QE and RS was more effective in restoring the antioxidant properties than either treatment was alone.
Reduced blood pH and HCO3− levels but elevated lactate and anionic gap levels were observed in the diabetic rats in this study, indicating hyperlactatemia and ketoacidosis (Go et al., 2015). Indeed, diabetic ketoacidosis and lactic acidosis are life-threatening complications of diabetes that occurs when insulin levels are low or deficient (Wilson, 2010). Interestingly, we observed that in the QE, RS, and particularly the QE and RS cotreated groups the values were maintained at normal levels. These results indicate that QE and RS promoted the utilization of glucose and ameliorated ketosis and metabolic acidosis. Furthermore, impairment of electrolyte balance such as that of Na+, Cl− and the anion gap are common features in patients with diabetes due to increased urinary loss. These symptoms were improved by QE, RS, and cotreatment, possibly due to the correction of hyperglycemia and insulin deficiency (Go et al., 2015). Finally, the elevated serum levels of ALT, AST, ALP, and GGT, as liver injury markers in diabetic rats indicate the induction of tissue injuries due to the diabetes-induced oxidative stress. These elevations were dramatically prevented by QE, RS, or cotreatment. Therefore, our test compounds may prevent the liver damage due to the diabetes. Serum CRE and BUN levels which are indicators for diabetic nephropathy (Wang et al., 2011) were significantly increased in diabetic rats while these effects were significantly inhibited by QE, RS, and the cotreatment. Thus, the compounds exhibited the potential to protect the kidney in the diabetic rats.
In conclusion, our results suggest that QE, RS, and especially their cotreatment have the potential to improve hyperglycemia, serum glucose dysfunction, and dyslipidemia in STZ-induced diabetic rats. We also demonstrated that treatment with QE and RS alone and in combination improved the pathological blood conditions of diabetic rats. Therefore, our study provides evidence that cotreatment with QE and RS is a potentially useful strategy for the treatment of diabetes. Furthermore, cotreatment of QE and RS is worth to evaluate their effects on the type 2 diabetes in the future.
Acknowledgments
This study was supported in part by the Brain Korea 21 Plus program of the National Research Foundation of Korea Grant and the Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (NRF-2015R1D1A3A01019245).
Footnotes
CONFLICT OF INTEREST
The authors have declared that there are no conflicts of interest associated with this manuscript.
REFERENCES
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias N, Macarulla MT, Aguirre L, Milton I, Portillo MP. The combination of resveratrol and quercetin enhances the individual effects of these molecules on triacylglycerol metabolism in white adipose tissue. Eur J Nutr. 2016;55:341–348. doi: 10.1007/s00394-015-0854-9. [DOI] [PubMed] [Google Scholar]
- Baquer NZ, Cascales M, McLean P, Greenbaum AL. Effects of thyroid hormone deficiency on the distribution of hepatic metabolites and control of pathways of carbohydrate metabolism in liver and adipose tissue of the rat. Eur J Biochem. 1976;68:403–413. doi: 10.1111/j.1432-1033.1976.tb10827.x. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic beta-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10:2–42. doi: 10.2174/1573399810666140214093600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Jiang H, Wu X, Fang J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediators Inflamm. 20162016:9340637. doi: 10.1155/2016/9340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Follo-Martinez A, Banerjee N, Li X, Safe S, Mertens-Talcott S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr Cancer. 2013;65:494–504. doi: 10.1080/01635581.2012.725194. [DOI] [PubMed] [Google Scholar]
- ElAttar TM, Virji AS. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anticancer Drugs. 1999;10:187–193. doi: 10.1097/00001813-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Go HK, Rahman MM, Kim GB, Na CS, Song CH, Kim JS, Kim SJ, Kang HS. Antidiabetic effects of yam (dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin-induced diabetes. Nutrients. 2015;7:8532–8544. doi: 10.3390/nu7105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaa KA, McLean P. The kinetic quantitation of ATP: D-glucose 6-phosphotransferases. FEBS Lett. 1972;27:293–297. doi: 10.1016/0014-5793(72)80644-6. [DOI] [PubMed] [Google Scholar]
- Gupta D, Raju J, Prakash J, Baquer NZ. Change in the lipid profile, lipogenic and related enzymes in the livers of experimental diabetic rats: effect of insulin and vanadate. Diabetes Res Clin Pract. 1999;46:1–7. doi: 10.1016/S0168-8227(99)00067-4. [DOI] [PubMed] [Google Scholar]
- Igura K, Ohta T, Kuroda Y, Kaji K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001;171:11–16. doi: 10.1016/S0304-3835(01)00443-8. [DOI] [PubMed] [Google Scholar]
- Kulkarni CR, Joglekar MM, Patil SB, Arvindekar AU. Antihyperglycemic and antihyperlipidemic effect of Santalum album in streptozotocin induced diabetic rats. Pharm Biol. 2012;50:360–365. doi: 10.3109/13880209.2011.604677. [DOI] [PubMed] [Google Scholar]
- Kurukulasuriya R, Link JT, Madar DJ, Pei Z, Rohde JJ, Richards SJ, Souers AJ, Szczepankiewicz BG. Prospects for pharmacologic inhibition of hepatic glucose production. Curr Med Chem. 2003;10:99–121. doi: 10.2174/0929867033368547. [DOI] [PubMed] [Google Scholar]
- Machha A, Achike FI, Mustafa AM, Mustafa MR. Quercetin, a flavonoid antioxidant, modulates endothelium-derived nitric oxide bioavailability in diabetic rat aortas. Nitric Oxide. 2007;16:442–447. doi: 10.1016/j.niox.2007.04.001. [DOI] [PubMed] [Google Scholar]
- McLellan AC, Thornalley PJ, Benn J, Sonksen PH. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 1994;87:21–29. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- Oshaghi EA, Goodarzi MT, Higgins V, Adeli K. Role of resveratrol in the management of insulin resistance and related conditions: mechanism of action. Crit Rev Clin Lab Sci. 2017;54:267–293. doi: 10.1080/10408363.2017.1343274. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Subramanian S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Chem Biol Interact. 2009;179:356–362. doi: 10.1016/j.cbi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim. Biophys. Acta. 2011;1812:719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Piwkowska A, Rogacka D, Audzeyenka I, Jankowski M, Angielski S. High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. J Cell Biochem. 2011;112:1661–1672. doi: 10.1002/jcb.23088. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Lee SJ, Mun AR, Adam GO, Park RM, Kim GB, Kang HS, Kim JS, Kim SJ, Kim SZ. Relationships between blood Mg2+ and energy metabolites/enzymes after acute exhaustive swimming exercise in rats. Biol Trace Elem Res. 2014;161:85–90. doi: 10.1007/s12011-014-9983-x. [DOI] [PubMed] [Google Scholar]
- Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9:562–566. doi: 10.1089/jmf.2006.9.562. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Roslan J, Giribabu N, Karim K, Salleh N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed Pharmacother. 2017;86:570–582. doi: 10.1016/j.biopha.2016.12.044. [DOI] [PubMed] [Google Scholar]
- Saisho Y, Kou K, Tanaka K, Abe T, Kurosawa H, Shimada A, Meguro S, Kawai T, Itoh H. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J. 2011;58:315–322. doi: 10.1507/endocrj.K10E-399. [DOI] [PubMed] [Google Scholar]
- Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Ann N Y Acad Sci. 2011;1215:34–39. doi: 10.1111/j.1749-6632.2010.05844.x. [DOI] [PubMed] [Google Scholar]
- Tahrani AA, Piya MK, Kennedy A, Barnett AH. Glycaemic control in type 2 diabetes: targets and new therapies. Pharmacol Ther. 2010;125:328–361. doi: 10.1016/j.pharmthera.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003;135C:357–364. doi: 10.1016/S1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig A, O’Rahilly S. Metabolism. Controlling the glucose factory. Nature. 2001;413:125–126. doi: 10.1038/35093198. [DOI] [PubMed] [Google Scholar]
- Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Alternat Med. 20112011:323171. doi: 10.1155/2011/323171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Wilson JF. In clinic. Diabetic ketoacidosis. Ann Intern Med. 2010;152 doi: 10.7326/0003-4819-152-1-201001050-01001. ITC1-1, ITC1-2, ITC1-3, ITC1-4, ITC1-5, ITC1-6, ITC1-7, ITC1-8, ITC1-9, ITC1-10, ITC1-11, ITC1-12, ITC1-13, ITC1-14, ITC1-15, table of contents; quiz ITC1-16. [DOI] [PubMed] [Google Scholar]
- Yim S, Malhotra A, Veves A. Antioxidants and CVD in diabetes: where do we stand now? Curr Diab Rep. 2007;7:8–13. doi: 10.1007/s11892-007-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonamine CY, Pinheiro-Machado E, Michalani ML, Freitas HS, Okamoto MM, Correa-Giannella ML, Machado UF. Resveratrol improves glycemic control in insulin-treated diabetic rats: participation of the hepatic territory. Nutr Metab (Lond) 2016;13:44. doi: 10.1186/s12986-016-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak B, Epstein E, Baginski ES. Determination of liver microsomal glucose-6-phosphatase. Ann Clin Lab Sci. 1977;7:169–177. [PubMed] [Google Scholar]
- Zamin LL, Filippi-Chiela EC, Dillenburg-Pilla P, Horn F, Salbego C, Lenz G. Resveratrol and quercetin cooperate to induce senescence-like growth arrest in C6 rat glioma cells. Cancer Sci. 2009;100:1655–1662. doi: 10.1111/j.1349-7006.2009.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zou H, Xu G. Clinical utility of serum cystatin c in predicting diabetic nephropathy among patients with diabetes mellitus: a meta-analysis. Kidney Blood Press Res. 2016;41:919–928. doi: 10.1159/000452593. [DOI] [PubMed] [Google Scholar]
- Zhou M, Wang S, Zhao A, Wang K, Fan Z, Yang H, Liao W, Bao S, Zhao L, Zhang Y, Yang Y, Qiu Y, Xie G, Li H, Jia W. Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. J Proteome Res. 2012;11:4961–4971. doi: 10.1021/pr3004826. [DOI] [PubMed] [Google Scholar]