Abstract
Spermidine is a naturally occurring polyamine compound that has recently emerged with anti-aging properties and suppresses inflammation and oxidation. However, its mechanisms of action on anti-inflammatory and antioxidant effects have not been fully elucidated. In this study, the potential of spermidine for reducing pro-inflammatory and oxidative effects in lipopolysaccharide (LPS)-stimulated macrophages and zebrafish was explored. Our data indicate that spermidine significantly inhibited the production of pro-inflammatory mediators such as nitric oxide (NO) and prostaglandin E2 (PGE2), and cytokines including tumor necrosis factor-α and interleukin-1β in RAW 264.7 macrophages without any significant cytotoxicity. The protective effects of spermidine accompanied by a marked suppression in their regulatory gene expression at the transcription levels. Spermidine also attenuated the nuclear translocation of NF-κB p65 subunit and reduced LPS-induced intracellular accumulation of reactive oxygen species (ROS) in RAW 264.7 macrophages. Moreover, spermidine prevented the LPS-induced NO production and ROS accumulation in zebrafish larvae and was found to be associated with a diminished recruitment of neutrophils and macrophages. Although more work is needed to fully understand the critical role of spermidine on the inhibition of inflammation-associated migration of immune cells, our findings clearly demonstrate that spermidine may be a potential therapeutic intervention for the treatment of inflammatory and oxidative disorders.
Keywords: Spermidine, Macrophages, Zebrafish, Anti-Inflammation, Anti-Oxidant
INTRODUCTION
The inflammatory response is a highly regulated self-limiting process to identify and destroy invading pathogens and restore normal tissue structure and function (Conti et al., 2004; Freire and Van Dyke, 2013). However, in many cases, an excessive inflammatory response has been recognized as the principal reason of chronic inflammation including cardiovascular disease, rheumatoid arthritis, inflammatory bowel disease, Alzheimer’s disease, and even cancer (Amin et al., 1999; Freire and Van Dyke, 2013). Moreover, under inflammatory conditions, the recruitment of macrophages and neutrophils to the inflammation site is crucial to the initiation of pathogenesis (Cunha et al., 2008; Williams et al., 2011; Zhang and Wang, 2014). When macrophages and neutrophils are over-activated by inflammatory stimulants including the gram-negative bacterial endotoxin lipopolysaccharides (LPS), the cells induce the production of inflammatory mediators including nitric oxide (NO) and prostaglandin E2 (PGE2), and inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), along with activation of several signaling pathways including nuclear factor-kappa B (NF-κB) (Czura et al., 2003; Muralidharan and Mandrekar, 2013).
Another important component of inflammation is oxidative stress, reflecting an imbalance between the production of reactive oxygen species (ROS) and the biological system’s ability to remove them (Brüne et al., 2013; Mills and O’Neill, 2016). The overproduced ROS by the activated macrophages and neutrophils act as an important contributor to the manifestation of inflammation (Varga et al., 2013; Mills and O’Neill, 2016) and are also involved in the production of inflammatory cytokines in the LPS-stimulated macrophages and neutrophils (Haddad and Land, 2002). Consequently, the suppression of the production of pro-inflammatory factors by blocking macrophage and neutrophil activation is emerging as a potential therapeutic approach to relieve the progression of inflammatory and oxidative disorders (Cunha et al., 2008; Zhang and Wang, 2014).
Naturally occurring polyamines, synthesized from both arginine and methionine, are thought to display important multi-functional characteristics in cells, ranging from basic DNA synthesis to the regulation of cell proliferation and differentiation (Löser, 2000; Larqué et al., 2007). Several previous studies have suggested that polyamines possess multiple pharmacological activities including anti-oxidant and anti-inflammatory benefits (Merentie et al., 2007; Minois, 2014). Among several polyamines, spermidine is a positively charged polyamine that is synthesized from putrescine and serves as a precursor of spermine (Moinard et al., 2005; Larqué et al., 2007). In particular, spermidine has emerged with anti-aging properties. Its supplementation prolongs the lifespan of several model organisms and resistance to stress (Eisenberg et al., 2009; Minois et al., 2012; Minois, 2014). It also reduces the age-related oxidative protein damage, free radical-scavenging activities, and the overproduction of ROS (Sava et al., 2006; Morselli et al., 2011), explaining its antioxidant ability. In addition, previous studies including our data indicated that spermidine had strong anti-inflammatory properties, associated with the inhibition of secretion of various pro-inflammatory mediators, cytokines and chemokines, and suppression of infiltration of leucocytes and epithelial damage (Choi and Park, 2012; Morón et al., 2013; Paul and Kang, 2013; Yang et al., 2016). Despite these encouraging studies, the effects and molecular mechanisms responsible for the anti-inflammatory and antioxidant potentials of spermidine are still complex and have remained elusive. Therefore, in this study, the anti-inflammatory and antioxidant effects of spermidine and its molecular mechanism of action in LPS-stimulated RAW 264.7 macrophage cells and a zebrafish model was examined. The protective effects of spermidine on zebrafish macrophage and neutrophil recruitment were also investigated.
MATERIALS AND METHODS
Cell culture and spermidine treatment cell viability assay
The murine RAW 264.7 macrophages were obtained from the Korean Cell Line Bank (Seoul, Korea) and cultured at 37°C in 5% CO2 containing Dulbecco’s modified Eagle’s medium (DMEM, WelGENE Inc., Daegu, Korea) supplemented with 10% fetal bovine serum (FBS, WelGENE Inc.), 100 u/mL of penicillin, and 100 mg/mL of streptomycin (Sigma-Aldrich Chemical Co., St. Louis, MO, USA). Spermidine (≥99% (GC) 1,8-Diamino-4-azaoctane, N-(3-Aminopropyl)-1,4-diaminobutane, CAS # 124-20-9) was purchased from Sigma-Aldrich Chemical Co. and dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich Chemical Co.) as a 1 M stock solution, and further diluted with DMEM. In all the experiments, cells were pretreated with the indicated concentrations of spermidine for 1 h before the addition of 500 ng/mL LPS (Sigma-Aldrich Chemical Co.).
Cell viability assay
A colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich Chemical Co.) assay was performed to measure the cell viability. Briefly, RAW 264.7 cells were treated with different concentrations of spermidine for 24 h or pretreated with spermidine for 1 h before stimulation with LPS for 24 h. After incubation, the medium was discarded, and MTT solution (5 mg/mL in phosphate-buffered saline, PBS) was added to each well and incubated for another 3 h at 37°C. The medium was removed, and DMSO was added to dissolve the formazan dye. The optical density was then read at 560 nm using a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) to determine the cell viability.
Measurement of NO production in RAW 264.7 macrophages
The production of NO in the culture supernatants was assayed using Griess reagent (Sigma-Aldrich Chemical Co.). For this assay, the supernatant was collected and mixed with the same volume of Griess reagent for 10 min at room temperature in the dark. The absorbance was measured at 540 nm using a microplate reader, and NO concentrations were calculated by referencing a standard curve generated from the known concentrations of sodium nitrite (Lee et al., 2015).
Measurement of PGE2, TNF-α, and IL-1β production in RAW 264.7 macrophages
To measure the production of PGE2, TNF-α, and IL-1β, the cells were cultured under the same conditions as for the NO measurement assay. The levels of PGE2, TNF-α, and IL-1β concentrations in the cultured media were determined using a selective enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions (Wang et al., 2015).
Protein extraction and western blot analysis
The cell extracts were prepared with extraction lysis buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 1% NP-40, 1 mM pheny-methylsulfonyl fluoride and 5 mM dithiothreitol] for 30 min at 4°C and centrifuged at 14,000×g for 15 min at 4°C. In a parallel experiment, nuclear and cytosolic proteins were separated using NE-PER nuclear and cytosolic extraction reagents (Pierce Biotechnology, Rockford, IL, USA), according to the manufacturer’s protocol. The protein concentration in the supernatant was quantified using a DC™ protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer’ instructions. Protein samples (50–100 μg per lane) were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.05% Tween-20 (TBST buffer) for 1 h at room temperature, incubated with the corresponding primary antibodies purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and Cell Signaling Technology, Inc. (Boston, MA, USA), and then incubated with appropriate secondary antibodies conjugated to horseradish peroxidase (Amersham Co., Arlington Heights, IL, USA) at room temperature for 2 h. The immunoreactive bands were visualized using an enhanced chemiluminescence (ECL, Amersham Co.) detection system (Lee et al., 2016).
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), according to the manufacturer’s instructions and reverse transcribed using an M-MLV reverse transcriptase kit (BioNEER, Daejeon, Korea) to produce cDNAs. The RT-generated cDNAs encoding iNOS, COX-2, TNF-α, and IL-1β genes were amplified by PCR using desired primers (BioNEER). Following amplification, the PCR products were separated on 1.5% agarose gel electrophoresis, stained with ethidium bromide (EtBr, Sigma-Aldrich Chemical Co.) and visualized under ultraviolet illumination. In a parallel experiment, glyceraldehyde 3-phosphate dehydrogenase (GPDH) was used as the internal control. The PCR primers were as follows: iNOS forward, 5′ATG TCC GAA GCA AAC ATCAC3′ and reverse, 5′TAA TGT CCA GGA AGT AGG TG3′; COX-2 forward, 5′-CAG CAA ATC CTT GCT GTT CC-3′ and reverse 5′-TGG GCA AAG AAT GCA AAC ATC-3′, TNFα forward, 5′TCT CAT CAG TTC TAT GGC CC3′ and reverse, 5′GGG AGT AGA CAA GGT ACA AC3′; IL1β forward, 5′GGG CTG CTT CCA AAC CTT TG3′ and reverse, 5′GCT TGG GAT CCA CAC TCT CC3′; and GAPDH forward, 5′AGG CCG GTG CTG AGT ATG TC3′ and reverse, 5′TGC CTG CTT CAC CAC CTT CT3′.
Immunofluorescent staining for NF-κB p65 in RAW 264.7 macrophages
The NF-κB p65 nuclear translocalization was detected by an immunofluorescence assay using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany). After designated treatments, the cells were fixed with 3.7% paraformaldehyde (Sigma-Aldrich Chemical Co.) in PBS for 10 min at 4°C, permeabilized with 0.4% Triton X-100 in PBS for 10 min, and blocked with 5% bovine serum albumin for 1 h. The cells were probed with anti-p65 NF-κB antibody (Santa Cruz Biotechnology, Inc.) overnight at 4°C and then incubated with fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 2 h at room temperature. The position of the cell nucleus was determined with 4,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich Chemical Co.) solution (1 mg/mL) for 15 min. After washing with PBS, the fluorescence was visualized using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Measurement of intracellular ROS generation in RAW 264.7 macrophages
To measure the intracellular ROS production, the cells were washed twice with PBS and lysed with 1% Triton X-100 in PBS for 10 min at 37°C. The cells were then stained with 10 μM 2′,7′-dichlorofluorescein diacetate (DCF-DA, Molecular Probes, Eugene, OR, USA) for 20 min at room temperature in the dark. The green fluorescence emitted by DCF was recorded at 515 nm using a flow cytometer (Becton Dickinson, SanJose, CA, USA), and 10,000 events were counted per sample (Eom et al., 2015). Image analysis for the generation of intracellular ROS was also acquired using a fluorescence microscope.
Zebrafish maintenance
Adult zebrafish were obtained from Dr. Hyo-Jong Lee, College of Pharmacy, Inje University (Gimhae, Korea) and maintained at 28.5°C with a 14:10-h light/dark cycle in a recirculating tank system using local tap water (pH 7.2–7.6, salinity 0.03%–0.04%). The embryos were obtained from natural spawning within 30 min and maintained at a density of ∼50 embryos per 100 mm2 in a Petri dish containing media as previously reported (Nirwane et al., 2016). The entire study design and experimental procedures were approved by the Dongeui University Animal Care and Use Committee (Busan, Korea).
Measurement of NO and ROS production in zebrafish larvae
From ∼3 days post-fertilization (dpf), embryos (n=25) were transferred to individual wells of a 24-well plate and maintained in embryo media containing sterile distilled water (vehicle control), 800 μg/mL spermidine (final concentration), 10 μg/mL LPS (final concentration) or 800 μg/mL spermidine for 1 h followed by the treatment with 10 μg/mL LPS, except larvae in the control group, for up to 4 dpf. The generation of NO and ROS in the zebrafish larvae was analyzed using fluorescent probe dyes, 4-amino-5-methylamino-2′7′ difluorofluorescein diacetate (DAF-FM-DA, Molecular Probes) and DCF-DA, respectively. After 4 dpf, the larvae were transferred to 24-well plates and incubated with DAF-FM-DA (5 μM) and DCF-DA (20 μg/mL) solution for 1 h in the dark at 28.5°C, and then anaesthetized using 1-phenoxy-2-propanol (1/500 dilution, Acros Organics, Morris Plains, NJ, USA). The images of stained larvae were observed for the NO and ROS generation under a fluorescence microscope, and the fluorescence intensity of individual larvae was quantified at an excitation wavelength of 485 nm and an emission wavelength of 535 nm using a spectrophotometer and ImageJ 1.46r software (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA), respectively. The generation of NO and ROS were calculated by comparing the fluorescence intensity of treatment larvae to the controls (Ko and Jeon, 2015).
Exposure of LPS by microinjection to zebrafish larvae
For exposure of LPS by microinjection, 3 dpf larvae were collected and incubated with embryo media containing sterile distilled water (vehicle control) or 800 μg/mL spermidine (final concentration) at 28.5°C. After 18 and 20 h incubation, larvae were anesthetized and 1 mg/mL or 0.125 mg/mL LPS was injected into the yolk using a microinjector (Harvard Apparatus, Inc., Cambridge, MA, USA) for sudan black or neutral red staining, respectively, according to the methods used by Yang et al (2014). Microinjection volume was 2 nL per larvae, and the control group was injected with an equal volume of PBS. The larvae from each group were further cultured for up to 4 dpf in the embryo media with or without 100 μg/mL spermidine for staining with sudan black and neutral red.
Sudan black and neutral red staining in zebrafish larvae
For staining of neutrophils in zebrafish larvae, 0.6 g of sudan black (Acros Organics, Morris Plains, NJ, USA) was dissolved in 200 mL of ethanol as a stock solution. The sudan black solution was then prepared by mixing 30 mL of stock solution with a buffer solution, which was made from phenol (16 g) dissolved in pure ethanol (30 ml) and Na2HPO4·12H2O (0.3 g) dissolved in distilled water (100 mL). Whole larvae were fixed with 4% methanol-free paraformaldehyde (Sigma-Aldrich Chemical Co.) in PBS for 2 h at room temperature, rinsed with PBS, and incubated in sudan black solution at 28.5°C in the dark for 40 min. The larvae were washed with 70% ethanol in water, and then progressively rehydrated with PBS plus 0.1% Tween-20 (Le Guyader et al., 2008). Optimal staining of macrophages was accomplished by incubating larvae in 2.5 μg/mL neutral red solution containing 0.003% 1-phenyl-2-thiourea (Sigma-Aldrich Chemical Co.) for 6 h (Herbomel et al., 2001). After staining, the recruitment of neutrophils and macrophages was observed using a dissecting microscope (Carl Zeiss).
Statistical analysis
All the data are presented as mean ± standard deviation (SD). Significant differences among groups were determined by the unpaired Student’s t-test. A value of p<0.05 was accepted as an indication of statistical significance. All the figures shown here are the data obtained from at least three independent experiments.
RESULTS
Cytotoxic effects of spermidine and LPS on RAW 264.7 macrophages
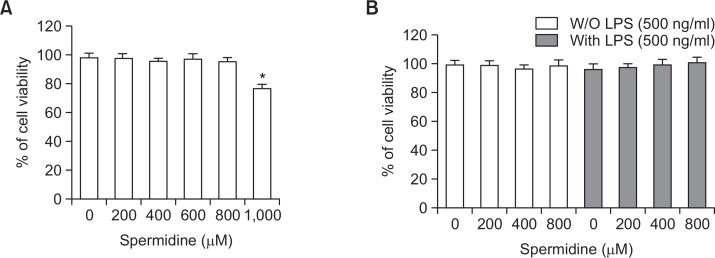
To exclude the cellular toxicity caused by spermidine treatment, RAW 264.7 cells were treated with spermidine and/or LPS for 24 h. The MTT assay showed that up to 800 μg/mL spermidine in the presence or absence of 500 ng/mL LPS was not cytotoxic; however, treatment with 1,000 μg/mL spermidine decreased the cell viability (Fig. 1). Therefore, 800 μg/mL spermidine was selected as the maximum concentration for further experiments in RAW 264.7 cells.
Fig. 1.
Effect of spermidine on the cell viability of RAW 264.7 macrophages. The cells were treated with various concentrations of spermidine for 24 h (A) or pretreated with the indicated concentrations of spermidine for 1 h prior to LPS (500 ng/mL) treatment for 24 h (B). The cell viability was assessed by the MTT reduction assay, and the results are expressed as the percentage of surviving cells over the control cells (no addition of spermidine and LPS). Values represent the means ± SD of three independent experiments (*p<0.05 vs. the control group).
Effects of spermidine on the LPS-induced NO and PGE2 production in RAW 264.7 macrophages
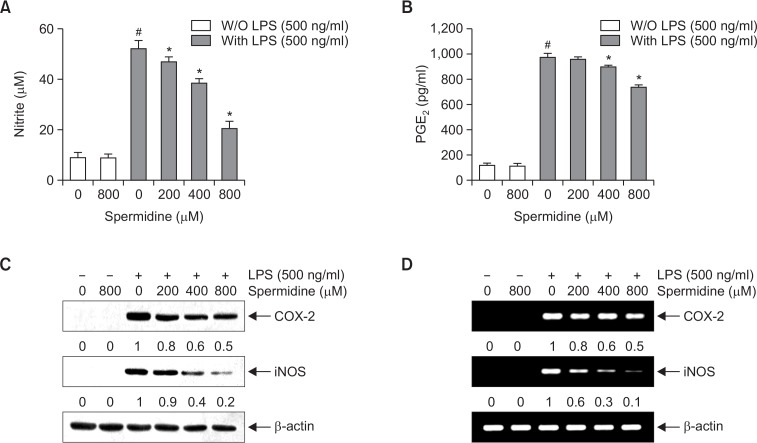
To determine the inhibitory properties of spermidine on LPS-induced NO and PGE2 production in RAW 264.7 cells, the cells were pretreated with the indicated concentrations of spermidine for 1 h and then stimulated with LPS for another 24 h. The levels of NO and PGE2 in the culture supernatants were determined by Griess reaction assay and ELISA, respectively. As shown in Fig. 2A and 2B, stimulation with LPS markedly induced the production of NO and PGE2 compared to not stimulating with LPS; however, spermidine significantly inhibited NO and PGE2 secretion in RAW 264.7 cells in a concentration-dependent manner.
Fig. 2.
Inhibition of NO and PGE2 production by spermidine in LPS-stimulated RAW 264.7 macrophages. The cells were pretreated with the indicated concentrations of spermidine for 1 h prior to incubation with 500 ng/mL LPS for 24 h. The levels of NO (A) and PGE2 (B) in the culture media were measured by Griess assay and a commercial ELISA kit, respectively. Each value indicates the mean ± SD and is representative of the results obtained from three independent experiments (#p<0.05 compared to the control; *p<0.05 compared to cells cultured with 500 ng/mL LPS). (C) Cell lysates were prepared for Western blot analysis with antibodies specific for murine iNOS and COX-2, and an ECL detection system. (D) The total RNAs were prepared for RT-PCR analysis of the iNOS and COX-2 mRNA expression using the indicated primers. The experiment was repeated thrice, and similar results were obtained. β-actin and GAPDH were used as the internal controls for the Western blot analysis and RT-PCR, respectively. The relative ratios of expression from Western blot analysis and RT-PCR are presented at the bottom of each of the results as relative values of the β-actin and GAPDH expression, respectively.
Inhibition of LPS-induced iNOS and COX-2 expression by spermidine in RAW 264.7 macrophages
Next, we investigated if the inhibitory effects of spermidine on NO and PGE2 production were related to the regulation of the expression of their synthesis enzymes, iNOS and COX-2, respectively. As shown in Fig. 2C and 2D, spermidine inhibited the protein and mRNA expression of iNOS and COX-2 in the LPS-stimulated RAW 264.7 cells in a concentration-dependent manner.
Effects of spermidine on the LPS-induced TNF-α and IL-1β production in RAW 264.7 macrophages
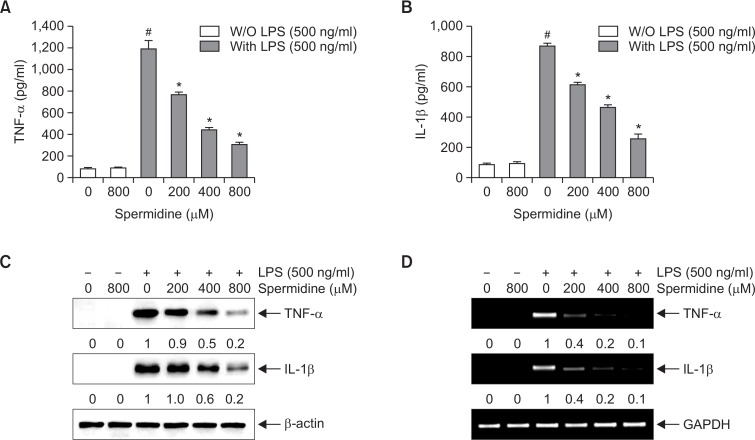
To determine the effect of spermidine on the production of pro-inflammatory cytokines, such as TNF-α and IL-1β, the cells were incubated with spermidine in the presence or absence of LPS, and the cytokine levels were measured by ELISA. As shown in Fig. 3A and 3B, the secretion of TNF-α and IL-1β markedly increased following the LPS treatment, whereas pretreatment with spermidine considerably attenuated the LPS-induced production of TNF-α and IL-1β.
Fig. 3.
Effects of spermidine on LPS-induced secretion and expression of TNF-α and IL-1β in RAW 264.7 macrophages. The cells were pretreated with different concentrations of spermidine for 1 h, followed by stimulation with 500 ng/mL LPS for 24 h. The amounts of TNF-α (A) and IL-1β (B) in the culture supernatants were measured by ELISA kits. Data are presented as the means ± SD of three independent experiments (#p<0.05 compared to the control; *p<0.05 compared to cells cultured with 500 ng/mL LPS). (C) Cell lysates were prepared for Western blot analysis with antibodies specific for murine TNF-α and IL-1β, and an ECL detection system. (D) The total RNAs were prepared for RT-PCR analysis of the TNF-α and IL-1β mRNA expression using the indicated primers. The experiment was repeated three times, and similar results were obtained. β-actin and GAPDH were used as the internal controls for the Western blot analysis and RT-PCR, respectively. The relative ratios of expression from Western blot analysis and RT-PCR are presented at the bottom of each of the results as relative values of the β-actin and GAPDH expression, respectively.
Inhibition of LPS-induced TNF-α and IL-1β expression by spermidine in RAW 264.7 macrophages
To elucidate the mechanisms responsible for the inhibitory effects of spermidine on TNF-α and IL-1β production, we confirmed whether the regulation of cytokine production by spermidine was related to a change in the cytokine expression. Consistent results were obtained as that from the cytokine production, and the LPS-induced protein and mRNA levels of TNF-α and IL-1β decreased by treatment with spermidine in a concentration-dependent manner (Fig. 3C, 3D).
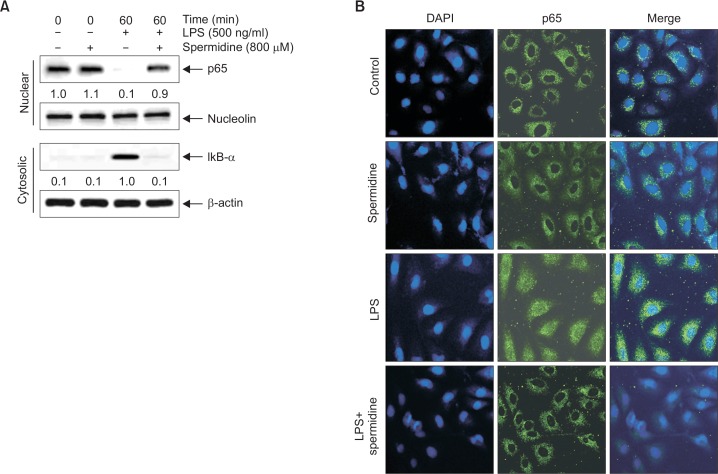
Suppression of LPS-induced NF-κB nuclear translocation by spermidine in RAW 264.7 macrophages
Next, we investigated the attenuating effect of spermidine on the LPS-induced nuclear translocation of NF-κB in RAW 264.7 cells. As shown in Fig. 4, the immunoblotting data using cytoplasmic and nuclear extracts indicated that spermidine pretreatment inhibited NF-κB p65 subunit nuclear accumulation, which was associated with the attenuation of IκBα degradation in LPS-stimulated RAW 264.7 cells (Fig. 4A). Consistent with these results, immunocytochemistry analysis also indicated that NF-κB p65 was normally sequestered in the cytoplasm following stimulation with LPS. However, LPS-mediated nuclear translocation of NF-κB was considerably blocked by pretreatment with spermidine (Fig. 4B).
Fig. 4.
Inhibition of NF-κB nuclear translocation by spermidine in LPS-stimulated RAW 264.7 macrophages. (A) The cells were pretreated with 800 μM spermidine for 1 h before 500 ng/mL LPS treatment for 1 h. The nuclear and cytosolic proteins were prepared for Western blot analysis using anti-NF-κB p65 and anti-IκB-α antibodies, and an ECL detection system. Nucleolin and β-actin were used as the internal controls for the nuclear and cytosolic fractions, respectively. The relative ratios of expression in the results of Western blotting are presented at the bottom of each of the results as relative values of the nucleolin and β-actin expression. (B) The cells were pretreated with 800 μM spermidine for 1 h before 500 ng/mL LPS treatment. After 1 h of incubation, the localization of NF-κB p65 was visualized with fluorescence microscopy after immunofluorescence staining with anti-NF-κB p65 antibody (green). The cells were also stained with DAPI to visualize the nuclei (blue). The results are representative of those obtained from three independent experiments.
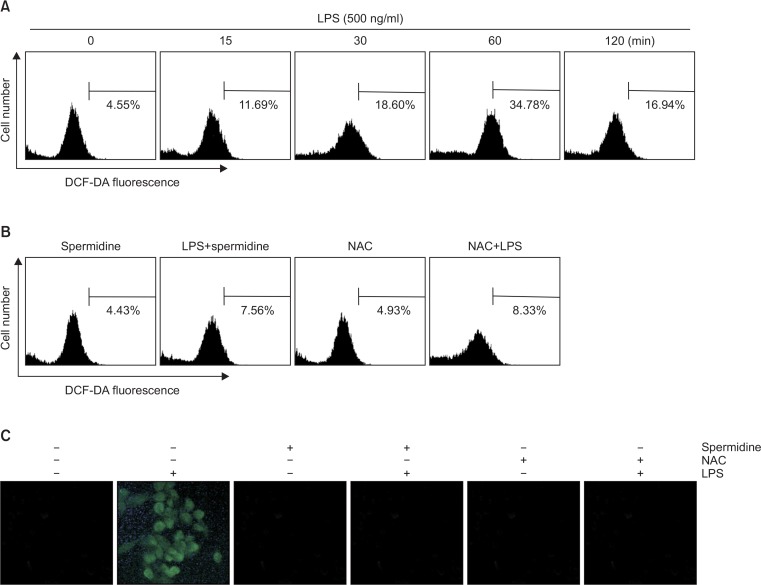
Suppression of LPS-induced accumulation of ROS by spermidine in RAW 264.7 macrophages
To determine the antioxidant effects of spermidine, whether spermidine reduces LPS-induced generation of ROS in RAW 264.7 cells was investigated by DCF-DA staining. The results of the flow cytometric assay indicate that the accumulation of intracellular ROS was observed within 0.25 h, and the levels continued to increase up to 1 h by the LPS treatment (Fig. 5A). However, the increase in the LPS-stimulated ROS production markedly attenuated by pretreatment with spermidine (Fig. 5B, 5C). As a positive control, the ROS scavenger N-acetyl-lcysteine (NAC) also effectively attenuated LPS-induced ROS generation, and spermidine itself did not increase the ROS generation.
Fig. 5.
Effects of spermidine on LPS-induced ROS production in RAW 264.7 macrophages. The cells were treated with 500 ng/ml LPS for the indicated time (A) or pre-incubated with or without 800 μM spermidine or 10 mM NAC for 1 h and then stimulated with 500 ng/ml LPS for 1 h (B and C). The cells were incubated with 10 μM DCF-DA for 30 min at 37°C. Cells were collected, and DCF fluorescence was measured by a flow cytometry. Values represent the means ± SD of two independent experiments. (C) Images were obtained using a fluorescence microscope and presented are from one experiment and are representative of at least 3 independent experiments.
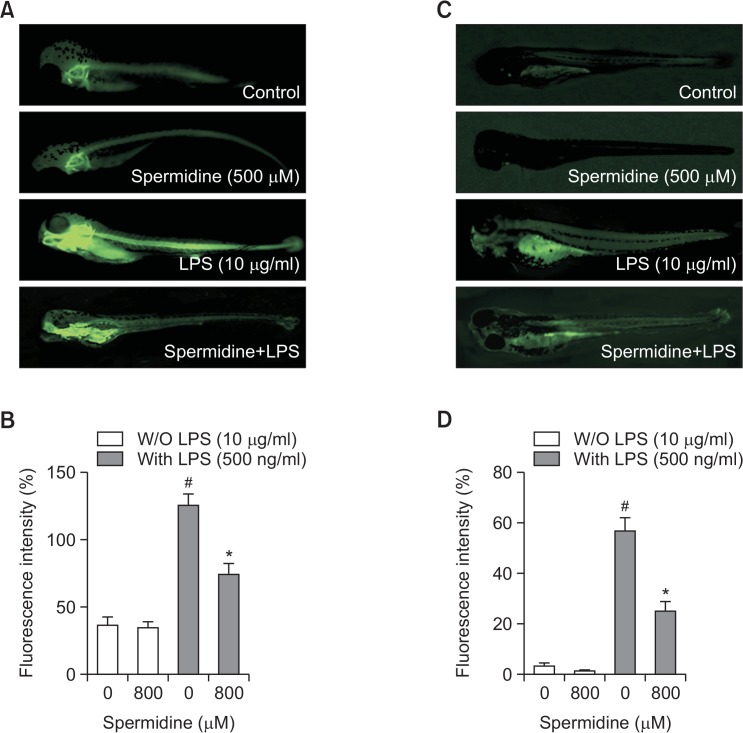
Downregulation of LPS-induced NO and ROS production by spermidine in zebrafish
To confirm the in vivo protective effects of spermidine against LPS-induced NO generation, DAF-FM-DA staining in zebrafish model was visualized. As shown in Fig. 6A and 6B, the control, which was not treated with LPS or spermidine, and spermidine alone groups generated clear image, indicating that spermidine alone did not affect the basal NO levels. However, stimulation of zebrafish larvae with LPS markedly generated fluorescence image, suggesting that NO was generated in the presence of LPS, and spermidine reduced the LPS-stimulated elevation of NO production. The inhibitory effect of spermidine on LPS-induced ROS accumulation was also evaluated, and the microphotographs of DCF-DA staining revealed excessive ROS accumulation after LPS stimulation. In contrast, when the zebrafish larvae were treated with spermidine prior to the LPS administration, the generation of ROS was effectively reduced (Fig. 6C, 6D).
Fig. 6.
Protective effect of spermidine on LPS-induced NO and ROS generation in zebrafish larvae. The zebrafish larvae were treated with 800 μM spermidine and 10 μg/mL LPS for 24 or pretreated with 800 μM spermidine for 1 h prior to incubation with 10 μg/mL LPS for 24 h. (A, B) The levels of NO and ROS generation were observed under a fluorescence microscope after staining with DAF-FM-DA and DCF-DA, respectively. (C and D) The fluorescence intensities of NO and ROS levels in individual zebrafish larvae were quantified, and values represent the means ± SD of three independent experiments (#p<0.05 compared to the control; *p<0.05 compared to the LPS-treated group).
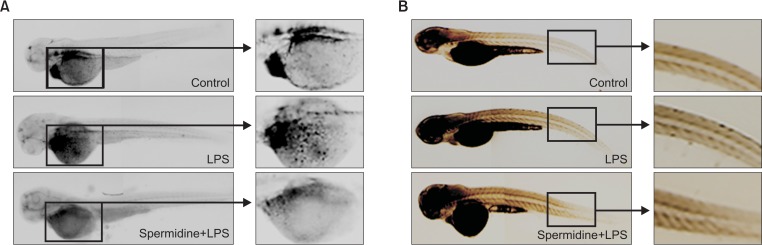
Prevention of LPS-induced recruitment of neutrophils and macrophages by spermidine in zebrafish
The effects of spermidine on the LPS-induced infiltration of neutrophils and macrophages in zebrafish larvae was further investigated using sudan black and neutral red staining, respectively. As illustrated in Fig. 7A, after being injected with LPS, large and clear cytolymph lipid droplets, indicating the recruitment of neutrophils, were markedly present in the yolk sac of larvae. However, pretreatment with spermidine reduced the LPS-induced neutrophil recruitment than the LPS treatment. In addition, neutral red staining showed that the macrophage numbers were predominately elevated in the epidermis in LPS-immersed larvae compared to the PBS-injected controls; however, the treatment with spermidine significantly reduced the accumulation of macrophages (Fig. 7B).
Fig. 7.
Attenuation of LPS-induced recruitment of neutrophils and macrophages by spermidine in zebrafish larvae. After incubation with or without 800 μM spermidine, the larvae were collected, and 10 μg/mL LPS was injected into the yolk using a microinjector. The larvae were stained sudan black or neutral red to detect neutrophils (A) and macrophages (B) migration, respectively. After staining, recruitment of neutrophils and macrophages was observed using a dissecting microscope. The representative pictures for neutrophils and macrophages (Control, PBS-injected negative controls; LPS, LPS-injected positive controls; spermidine + LPS, spermidine-treated and LPS-injected larvae) are shown.
DISCUSSION
Inflammation is a host defense mechanism against pathogenic challenges involving multiple events in the development of inflammation. During infection by gram-negative bacterial LPS, membrane-bound pattern recognition receptor Toll-like receptor 4 (TLR4) plays a critical driver of immune responses (Aderem and Ulevitch, 2000; Nikaido, 2003). The activation of TLR4 pathway leads to intracellular signaling pathways that culminate in the activation of several intracellular signaling pathways including NF-κB. The consequent activation of macrophages promotes inflammation through the aberrant production of pro-inflammatory mediators and cytokines (Aderem and Ulevitch, 2000; Nikaido, 2003). The pro-inflammatory factors induce the influx of neutrophils that in turn produce the intracellular accumulation of ROS (Brüne et al., 2013; Mills and O’Neill, 2016). Importantly, neutrophil-derived myeloperoxidase, a bacteriolytic enzyme, induces bacterial lysis and increases the LPS release during gram-negative bacteria infection (Ginsburg and Koren, 2008; Mitroulis et al., 2015). Therefore, the inhibition of macrophage activation could be a potential therapeutic target in inflammatory and oxidative diseases.
In this study, spermidine significantly attenuated the LPS-induced production of NO and PGE2 in RAW 264.7 macrophages by downregulating iNOS and COX-2 expression on both the protein and mRNA levels without cytotoxicity. Consistent with our previous results in a LPS-stimulated microglial cell model (Choi and Park, 2012), spermidine also attenuated the LPS-induced mRNA upregulation and secretion of TNF-α and IL-1β in RAW 264.7 cells. These data indicate that spermidine suppresses the production of pro-inflammatory mediators and cytokines by reducing the expression of their encoding genes. Thus, the results support that spermidine is a promising target for inhibiting early steps in inflammatory pathways.
NF-κB has been shown to play an important role in various inflammatory states as a transcription factor for many inflammation-mediated genes (Lu et al., 2011; Rigoglou and Papavassiliou, 2013). NF-κB, a dimer of p65 and p50 subunits, is normally retained in the cytoplasm because of its association with its endogenous inhibitor, IκB-α. Once activated by inflammatory stimulants including LPS, IκB-α is rapidly phosphorylated and degraded through a proteasome-mediated pathway followed by a nuclear translocation of NF-κB, resulting in transcriptional induction of inflammation-associated genes (Nikaido, 2003; Rigoglou and Papavassiliou, 2013). Therefore, pharmacological agents that effectively modulate NF-κB activation are promising candidates for treating various inflammatory diseases. In this study, spermidine effectively suppressed the nuclear translocation of NF-κB and degradation of IκB-α in the LPS-stimulated RAW 264.7 macrophages. These findings elucidate that the inactivation of NF-κB by spermidine might downregulate pro-inflammatory genes expression; hence, spermidine possesses anti-inflammatory potential.
In contrast, oxidative stress, representing the over-production of ROS, is strongly associated with many other pathological statuses including inflammation (Brüne et al., 2013; Mills and O’Neill, 2016). Moreover, during chronic inflammation, ROS amplify inflammatory signals in macrophages through activation of NF-κB signaling pathway and over-expression of inflammation-associated genes (Kauppinen et al., 2013; Tan et al., 2016). Therefore, the inhibitory effect of spermidine on LPS-induced ROS generation was investigated, and the ROS accumulation significantly reduced after pretreatment with spermidine in the LPS-stimulated RAW 264.7 macrophages. The data suggest that spermidine-mediated inhibition of ROS generation might potentially inhibit NF-κB signaling pathway-dependent expression of pro-inflammatory mediators, thereby resulting in an anti-inflammatory efficacy.
The protective effect of spermidine against LPS-induced NO and ROS generation was further investigated by DAF-FM-DA and DCF-DA staining in zebrafish as an alternative in vivo animal model system. In agreement with previous results (Wijesinghe et al., 2014; Cheong et al., 2016), the fluorescence signals dramatically increased in the LPS-exposured group as compared to the unstimulated control group, indicating that the NO and ROS generation occurred during the LPS treatment in the zebrafish larvae. However, similar to our in vitro results, zebrafish treated with spermidine prior to the LPS treatment showed a significant reduction in the amount of NO and ROS, indicating strong anti-inflammatory and antioxidant potentials of spermidine.
In addition to the activation of macrophages, neutrophils also play an important role in inflammatory processes. The accumulation and interaction of neutrophils with resident cells at the site of inflammation is a defining early event of innate immunity, and local inflammatory mediators amplify the inflammatory response through the release of pro-inflammatory mediators and cytokines, further inducing neutrophil influx (Cunha et al., 2008; Zhang and Wang, 2014). The pro-inflammatory cytokines also help upregulate the expression of adhesion molecules promoting neutrophil recruitment to the inflammatory tissue (Williams et al., 2011; Mitroulis et al., 2015) and increase tissue damage by extending the survival of neutrophils (Dibbert et al., 1999; Sikora, 2002). Moreover, the activation of neutrophils produced oxygen radicals at an increased rate, and the release of granular enzymes during the LPS-induced inflammatory cascade (Matthy and Zimmerman, 2005; Tan et al., 2016). In this study, a marked inhibition of LPS-stimulated NO production and ROS accumulation by spermidine in the zebrafish model was connected with the inhibition of neutrophil and macrophage recruitment. Consistent with our results, spermidine is reported to reduce the severity of dermal edema in mice by decreasing neutrophil infiltrations and systemic inflammation (Paul and Kang, 2013). Moreover, spermidine also significantly ameliorated experimental autoimmune encephalomyelitis in mouse multiple sclerosis model by reducing the infiltration of macrophages (Yang et al., 2016). Although our results indicate that the potentially anti-inflammatory effects of spermidine on an LPS instillation-induced zebrafish model was achieved via the reduction of inflammatory cells, including neutrophils and macrophages, further studies are needed to evaluate a possible direct effect mediated by the NO production and ROS accumulation in recruited neutrophils and macrophages. The detailed mechanism by which spermidine affects the pro-inflammatory mediators and cytokines production in activated neutrophils also needs further study.
In summary, the results presented here demonstrate that spermidine exerts potent anti-inflammatory effects in RAW 264.7 macrophages and zebrafish. In LPS-stimulated RAW 264.7 macrophages, spermidine significantly attenuated the production of pro-inflammatory mediators by reducing their corresponding gene expression. These anti-inflammatory effects of spermidine were associated with the suppression of LPS-induced NF-κB nuclear translocalization and ROS accumulation. Spermidine also significantly decreased the elevation of NO and ROS levels in an LPS-stimulated zebrafish model and reduced the inflammation-associated migration of immune cells such as neutrophils and macrophages. Based on the results of this study, spermidine could be a natural drug that is extremely useful in improving anti-inflammatory and antioxidant treatment.
Acknowledgments
This research was a part of the project titled ‘Omics based on fishery disease control technology development and industrialization,’ funded by the Ministry of Oceans and Fisheries (20150242) and National Marine Biodiversity Institute Research Program (2016M00600).
REFERENCES
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Amin AR, Attur M, Abramson SB. Nitric oxide synthase and cyclooxygenases: distribution, regulation, and intervention in arthritis. Curr Opin Rheumatol. 1999;11:202–209. doi: 10.1097/00002281-199905000-00009. [DOI] [PubMed] [Google Scholar]
- Brüne B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, von Knethen A, Weigert A. Redox control of inflammation in macrophages. Antioxid Redox Signal. 2013;19:595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong SH, Yang HW, Ko EY, Ahn G, Lee W, Kim D, Jeon YJ, Kim KN. Anti-inflammatory effects of trans-1,3-diphenyl-2,3-epoxypropane-1-one in zebrafish embryos in vivo model. Fish Shellfish Immunol. 2016;50:16–20. doi: 10.1016/j.fsi.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Choi YH, Park HY. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J Biomed Sci. 2012;19:31. doi: 10.1186/1423-0127-19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83:824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- Czura CJ, Friedman SG, Tracey KJ. Neural inhibition of inflammation: the cholinergic anti-inflammatory pathway. J Endotoxin Res. 2003;9:409–413. doi: 10.1177/09680519030090060401. [DOI] [PubMed] [Google Scholar]
- Dibbert B, Weber M, Nikolaizik WH, Vogt P, Schöni MH, Blaser K, Simon HU. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci USA. 1999;96:13330–13335. doi: 10.1073/pnas.96.23.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Böttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Fröhlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Eom SA, Kim DW, Shin MJ, Ahn EH, Chung SY, Sohn EJ, Jo HS, Jeon SJ, Kim DS, Kwon HY, Cho SW, Han KH, Park J, Eum WS, Choi SY. Protective effects of PEP-1-catalase on stress-induced cellular toxicity and MPTP-induced Parkinson’s disease. BMB Rep. 2015;48:395–400. doi: 10.5483/BMBRep.2015.48.7.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire MO, Van Dyke TE. Natural resolution of inflammation. Periodontol. 2000. 2013;63:149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I, Koren E. Are cationic antimicrobial peptides also ‘double-edged swords’? Expert Rev Anti Infect Ther. 2008;6:453–462. doi: 10.1586/14787210.6.4.453. [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Land SC. Redox signaling-mediated regulation of lipopolysaccharide-induced proinflammatory cytokine biosynthesis in alveolar epithelial cells. Antioxid Redox Signal. 2002;4:179–193. doi: 10.1089/152308602753625942. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev Biol. 2001;238:274–288. doi: 10.1006/dbio.2001.0393. [DOI] [PubMed] [Google Scholar]
- Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Ko SC, Jeon YJ. Anti-inflammatory effect of enzymatic hydrolysates from Styela clava flesh tissue in lipopolysaccharide-stimulated RAW 264.7 macrophages and in vivo zebrafish model. Nutr Res Pract. 2015;9:219–226. doi: 10.4162/nrp.2015.9.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larqué E, Sabater-Molina M, Zamora S. Biological significance of dietary polyamines. Nutrition. 2007;23:87–95. doi: 10.1016/j.nut.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, Mordelet E, Zapata A, Shinomiya H, Herbomel P. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- Lee H, Pyo MJ, Bae SK, Heo Y, Kim CG, Kang C, Kim E. Improved therapeutic profiles of PLA2-free bee venom prepared by ultrafiltration method. Toxicol Res. 2015;31:33–40. doi: 10.5487/TR.2015.31.1.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IC, Lee SM, Ko JW, Park SH, Shin IS, Moon C, Kim SH, Kim JC. Role of mitogen-activated protein kinases and nuclear factor-κB in 1,3-dichloro-2-propanol-induced hepatic injury. Lab Anim Res. 2016;32:24–33. doi: 10.5625/lar.2016.32.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löser C. Polyamines in human and animal milk. Br J Nutr. 2000;84:S55–S58. doi: 10.1017/S0007114500002257. [DOI] [PubMed] [Google Scholar]
- Lu YC, Jayakumar T, Duann YF, Chou YC, Hsieh CY, Yu SY, Sheu JR, Hsiao G. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-κB signaling in activated SW1353 cells. J Agric Food Chem. 2011;59:4969–4978. doi: 10.1021/jf1046738. [DOI] [PubMed] [Google Scholar]
- Matthy MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merentie M, Uimari A, Pietilä M, Sinervirta R, Keinänen TA, Vepsäläinen J, Khomutov A, Grigorenko N, Herzig KH, Jänne J, Alhonen L. Oxidative stress and inflammation in the pathogenesis of activated polyamine catabolism-induced acute pancreatitis. Amino Acids. 2007;33:323–330. doi: 10.1007/s00726-007-0522-3. [DOI] [PubMed] [Google Scholar]
- Mills EL, O’Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol. 2016;46:13–21. doi: 10.1002/eji.201445427. [DOI] [PubMed] [Google Scholar]
- Minois N. Molecular basis of the ‘anti-aging’ effect of spermidine and other natural polyamines a mini-review. Gerontology. 2014;60:319–326. doi: 10.1159/000356748. [DOI] [PubMed] [Google Scholar]
- Minois N, Carmona-Gutierrez D, Bauer MA, Rockenfeller P, Eisenberg T, Brandhorst S, Sisgrist SJ, Kroemer G, Madeo F. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis. 2012;3:e401. doi: 10.1038/cddis.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitroulis I, Alexaki VI, Kourtzelis I, Ziogas A, Hajishengallis G, Chavakis T. Leukocyte integrins: role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 2015;147:123–135. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moinard C, Cynober L, de Bandt JP. Polyamines: metabolism and implications in human diseases. Clin Nutr. 2005;24:184–197. doi: 10.1016/j.clnu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Morón B, Spalinger M, Kasper S, Atrott K, Frey-Wagner I, Fried M, McCole DF, Rogler G, Scharl M. Activation of protein tyrosine phosphatase non-receptor type 2 by spermidine exerts anti-inflammatory effects in human THP-1 monocytes and in a mouse model of acute colitis. PLoS ONE. 2013;8:e73703. doi: 10.1371/journal.pone.0073703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Benit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, Lopez-Otin C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013;94:1167–1184. doi: 10.1189/jlb.0313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirwane A, Sridhar V, Majumdar A. Neurobehavioural changes and brain oxidative stress induced by acute exposure to GSM900 mobile phone radiations in zebrafish (Danio rerio) Toxicol Res. 2016;32:123–132. doi: 10.5487/TR.2016.32.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Kang SC. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflamm Res. 2013;62:681–688. doi: 10.1007/s00011-013-0620-5. [DOI] [PubMed] [Google Scholar]
- Rigoglou S, Papavassiliou AG. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45:2580–2584. doi: 10.1016/j.biocel.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Sava IG, Battaglia V, Rossi CA, Salvi M, Toninello A. Free radical scavenging action of the natural polyamine spermine in rat liver mitochondria. Free Radic Biol Med. 2006;41:1272–1281. doi: 10.1016/j.freeradbiomed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Sikora JP. Immunotherapy in the management of sepsis. Arch Immunol Ther Exp (Warsz) 2002;50:317–324. [PubMed] [Google Scholar]
- Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longeva. 20162016:2795090. doi: 10.1155/2016/2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A, Budai MM, Milesz S, Bácsi A, Tőzsér J, Benkő S. Ragweed pollen extract intensifies lipopolysaccharide-induced priming of NLRP3 inflammasome in human macrophages. Immunology. 2013;138:392–401. doi: 10.1111/imm.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xu ML, Liu J, Wang Y, Hu JH, Wang MH. Sonchus asper extract inhibits LPS-induced oxidative stress and pro-inflammatory cytokine production in RAW264.7 macrophages. Nutr Res Pract. 2015;9:579–585. doi: 10.4162/nrp.2015.9.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesinghe WA, Kim EA, Kang MC, Lee WW, Lee HS, Vairappan CS, Jeon YJ. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ Toxicol Pharmacol. 2014;37:110–117. doi: 10.1016/j.etap.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Bu L, Sun W, Hu L, Zhang S. Functional characterization of mannose-binding lectin in zebrafish: implication for a lectin-dependent complement system in early embryos. Dev Comp Immunol. 2014;46:314–322. doi: 10.1016/j.dci.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Yang Q, Zheng C, Cao J, Cao G, Shou P, Lin L, Velletri T, Jiang M, Chen Q, Han Y, Li F, Wang Y, Cao W, Shi Y. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016;23:1850–1861. doi: 10.1038/cdd.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang CC. Inflammatory response of macrophages in infection. HBPD INT. 2014;13:138–152. doi: 10.1016/s1499-3872(14)60024-2. [DOI] [PubMed] [Google Scholar]