Abstract
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) is a common clinical syndrome of diffuse lung inflammation with high mortality rates and limited therapeutic methods. Diosmetin, an active component from Chinese herbs, has long been noticed because of its antioxidant and anti-inflammatory activities. The aim of this study was to evaluate the effects of diosmetin on LPS-induced ALI model and unveil the possible mechanisms. Our results revealed that pretreatment with diosmetin effectively alleviated lung histopathological changes, which were further evaluated by lung injury scores. Diosmetin also decreased lung wet/dry ratios, as well as total protein levels, inflammatory cell infiltration and proinflammatory cytokine (eg. TNF-α, IL-1β and IL-6) overproduction in bronchoalveolar lavage fluid (BALF). Additionally, increased MPO, MDA and ROS levels induced by LPS were also markly suppressed by diosmetin. Furthermore, diosmetin significantly increased the expression of Nrf2 along with its target gene HO-1 and blocked the activation of NLRP3 inflammasome in the lung tissues, which might be central to the protective effects of diosmetin. Further supporting these results, in vitro experiments also showed that diosmetin activated Nrf2 and HO-1, as well as inhibited the NLRP3 inflammasome in both RAW264.7 and A549 cells. The present study highlights the protective effects of diosmetin on LPS-induced ALI via activation of Nrf2 and inhibition of NLRP3 inflammasome, bringing up the hope of its application as a therapeutic drug towards LPS-induced ALI.
Keywords: Acute lung injury, Lipopolysaccharide, Diosmetin, Nrf2, NLRP3
INTRODUCTION
Acute lung injury (ALI) is a severe clinical occurrence characterized by increased permeability of pulmonary capillary and severely impaired gas exchange (Kim and Hong, 2016). Despite improvements in antibiotic therapy and mechanical ventilation, the most severe form of ALI, acute respiratory distress syndrome (ARDS) still has a high mortality, which indicates that better interventions remain elusive (Mehla et al., 2013). In consideration of this urgent need, the pathogenesis and interventions of ALI are under study. Related studies mainly concentrate on the involvement of oxidative stress and inflammation response (Lykens et al., 1992; Baetz et al., 2005), which are inseparably linked as each begets and amplifies the others.
Oxidative stress represents an imbalance between production of free radicals and reactive metabolites, so-called oxidants or reactive oxygen species (ROS), and their elimination by protective mechanisms, referred to as antioxidants. As reported, excessive ROS under such imbalance is central to severe pulmonary inflammation that results in ALI/ARDS (Chabot et al., 1998; Ware and Matthay, 2000). In order to counterbalance the negative effects of ROS, Nrf2 and its downstream target antioxidant genes have been well recognized in recent years. Under normal condition, Nrf2 constitutively resides in the cytoplasm and binds to Kelch-like ECH-associated protein1 (Keap1), which ubiquitylates Nrf2 and leads to its degradation. In response to diverse stimuli, Nrf2 is released from Keap1. Released Nrf2 accumulates in the nucleus and binds to the antioxidant responsive element (ARE) in the promoter region of phase II detoxifying enzymes and cytoprotective genes (Nguyen et al., 2009). HO-1 is precisely one such antioxidant enzyme that is mainly regulated by Nrf2. Its ability to combat oxidative stress and maintain redox homeostasis has been well clarified (Alam et al., 1999). Nevertheless, the pathways by which ROS inflicts tissue damages are more than direct effects. The relationship between oxidative stress and inflammation is fairly important in the pathogenesis of various diseases. Recent studies reveals that ROS also provides necessary signaling to the activation of nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3) inflammasome, which is already proved to play a critical role in the inflammation response during ALI (Grailer et al., 2014). NLRP3 inflammasome is a major intracellular multiprotein inflammatory pathway composed of NOD like receptor (NLRP3), the adaptor protein ASC and caspase-1 (Kim and Lee, 2013). Upon diverse stimuli (microbial- and stress-substances, etc.), NLRP3 regulates the maturation of proinflammatory cytokines IL-1β and IL-18 through a continuous process of NLRP3 activation, ASC and pro-caspase-1 recruitment, caspase-1 activation and the subsequent process of pro-IL-1β and pro-IL-18 (Grailer, Canning, Kalbitz, Haggadone, Dhond, Andjelkovic, Zetoune and Ward, 2014; Tianzhu et al., 2014). These activated cytokines are central to mediate lung inflammation. A recent study revealed that NLRP3 activation could be inhibited through ROS reduction, indicating that exist of oxidative stress contributes to the aberrant inflammation (Ren et al., 2016). Therefore, NLRP3 inhibition may also be achieved by antioxidants to alleviate lung injury of ALI/ARDS.
Diosmetin (3′, 5, 7-trihydroxy-4′-methoxyflavone) is the aglycone of the flavonoid glycoside diosmin, which can be found in citrus species (Roowi and Crozier, 2011) and olive leaves (Meirinhos et al., 2005). It has been reported that diosmetin possesses different biological properties, such as anticancer, antimicrobial, antioxidant and anti-inflammatory activities (Androutsopoulos et al., 2009; Chan et al., 2013; Liao et al., 2014; Yu et al., 2014). However, limited studies have discussed about how it exerts antioxidant and anti-inflammatory activities. As lipopolysaccharide (LPS) has both the properties of promoting inflammation and oxidative stress, and is recognized as a major risk factor for ALI, intranasal administration of LPS has been used to investigate the protective effects of diosmetin on LPS-induced ALI as well as the involved mechanisms such as Nrf2 activation and NLRP3 inhibition in this study. In addition, in vitro studies were also carried out to further identify the effects of diosmetin on the Nrf2/HO-1 pathway and NLRP3 inflammasome.
MATERIALS AND METHODS
Drug and reagents
Diosmetin, purity >98%, was purchased from Chengdu Pufei De Biotech Co., Ltd. Dexamethasone was purchased from TianJin KingYork Group HuBei TianYao Pharmaceutical Co., Ltd. LPS (Escherichia coli 055:B5) and Dimethylsulfoxide (DMSO) were purchased from Sigma Chemical Co (St. Louis, MO, USA). Penicillin and streptomycin, foetal bovineserum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) for cell culture use were purchased from Invitrogen-Gibco (GrandIsland, NY, USA). MPO and MDA determination kit were provided by the Jiancheng Bioengineering Institute of Nanjing (Jiangsu, China). Mouse TNF-α, IL-1β and IL-6 ELISA kits were provided by Biolegend (San Diego, CA, USA). Antibodies against NLRP3, ASC, caspase-1, IL-1β, Nrf2, HO-1 and β-actin were purchased from Cell Signaling (Boston, MA, USA). The horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse IgG were obtained from protein-tech (Boston, MA, USA). All other chemicals, unless specifically stated elsewhere, were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animal
Adult female BALB/c mice (18–20 g) were obtained from Liaoning Changsheng Technology Industrial Co., LTD (Certificate SCXK2010-0001; Liaoning, China). The animals were fed with food and water ad libitum and housed in certified, standard laboratory cages before experiments. All of the experiments were approved by Animal Use Committee of Jilin University (Changchun, China), in accordance with International Guiding Principles for Biomedical Research Involving Animals.
Murine model and grouping of LPS-induced ALI
To induce ALI model, the mice received an intranasal instillation of LPS (0.5 mg/kg). As for drug-treated groups, the mice received two doses of diosmetin as well as dexamethasone (Dex) by intraperitoneal injection 1 h before LPS challenge or not. More exactly, mice were randomly divided into six groups, i.e., (1) control group, (2) diosmetin (25 mg/kg), (3) LPS group (0.5 mg/kg), (4) LPS+diosmetin (5 mg/kg), (5) LPS+diosmetin (25 mg/kg) and (6) LPS+dexamethasone (5 mg/kg). Mice were sacrificed under diethyl ether anesthesia 12 h after LPS challenge for further experiments.
Histological evaluation
Lung tissues for histological evaluation were from mice that were not used for BALF collection. Mice were killed 12 h after LPS administration, lower lobe from left lungs were fixed in 4% formalin, dehydrated with ethanol, followed by embedded in paraffin and cut into 5 μm sections. After deparaffinization, the tissues were stained with hematoxylin and eosin (H&E). The hematoxylin and eosin staining process was the same as previous description (Yunhe et al., 2012). Pathological changes among different groups were evaluated under a light microscope. Lung injury scores were assessed using a semi-quantitative method.
Measurement of lung wet/dry weight ratios
The right lungs were removed 12 h after LPS administration. After blunt dissection, the lungs were separated, and the wet weights were determined. For dry weight mearsurement, the lungs were incubated at 60°C for 3 days. Then the ratios of wet to-dry weight were calculated.
Bronchoalveolar lavage fluid (BALF) analysis
After LPS was administrated for 12 h, bronchoalveolar lavage fluid (BALF) was obtained by intratracheal injection of 0.5 ml PBS and gentle aspiration for 3 times. A small portion of BALF was used to determine the total protein concentration using BCA (Bicinchoninic acid) method. Rest liquid was centrifuged at 3000 rpm for 10 min at 4°C, and then the supernatants were preserved at −80°C for detection of cytokines by ELISA according to the manufacturer’s instructions. The sediment cells were re-suspended in PBS for total and differential inflammatory cell counting as well as ROS detection.
Enzyme-linked immunosorbent assay (ELISA)
Levels of TNF-α, IL-1β and IL-6 in BALF were quantified using a commercially available ELISA kits according to the manufacturer’s instructions. The absorbance of each well was read at 450 nm with a microplate reader.
ROS detection in BALF
The sediment cells in BALF were washed and incubated with 10 μM DCF-DA (20, 70-Dichlorofluorescein diacetate), a ROS-sensitive fluorescent dye for 40 min at 37°C. ROS production was analyzed by flow cytometry.
Measure of MPO and MDA contents
The mice were sacrificed under diethyl ether anesthesia 12 h after LPS challenge, and the right lungs were excised. The lungs were homogenized, and levels of MPO and MDA were determined using test kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Procedures were according to the manufacturer’s kit protocol.
Cell culture and treatment
RAW264.7 and A549 cells were obtained from the China Cell Line Bank (Beijing, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin and 100 U/ml of streptomycin in a humidified incubator containing 5% CO2 at 37°C prior to experiments. Before any treatment, cells were plated in 6-well plates and allowed to acclimate for 24 h. For the activation of Nrf2/HO-1 pathway, the cells were stimulated with diosmetin (40 μM) for the indicated time. While for the stimulation of NLRP3 inflammasome, cells were pre-treated with diosmetin (40 μM) for 1 h, followed by stimulation with LPS (1 μg/ml) for 6 h and ATP (5 mM) for 40 min. And then the cells were harvested at 8000 rpm for 5 min at 4°C for Western blot analysis.
Western blots
Lung tissues for western blot were obtained 12 h after LPS administration and stored at −80°C. And cells treated with diosmetin in the present or absence of LPS were collected. Tissue homogenates or the cells were lysed in RIPA buffer with protease and phosphatase inhibitors for 30 min and centrifuged. The supernatants were collected, and protein concentrations were determined using BCA method. Protein extracts were separated by 10% SDS-PAGE, and then electro-transferred to PVDF membrane. The membrane was blocked in 5% skim milk at room temperature for 1 h, followed by incubated with each primary antibody overnight and corresponding secondary antibody for 1 h. Finally the bands were detected with an enhanced chemiluminescence (ECL) Western blot detection system according to the manufacturer’s instructions.
Statistical analysis
Values are presented as means ± SEM. For comparison among groups, one-way analysis of variance (ANOVA) tests were used. Statistical significance was accepted when p<0.05 or p<0.01.
RESULTS
Diosmetin ameliorates lung injury of LPS-challenged BALB/c mice
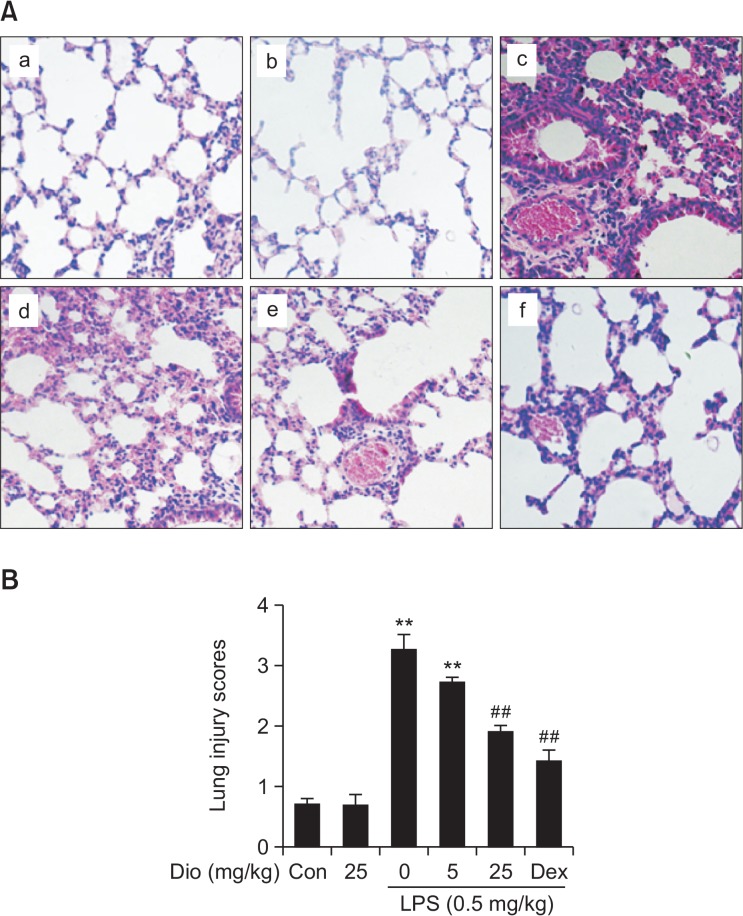
LPS instillation is known to mediate structural ravage in the mice lung tissues. To determine LPS-induced lung injury in our ALI model, lower lobe of left lung was taken for histology 12 h after LPS challenge (Fig. 1A). In the control group, normal pulmonary histology was observed. LPS treatment was associated with notable inflammatory cell infiltration and alveolar hemorrhage. Pretreatment with diosmetin significantly attenuated such pathological changes induced by LPS. The positive control drug dexamethasone also improved the histopathological conditions in LPS-induced ALI. For semiquantitative evalution, the changes were also evaluated by calculating a lung injury score (Fig. 1B). Such results indicated that diosmetin alleviated pathological damages caused by LPS challenge in ALI.
Fig. 1.
Effects of diosmetin on LPS-induced lung injury in BALB/c mice. (A) H&E staining of lung tissue section (original magnification, ×200). Lungs from each experimental group (n=5) were obtained 12 h after LPS challenge, prepared and stained with H&E: (a) control, (b) diosmetin (25 mg/kg), (c) LPS (0.5 mg/kg), (d) LPS+diosmetin (5 mg/kg), (e) LPS+diosmetin (25 mg/kg) and (f) LPS+Dex (5 mg/kg). (B) Lung mean injury score. The lung injury score was determined following a five-point scale from 0 to 4 as follows: 0, 1, 2, 3, and 4 represent no damage, mild damage, moderate damage, severe damage, and very severe damage, respectively. Data was expressed as means ± SEM. n=5. **p<0.01 vs the control group. ##p<0.01 vs the LPS group.
Diosmetin reduces lung edema and increase of capillary permeability during inflammation
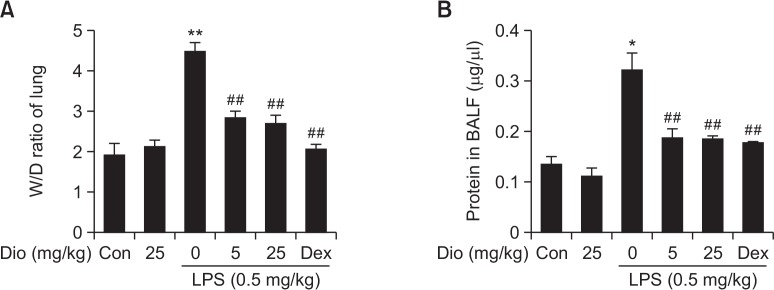
To evaluate changes in lung edema, lung wet/dry weight ratios were measured. A notable increase in lung wet/dry weight ratio was caused by LPS instillation compared with control group. Diosmetin exhibited inhibitory effects on LPS-induced rise of lung wet/dry weight ratio as well as Dex did (Fig. 2A).
Fig. 2.
Effects of diosmetin on lung wet/dry ratios and protein leakage. (A) Effects of diosmetin on LPS-induced variability in lung wet-to-dry weight ratios. (B) Effects of diosmetin on LPS-induced protein leakage in BALF. Data was expressed as means ± SEM. n=5. *p<0.05 and **p<0.01 vs the control group. ##p<0.01 vs the LPS group.
Total protein concentration change in BALF is a character of capillary permeability increase. A sharp rise of total protein in BALF was observed in LPS group. While such rise was blocked by diosmetin pretreatment as well (Fig. 2B).
Diosmetin alleviates myeloperoxidase activation, lipid peroxidation and ROS production
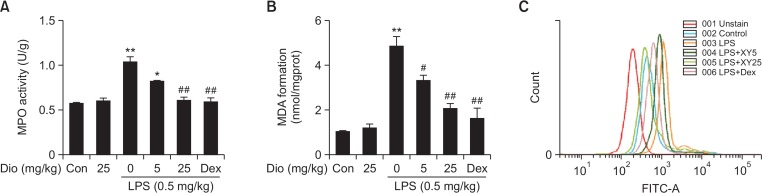
Myeloperoxidase (MPO) is a heme-containing enzyme that generates during the infiltration and activation processes of neutrophils. Although MPO acts as a killer of microorganisms, MPO and MPO-derived oxidants indeed result in tissue damages. Our results illustrated that pretreatment with diosmetin markly blocked LPS-induced increase in MPO activity in lung tissues (Fig. 3A). MDA, a major product of lipid peroxidation, is often used as a marker of oxidative stress because its levels correspond to the amount of oxidative stress. LPS stimulation significantly induced the generation of MDA, however, diosmetin pretreatment effectively attenuated MDA content in lung tissues in LPS-induced ALI (Fig. 3B). Furthermore, LPS-induced overproduction of ROS is an essential and direct source of oxidative injury, thus, we also evaluated the effects of diosmetin on ROS levels. Treatment with LPS significantly increased the ROS levels of sediment cells in BALF after 12 h, and such increase was effectively suppressed by the diosmetin treatment (Fig. 3C). These results revealed that diosmetin alleviated lung injury by reducing the degree of oxidative stress.
Fig. 3.
Effects of diosmetin on MPO, MDA and ROS levels in LPS-induced ALI. (A) MPO activation. Lung tissues were obtained and homogenized to measure MPO activity. (B) MDA contents. Lung tissues were obtained and homogenized to measure MDA levels. Data was expressed as means ± SEM. n=5. *p<0.05 and **p<0.01 vs the control group. #p<0.05 and ##p<0.01 vs the LPS group. (C) ROS generation. The sediment cells in BALF were incubated with 10 μM DCF-DA for 40 min, and ROS production induced by LPS was detected by flow cytometry.
Diosmetin reduces the infiltration of inflammatory cells in BALF
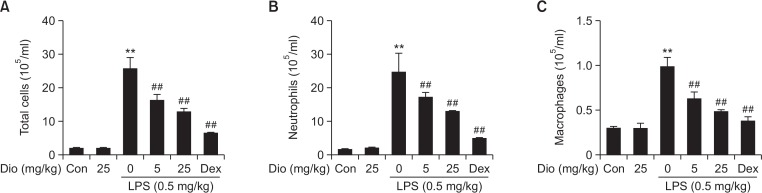
To further verify the effects of diosmetin on lung inflammation, the number of total and differential inflammatory cells in BALF were determined. LPS instillation markly raised the levels of total cells, neutrophils and macrophages in BALF. While compared with LPS stimulation alone, treatment with diosmetin significantly reduced the infiltration of total cells, neutrophils and macrophages (Fig. 4).
Fig. 4.
Effects of diosmetin on inflammatory cell infiltration in LPS-induced ALI. BALF was collected 12 h after LPS challenge, and the numbers of total cells, neutrophils and macrophages were measured. Data was expressed as means ± SEM. n=5. **p<0.01 vs the control group. ##p<0.01 vs the LPS group.
Diosmetin reduces the levels of proinflammatory cytokines in BALF
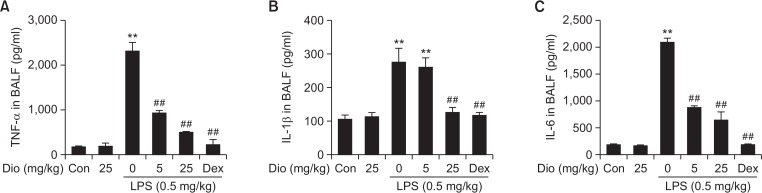
Proinflammatory cytokines involved in the recruitment of neutrophils, such as TNF-α, IL-1β and IL-6, were shown to take part in the pathogenesis of LPS-induced ALI. So the levels of these three inflammatory mediators in BALF were also measured (Fig. 5). Dramatic increases of TNF-α, IL-1β, and IL-6 were recorded in LPS-treated group. While pretreatment with diosmetin downregulated these proinflammatory cytokines levels, which in turn improved the tissue damage. Dexamethasone had the similar effects as well. Such results demonstrated that diosmetin improved lung injury by reducing the expression of proinflammatory cytokines in LPS-induced ALI.
Fig. 5.
Effects of diosmetin on LPS-induced proinflammatory cytokine secretion. The supernatant of BALF was collected to detect levels of proinflammatory cytokines by ELISA. Data was expressed as means ± SEM. n=5. **p<0.01 vs the control group. ##p<0.01 vs the LPS group.
Diosmetin upregulates Nrf2/HO-1 pathway in LPS-induced ALI and in vitro
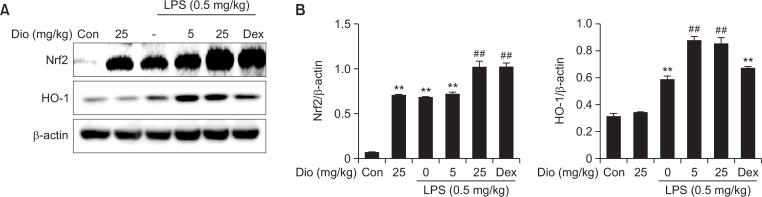
HO-1, an endogenous cytoprotective enzyme, is expressed during the amelioration of ALI. It is under the regulation of a redox sensitive transcription factor-Nrf2, which acts as a key transcriptional regulator through its binding to ARE. The effect of diosmetin on HO-1 expression was analyzed by Western blotting. Although LPS alone also induced increase in the protein levels of Nrf2 and HO-1, diosmetin treatment evoked a more significant rise of total Nrf2 and HO-1 in the lung homogenates. The results indicated that diosmetin markly reduced LPS-induced oxidative stress by activation of Nrf2/HO-1 pathway (Fig. 6).
Fig. 6.
Effect of diosmetin on Nrf2/HO-1 pathway in vivo. (A) Lung tissues were obtained and homogenized, and then protein expression of Nrf2 and HO-1 was determined by western blot. (B) Densitometry quantitation of protein expression. β-actin acted as an internal control. Data was expressed as means ± SEM. n=5. **p<0.01 vs the control group. ##p<0.01 vs the LPS group.
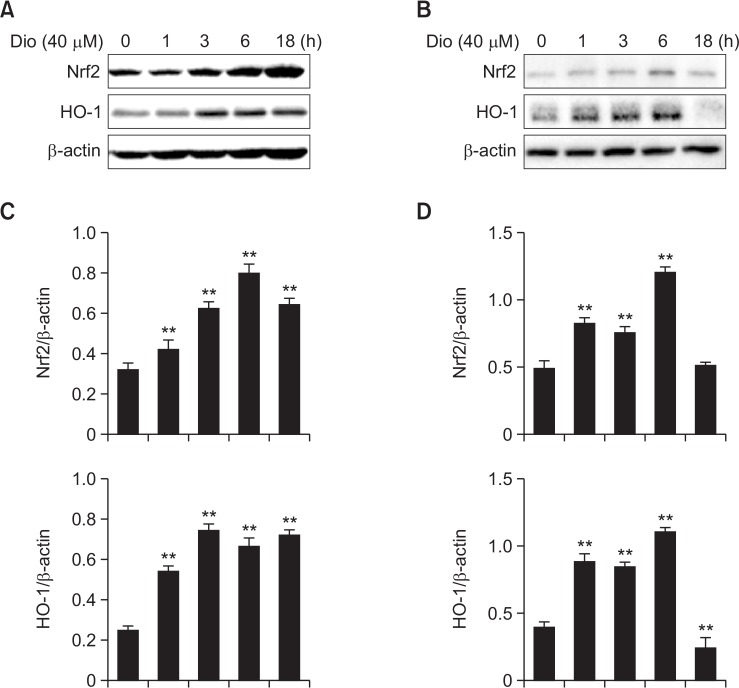
Additionally, cells and cellular components of lung as well as inflammatory cells are essential in the defense of oxidative stress and inflammation. To further figure out the exact effects of diosemtin on Nrf2 and HO-1 expression, we stimulated RAW264.7 and A549 cells with diosmetin (40 μM) for the indicated time and then tested the activation of Nrf2/HO-1 pathway. Similar to the results in vivo, diosmetin induced Nrf2 activation associated with HO-1 upregulation (Fig. 7). Intriguingly, at the time point of 18 h, the expressions of Nrf2 and HO-1 were slightly reduced in A549 cells.
Fig. 7.
Effect of diosmetin on Nrf2/HO-1 pathway in vitro. (A, B) RAW264.7 and A549 cells were treated with diosmetin (40 μM) for the indicated time, and then the cells were collected. Protein expression of Nrf2 and HO-1 in total cell lysates was determined by western blot in RAW264.7 (A) and A549 (B) cells. (C, D) Densitometry quantitation of protein expression in RAW264.7 (C) and A549 (D) cells. β-actin acted as an internal control. Data was expressed as means ± SEM. n=5. **p<0.01 vs the control group.
Diosmetin represses NLRP3 inflammation activation in LPS-induced ALI and in vitro
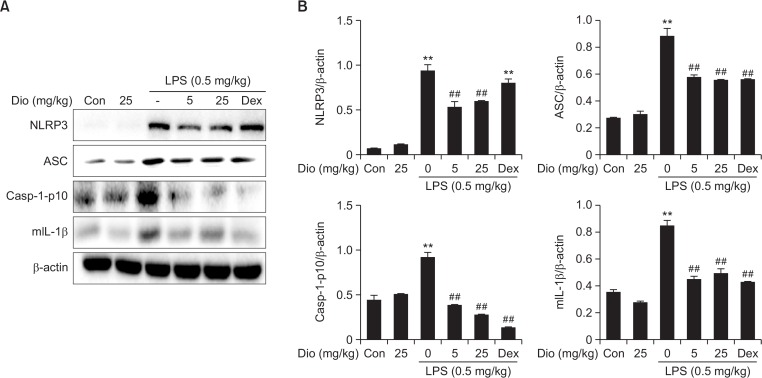
The development of ALI also requires the participation of NLRP3 inflammasome, which is essential for the maturation of IL-1β. Consistent with this, western blot analysis in this study revealed that LPS administration significantly increased the protein levels of all 3 inflammasome components, NLRP3, ASC and caspase-1 in the lung tissue. Diosmetin blocked such increase caused by LPS. Mature IL-1β (17.5 kDa) also increased 12h after LPS challenge, which was in accordance with its concentration in BALF, and was reduced by diosmetin (Fig. 8).
Fig. 8.
Effect of diosmetin on NLRP3 inflammasome protein expression in vivo. (A) Lung tissues were obtained and homogenized, and then protein expression of NLRP3, ASC, caspase-1-p10 and mature IL-1β was determined by western blot. (B) Densitometry quantitation of protein expression. β-actin acted as an internal control. Data was expressed as means ± SEM. n=5. **p<0.01 vs the control group. ##p<0.01 vs the LPS group.
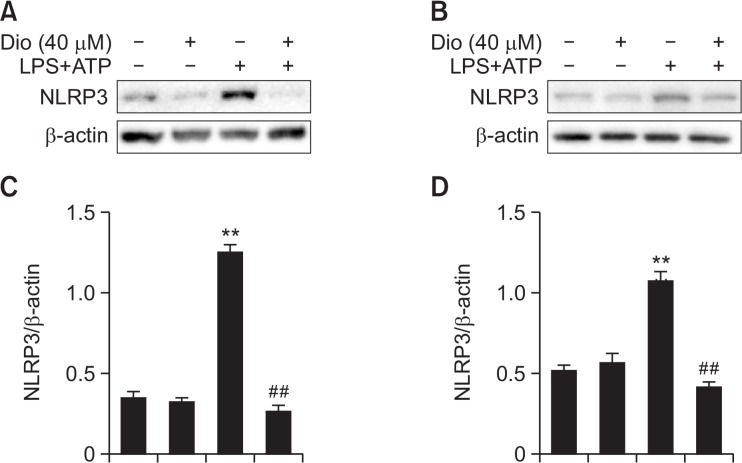
In addition, to further elucidate and verify the anti-inflammasome property of diosmetin, we stimulated RAW264.7 and A549 cells with diosmetin followed by LPS plus ATP, and then western blot analysis of NLRP3 was carried out. Stimulation by LPS plus ATP significantly increased the expression of NLRP3 inflammasome, while pretreatment with diosmetin effectively blocked such increase (Fig. 9). These results in vitro precisely supported the findings in our mouse model of ALI, indicating that the inhibition of NLRP3 inflammasome may be important to alleviate LPS-induced inflammation.
Fig. 9.
Effect of diosmetin on NLRP3 inflammasome protein expression in vitro. (A, B) RAW264.7 and A549 cells were pre-treated with diosmetin (40 μM) for 1 h, followed by stimulation with LPS (1 μg/ml) for 6 h and ATP (5 mM) for 40 min, and then protein expression of NLRP3 was determined by western blot in RAW264.7 (A) and A549 (B) cells. (C, D) Densitometry quantitation of protein expression in RAW264.7 (C) and A549 (D) cells. β-actin acted as an internal control. Data was expressed as means ± SEM. n=5. **p<0.01 vs the control group. ##p<0.01 vs the LPS plus ATP group.
DISCUSSION
Acute lung injury (ALI) and its most severe form, acute respiratory distress syndrome (ARDS), represents a heterogeneous syndrome of diffuse lung injury and respiratory failure. ALI may result in a high morbidity and mortality associated with several clinical disorders including pneumonia, non-pulmonary sepsis and severe trauma (Kim and Hong, 2016). For the past few years, people have acquired more in-depth understanding in the pathogenesis of ALI via application of cytology, molecular biology and genetics. However, no pharmacologic interventions have been effectively used in reducing mortality. As studies of phytochemical and pharmacological developed, people have identified a mass of natural products that exert diverse biological activities such as anti-oxidant, anti-inflammatory and anti-microbial. Therefore, components from plants in traditional medicine are drawing increasing attention as sources of the prevention and treatment for ALI (Chen et al., 2013; Yeh et al., 2014). Diosmetin, a natural product isolated from Citrus limon (L.) Burm, has been reported to exert antioxidant and anti-inflammatory effects. The present study was designed to explore the therapeutic effects and mechanisms of diosmetin in the defense of LPS-induced ALI.
Histopathology is a visualized reflection of lung injury in ALI. With the help of histopathology analysis, we observed that diometin indeed ameliorated LPS-induced symptoms such as inflammatory cell infiltration and alveolar congestion. And the improvements of histopathological changes were also proved by lung injury scores. In addition, diosmetin significantly attenuated lung wet/dry weight ratios and protein leakage in BALF. These findings indicated the potential therapeutic effects of diosmetin against ALI. For the further experiments, we explored the underlying protective mechanisms of diosmetin against ALI. As oxidative stress and inflammation, which closely intersects with each other, are particularly important mechanisms to explain the occurrence of ALI, we sought to explore the related indexes and pathways in our study.
Oxidative stress charactered by excessive ROS is central to severe pulmonary inflammation that results in ALI/ARDS (Quinlan et al., 1997). Production of intracellular ROS could be significantly inhibited by diosmetin. This observation indicated that diosmetin could exert protective effects through eliminating ROS induced by LPS stimulation directly or indirectly. Myeloperoxidase (MPO), a marker of neutrophil accumulation, mainly generates from neutrophils (Klebanoff, 2005). Despite its positive effects on the organism, MPO and MPO-derived oxidants may further lead to tissue damages (Kisic et al., 2016). MDA, a product of lipid peroxidation, is a widely applied marker of oxidative stress. The increase in MDA levels indicated the destruction and damage of cell membranes caused by ROS. The reduction of MPO and MDA contents by diosmetin pretreatment in LPS-induced ALI verifed its antioxidant effects from another point of view.
At the inflammatory site, inflammatory cell infiltration and proinflammatory cytokine generation are both important characteristics in the exudative phase of LPS-induced ALI (Matute-Bello et al., 2008; Grommes and Soehnlein, 2011). It has already been proved that diosmetin ameliorates proinflammatory cytokine generation (eg. TNF-α, IL-1β and IL-6) by inhibiting the activation of nuclear factor-kappa B (NF-κB) in a previous study (Yu et al., 2014). While in the present study, we observed that diosmetin effectively suppressed the infiltration of total cells, neutrophils and macrophages, while the inhibited inflammatory cell counts were also associated with reduced proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 in BALF. TNF-α and IL-1β are early response cytokines secreted from activated alveolar macrophages. These cytokines further induce neighboring cells to produce more effective proinflammatory cytokines and chemokines, such as IL-6 and so on, which may recruit neutrophils and macrophages in turn. These findings indicated that diosmetin ameliorated ALI by preventing inflammatory cell sequestration and proinflammatory cytokines generation. Furthermore, neutrophils is a pivotal source of MPO, and their respiratory burst produces excessive reactive oxygen species (ROS). The inhibition of MPO and ROS by diosmetin further demonstrated the close relationship between oxidative stress and inflammation in LPS-induced ALI.
To counterbalance excessive ROS, nuclear factor erythroid-2 related factor 2 (Nrf2), a main regulator of various cytoprotective genes that combat oxidative damage, plays a critical role in the indirect elimination of ROS (Wang et al., 2012). Among these cytoprotective enzymes, HO-1 is a major one that maintains redox homeostasis. HO-1 catalyzes the degradation of heme, producing carbon monoxide (CO), ferrous iron, and biliverdin. Both CO and biliverdin contribute to the cytoprotective functions of HO-1 (Ryter and Tyrrell, 2000). On the other side, previous studies showed that HO-1 also plays a pivotal role in the control of lung inflammation, and abundant herb compounds were observed to exert anti-inflammatory effect by inducing HO-1 (Joo Choi et al., 2014). Present study found that diosmetin pretreatment markly increased Nrf2 and HO-1 levels in the lung homogenates, suggesting that HO-1 increase mediated by Nrf2 at least partially underlies the protective effects of diosmetin on LPS-induced ALI. Furthermore, the effect of diosmetin on Nrf2/HO-1 pathway were supported by in vitro study, which showed a significant upregulation with diosmetin treatment in both RAW264.7 and A549 cell line. However, an intriguingly phenomenon was that, despite the essential roles of these two cells in the defense of oxidative stress and inflammation, their regulations of Nrf2/HO-1 pathway had a littlle difference. That is, the expressions of Nrf2 and HO-1 were slightly reduced at the time point of 18 h in A549 cells, but not in RAW264.7 cells. Such finding indicated that different cells in the lung may act differently in the period and type of cellular defense.
However, ROS-induced tissue destruction derives from more than direct effects. The relationship between oxidative stress and inflammation plays a critical role in the occurrence of diverse diseases. Recent studies showed that increased ROS promoted the expression of NLRP3, a major inflammatory pathway of the innate immune system (Ren et al., 2016). NLRP3 inflammasome is consisted of a NLR (nucleotide-binding domain, leucine-rich repeat-containing) family member, ASC (apoptosis-associated speck-like protein containing a CARD), and caspase-1. It can be activated by various stimuli such as bacteria, virus, fungi, and components of dying cells (Strowig et al., 2012). Stimulation with LPS resulted in a significant increase of NLRP3 in cultured hepatocytes (Boaru et al., 2015). In vivo evidences also indicated that LPS can activate NLRP3 inflammasome to induce inflammatory responses by mediating immune cell infiltration and aggravating injury (Luo et al., 2014; Zhang et al., 2016). To be more detailed, after activation, NLRP3 provides a multi-protein platform for caspase-1 activation and IL-1β processing. In the early phase of ALI, IL-1β is a fairly active cytokines that causes release of proinflammatory mediators such as IL-6 and IL-8 (Cross and Matthay, 2011). Draw support from these mediators, inflammatory cells are recruited, and excessive inflammation finally leads to subsequent lung injury (Goodman et al., 2003).
In the present study, LPS significantly increased the protein levels of NLRP3, ASC and caspase-1, as well as IL-1β. Pretreatment with diosmetin reduced the protein levels of these 3 inflammasome components and IL-1β. The inhibition of NLRP3 was again supported by in vitro results, which demonstrated the blockade of NLRP3 activation by diosmetin in a cell model stimulated by LPS plus ATP. More interestingly, several investigations indicated that the anti-inflammatory effects of HO-1 may result from its ability to repress NLRP3 inflammasome (Kim and Lee, 2013). From the above evidences, the protective effects of diosmetin may derive from its direct or indirect suppression of NLRP3 activation.
In conclusion, we demonstrated that diosmetin significantly improved LPS-induced lung injury in mice, which at least partially contributed to the restriction of oxidative injury, as well as inflammatory cell accumulation and proinflammatory cytokine secretion. In vivo and in vitro experiments indicated that scavenging ROS via Nrf2 activation and restricting inflammation via NLRP3 inhibition may be two important mechanisms by which diosmetin treats ALI. The results support the potential use of diosmetin for the treatment of ALI associated with gram-negative bacteria infection. While further studies are required to acquire more insight into the mechanisms underlying how diosmetin leads to Nrf2 activation and NLRP3 inhibition, as well as the relationship between these two pathways.
Acknowledgments
This work supported by a grant from the Natural Science Foundation of Jilin (no. 20150520050JH).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Androutsopoulos VP, Mahale S, Arroo RR, Potter G. Anticancer effects of the flavonoid diosmetin on cell cycle progression and proliferation of MDA-MB 468 breast cancer cells due to CYP1 activation. Oncol Rep. 2009;21:1525–1528. doi: 10.3892/or_00000384. [DOI] [PubMed] [Google Scholar]
- Baetz D, Shaw J, Kirshenbaum LA. Nuclear factor-κB decoys suppress endotoxin-induced lung injury. Mol Pharmacol. 2005;67:977–979. doi: 10.1124/mol.105.011296. [DOI] [PubMed] [Google Scholar]
- Boaru SG, Borkham-Kamphorst E, Van de Leur E, Lehnen E, Liedtke C, Weiskirchen R. NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem Biophys Res Commun. 2015;458:700–706. doi: 10.1016/j.bbrc.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J. 1998;11:745–757. [PubMed] [Google Scholar]
- Chan BC, Ip M, Gong H, Lui SL, See RH, Jolivalt C, Fung KP, Leung PC, Reiner NE, Lau CB. Synergistic effects of diosmetin with erythromycin against ABC transporter over-expressed methicillin-resistant Staphylococcus aureus (MRSA) RN4220/UL5054 and inhibition of MRSA pyruvate kinase. Phytomedicine. 2013;20:611–614. doi: 10.1016/j.phymed.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Chen XJ, Zhang B, Hou SJ, Shi Y, Xu DQ, Wang YX, Liu ML, Dong HY, Sun RH, Bao ND, Jin FG, Li ZC. Osthole improves acute lung injury in mice by up-regulating Nrf-2/thioredoxin 1. Respir Physiol Neurobiol. 2013;188:214–222. doi: 10.1016/j.resp.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Cross LJ, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin. 2011;27:355–377. doi: 10.1016/j.ccc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev. 2003;14:523–535. doi: 10.1016/S1359-6101(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192:5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo Choi R, Cheng MS, Kim YS. Desoxyrhapontigenin up-regulates Nrf2-mediated heme oxygenase-1 expression in macrophages and inflammatory lung injury. Redox Biol. 2014;2:504–512. doi: 10.1016/j.redox.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee SM. NLRP3 inflammasome activation in D-galactosamine and lipopolysaccharide-induced acute liver failure: role of heme oxygenase-1. Free Radic Biol Med. 2013;65:997–1004. doi: 10.1016/j.freeradbiomed.2013.08.178. [DOI] [PubMed] [Google Scholar]
- Kim WY, Hong SB. Sepsis and acute respiratory distress syndrome: recent update. Tuberc Respir Dis (Seoul) 2016;79:53–57. doi: 10.4046/trd.2016.79.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisic B, Miric D, Dragojevic I, Rasic J, Popovic L. Role of myeloperoxidase in patients with chronic kidney disease. Oxid Med Cell Longev. 20162016:1069743. doi: 10.1155/2016/1069743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Liao W, Ning Z, Chen L, Wei Q, Yuan E, Yang J, Ren J. Intracellular antioxidant detoxifying effects of diosmetin on 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. J Agric Food Chem. 2014;62:8648–8654. doi: 10.1021/jf502359x. [DOI] [PubMed] [Google Scholar]
- Luo YP, Jiang L, Kang K, Fei DS, Meng XL, Nan CC, Pan SH, Zhao MR, Zhao MY. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int Immunopharmacol. 2014;20:24–32. doi: 10.1016/j.intimp.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Lykens MG, Davis WB, Pacht ER. Antioxidant activity of bronchoalveolar lavage fluid in the adult respiratory distress syndrome. Am J Physiol. 1992;262:L169–L175. doi: 10.1152/ajplung.1992.262.2.L169. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla K, Balwani S, Agrawal A, Ghosh B. Ethyl gallate attenuates acute lung injury through Nrf2 signaling. Biochimie. 2013;95:2404–2414. doi: 10.1016/j.biochi.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Meirinhos J, Silva BM, Valentao P, Seabra RM, Pereira JA, Dias A, Andrade PB, Ferreres F. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat Prod Res. 2005;19:189–195. doi: 10.1080/14786410410001704886. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan GJ, Lamb NJ, Tilley R, Evans TW, Gutteridge JM. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am J Respir Crit Care Med. 1997;155:479–484. doi: 10.1164/ajrccm.155.2.9032182. [DOI] [PubMed] [Google Scholar]
- Ren JD, Wu XB, Jiang R, Hao DP, Liu Y. Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochim Biophys Acta. 20161863:50–55. doi: 10.1016/j.bbamcr.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Roowi S, Crozier A. Flavonoids in tropical citrus species. J Agric Food Chem. 2011;59:12217–12225. doi: 10.1021/jf203022f. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. doi: 10.1016/S0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Tianzhu Z, Shihai Y, Juan D. The effects of morin on lipopolysaccharide-induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation. 2014;37:1976–1983. doi: 10.1007/s10753-014-9930-1. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhu X, Kim Y, Li J, Huang S, Saleem S, Li RC, Xu Y, Dore S, Cao W. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med. 2012;52:928–936. doi: 10.1016/j.freeradbiomed.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Yang JJ, Yang ML, Li YC, Kuan YH. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free Radic Biol Med. 2014;69:249–257. doi: 10.1016/j.freeradbiomed.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Yu G, Wan R, Yin G, Xiong J, Hu Y, Xing M, Cang X, Fan Y, Xiao W, Qiu L, Wang X, Hu G. Diosmetin ameliorates the severity of cerulein-induced acute pancreatitis in mice by inhibiting the activation of the nuclear factor-κB. Int J Clin Exp Pathol. 2014;7:2133–2142. [PMC free article] [PubMed] [Google Scholar]
- Yunhe F, Bo L, Xiaosheng F, Fengyang L, Dejie L, Zhicheng L, Depeng L, Yongguo C, Xichen Z, Naisheng Z, Zhengtao Y. The effect of magnolol on the Toll-like receptor 4/nuclear factor κB signaling pathway in lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2012;689:255–261. doi: 10.1016/j.ejphar.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li X, Grailer JJ, Wang N, Wang M, Yao J, Zhong R, Gao GF, Ward PA, Tan DX, Li X. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res. 2016;60:405–414. doi: 10.1111/jpi.12322. [DOI] [PubMed] [Google Scholar]