Abstract
Alterations in sulfur amino acid metabolism are associated with an increased risk of a number of common late-life diseases, which raises the possibility that metabolism of sulfur amino acids may change with age. The present study was conducted to understand the age-related changes in hepatic metabolism of sulfur amino acids in 2-, 6-, 18- and 30-month-old male C57BL/6 mice. For this purpose, metabolite profiling of sulfur amino acids from methionine to taurine or glutathione (GSH) was performed. The levels of sulfur amino acids and their metabolites were not significantly different among 2-, 6- and 18-month-old mice, except for plasma GSH and hepatic homocysteine. Plasma total GSH and hepatic total homocysteine levels were significantly higher in 2-month-old mice than those in the other age groups. In contrast, 30-month-old mice exhibited increased hepatic methionine and cysteine, compared with all other groups, but decreased hepatic S-adenosylmethionine (SAM), S-adenosylhomocysteine and homocysteine, relative to 2-month-old mice. No differences in hepatic reduced GSH, GSH disulfide, or taurine were observed. The hepatic changes in homocysteine and cysteine may be attributed to upregulation of cystathionine β-synthase and down-regulation of γ-glutamylcysteine ligase in the aged mice. The elevation of hepatic cysteine levels may be involved in the maintenance of hepatic GSH levels. The opposite changes of methionine and SAM suggest that the regulatory role of SAM in hepatic sulfur amino acid metabolism may be impaired in 30-month-old mice.
Keywords: Aging, Mice, Sulfur amino acids, Metabolomics
INTRODUCTION
A 42% increase in mean life span and a 44% increase in maximum life span were observed in male F344 rats fed a methionine-restricted diet (0.86 to 0.17% methionine) that was devoid of cysteine (Orentreich et al., 1993). Increased maximal lifespan was also observed in female CB6F1 mice that were fed a methionine-deficient diet (Miller et al., 2005). Methionine restriction resulted in increased blood glutathione (GSH) and decreased hepatic GSH (Richie et al., 1994). Ames dwarf mice, which are deficient in growth hormone, prolactin, and thyroid-stimulating hormone, exhibit an extended lifespan and increased methionine transsulfuration relative to control mice (Uthus and Brown-Borg, 2003, 2006). These findings highlight the role of sulfur amino acids and their metabolites in aging.
Hyperhomocysteinemia may be associated with an increased risk of chronic diseases, including occlusive vascular disease, cognitive decline, senile osteoporosis and presbyopia (Krumdieck and Prince, 2000). In fact, blood homocysteine levels increase with age in humans, which may be associated with lower levels of folate and vitamin B12 (Selhub et al., 1993; Brattström et al., 1994). In contrast, plasma homocysteine levels decrease with age in rats (Giménez and Aguilar, 2003; Andersen et al., 2004; Martins et al., 2005), suggesting the presence of species differences in homocysteine regulation between humans and rats. GSH, a major antioxidant, plays a protective role in the aging process; decreased GSH was associated with the pathogenesis of chronic diseases (Reid and Jahoor, 2001). GSH and the enzymes involved in its synthesis decline with age (Suh et al., 2004). However, information concerning changes in the levels of sulfur amino acids and their metabolites as well as activities of enzymes involved in sulfur amino acid metabolism in mice is limited, particularly over the entire life span.
In mammals, the liver plays a central role in sulfur amino acid metabolism, where almost 50% of methionine metabolism and up to 85% of methylation reactions occur (Stipanuk, 2004). The first step in methionine metabolism is the formation of S-adenosylmethionine (SAM), which is catalyzed by methionine adenosyltransferase I/III (MAT I/III). SAM serves as a methyl donor for biological methylation reactions, and the co-product of transmethylation, S-adenosylhomocysteine (SAH), is hydrolyzed by SAH hydrolase (SAHH) to yield homocysteine that is either competitively remethylated to methionine or condensed into cystathionine with serine (Kim and Kim, 2005). Two enzymes independently mediate remethylation: methionine synthase (MS) and betaine-homocysteine methyltransferase (BHMT). MS is a cobalamin-dependent enzyme that requires methylenetetrahydrofolate reductase (MTHFR) as a folate cycle enzyme to generate the methyl donor, 5-methyltetrahydrofolate, for homocysteine remethylation by MS. BHMT uses betaine as a methyl donor, and is mainly expressed in the liver, although BHMT activity is also observed in the kidney. Transsulfuration of homocysteine to cysteine via cystathionine is mediated by the consecutive actions of cystathionine β-synthase (CβS) and cystathionine γ-lyase. Homocysteine distribution between the two competitive reactions provides a major regulatory locus for the hepatic metabolism of sulfur amino acids (Finkelstein and Martin, 2000). Cysteine is metabolized in the liver to yield either taurine, inorganic sulfate, or GSH. Cysteine dioxygenase (CDO) catalyzes the oxidation of this amino acid to cysteine sulfonate, which is converted mainly to hypotaurine by cysteine sulfinate decarboxylase (CDC) activity. Hypotaurine is non-enzymatically transformed into the end product taurine. GSH synthesis is consecutively mediated by γ-glutamylcysteine ligase (GCL) and GSH synthetase (Stipanuk, 1986).
Changes in sulfur amino acid metabolism can be associated with an increased risk of a number of common late-life diseases, which raises the possibility that metabolism of sulfur amino acid may change with age. The aim of the present study was to investigate the effect of aging on the hepatic metabolism of sulfur-containing amino acids in 2-month-old (young), 6-month-old (adult), 18-month-old (middle-aged), and 30-month-old (aged) male mice. For this purpose, we compared sulfur amino acid metabolite profiles (i.e., methionine to taurine or GSH) in plasma and liver in mice of four ages. Moreover, we examined the hepatic levels and activities of enzymes involved in sulfur amino acid metabolism to determine whether differences in sulfur amino acid metabolic profiles reflected changes in the activities of their metabolizing enzymes.
MATERIALS AND METHODS
Reagents
Chemicals including amino acid standards, dl-homocysteine, l-cysteine, l-methionine, GSH, GSH disulfide (GSSG), SAH, SAM, taurine, hypotaurine, l-serine, cystathionine, betaine hydrochloride, adenosine, homocysteine thiolactone, N-(2-mercaptopropionyl)-glycine (MPG), NAD+, ATP, MgCl2, ethylenediaminetetraacetic acid (EDTA), pyridoxal-5-phosphate, 5-methyl-tetrahydrofolate, hydroxocobalamin, 1-heptanesulfonic acid, trichloroacetic acid (TCA), tris-(2-carboxyethyl)-phosphine hydrochloride (TCEP), ammonium-7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonic acid (SBD-F), 2-mercaptoethanol, dithiothreitol (DTT), chloroform, potassium chloride, hydrochloric acid, sodium acetate, methanol, acetonitrile, tris(hydroxymethyl)aminomethane, ferrous ammonium sulfate, 5,5-dithiobis-2-nitrobenzoic acid (DTNB), hydroxylamine, 2-vinyl pyridine, sodium hydroxide, sodium carbonate, boric acid, bovine serum albumin (BSA), and sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Perchloric acid (PCA), sodium dihydrogen phosphate and disodium hydrogen phosphate were purchased from Junsei Chemical (Tokyo, Japan). Antibodies against MATI/III and MTHFR were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and the anti-SAHH, anti-MS, anti-CDO and anti-β-actin antibodies were purchased from Abcam (Cambridge, UK). The anti-BHMT antibody was purchased from Everest Biotech (Oxfordshire, UK). The anti-GCL catalytic subunit (GCLC) and anti-GCL modifier subunit (GCLM) antibodies were purchased from Neo-Marker Inc. (Fremont, CA). The anti-CβS antibody was kindly provided by Dr. Matherly (Wayne State University School of Medicine, Detroit, MI, USA). Horseradish peroxidase-conjugated goat anti-rabbit and rabbit anti-goat antibodies were purchased from BioRad Laboratories (Hercules, CA, USA). Enhanced chemiluminescence detection reagent solutions were purchased from Thermo Scientific (Rockford, IL, USA). All chemicals and solvents used in this study were reagent grade or higher.
Animal experiments
This study used male C57BL/6 mice, and all animals were maintained at the Korea Research Institute of Bioscience and Biotechnology (Ochang, Korea). Mice aged 2-, 6-, 18-, or 30-months-old were classified as young, adult, middle-aged, and aged, respectively (Yang et al., 2013), and each group consisted of five mice. The mice were housed in 22 ± 2°C and 50 ± 5% humidity controlled rooms with a 12-h light/dark cycle for at least 1 week prior to experimentation. Food and tap water were provided ad libitum. Mice were fed a 2018S Teklad global 18% protein rodent diet that contained the following percentages of calories 18.6% protein, 44.2% carbohydrate and 6.2% fat. Methionine and cysteine content in the diet was approximately 0.6 and 0.3%, respectively. The mice were euthanized using CO2. No animals used in this study died before they were euthanized. All animal experiments were approved by the Institutional Animal Care and Use Committee, and were performed in accordance with institutional guidelines.
Preparation of plasma and hepatic samples
Blood samples were obtained by heart puncture immediately after euthanasia from 9 to 10 am to minimize variations due to the circadian cycle. The standard protocol entailed euthanizing the mice by CO2 inhalation followed by maximal collection (approximately 1 mL) by cardiocentesis with a 1-mL syringe and a 25-gauge, 5/8-in. tuberculin needle. Plasma was obtained by centrifugation of the blood at 10,000×g for 15 min at 4°C. The plasma was stored at −70°C until analysis. The liver was removed rapidly and homogenized in a three-fold volume of ice-cold buffer consisting of 0.154 M KCl, 50 mM Tris-HCl, and 1 mM EDTA (pH 7.4). All subsequent steps were performed at 0–4°C. The liver homogenates were deproteinized in a three-fold volume of ice-cold methanol to measure methionine, hypotaurine, and taurine, or in an equal volume of 10% PCA to measure SAM, SAH, homocysteine, cysteine, GSH, GSSG, putrescine, spermidine, and spermine. After centrifugation at 10,000×g for 20 min, the supernatant fraction was collected and stored at −70°C.
For enzyme assay and immunoblot analysis, the liver homogenates were centrifuged at 10,000×g for 20 min. The supernatant was further centrifuged at 104,000×g for 65 min. The supernatant fraction was collected and refrigerated in −70°C (cytosol samples). Total protein concentration was measured using a bicinchoninic acid protein assay kit (Thermo Scientific).
Determination of sulfur amino acid content and their metabolites in plasma and tissues
The amount of methionine, hepatic SAM and SAH, total plasma and liver homocysteine, GSH, cysteine, hypotaurine, taurine, polyamines were determined according to our previous methods (Kwak et al., 2015).
Immunoblot analyses
The expression of β-actin, MAT I/III, SAHH, BHMT, MS, MTHFR, CβS, CDO, GCLC and GCLM, the cytosol samples were determined according to our previous methods (Kwak et al., 2015). The antibodies were diluted with PBS-T containing 3–5% BSA: β-actin (1:2,000), MATI/III (1:3,000), SAHH (1:500), BHMT (1:5,000), MS (1:200), MTHFR (1:200), CβS (1:2,000), CDO (1:1,000), GCLC (1:3,000) and GCLM (1:2,000).
Enzyme assays
Activities of MAT, SAHH, BHMT, MS, CβS, CDO and GCL were determined according to our previous methods (Kim et al., 2003; Kwak et al., 2015).
Statistical analyses
Differences between groups were determined using analysis of variance followed by the Newman-Keuls multiple range test (p<0.05). All data are presented as mean ± standard deviation for five samples. Linear regression was calculated using GraphPad Prism, ver. 4.0 software (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Body weight and liver weight
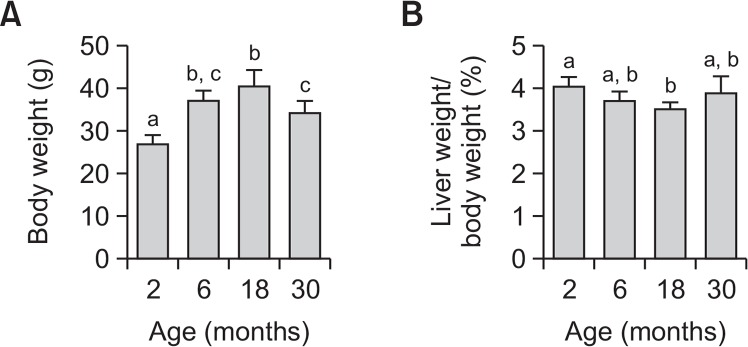
Body weight increased with age, peaking in 18-monthold mice, and subsequently decreasing in 30-month-old mice (Fig. 1A). Body weight of 2-month-old mice was significantly different from all other groups, and body weight of 30-month-old mice decreased significantly, relative to that of 18-month-old mice. Liver weight per body weight was the lowest in 18-month-old mice (Fig. 1B). The relative liver weight of 18-month-old-mice was significantly different from that of 2-month-old mice. These results are consistent with previous findings (Anisimov et al., 2010).
Fig. 1.
Changes in body weight and liver weight per body weight with increasing age in mice. Each value represents mean ± SD for five mice. Comparisons among the four different age groups were made using a one-way ANOVA followed by Newman-Keuls multiple range test. Values with different letters are significantly different from each other, p<0.05.
Sulfur amino acid concentrations in plasma
Cysteine, homocysteine, GSH, and methionine concentrations were determined in plasma collected from mice at 2, 6, 18, or 30 months of age (Table 1). There were no significant differences in plasma total cysteine or homocysteine levels in mice. Plasma total GSH levels decreased with age, and thus the GSH levels in 30-month-old mice decreased to 45% relative to that in 2-month-old mice. There were no significant differences in plasma methionine levels in mice.
Table 1.
Age-related changes in cysteine, homocysteine, GSH and methionine in plasma
| Age (months) | 2 | 6 | 18 | 30 |
|---|---|---|---|---|
| Cysteine (μM) | 162 ± 19 | 155 ± 17 | 156 ± 22 | 200 ± 96 |
| Homocysteine (μM) | 1.47 ± 0.33 | 1.21 ± 0.22 | 1.31 ± 0.31 | 1.67 ± 0.88 |
| GSH (μM) | 56.0 ± 12.8a | 45.0 ± 7.1b | 31.4 ± 11.5b | 25.4 ± 8.8b |
| Methionine (μM) | 62.3 ± 8.3 | 53.4 ± 6.6 | 48.9 ± 5.6 | 69.5 ± 22.7 |
GSH, glutathione.
Each value represents the mean ± SD for five mice. Comparisons among the four different age groups were made using a one-way ANOVA followed by Newman-Keuls multiple range test. Values with different letters are significantly different from each other, p<0.05.
Sulfur amino acid concentrations in liver
Hepatic concentrations of sulfur amino acids and their metabolites were monitored with increasing age in mice (Table 2). Hepatic methionine and cysteine levels were increased in 30-month-old mice relative to 2-, 6-, and 18-month-old mice. In contrast, hepatic total homocysteine levels were decreased in 6-, 18-, and 30-month-old mice relative to those in 2-month-old mice. There were no significant differences in hepatic total GSH, reduced GSH, GSSG levels or GSH/GSSG ratio. Hepatic SAM and SAH levels decreased with age, and thus the SAM/SAH ratio, which serves as an index of transmethylation potential, did not significantly change with age. Hepatic hypotaurine levels in 6-, 18-, or 30-month-old mice were more than twofold those observed in 2-month-old mice, and hepatic taurine levels in 6-, 18- and 30-month-old mice were approximately 52, 165, and 143%, respectively, relative to those in 2-month-old mice, despite the lack of significant differences.
Table 2.
Age-related changes in sulfur amino acids and their metabolites in liver
| Age (months) | 2 | 6 | 18 | 30 |
|---|---|---|---|---|
| Methionine (nmol/g liver) | 40.6 ± 2.3a | 38.7 ± 3.6a | 35.7 ± 4.0a | 49.4 ± 4.7b |
| Cysteine (nmol/g liver) | 120 ± 17a | 138 ± 18a | 119 ± 23a | 189 ± 29b |
| Homocysteine (nmol/g liver) | 7.19 ± 1.24a | 4.89 ± 1.26b | 5.36 ± 1.07b | 3.54 ± 0.95b |
| Total GSH (μmol/g liver) | 5.99 ± 1.26 | 6.71 ± 0.79 | 5.96 ± 0.73 | 5.36 ± 1.10 |
| Reduced GSH (μmol/g liver) | 5.63 ± 1.14 | 6.41 ± 0.78 | 5.63 ± 0.74 | 5.04 ± 1.13 |
| GSSG (nmol/g liver) | 183 ± 86 | 150 ± 30 | 164 ± 42 | 159 ± 41 |
| GSH/GSSG | 36.3 ± 11.7 | 46.2 ± 10.9 | 38.8 ± 12.8 | 26.3 ± 11.2 |
| SAH (nmol/g liver) | 44.8 ± 9.5a | 38.2 ± 6.3a,b | 35.4 ± 9.2a,b | 26.3 ± 11.2b |
| SAM (nmol/g liver) | 182 ± 36a | 196 ± 56a | 130 ± 61a,b | 85 ± 39b |
| SAM/SAH | 4.33 ± 1.61 | 5.41 ± 2.11 | 3.64 ± 1.87 | 4.31 ± 3.47 |
| Taurine (μmol/g liver) | 4.49 ± 3.23 | 2.35 ± 2.18 | 7.43 ± 3.61 | 6.42 ± 4.05 |
| Hypotaurine (nmol/g liver) | 87 ± 35 | 199 ± 91 | 211 ± 101 | 213 ± 223 |
| Spermidine (nmol/g liver) | 770 ± 117 | 577 ± 75 | 629 ± 93 | 697 ± 205 |
| Spermine (nmol/g liver) | 758 ± 51 | 724 ± 83 | 693 ± 80 | 689 ± 138 |
GSH, glutathione; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; GSSG, glutathione disulfide.
Each value represents the mean ± SD for five mice. Comparisons among the four different age groups were made using a one-way ANOVA followed by Newman-Keuls multiple range test. Values with different letters are significantly different from each other, p<0.05.
SAM is a source of the propylamine group for synthesis of spermidine and spermine, following its decarboxylation by SAM decarboxylase (Grillo, 1985). Hepatic spermidine and spermine levels were determined to test whether polyamine synthesis may be associated with the decrease in hepatic SAM levels. No differences in hepatic spermidine or spermine levels were detected in mice.
Immunoblot analysis of sulfur amino acid metabolizing enzymes
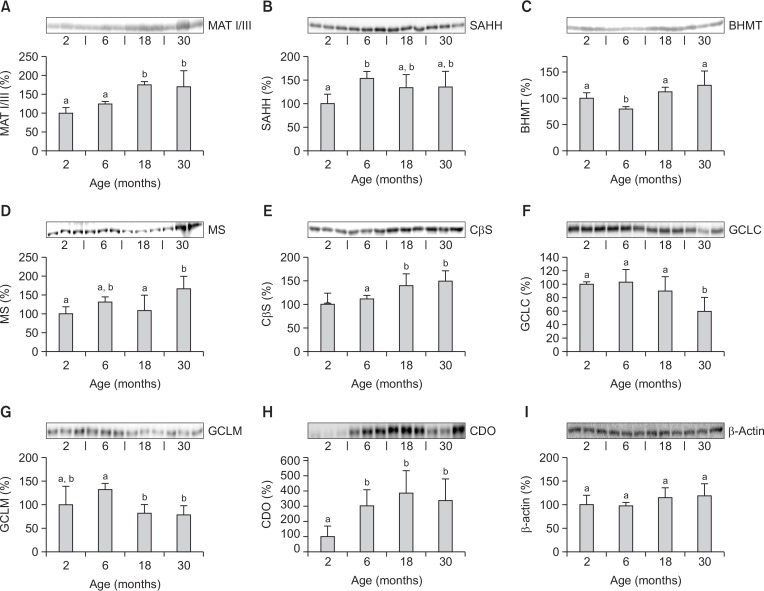
The relative level of hepatic enzymes involved in sulfur amino acid metabolism was determined using immunoblot analysis with specific antibodies in mice aged 2, 6, 18, and 30 months (Fig. 2). MAT I/III and CβS were increased in 18- and 30-month-old mice relative to 2- and 6-month-old mice (Fig. 2A, 2E). SAHH was higher in 6-month-old mice than in 2-month-old mice (Fig. 2B), while BHMT was minimal in 6-month-old mice (Fig. 2C). MS was increased in 30-month-old mice relative to 2- and 18-month-old mice (Fig. 2D). GCLC and GCLM levels were lower in 30-month-old mice than in 6-month-old mice level (Fig. 2F, 2G). CDO levels were markedly lower in 2-month-old mice (Fig. 2H). No age-related differences were observed in hepatic β-actin levels used as a loading control (Fig. 2I).
Fig. 2.
Changes in hepatic levels of enzymes involved in sulfur amino acid metabolism with increasing age in mice by immunoblot analysis. Values are percentages of the hepatic level in 2-month-old mice. Each value represents the mean ± SD for five mice. Comparisons among the four different age groups were made using a one-way ANOVA followed by Newman-Keuls multiple range test. Values with different letters are significantly different from each other, p<0.05. MAT I/III, methionine adenosyltransferase I/III; SAHH, Sadenosylhomocysteine hydrolase; BHMT, betaine homocysteine methyltransferase; MS, methionine synthase; CβS, cystathionine β-synthase; GCLC, γ-glutamylcysteine ligase catalytic subunit; GCLM, γ-glutamylcysteine ligase modifier subunit; CDO, cysteine dioxygenase.
Activities of sulfur amino acid metabolizing enzymes
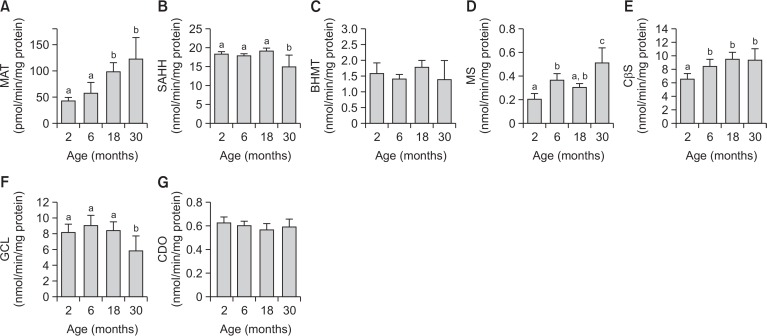
The activities of hepatic enzymes involved in sulfur amino acid metabolism are regulated by post-translational modification and allosteric regulation (Mato and Lu, 2007). Thus, the activities of MAT, SAHH, MS, BHMT, CβS, GCL and CDO were measured using a HPLC system equipped with a fluorescence detector, an ultraviolet detector, or a tandem mass spectrometry (MS/MS) system (Fig. 3). MAT, MS, CβS and GCL activity patterns were similar to their immunoblot assay results (Fig. 3A, 3D, 3E, 3F). SAHH activity was decreased in 30-month-old mice relative to 2-, 6-, and 18-month-old mice (Fig. 3B). There were no significant differences in BHMT or CDO activity (Fig. 3C, 3G).
Fig. 3.
Changes in hepatic activities of sulfur amino acid-metabolizing enzymes with increasing age in mice. Each value represents the mean ± SD for five mice. Comparisons among the four different age groups were made using a one-way ANOVA followed by Newman-Keuls multiple range test. Values with different letters are significantly different from each other, p<0.05. MAT, methionine adenosyltransferase; SAHH, Sadenosylhomocysteine hydrolase; BHMT, betaine homocysteine methyltransferase; MS, methionine synthase; CβS, cystathionine β-synthase; GCL, γ-glutamylcysteine ligase; CDO, cysteine dioxygenase.
DISCUSSION
The present study demonstrated sulfur amino acid metabolism changes with increasing age in mice. With the exception of plasma GSH and hepatic homocysteine, the levels of the other sulfur amino acids examined and their metabolites in plasma and liver did not differ significantly among 2-, 6- and 18-month-old mice. The plasma GSH level decreased with age, and the hepatic total homocysteine level was significantly higher in 2-month-old mice than in older mice. In contrast, we observed changes in hepatic methionine, cysteine, SAM and SAH in 30-month-old mice, suggesting that alterations in hepatic sulfur amino acid metabolism may occur in aged mice. Neither GSH nor taurine level was significantly different in liver, although hepatic taurine levels were 3.1- and 2.7-fold in 18- and 30-month-old mice, respectively, relative to 6-month-old mice.
There were no significant differences in plasma total cysteine or homocysteine levels with increasing age in mice, but plasma total GSH level decreased with age. Decrease in plasma GSH was observed in aged humans (Yang et al., 1995), as well as in experimental animals, including monkeys (Paredes et al., 2014) and mice (Iciek et al., 2004). In contrast, age-related changes in hepatic GSH levels are controversial. In 24-month-old Fisher 344 rats, hepatic GSH levels were decreased by GCL down-regulation (Liu and Choi, 2000). Moreover, nuclear factor erythroid 2-related factor 2-mediated activation of antioxidant enzyme expression, including GCL, was reduced in rats greater than 24 months of age (Suh et al., 2004). In contrast, hepatic GSH was similar among male rats at 4–5, 14–15, or 24–25 months of age (Rikans and Kosanke, 1984). These results are supported by findings from 2- and 27-month-old Wistar rats (Barja de et al., 1990) and from 5-week-old to 30-month-old Fisher rats (Nakata et al., 1996). In this study, hepatic total GSH, reduced GSH, GSSG and GSH/GSSG did not differ significantly among the groups. These results are consistent with previous findings using 3-, 12-, 24-, and 30- or 31-month-old C57BL/6 mice (Chen et al., 2000), the same strain of mice used in this study. The reason for this discrepancy in the effect of aging on hepatic GSH levels remains unknown, but the rate of de novo GSH synthesis is controlled by cysteine availability as well as GCL activity. Elevated hepatic cysteine levels may be associated with the maintenance of hepatic GSH levels despite decreased GCL activity.
In the present study, GSH levels in plasma, but not liver, decreased with age. Plasma GSH levels are controlled by hepatic GSH efflux regulation and GSH utilization in extra-hepatic tissues (Lu et al., 1990). The hepatic efflux of GSH is the source of almost 85% of the GSH plasma concentration in the rat (Kaplowitz et al., 1985). Plasma GSH is used as a continuous source of cysteine in extrahepatic tissues via the gamma-glutamyl cycle (Lu, 2013). Thus, plasma GSH levels can be decreased by both or either of the following: an increase in extra-hepatic utilization of plasma GSH and/or a decrease in hepatic efflux of GSH into plasma. Interestingly, serum gamma-glutamyltranspeptidase activity increased with age in humans (Strømme et al., 2004), suggesting that plasma GSH can be decreased by aging via a tissue-independent mechanism.
Cysteine and methionine increased in the livers of 30-month-old mice, compared with those in all other groups, but total homocysteine, SAH and SAM decreased, relative to those in 2-month-old mice. Decreased hepatic homocysteine and increased hepatic cysteine may be attributable, in part, to induction of CβS, which produces cysteine from homocysteine via cystathionine, and inhibition of GCL, which utilizes cysteine to produce GSH via gamma-glutamylcysteine. Previous studies have shown a direct repressing effect of insulin on CβS in human and rat hepatocytes (Ratnam et al., 2002), and upregulation of hepatic CβS in streptozotocin-induced diabetes (Jacobs et al., 1998). These results warrant further investigation of the role of insulin production and/or insulin signaling on hepatic sulfur amino acid metabolism in aged animals. Despite the increase in methionine, a SAM precursor, and MAT I/III up-regulation, a SAM synthesizing enzyme, hepatic SAM levels were markedly decreased in 30-month-old mice. SAM serves as a methyl donor for methylation reactions and is a source for the propylamine group in polyamine synthesis. Hepatic concentrations of the polyamines including spermidine and spermine were not different among 2-, 6-, 18- and 30-month-old mice. Thus, the underlying mechanism for SAM regulation in 30-month-old mice remains to be determined.
Hepatic sulfur amino acid metabolism plays a major role in the maintenance of methionine concentration. Hepatic SAM serves as a regulator of hepatic sulfur amino acid metabolism. SAM exerts feedback activation on MAT (Cabrero et al., 1987). SAM is also an allosteric activator of CβS and an allosteric inhibitor of MTHFR (Brosnan and Brosnan, 2006). Moreover, SAM inactivates BHMT in rat liver (Finkelstein and Martin, 1986) and down-regulates BHMT expression in HepG2 cells (Ou et al., 2007). Elevated hepatic SAM can accelerate the consumption of excessive methionine to produce cysteine via increased homocysteine transsulfuration and decreased homocysteine remethylation. Thus, these previous findings in conjunction with the present results indicate that the regulation of hepatic sulfur amino acid metabolism is impaired in 30-month-old mice, although the mechanism underlying the decreased hepatic SAM level remains to be determined.
High methionine and cysteine concentrations can be quite toxic (Benevenga and Steele, 1984). Excessive methionine leads to growth retardation and histopathological changes in the liver, kidney, and spleen (Harper et al., 1970). In addition, mice fed methionine-rich diets had significant atheromatous pathology in the aortic arch, even with normal plasma homocysteine levels (Troen et al., 2003). Moreover, hyperhomocysteinemia can be the result of high methionine diets up to 12–20 g/kg. In fact, animals with hyperhomocysteinemia induced by a high-methionine diet develop vascular diseases, neurological disorders, and aortic diseases (Dayal and Lentz, 2008). The potent toxicity of excess cysteine was also demonstrated in humans as well as in experimental animals (Baker, 2006; Stipanuk et al., 2006). Cysteine undergoes auto-oxidation in the presence of iron, resulting in reactive oxygen species, and consequently leads to oxidative damage (Iyamu et al., 2008). Chronic excessive cysteine exposure is closely associated with an increased risk of a number of common diseases, including rheumatoid arthritis, Parkinson’s disease, Alzheimer’s disease, and increased risk of cardiovascular disease (Heafield et al., 1990; Mills et al., 2000). Thus, excessive hepatic methionine and cysteine can lead to toxic reactions in aged mice, exhibiting impaired hepatic sulfur amino acid metabolism.
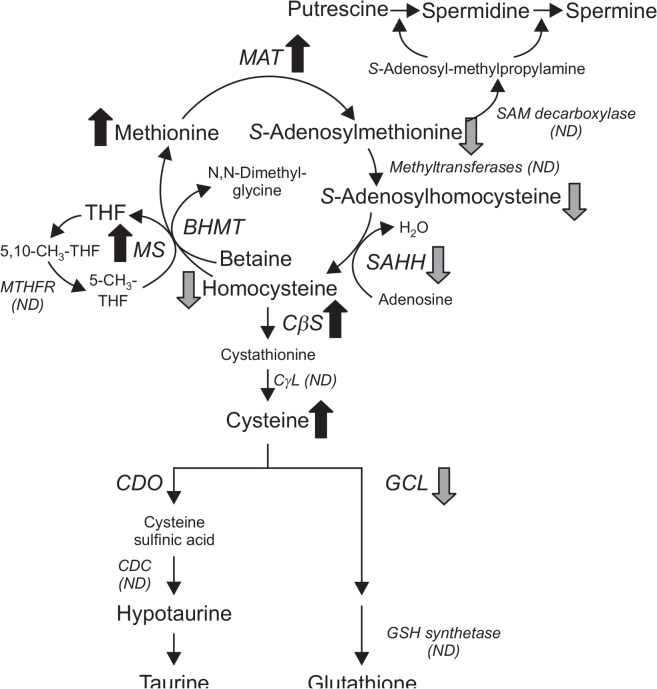
In conclusion, a simplified scheme of hepatic sulfur amino acid metabolism and changes in sulfur amino acids and their metabolites in 30-month-old mice is illustrated in Fig. 4. The 30-month-old mice exhibited alterations in hepatic sulfur amino acid metabolism, such as elevated hepatic cysteine and methionine, and decreased SAM, SAH, and total homocysteine. Changes in liver homocysteine and cysteine may be attributable to up-regulation of CβS and down-regulation of GCL. Despite the increase in methionine and up-regulation of MAT I/III, hepatic SAM levels were markedly decreased in 30-month-old mice. Opposing changes between methionine and SAM in liver raise the possibility that the regulatory role of SAM in hepatic sulfur amino acid metabolism may be impaired in 30-month-old mice. These results provide a rationale for an increased life span in animals fed a methionine-restricted diet.
Fig. 4.
Differences in hepatic sulfur amino acid metabolism between 2- and 30-month-old mice. MAT, methionine adenosyltransferase; SAHH, S-adenosylhomocysteine hydrolase; BHMT, betaine homocysteine methyltransferase; MS, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; CβS, cystathionine β-synthase; CγL, cystathionine γ-lyase; CDO, cysteine dioxygenase; CDC, cysteine sulfinic acid decarboxylase; GCL, γ-glutamylcysteine ligase; THF, tetrahydrofolate; ND, not determined. Arrows represent situation in 30-month-old mice relative to 2-month-old mice.
Acknowledgments
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (2016R1A2B4008382).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Andersen ML, Martins PJ, D’Almeida V, Santos RF, Bignotto M, Tufik S. Effects of paradoxical sleep deprivation on blood parameters associated with cardiovascular risk in aged rats. Exp Gerontol. 2004;39:817–824. doi: 10.1016/j.exger.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, Poroshina TE, Berstein LM. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2010;2:945–958. doi: 10.18632/aging.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DH. Comparative species utilization and toxicity of sulfur amino acids. J Nutr. 2006;136:1670S–1675S. doi: 10.1093/jn/136.6.1670S. [DOI] [PubMed] [Google Scholar]
- Barja de QG, Pérez-Campo R, López TM. Anti-oxidant defences and peroxidation in liver and brain of aged rats. Biochem J. 1990;272:247–250. doi: 10.1042/bj2720247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevenga NJ, Steele RD. Adverse effects of excessive consumption of amino acids. Annu Rev Nutr. 1984;4:157–181. doi: 10.1146/annurev.nu.04.070184.001105. [DOI] [PubMed] [Google Scholar]
- Brattström L, Lindgren A, Israelsson B, Andersson A, Hultberg B. Homocysteine and cysteine: determinants of plasma levels in middle-aged and elderly subjects. J Intern Med. 1994;236:633–641. doi: 10.1111/j.1365-2796.1994.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- Cabrero C, Puerta J, Alemany S. Purification and comparison of two forms of S-adenosyl-L-methionine synthetase from rat liver. Eur J Biochem. 1987;170:299–304. doi: 10.1111/j.1432-1033.1987.tb13699.x. [DOI] [PubMed] [Google Scholar]
- Chen TS, Richie JP, Nagasawa HT, Lang CA. Glutathione monoethyl ester protects against glutathione deficiencies due to aging and acetaminophen in mice. Mech Ageing Dev. 2000;120:127–139. doi: 10.1016/S0047-6374(00)00214-1. [DOI] [PubMed] [Google Scholar]
- Dayal S, Lentz SR. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler Thromb Vasc Biol. 2008;28:1596–1605. doi: 10.1161/ATVBAHA.108.166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Adaptation to methionine excess. J Biol Chem. 1986;261:1582–1587. [PubMed] [Google Scholar]
- Finkelstein JD, Martin JJ. Homocysteine. Int J Biochem Cell Biol. 2000;32:385–389. doi: 10.1016/S1357-2725(99)00138-7. [DOI] [PubMed] [Google Scholar]
- Giménez R, Aguilar J. Effects of cytidine 5′-diphosphocholine on plasma homocysteine levels in rat. Comp Biochem Physiol B, Biochem Mol Biol. 2003;134:271–276. doi: 10.1016/S1096-4959(02)00258-0. [DOI] [PubMed] [Google Scholar]
- Grillo MA. Metabolism and function of polyamines. Int J Biochem. 1985;17:943–948. doi: 10.1016/0020-711X(85)90238-1. [DOI] [PubMed] [Google Scholar]
- Harper AE, Benevenga NJ, Wohlhueter RM. Effects of ingestion of disproportionate amounts of amino acids. Physiol Rev. 1970;50:428–558. doi: 10.1152/physrev.1970.50.3.428. [DOI] [PubMed] [Google Scholar]
- Heafield MT, Fearn S, Steventon GB, Waring RH, Williams AC, Sturman SG. Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson’s and Alzheimer’s disease. Neurosci Lett. 1990;110:216–220. doi: 10.1016/0304-3940(90)90814-P. [DOI] [PubMed] [Google Scholar]
- Iciek M, Chwatko G, Lorenc-Koci E, Bald E, Włodek L. Plasma levels of total, free and protein bound thiols as well as sulfane sulfur in different age groups of rats. Acta Biochim Pol. 2004;51:815–824. [PubMed] [Google Scholar]
- Iyamu EW, Perdew H, Woods GM. Cysteine-iron promotes arginase activity by driving the Fenton reaction. Biochem Biophys Res Commun. 2008;376:116–120. doi: 10.1016/j.bbrc.2008.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RL, House JD, Brosnan ME, Brosnan JT. Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes. 1998;47:1967–1970. doi: 10.2337/diabetes.47.12.1967. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N, Aw TY, Ookhtens M. The regulation of hepatic glutathione. Ann Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- Kim SK, Kim YC. Effects of betaine supplementation on hepatic metabolism of sulfur-containing amino acids in mice. J Hepatol. 2005;42:907–913. doi: 10.1016/j.jhep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Kim SK, Seo JM, Jung YS, Kwak HE, Kim YC. Alterations in hepatic metabolism of sulfur-containing amino acids induced by ethanol in rats. Amino Acids. 2003;24:103–110. doi: 10.1007/s00726-002-0324-6. [DOI] [PubMed] [Google Scholar]
- Krumdieck CL, Prince CW. Mechanisms of homocysteine toxicity on connective tissues: implications for the morbidity of aging. J Nutr. 2000;130:365S–368S. doi: 10.1093/jn/130.2.365S. [DOI] [PubMed] [Google Scholar]
- Kwak HK, Kim YM, Oh SJ, Kim SK. Sulfur amino acid metabolism in Zucker diabetic fatty rats. Biochem Pharmacol. 2015;96:256–266. doi: 10.1016/j.bcp.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Liu R, Choi J. Age-associated decline in γ-glutamylcysteine synthetase gene expression in rats. Free Radic Biol Med. 2000;28:566–574. doi: 10.1016/S0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- Lu SC, Garcia-Ruiz C, Kuhlenkamp J, Ookhtens M, Salas-Prato M, Kaplowitz N. Hormonal regulation of glutathione efflux. J Biol Chem. 1990;265:16088–16095. [PubMed] [Google Scholar]
- Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins PJ, Galdieri LC, Souza FG, Andersen ML, Benedito-Silva AA, Tufik S, D’Almeida V. Physiological variation in plasma total homocysteine concentrations in rats. Life Sci. 2005;76:2621–2629. doi: 10.1016/j.lfs.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Mato JM, Lu SC. Role of S-adenosyl-L-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- Mills BJ, Weiss MM, Lang CA, Liu MC, Ziegler C. Blood glutathione and cysteine changes in cardiovascular disease. J Lab Clin Med. 2000;135:396–401. doi: 10.1067/mlc.2000.105976. [DOI] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Kawase M, Ogino S, Kinoshita C, Murata H, Sakaue T, Ogata K, Ohmori S. Effects of age on levels of cysteine, glutathione and related enzyme activities in livers of mice and rats and an attempt to replenish hepatic glutathione level of mouse with cysteine derivatives. Mech Ageing Dev. 1996;90:195–207. doi: 10.1016/0047-6374(96)01771-X. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Ou X, Yang H, Ramani K, Ara AI, Chen H, Mato JM, Lu SC. Inhibition of human betaine-homocysteine methyltransferase expression by S-adenosylmethionine and methylthio-adenosine. Biochem J. 2007;401:87–96. doi: 10.1042/BJ20061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes J, Jones DP, Wilson ME, Herndon JG. Age-related alterations of plasma glutathione and oxidation of redox potentials in chimpanzee (Pan troglodytes) and rhesus monkey (Macaca mulatta) Age (Dordr) 2014;36:719–732. doi: 10.1007/s11357-014-9615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam S, Maclean KN, Jacobs RL, Brosnan ME, Kraus JP, Brosnan JT. Hormonal regulation of cystathionine β-synthase expression in liver. J Biol Chem. 2002;277:42912–42918. doi: 10.1074/jbc.M206588200. [DOI] [PubMed] [Google Scholar]
- Reid M, Jahoor F. Glutathione in disease. Curr Opin Clin Nutr Metab Care. 2001;4:65–71. doi: 10.1097/00075197-200101000-00012. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Rikans LE, Kosanke SD. Effect of aging on liver glutathione levels and hepatocellular injury from carbon tetrachloride, allyl alcohol or galactosamine. Drug Chem Toxicol. 1984;7:595–604. doi: 10.3109/01480548409042822. [DOI] [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. 1993;270:2693–2698. doi: 10.1001/jama.1993.03510220049033. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH. Metabolism of sulfur-containing amino acids. Annu Rev Nutr. 1986;6:179–209. doi: 10.1146/annurev.nu.06.070186.001143. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Dominy JE, Jr, Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr. 2006;136:1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- Strømme JH, Rustad P, Steensland H, Theodorsen L, Urdal P. Reference intervals for eight enzymes in blood of adult females and males measured in accordance with the International Federation of Clinical Chemistry reference system at 37 degrees C: part of the Nordic Reference Interval Project. Scand J Clin Lab Invest. 2004;64:371–384. doi: 10.1080/00365510410002742. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci USA. 2003;100:15089–15094. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Altered methionine metabolism in long living Ames dwarf mice. Exp Gerontol. 2003;38:491–498. doi: 10.1016/S0531-5565(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech Ageing Dev. 2006;127:444–450. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MJ, Sim S, Jeon JH, Jeong E, Kim HC, Park YH, Kim IB. Mitral and tufted cells are potential cellular targets of nitration in the olfactory bulb of aged mice. PLoS ONE. 2013;8:e59673. doi: 10.1371/journal.pone.0059673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Chou ST, Liu L, Tsai PJ, Kuo JS. Effect of ageing on human plasma glutathione concentrations as determined by high-performance liquid chromatography with fluorimetric detection. J Chromatogr B, Biomed Appl. 1995;674:23–30. doi: 10.1016/0378-4347(95)00287-8. [DOI] [PubMed] [Google Scholar]