Abstract
Carbon monoxide (CO) is well-known as toxic gas and intrinsic signaling molecule such as neurotransmitter and blood vessel relaxant. Recently, it has been reported that low concentration of CO exerts therapeutic actions under various pathological conditions including liver failure, heart failure, gastric cancer, and cardiac arrest. However, little has been known about the effect of CO in neurodegenerative diseases like Parkinson’s disease (PD). To test whether CO could exert a beneficial action during oxidative cell death in PD, we examined the effects of CO on 6-hydroxydopamine (6-OHDA)-induced cell death in C6 glioma cells. Treatment of CO-releasing molecule-2 (CORM-2) significantly attenuated 6-OHDA-induced apoptotic cell death in a dose-dependent manner. CORM-2 treatment decreased Bax/Bcl2 ratio and caspase-3 activity, which had been increased by 6-OHDA. CORM-2 increased phosphorylation of NF-E2-related factor 2 (Nrf2) which is a transcription factor regulating antioxidant proteins. Subsequently, CORM-2 also increased the expression of heme oxygenase-1 and superoxide dismutases (CuZnSOD and MnSOD), which were antioxidant enzymes regulated by Nrf2. These results suggest that CO released by CORM-2 treatment may have protective effects against oxidative cell death in PD through the potentiation of cellular adaptive survival responses via activation of Nrf2 and upregulation of heme oxygenase-1, leading to increasing antioxidant defense capacity.
Keywords: CO, PD, Neuroprotection, Nrf2, HO-1, SOD
INTRODUCTION
Parkinson’s disease (PD) is one of neurodegenerative diseases with selective death of dopaminergic neurons in the substantia nigra of the brain, leading to the movement disorder (Kalia and Lang, 2016). Oxidative stress has been implicated in the pathogenesis of PD by exposure of free radical or other reactive species and defect of antioxidant defense mechanisms (Kikuchi et al., 2002; Halliwell, 2006). To develop in vitro or in vivo models of PD, the oxidative stress can be induced by 6-hydroxydopamine (6-OHDA) which destroys dopaminergic neurons through free radical-mediated mechanisms (Shiraga et al., 1993). Thus, the 6-OHDA model has been widely used for replicating a PD-like loss of dopaminergic neurons (Blandini et al., 2008).
Carbon monoxide (CO) is widely known as a virulent gas. CO poisoning causes various toxic symptoms such as nausea, vomiting, dizziness, fatigue and oxygen deficiency (Pietrus et al., 2015). It has been also reported that CO is endogenously generated in a mammalian cell by activity of heme oxygenase (HO) enzyme (Tenhunen et al., 1968). The past decade has witnessed an increase in research into the role of CO as neurotransmitter modulating inflammatory responses in the body (Verma et al., 1993; Herman, 1997; McCoole et al., 2012; Christie et al., 2014). Converging lines of evidence revealed that low dose of CO exhibits beneficial effects in an array of pathophysiological conditions (Choi, 2017). Carbon monoxide releasing molecules (CORMs) is widely used as a CO donor to perform the CO-related studies. CORM-2 that was used in this study is a lipid-soluble metal carbonyl complex tricarbonyldichlororuthenium (II) dimer ([Ru(CO)3Cl2]2). This synthetic metal carbonyl complexes can release controlled amounts of CO to cell and tissues and be developed as a promising therapeutic agents.
A variety of therapeutic studies about administration of CO has been reported to be beneficial in bacterial infection, cancer, stroke, erectile dysfunction, cardiac arrest, and transplant. Most recently, co-treatment of hydrogen sulfide and carbon monoxide protects gastric mucosa against alendronate compromised by mild stress (Magierowski et al., 2016) and CO significantly reduces endothelial cell proliferation in the gastric cancer cells (Lian et al., 2016). Interestingly, CO sensitizes cancer cells, not normal cells, to the genotoxin doxorubicin (Suliman et al., 2007; Kim et al., 2009) and spares normal cells compared by cancer cell in the cancer-laden tissue (Wegiel et al., 2013). In another studies, CO and HO-1 prevented from intestinal inflammation by promoting bacterial clearance (Onyiah et al., 2013) and attenuated aeroallergen-induced inflammation in mice (Chapman et al., 2001). CO also prevents ischemia-reperfusion injury during kidney transplantation (Caumartin et al., 2011). CO protects cardiac mitochondrial function by decreasing the production of reactive oxygen species in a rat model of cardiac arrest (Yao et al., 2015). Although accumulating evidence revealed the potential therapeutic effects of CO under the various pathological conditions, the role of CO in the neurodegenerative diseases like PD has not been elucidated yet. In this study, we examined the effects of CO on 6-OHDA-induced cell death in C6 glioma cells. The results of this study will shed an insight on whether CO could be applied as therapeutic agents for the treatments and/ or prevention of PD.
MATERIALS AND METHODS
Materials
Tricarbonyldichlororuthenuim (II) dimer (CORM-2), 6-hydroxydopamine hydrochloride, MTT[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], anti-actin antibody and other chemicals were provided from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Hyclone (GE Healthcare life sciences, Logan, Utah, USA). Fetal bovine serum (FBS) and penicillin-streptomycin antibiotic were purchased from Gibco BRL (Grand Island, NY, USA). Anti-bodies against Bcl-2, Bax, capase-3, NF-E2-related factor 2 (Nrf2), manganese superoxide dismutase (MnSOD) and copper-zinc superoxide dismutase (CuZnSOD) were provided from Santa cruz Biotechnology (Santa Cruz, CA, USA). Anti-heme oxygenase-1 (HO-1) antibody was supplied by Enzo life sciences (Farmingdale, NY, USA) and Anti-phospho Nrf2 antibody was supplied by Abcam (Cambridge, UK). Anti-cleaved caspase-3 was purchased from Cell signaling (Danvers, MA, USA).
Cell culture
C6 glioma cells were cultured in DMEM supplement with 10% FBS, penicillin (100 U/ml) and streptomycin (100 U/ml). Cells were incubated at 37°C in a humidified 5% CO2 incubator and sub-cultured at appropriate density for each experiment.
Cell viability assay
Cell viability was analyzed using thiazolyl blue tetrazolium bromide (MTT) reduction assays. Cellular density was 1.0× 105 cells/200 μl in 48-well plates. After cells were treated with 6-OHDA and CORM-2, MTT solution was added and further incubated for 3 h. Then dimethyl sulfoxide was added to solubilize the formazan products formed by viable cells. Absorbance was measured at 570 nm using an ELISA microplate reader from Tecan (Mannedorf, Switzerland).
Detection of cell apoptosis
To measure apoptosis, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining was conducted under the protocol named In situ Cell Death Dectection Kit (Roche Diagnostics GmbH, Rotkreuz, Switzerland). Cells were cultured at a density of 1×105 cells/400 μl in 4-well chamber slide and treated with 6-OHDA for 24 h in the presence or absence of CORM-2. After treatment, cells were fixed in 4% paraformaldehyde in PBS, pH 7.4 and then incubated with 3% H2O2 in methanol for 10 min at room temperature (RT). Cells were incubated in 0.1% triton X-100 in 0.1% sodium citrate for 2 min on ice and then reacted with TUNEL reaction mixture according to the protocol for 60 min at 37°C. Anti-fluorescein antibody (converter-POD) and 3,3-diaminobenzidine (DAB, VECTOR Lab., CA, USA) were added for 10 min to visualized the TUNEL-positive cells. Apoptotic cells were analyzed under a light microscope (Leica Co., Welzlar, Germany).
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential was measured by using tetramethylrhodamine ethyl ester perchlorate (TMRE) fluorescent dye. Cells were cultured at a density of 1×105 cells/400 μl in 4-well chamber slides. C6 cells were incubated with 6-OHDA in the presence or absence of 6-OHDA for 24 h and TMRE solution (150 nM) was added for 30 min. Images were acquired under a fluorescence microscope (Leica Co, Welzlar, Germany).
Western blotting
The expression of proteins was measured by Western blot analysis. After treatment of 6-OHDA in the presence or absence of CORM-2, protein samples were isolated by RIPA buffer (Sigma-Aldrich). Protein samples were separated in 10% or 12% SDS-polyacrylamide gels and transferred to a polyvinylidene fluoride (PVDF) membrane (Pall Co., MI, USA). The membranes were blocked by 0.1% Tween 20 in PBS (PBST) containing 5% non-fat milk for 30 min at RT and then incubated with primary antibodies [anti-actin (1:1000), antibcl-2 (1:1000), anti-bax (1:1000), anti-caspase-3 (1:1000), anti-cleaved caspase-3 (1:1000), anti-phospho-Nrf2 (1:1000), anti-HO-1 (1:1000), anti-MnSOD (1:1000) and anti-CuZnSOD (1:1000)] in PBST containing 5% non-fat milk at 4°C overnight. After three times of wash with PBST, the blot were reacted with horse-radish peroxidase (HRP)-conjugated anti-rabbit (1:10000, Sigma-Aldrich) or anti-mouse secondary antibody (1:10000, Santa Cruz Biotechnology). The specific bands were detected by using enhanced chemiluminescence (ECL) Western blotting detection reagent (Thermo, Rockford, IL, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
The level of mRNAs was measured by RT-PCR. Total RNA samples were extracted by using Trizol Reagent (Life Technologies, Carlsbad, CA, USA). Reverse transcription to DNA was conducted by using M-MLV reverse transcriptase (Promega, WI, USA). cDNA was amplified by PCR using specific primers for HO-1, MnSOD, CuZnSOD, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as follows. HO-1 : 5′-ACT TTC AGA AGG GTC AGG TGT CC-3′ (sense) and 5′-TTG AGC AGG AAG GCG GTC TTA G-3′ (antisense), MnSOD : 5′-TGA CCT GCC TTA CGA CTA TG-3′ (sense) and 5′-CGA CCT TGC TCC TTA TTG AA-3′ (antisense), CuZnSOD : 5′-CCA TCA ATA TGG GGA CAA TAC AC-3′ (sense) and -5′ACA CGA TCT TCA ATG GAC AC-3′ (antisense), GAPDH : 5′-GCC AAG GTC ATC CAT GAC AAC-3′ (sense) and 5′-AGT GTA GCC CAG GAT GCC CTT-3′. The PCR products were separated by 1% agarose gel electrophoresis in Tris-borate-EDTA buffer and visualized by staining with Eco green dye (Biofact, Daejeon, Korea). The specific bands were visualized by UV lighting using gel documentation system (Bio-rad laboratories, Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using Graphpad prism 5.0 (San Diego, CA, USA) and IBM SPSS statistics for windows (SPSS Inc. Chicago, IL, USA). The data were expressed as mean ± standard error of the mean (SEM). Multiple group comparisons were done by ANOVA followed by post-hoc analysis.
RESULTS
Effect of CO on 6-OHDA-induced cell death in C6 glioma cells
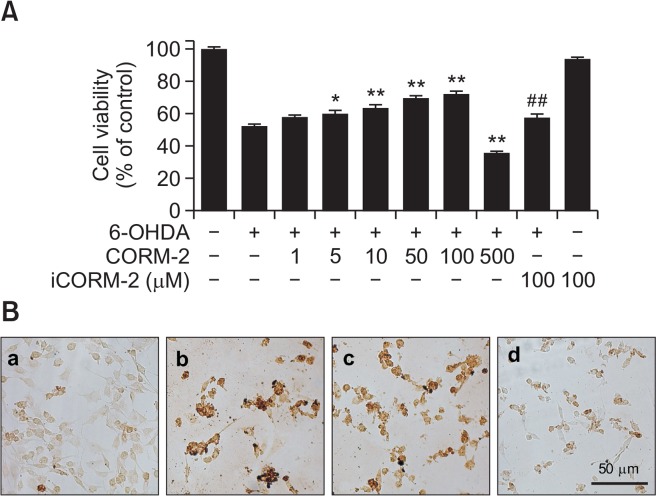
To investigated the effect CO on 6-OHDA-induced oxidative cell death in C6 cells, various concentrations of CORM-2 (0, 10, 20, 50, 100, 500 μM) were treated for 24 h at 37°C and cell viability was measured by MTT assay (Fig. 1A). Low doses of CORM-2 increased the cell viability in a concentration-dependent manner upto 100 μM. However high dose of CORM-2 (500 μM) showed cytotoxicity. Inactive CORM-2 (iCORM-2), a CO-depleted molecule was of no significant effect on 6-OHDA-induced cell deaths. To further characterize the cell deaths which was rescued by CO, TUNEL staining was carried out to measured DNA fragmentation formed by apoptotic signaling cascade (Fig. 1B). 6-OHDA increased the production of DNA fragmentation as an index of apoptotic cell whereas CORM-2 decreased it. Collectively, CORM-2 significantly attenuated the apoptotic cell death caused by 6-OHDA in C6 glioma cells.
Fig. 1.
Protective effect of CO against 6-OHDA-induced cytotoxicity and apoptosis in C6 cells (A) Cells were treated with 6-OHDA (150 μM) and various concentrations of CORM-2 for 24 h and cell viability was measured by MTT assay. iCORM stands for inactive CORM which is CO-depleting molecule. (B) Apoptotic cell death was measured by TUNEL staining. (a) control; (b) 6-OHDA (150 μM) alone; (c) 6-OHDA (150 μM)+CORM-2 (10 μM); (d) 6-OHDA (150 μM)+CORM-2 (100 μM). *p<0.05 and **p<0.01 compared by 6-OHDA treatment alone and ##p<0.01 compared by co-treatment of 6-OHDA and CORM-2.
Effect of CO on 6-OHDA-induced apoptotic signaling
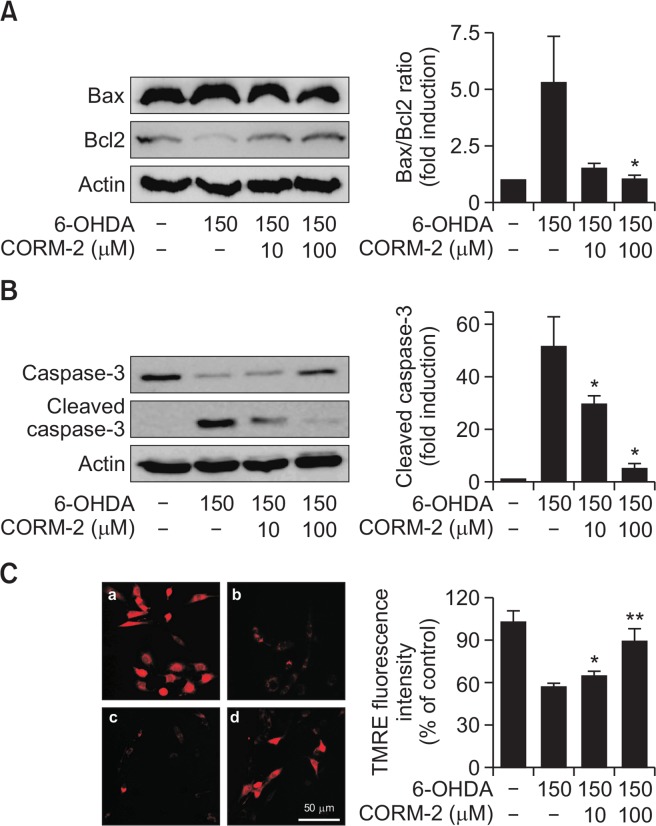
Since we demonstrated that CO attenuated the 6-OHDA-induced apoptotic cell death in C6 cells, we examined the expression levels of some of the distinct markers such as Bax and Bcl2 for apoptotic signaling. Treatment of 6-OHDA in C6 cells increased the ratio of Bax/Bcl2 expression, which was an indicator of pro-apoptotic signal whereas CORM-2 significantly suppressed the Bax/Bcl2 ratio (Fig. 2A). In addition, 6-OHDA increased the level of cleaved caspase-3, one of executor of apoptosis, but CORM-2 inhibited cleavage of caspase-3 induced by 6-OHDA toxicity (Fig. 2B).
Fig. 2.
Effect of CO on 6-OHDA-induced activation of apoptotic signals in C6 cells Cells were treated with 6-OHDA (150 μM) and CORM-2 (10 μM or 100 μM) for 24 h. (A) Expression of proapoptotic protein, Bax and anti-apoptotic protein. Quantitative data for the relative ratio of Bax to Bcl2 was shown on the right panel. (B) Activation of caspase-3 protein by cleavage. Quantitative data for the expression levels of total and cleaved forms of caspase-3 was shown on the right panel. *p<0.05 compared by 6-OHDA alone group. (C) Alterations in mitochondrial transmembrane potential. The TMRE staining images were acquired by using a fluorescence microscope. (a) control; (b) 6-OHDA (150 μM) alone; (c) 6-OHDA (150 μM)+CORM-2 (10 μM); (d) 6-OHDA (150 μM)+CORM-2 (100 μM). Quantitative fluorescence intensity was shown on the right panel. *p<0.05, **p<0.001 was compared by 6-OHDA alone group.
Effect of CO on mitochondrial membrane potential disturbed by 6-OHDA
Mitochondrial transmembrane potential was measured by using TMRE fluorescent dye, which rapidly equilibrates between cellular compartments due to potential differences. When apoptotic events progress, mitochondria undergo major changes in membrane integrity followed by manifestation of apoptotic-associated molecules such as Bcl-2 family, cytochrome c, and caspases. Mitochondrial transmembrane potential is highly related in mitochondrial process such as ATP synthesis, generation of ROS, import of proteins into the mitochondrion and mitochondrial membrane dynamics. Pharmacological change in mitochondrial transmembrane potential is regarded as a multitude of mitochondrial pathological parameters. In the present study, mitochondrial transmembrane potential was significantly decreased by 6-OHDA treatment. Conversely, CORM-2 treatment restrored 6-OHDA-disturbed mitochondrial transmembrane potentials as measured by red fluorescence of TMRE (Fig. 2C).
Effect of CO on intracellular accumulation of reactive oxygen species
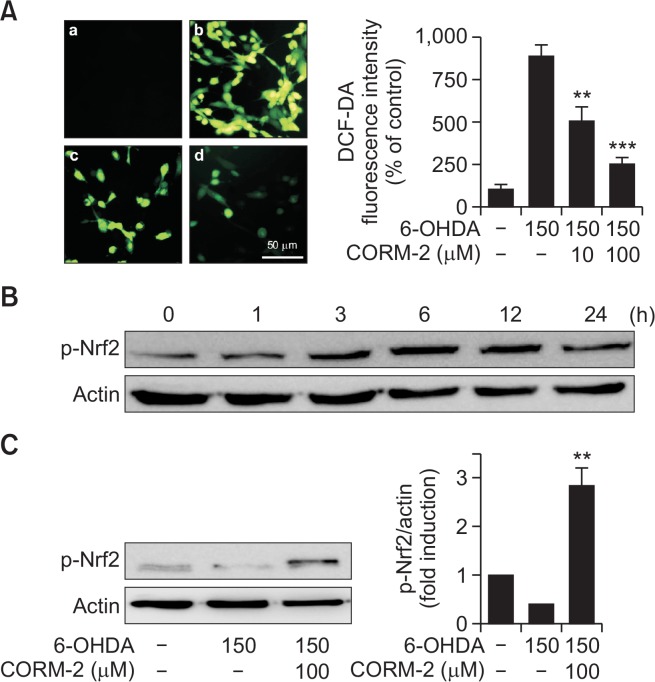
To determine the possible involvement of oxidative stress in 6-OHDA-induced apoptotic cell death in C6 cells, reactive oxygen species (ROS) was measured by DCF-DA fluorescent staining which detected hydroxyl radical, peroxide and other ROS activity within the cell. 6-OHDA increased the intensity of the typical green fluorescence of DCF-DA indicating the accumulation of ROS, whereas CORM-2 significantly decreased the fluorescent intensity (Fig. 3A).
Fig. 3.
Protective effect of CO against 6-OHDA-induced oxidative stress via activation of redox-sensitive transcription factor Nrf2 (A) Effect of CO on 6-OHDA-induced accumulation of ROS in C6 cells. Cells were co-treated with 6-OHDA (150 μM) and CORM-2 (10 μM or 100 μM) for 6 h. DCF-DA staining images were acquired by using a fluorescence microscope. (a) control; (b) 6-OHDA (150 μM) alone; (c) 6-OHDA (150 μM)+CORM-2 (10 μM); (d) 6-OHDA (150 μM)+CORM-2 (100 μM). Quantitative fluorescence intensity was shown on the right panel. **p<0.01, ***p<0.001 compared by 6-OHDA alone group. (B–C) Effect of CO on phosphorylation of Nrf2 (p-Nrf2) in 6-OHDA-treated C6 cells. Cells were treated with 6-OHDA (150 μM) and CORM-2 (100 μM). Phosphorylation of Nrf2 for indicated time period (B) and at 6 h, the peak time (C) was examined by Western blot analysis. Statistical significance was denoted by **p<0.01 compared with the 6-OHDA treatment alone.
Effect of CO on redox signaling molecules mediating antioxidant defense capacity
To further explore the possible mechanisms by which CO attenuates 6-OHDA-induced oxidative cell death, we examine the expression of antioxidant response element, Nrf2. It has been investigated Nrf2 regulates the expression of antioxidant proteins in response to oxidative damages. In this study, treatment of CORM-2 increased phosphorylation of Nrf2, which increased at 3 h, peaked at 6 h, and lasted until 12 h (Fig. 3B). The expression of phosphorylation of Nrf2 was decreased by 6-OHDA treatment whereas CORM-2 significantly increased the phosphorylation of Nrf2 at 6 h (Fig. 3C).
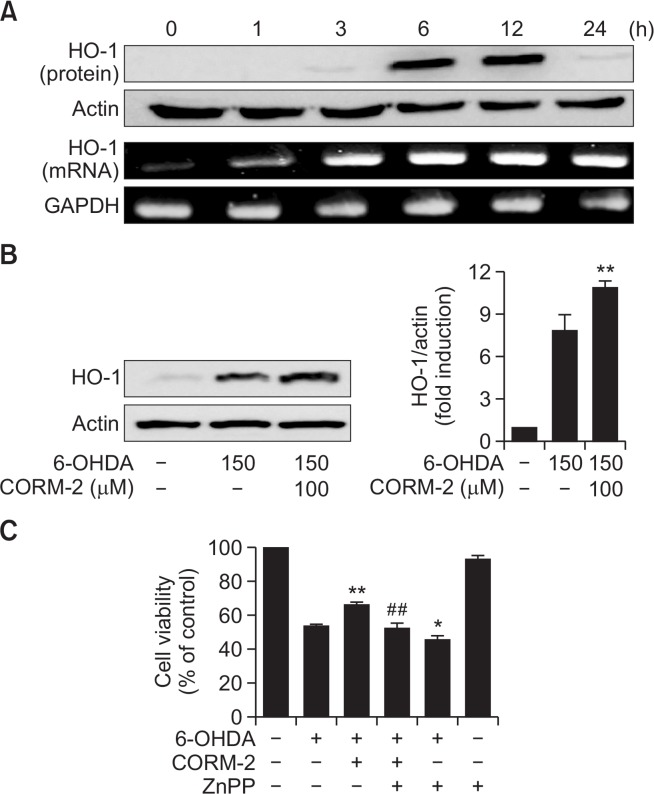
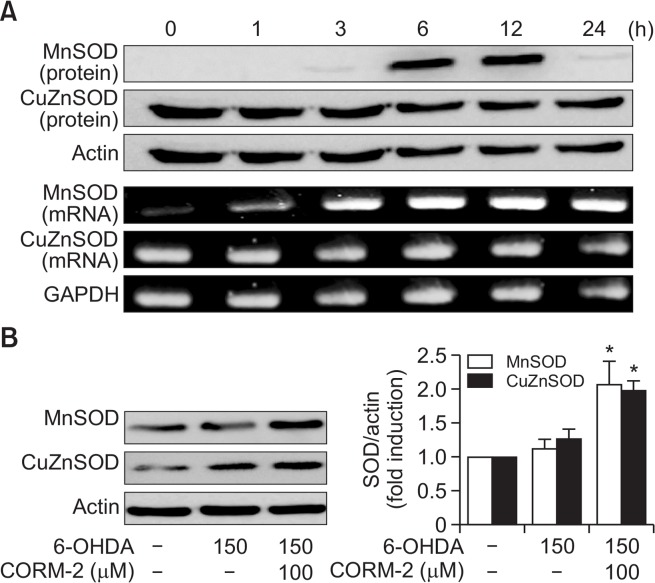
To test whether the antioxidant enzymes might be involved in the protective effects of CO against 6-OHDA-induced cell death in C6 cells, we examined the expression of key antioxidant enzymes for instance HO-1, MnSOD, and CuZnSOD. After cells were treated with CORM-2 (100 μM) and 6-OHDA (150 μM), the levels of mRNA and protein of HO-1 were measured in a time-dependent manner by RT-PCR and western blot analysis, respectively. The mRNA level of HO-1 began to be elevated at 6 h after treatment of CORM-2 and lasted until 24 h) (Fig. 4A, 4B). To demonstrate the role of HO-1 in CO-mediated protection against 6-OHDA-induced cell death in C6 cells, we used ZnPP, a chemical inhibitor of HO-1. As demonstrated before, CORM-2 treatment protected cells against 6-OHDA-induced cell death. On the contrary, protective effects of CORM-2 were abrogated by pretreatment of ZnPP (Fig. 4C). These results suggest that the protective effect of CORM-2 against 6-OHDA might be mediated through HO-1 activation. Another anti-oxidative enzymes, SODs were also examined in the same way. The mRNA and protein expression of MnSOD and CuZnSOD (Fig. 5) was increased from 6 h after treatment of CORM-2 and lasted until 24 h.
Fig. 4.
Effect of CO on expression of HO-1 in 6-OHDA-treated C6 cells (A–B) Cells were treated with 6-OHDA (150 μM) in the presence and absence with CORM-2 (100 μM) for indicated times (A) and 12 h, the peak time (B). Expression of HO-1 in either protein and mRNA levels was examined by Western blot and RT-PCR. Statistical significance was denoted by **p<0.01 compared with the 6-OHDA treatment alone. (C) A possible role of HO-1 mediating protective effect of CO against 6-OHDA-induced cytotoxicity. The cells were pre-incubated with ZnPP (0.1 μM) and then treated with 6-OHDA (150 μM) and CORM-2 (100 μM). Cell viability was examined by MTT assay. Statistical significance was *p<0.05 and **p<0.01 compared by 6-OHDA treatment alone and ##p<0.01 compared by co-treatment of 6-OHDA and CORM-2.
Fig. 5.
CO-induced expression of antioxidant enzyme SOD in 6-OHDA-treated C6 cells (A) Effect of CORM-2 on the protein and mRNA levels of MnSOD and CuZnSOD in combination with 6-OHDA for indicated periods. (B) C6 cells were treated 6-OHDA (150 μM) in the presence or absence with CORM-2 (100 μM) for 24 h which was the peak time of the expression of SOD. Statistical significance was denoted by *p<0.05 compared with the 6-OHDA treatment alone.
DISCUSSION
In this study, we examine the effects of CO on 6-OHDA-induced oxidative cell death in C6 cells. CO released from CORM-2 attenuated oxidative cell death by reducing apoptotic death signals and fortifying the adaptive survival responses which were mediated by Nrf2, HO-1, and SODs. These findings suggest that CO might exert beneficial actions rather than toxic ones in neuronal disorders such as Alzheimer’s disease, PD, stroke, etc.
Consistent with this idea from this study, it has also been demonstrated that CORM-2 protects mice from doxorubicin-induced cardiotoxicity and decreases hepatic ischemia reperfusion injury in rats against apoptosis (Wei et al., 2010; Soni et al., 2011). As such, protective effect of CO against 6-OH-DA-induced cell death has been examined as measured by MTT reduction assay and TUNEL staining. Representative anti-apoptotic protein is Bcl-2, which promotes cellular survival and inhibits the actions of pro-apoptotic proteins (Ruvolo et al., 1998). CORM treatment increases the expression of anti-apoptotic Bcl-2 and decreases the expression of apoptotic cleaved caspase-3 in acute hepatic ischemia reperfusion injury (Wei et al., 2010). CORM-2 treatment causes up-regulation of anti-apoptotic Bcl-2 whereas it does down-regulation of pro-apoptotic Bax and cleaved caspase-3 on iron overload induced apoptosis in mouse neuronal stem cell (Xie et al., 2016). In agreement with the previous findings, CORM-2 treatment in 6-OHDA-treated C6 glioma cell increased the expression of bax/bcl2 ratio and cleave caspase-3 in the present study.
Up-stream transcription factor of antioxidant enzyme, Nrf-2 has been reported to protect cells or tissues against oxidative stress in many disease such as cancer, kidney injury, brain inflammation and neurodegenerative disorders (Innamorato et al., 2008; Joshi and Johnson, 2012; Fledderus and Goldschmeding, 2013; Zhou et al., 2013). In the same manner, some researches have documented that CORM-2 protects cells from oxidative stress via increasing Nrf-2. CORM-2 activates Nrf-2 which regulates antioxidant signal against oxidative stress and inflammation-related disorders (Qin et al., 2015). CORM-2 increases formation of Nrf-2 and other antioxidant elements, c-Jun and Sp1 to protect astrocyte of rat brain (Chi et al., 2015). In this study, CORM-2 also elevated the expression of Nrf-2 to defend C6 cell against 6-OHDA induced toxicity which is known as PD model.
It has been reported that HO-1 is induced by oxidative damage to protect against oxidative injury (Schipper, 1999; Ghattas et al., 2002). There is a study on the neuroprotective effects of HO-1 against oxidative damage in HT22 cells, a mouse hippocampal cell line (Kaizaki et al., 2006). SOD families have been known to catalyze the dismutation of the superoxide radical into either molecular oxygen or hydrogen peroxide and considered as important antioxidant enzyme in living cells exposed to reactive oxygen species (Michiels et al., 1994). In this study, CO released from CORM-2 upregulated both MnSOD (SOD-2) located in mitochondrial matrix and CuZnSOD (SOD-1) located in mitochondrial intermembrane, cytosol, extracellular space. There has been other studies supporting that CORM-2 increases HO-1, SOD expression leading to increase in cell survival. It is reported on the antioxidant effect of CORM-A1 on TNF-alpha/cycloheximide-induced oxidative stress in murine intestinal epithelial MODE-K cells (Babu et al., 2015). Protective effects of CORM-2 has been studied on hepatic ischemia reperfusion injury against oxidative stress (Soni et al., 2011). CORM-A1 prevents dysfunction of blood-brain barrier induced by ionotropic glutamate receptor-mediated oxidative stress and apoptosis (Basuroy et al., 2013). CORM-2 attenuates beta-amyloid-induced cell death and increased antioxidant signal (Hettiarachchi et al., 2014). Co-treatment of CORM-2 and hydrogen sulfide (H2S) protects against alendronate-induced toxicity via increasing the mRNA level of HO-1 and SOD (Magierowski et al., 2016). Another carbon monoxide releasing molecule, CORM-3 significantly increases total cell-associated SOD activity (Mizuguchi et al., 2010).
To elucidate the mechanisms by which CO exerts antioxidant actions in 6-OHDA-treated C6 cells, we examined whether antioxidant molecules can be regulated by CORM-2. One of the candidates is HO-1, which is induced by oxidative stress and protects against oxidative damage in PD (Schipper et al., 1998). CORMs can induce HO-1 expression and then inhibit STAT3 phosphorylation by using HO-1 siRNA (Yang et al., 2014). CO and HO-1 induction regulates IRG1 and A20 expression which have crucial functions in embryonic implantation and neurodegeneration, leading to inhibition of inflammation by using HO-1 siRNA and ZnPP as HO-1 inhibitors (Uddin et al., 2016). In accordance with previous findings, we demonstrated that expression of HO-1 was increased by CORM-2 treatment compared with 6-OHDA alone and inhibition of HO-1 by ZnPP has significantly decreased the cell viability as well as the expression of HO-1, which had been increased by CORM-2. Interestingly, it seems that CO released from CORM-2 can induce the expression of HO-1 and HO-1 also can generate CO as a byproduct while degrading heme molecule. This makes a positive feedback loop by which CO protect C6 cells against 6-OHDA-induced oxidative cell deaths.
Taken together, CORM-2 protected C6 cells against 6-OHDA induced cell death by inhibition of apoptotic cellular signal and increase of anti-apoptotic and by potentiating antioxidative defense capacity via activating Nrf-2 and upregulating antioxidant enzymes such as HO-1 and SOD. These results suggest that CO inhalation or administration of CORM-2 could be considered when developing promising therapeutic strategies to treat and/or prevent neurodegenerative diseases like PD.
Acknowledgments
This work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu (2016).
REFERENCES
- Babu D, Leclercq G, Goossens V, Remijsen Q, Vandenabeele P, Motterlini R, Lefebvre RA. Antioxidant potential of CORM-A1 and resveratrol during TNF-alpha/cycloheximide-induced oxidative stress and apoptosis in murine intestinal epithelial MODE-K cells. Toxicol Appl Pharmacol. 2015;288:161–178. doi: 10.1016/j.taap.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Basuroy S, Leffler CW, Parfenova H. CORM-A1 prevents blood-brain barrier dysfunction caused by ionotropic glutamate receptor-mediated endothelial oxidative stress and apoptosis. Am J Physiol Cell Physiol. 2013;304:C1105–C1115. doi: 10.1152/ajpcell.00023.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F, Armentero MT, Martignoni E. The 6-hydroxydopamine model: news from the past. Parkinsonism Relat Disord. 2008;14:S124–S129. doi: 10.1016/j.parkreldis.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Caumartin Y, Stephen J, Deng JP, Lian D, Lan Z, Liu W, Garcia B, Jevnikar AM, Wang H, Cepinskas G, Luke PP. Carbon monoxide-releasing molecules protect against ischemia-reperfusion injury during kidney transplantation. Kidney Int. 2011;79:1080–1089. doi: 10.1038/ki.2010.542. [DOI] [PubMed] [Google Scholar]
- Chapman JT, Otterbein LE, Elias JA, Choi AM. Carbon monoxide attenuates aeroallergen-induced inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L209–L216. doi: 10.1152/ajplung.2001.281.1.L209. [DOI] [PubMed] [Google Scholar]
- Chi PL, Lin CC, Chen YW, Hsiao LD, Yang CM. CO induces Nrf2-dependent heme oxygenase-1 transcription by cooperating with Sp1 and c-Jun in rat brain astrocytes. Mol Neurobiol. 2015;52:277–292. doi: 10.1007/s12035-014-8869-4. [DOI] [PubMed] [Google Scholar]
- Choi YK. Role of carbon monoxide in neurovascular repair processing. Biomol Ther (Seoul) 2017 doi: 10.4062/biomolther.2017.144. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie AE, Fontanilla TM, Roncalli V, Cieslak MC, Lenz PH. Diffusible gas transmitter signaling in the copepod crustacean Calanus finmarchicus: identification of the biosynthetic enzymes of nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) using a de novo assembled transcriptome. Gen Comp Endocrinol. 2014;202:76–86. doi: 10.1016/j.ygcen.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledderus JO, Goldschmeding R. Nrf2 implicated as a novel therapeutic target for renal regeneration after acute kidney injury. Nephrol Dial Transplant. 2013;28:1969–1971. doi: 10.1093/ndt/gft202. [DOI] [PubMed] [Google Scholar]
- Ghattas MH, Chuang LT, Kappas A, Abraham NG. Protective effect of HO-1 against oxidative stress in human hepatoma cell line (HepG2) is independent of telomerase enzyme activity. Int J Biochem Cell Biol. 2002;34:1619–1628. doi: 10.1016/S1357-2725(02)00097-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Herman ZS. Carbon monoxide: a novel neural messenger or putative neurotransmitter? Pol J Pharmacol. 1997;49:1–4. [PubMed] [Google Scholar]
- Hettiarachchi N, Dallas M, Al-Owais M, Griffiths H, Hooper N, Scragg J, Boyle J, Peers C. Heme oxygenase-1 protects against Alzheimer’s amyloid-beta(1–42)-induced toxicity via carbon monoxide production. Cell Death Dis. 2014;5:e1569. doi: 10.1038/cddis.2014.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- Jamal Uddin M, Joe Y, Kim S-K, Jeong SO, Ryter SW, Pae H-O, Chung HT. IRG1 induced by heme oxygenase-1/carbon monoxide inhibits LPS-mediated sepsis and pro-inflammatory cytokine production. Cell Mol Immunol. 2016;13:170–179. doi: 10.1038/cmi.2015.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G, Johnson JA. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discov. 2012;7:218–229. doi: 10.2174/157488912803252023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizaki A, Tanaka S, Ishige K, Numazawa S, Yoshida T. The neuroprotective effect of heme oxygenase (HO) on oxidative stress in HO-1 siRNA-transfected HT22 cells. Brain Res. 2006;1108:39–44. doi: 10.1016/j.brainres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson disease in 2015: evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol. 2016;12:65–66. doi: 10.1038/nrneurol.2015.249. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, Nunomura A, Castellani RJ, Perry G, Smith MA, Itoyama Y. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol Dis. 2002;9:244–248. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- Kim DS, Chae SW, Kim HR, Chae HJ. CO and bilirubin inhibit doxorubicin-induced cardiac cell death. Immunopharmacol Immunotoxicol. 2009;31:64–70. doi: 10.1080/08923970802354762. [DOI] [PubMed] [Google Scholar]
- Lian S, Xia Y, Ung TT, Khoi PN, Yoon HJ, Kim NH, Kim KK, Jung YD. Carbon monoxide releasing molecule-2 ameliorates IL-1beta-induced IL-8 in human gastric cancer cells. Toxicology. 2016;361–362:24–38. doi: 10.1016/j.tox.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Magierowski M, Magierowska K, Szmyd J, Surmiak M, Sliwowski Z, Kwiecien S, Brzozowski T. Hydrogen sulfide and carbon monoxide protect gastric mucosa compromised by mild stress against alendronate injury. Dig Dis Sci. 2016;61:3176–3189. doi: 10.1007/s10620-016-4280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoole MD, D’Andrea BT, Baer KN, Christie AE. Genomic analyses of gas (nitric oxide and carbon monoxide) and small molecule transmitter (acetylcholine, glutamate and GABA) signaling systems in Daphnia pulex. Comp Biochem Physiol Part D Genomics Proteomics. 2012;7:124–160. doi: 10.1016/j.cbd.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Michiels C, Raes M, Toussaint O, Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994;17:235–248. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Mizuguchi S, Capretta A, Suehiro S, Nishiyama N, Luke P, Potter RF, Fraser DD, Cepinskas G. Carbon monoxide-releasing molecule CORM-3 suppresses vascular endothelial cell SOD-1/SOD-2 activity while up-regulating the cell surface levels of SOD-3 in a heparin-dependent manner. Free Radic Biol Med. 2010;49:1534–1541. doi: 10.1016/j.freeradbiomed.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Onyiah JC, Sheikh SZ, Maharshak N, Steinbach EC, Russo SM, Kobayashi T, Mackey LC, Hansen JJ, Moeser AJ, Rawls JF, Borst LB, Otterbein LE, Plevy SE. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology. 2013;144:789–798. doi: 10.1053/j.gastro.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrus M, Paprota P, Radziszewska R, Huras H, Ludwin A, Wiechec M, Nocun A, Ossowski P, Knafel A, Kialka M, Klyszejko-Molska J, Pitynski K, Zalustowicz A, Banas T. Carbon monoxide poisoning in pregnant woman. Przegl Lek. 2015;72:482–484. [PubMed] [Google Scholar]
- Qin S, Du R, Yin S, Liu X, Xu G, Cao W. Nrf2 is essential for the anti-inflammatory effect of carbon monoxide in LPS-induced inflammation. Inflamm Res. 2015;64:537–548. doi: 10.1007/s00011-015-0834-9. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase Calpha in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Glial HO-1 expression, iron deposition and oxidative stress in neurodegenerative diseases. Neurotox Res. 1999;1:57–70. doi: 10.1007/BF03033339. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp Neurol. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- Shiraga H, Pfeiffer RF, Ebadi M. The effects of 6-hydroxydopamine and oxidative stress on the level of brain metallothionein. Neurochem Int. 1993;23:561–566. doi: 10.1016/0197-0186(93)90104-D. [DOI] [PubMed] [Google Scholar]
- Soni H, Pandya G, Patel P, Acharya A, Jain M, Mehta AA. Beneficial effects of carbon monoxide-releasing molecule-2 (CORM-2) on acute doxorubicin cardiotoxicity in mice: role of oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2011;253:70–80. doi: 10.1016/j.taap.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Wegiel B, Gallo D, Csizmadia E, Harris C, Belcher J, Vercellotti GM, Penacho N, Seth P, Sukhatme V, Ahmed A, Pandolfi PP, Helczynski L, Bjartell A, Persson JL, Otterbein LE. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73:7009–7021. doi: 10.1158/0008-5472.CAN-13-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Chen P, de Bruyn M, Zhang W, Bremer E, Helfrich W. Carbon monoxide-releasing molecule-2 (CORM-2) attenuates acute hepatic ischemia reperfusion injury in rats. BMC Gastroenterol. 2010;10:42. doi: 10.1186/1471-230X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Han P, Cui Z, Wang B, Zhong Z, Sun Y, Yang G, Sun Q, Bian L. Pretreatment of mouse neural stem cells with carbon monoxide-releasing molecule-2 interferes with NF-κB p65 signaling and suppresses iron overload-induced apoptosis. Cell Mol Neurobiol. 2016;36:1343–1351. doi: 10.1007/s10571-016-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Huang YT, Hsieh CW, Yang PM, Wung BS. Carbon monoxide induces heme oxygenase-1 to modulate STAT3 activation in endothelial cells via S-glutathionylation. PLoS ONE. 2014;9:e100677. doi: 10.1371/journal.pone.0100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Wang P, Chen M, Liu Y, Zhou L, Fang X, Huang Z. Carbon monoxide-releasing molecules attenuate postresus-citation myocardial injury and protect cardiac mitochondrial function by reducing the production of mitochondrial reactive oxygen species in a rat model of cardiac arrest. J Cardiovasc Pharmacol Ther. 2015;20:330–341. doi: 10.1177/1074248414559837. [DOI] [PubMed] [Google Scholar]
- Zhou S, Ye W, Shao Q, Zhang M, Liang J. Nrf2 is a potential therapeutic target in radioresistance in human cancer. Crit Rev Oncol Hematol. 2013;88:706–715. doi: 10.1016/j.critrevonc.2013.09.001. [DOI] [PubMed] [Google Scholar]