Abstract
Chalcone, (2E)-1,3-Diphenylprop-2-en-1-one, and its synthetic derivatives are known to possess anti-oxidative and anti-inflammatory properties. In the present study, we prepared a novel synthetic chalcone compound, (E)-1-(4-hydroxyphenyl)-3-(2-(trifluoromethoxy)phenyl)prop-2-en-1-one name (YJI-7), and investigated its inhibitory effects on endotoxin-stimulated production of reactive oxygen species (ROS) and expression of inflammatory mediators in macrophages. We demonstrated that treatment of RAW 264.7 macrophages with YJI-7 significantly suppressed lipopolysaccharide (LPS)-stimulated ROS production. We also found that YJI-7 substantially decreased NADPH oxidase activity stimulated by LPS, indicating that YJI-7 regulates ROS production via modulation of NADPH oxidase in macrophages. Furthermore, YJI-7 strongly inhibited the expression of a number of inflammatory mediators in a gene-selective manner, suggesting that YJI-7 possesses potent anti-inflammatory properties, as well as anti-oxidative activity. In continuing experiments to investigate the mechanisms that could underlie such biological effects, we revealed that YJI-7 suppressed phosphorylation of p38MAPK and JNK stimulated by LPS, whereas no significant effect on ERK was observed. Furthermore, LPS-stimulated production of ROS, activation of NADPH oxidase and expression of inflammatory mediators were markedly suppressed by treatment with selective inhibitor of p38MAPK (SB203580) and JNK (SP600125). Taken together, these results demonstrated that YJI-7, a novel synthetic chalcone derivative, suppressed LPS-stimulated ROS production via modulation of NADPH oxidase and diminished expression of inflammatory mediators, at least in part, via down-regulation of p38MAPK and JNK signaling in macrophages.
Keywords: Chalcone, NADPH oxidase, Reactive oxygen species, p38MAPK, JNK
INTRODUCTION
Reactive oxygen species (ROS) are unstable oxygen-containing molecules that are highly reactive towards various intracellular constituents, such as proteins, lipids and DNA (Rojkind et al., 2002). ROS are constantly generated in cells and removed via multiple mechanisms. ROS production is regulated by various organelles, including mitochondria, peroxisome and endoplasmic reticulum (Tse et al., 2016). In addition, a number of enzyme systems, such as NADPH oxidase, cytochrome P450 and xanthine oxidase, are involved in ROS production (Holmstrom and Finkel, 2014). The major source of ROS depends on the cell types and cellular environments. Whereas low and physiological levels of ROS function as signaling molecule and are implicated in a number of beneficial biological responses, upon exposure to the stressful environments, intracellular ROS levels get significantly elevated and excessive ROS production causes critical damages in the cells, by inducing conditions collectively referred to as oxidative stress. ROS is implicated in a wide range of pathological conditions, including aging, cancer and cell death (Hermes-Lima et al., 2015). Therefore, modulation of excessive ROS production would be a promising strategy for dealing with various diseases.
Inflammation is a complicated biological response to the various deleterious stimuli that generally commences as part of the innate immune response to remove harmful stimuli and start the healing process (Serhan and Savill, 2005). However, chronic and/or excess inflammation causes various patho-physiological conditions, including cancer, neurodegenerative diseases and auto-immune diseases (Eikelenboom et al., 1994; Coussens and Werb, 2002). The inflammatory process is tightly regulated via a coordinated action between pro- and anti-inflammatory signals. An imbalance between the signals, in particular, excessive activation of pro-inflammatory signals results in hyper inflammation and damage to the tissues. Inflammatory response is intensified and propagated by the release of inflammatory mediators, such as prostaglandins, nitric oxide, interleukins and tumor necrosis factor-α (Ben-Baruch et al., 1995). The signaling mechanisms underlying the production of inflammatory mediators have been extensively studied and the mechanisms are now well established. However, efficient tools for the management of inflammatory diseases and the regulation of inflammatory mediators in clinic remain limited.
ROS production is regulated by coordinated actions of oxidant and anti-oxidant machinery, which depends on cellular environments. Macrophages, which act as a first line of the defense system, are responsible for ROS production in the beginning of the exposure to the inflammatory stimulus (Gordon, 1998). Although moderate level of ROS is required for the removal of pathogens and maintenance of cellular homeostasis, excessive ROS generation is a key signaling event leading to the production of various inflammatory mediators and progression of inflammatory tissue injury (Mittal et al., 2014). Thus, modulation of excessive ROS production and oxidative stress would be a promising strategy for the treatment of inflammatory disorders.
Chalcone participates in the biosynthesis of flavonoids and is considered as metabolic precursors of flavonoes. Synthetic and natural derivatives of chalcone have a diverse spectrum of pharmacological actions, including anti-tumor, anti-bacterial/fungal, anti-oxidant and anti-inflammatory effects (Matos et al., 2015). Chalcones have originally received much attention as promising anti-cancer agents. Their anti-tumor activity is mediated through various mechanisms, including apoptosis induction, cell cycle arrest and topoisomerase inhibition (Mahapatra et al., 2015). In addition to its anti-cancerous activity, there is a growing appreciation that chalcones possess potent anti-inflammatory properties. Chalcones suppress the transcriptional activities of AP-1, NF-κB and STAT3, and inhibit the expression of various pro-inflammatory mediators, including TNF-α, interleukins, cell adhesion molecules and cyclo-oxygenase-2 (Kontogiorgis et al., 2008; Yadav et al., 2011; Zhang et al., 2014). Furthermore, chalcones activate anti-inflammatory mechanisms, e.g., heme oxygenase-1 induction (Kim et al., 2014; Kaufmann et al., 2016). Based on previous reports, there is a growing appreciation that chalcone and its synthetic derivatives are promising for the treatment of the various diseases associated with inflammation. However, no chalcone derivative has been successfully applied to the clinic yet. In the present study, in an attempt to develop optimal therapeutic agents, we prepared, ((E)-1-(4-hydroxyphenyl)-3-(2-(trifluoromethoxy)phenyl)prop-2-en-1-one) (YJI-7), a fluorine containing phenyl propenone intermediate, and evaluated its effects on the ROS production and inflammatory mediators expression in macrophages. Herein, we found that YJI-7, a novel synthetic chalcone derivative, potently suppressed LPS-stimulated production of ROS and expression of inflammatory mediators. In addition, we demonstrated that these effects are mediated, at least in part, via modulation of NADPH oxidase and MAPK signaling.
MATERIALS AND METHODS
Materials
All the reagents used for cell culture were purchased from Hyclone Laboratories (South Logan, UT, USA) unless indicated otherwise. Luciferase assay and MTS assay kits were obtained from Promega (Madison, WI, USA). 5-Chloromethyl-2,7-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) was obtained from Molecular Probes (Eugene, OR, USA). Lucigenin, NADPH and diphenyleneiodonium chloride (DPI) were procured from Enzo Life Sciences (Farmingdale, NY, USA). Antibodies against p38MAPK (phosphor-specific and total), ERK (phosphor-specific and total) and JNK (phosphor-specific and total) were purchased from Cell Signaling Technology Inc (Beverly, MA, USA). Horse radish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit antibodies were purchased from Pierce (Rockford, IL, USA).
Cell culture
RAW 264.7 macrophage cell line was purchased from the Korean cell line bank (KCLB, Seoul, Korea) and cultured in Dulbecco’s Modified Eagle Medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin. Cells were routinely maintained at 37°C in a humidified incubator.
Synthetic procedure of YJI-7
The structure of YJI-7 ((E)-1-(4-hydroxyphenyl)-3-(2-(trifluoromethoxy)phenyl)prop-2-en-1-one) is shown in Fig. 1. Synthetic procedure for YJI-7 was as follows. To a stirred solution of 4-hydroxyphenyl methyl ketone (0.14 g, 1.0 mmol, 1.0 equiv) and 2-(trifluoromethoxy)benzaldehyde (0.17 mL, 1.2 mmol, 1.2 equiv) in ethanol (3 mL), 50% aqueous KOH solution (9.0 eq., 1.0 mL) was added drop wise at room temperature, and the mixture was stirred for 1 h. Ice was added to the reaction mixture and neutralized by 6M aqueous HCl solution (pH adjusted to 2) to give precipitation. The precipitate was filtered off and washed with excess water to obtain a solid residue. The solid residue was further purified by recrystallization using ethyl acetate and n-hexane to afford compound YJI-7 to yield 219.90 mg (71.3%, 0.71 mmol) as a light yellow solid.
Fig. 1.

Chemical structures of YJI-7.
Rf (ethylacetate/n-hexane=1:3 v/v): 0.22, mp: 153.5-154.5°C, HPLC: Retention time: 5.12 min, purity: 99.67%, ESI LC/MS: m/z calcd for C16H11F3O3 [MH]+ 309.08; found 309.2.
1H NMR (250 MHz, DMSO-d6) δ 8.21 (dd, J=7.73, 1.48 Hz, 1H, 3-phenyl H-6), 8.05 (d, J=8.72 Hz, 2H, 1-phenyl H-2, H-6), 7.97 (d, J=15.65 Hz, 1H, -CO-C=CH-), 7.80 (d, J=15.65 Hz, 1H, -CO-CH=C-), 7.59-7.42 (m, 3H, 3-phenyl H-3, H-4, H-5), 6.90 (d, J=8.68 Hz, 2H, 1-phenyl H-3, H-5).
13C NMR (62.5 MHz, DMSO-d6) δ 187.09, 162.70, 147.07, 134.21, 132.18, 131.52 (2C), 128.90, 128.70, 128.21, 128.15, 125.23, 121.93, 120.30 (d, J=255.56 Hz), 115.70 (2C).
Measurement of cell viability (MTS assay)
Effect of YJI-7 on cell viability was examined using a CellTiter 96 Aqueous One kit (Promega) essentially as described previously (Nepal et al., 2015). Briefly, RAW 264.7 macrophages were seeded at a density of 5×104 cells/well in 96-well plates. After overnight incubation, cells were treated with indicated concentration of YJI-7 for 24 h and further incubated with 20 μl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTS) solution for additional 2 h. Cell viability was determined by the reduction of MTS tetrazolium to a formazan product and monitored using a SPEC-TROstar Nano microplate reader (BMG Labtech Inc., Ortenberg, Germany) by measuring the absorbance at 490 nm.
Measurement of reactive oxygen species (ROS) production
For the measurement of intracellular ROS production, RAW 264.7 macrophages were initially seeded at a density of 5×104 cells/well in 96-well black plates. Cells were pretreated with YJI-7 for 1 h, further stimulated with LPS for additional 24 h and incubated with 10 μM 5-chloromethyl-2,7-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) in the dark for 30 min. Cells were then washed with HBSS to remove the excess dye. Intracellular ROS production was assessed using a FLUOstar OPTIMA fluoremeter (BMG Labtech Inc.) and analyzed by FLUOstar OPTIMA software from BMG. The excitation and emission wavelengths were set to 485 nm and 520 nm, respectively.
Quantitative polymerase chain reaction (qPCR)
After treatment with LPS and/or YJI-7, total cellular RNA was extracted using Qiagen lysis solution (Qiagen, Hilden, Germany). Two hundred to three hundred nanograms of total RNAs were reverse transcribed into cDNA using the GoScript reverse transcription system (Promega) as previously described (Nepal and Park, 2013). Quantitative real-time PCR was then performed with LightCycler 1.5 (Roche Diagnostics, Mannheim, Germany) by using a qPCR SYBR Green Capillary Mix. The conditions for PCR amplification were as follows; 95°C for 15 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 30 s. The amount of target mRNA was determined via comparative threshold (Ct) method. Then, the value was normalized to the value of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The sequences of the primers used are listed in Table 1.
Table 1.
Sequences of the primers used for quantitative RT-PCR
| Target gene | Primer | Sequence |
|---|---|---|
| IL-1β | Forward | 5′-GCCTCGTGCTGTCGGACCCATAT-3′ |
| Reverse | 5′-TCCTTTGAGGCCCAAGGCCACA-3′ | |
| iNOS | Forward | 5′-GCTCGCTTTGCCACGGACGA-3′ |
| Reverse | 5′-AAGGCAGCGGGCACATGCAA-3′ | |
| IL-18 | Forward | 5′- GACTCTTGCGTCAACTTCAAGG -3′ |
| Reverse | 5′- CAGGCTGTCTTTTGTCAACGA -3′ | |
| IFN-β | Forward | 5′-AACTCCACCAGCAGACAGTG-3′ |
| Reverse | 5′-TGAGGACATCTCCCACGTCA-3′ | |
| TNF-α | Forward | 5′-CCCTCACACTCAGATCATCTTCT-3′ |
| Reverse | 5′-GCTACGACGTGGGCTACAG-3′ | |
| IL-6 | Forward | 5′-ACAACCACGGCCTTCCCTAC-3′ |
| Reverse | 5′-CACGATTTCCCAGAGAACAT-3′ | |
| COX-2 | Forward | 5′-GGGCTCAGCCAGGCAGCAAAT-3′ |
| Reverse | 5′-GCACTGTGTTTGGGGTGGGCT-3′ | |
| IL-8 | Forward | 5′-TTGCCTTGACCCTGAAGCCCCC-3′ |
| Reverse | 5′-GGCACATCAGGTACGATCCAGGC-3′ |
Transient transfection and luciferase assay
Transcriptional activity of AP-1 and NF-κB was determined by the luciferase reporter assay. RAW 264.7 macrophages were initially seeded in 24-well plates at a density of 5×105 cells/well. After overnight culture, cells were co-transfected with a control vector (pRL-TK, 0.01 μg/well) expressing wild type Renilla luciferase under the control of the thymidine kinase promoter, as a control for transfection efficiency, and AP-1-dependent (or NF-κB-dependent) luciferase expressing vectors (pGL4/NF-κB or pTL/AP-1, 0.05 μg/well) using Fugene HD (Promega) according to the manufacturer’s instruction. After 4 h of incubation, the cells were then treated with YJI-7 for 2 h followed by further incubation with LPS for additional 6 h. Cellular extracts were prepared using a passive lysis buffer (PLB, 100 μl/well) by incubation for 15 min. Levels of Firefly and Renilla luciferase activity was measured by the Dual Luciferase Reporter Assay System (Promega). Relative luciferase activity was normalized to the value of Renilla luciferase.
Preparation of cellular extracts and Western blot analysis
RAW 264.7 macrophages (1×106 cells) were initially seeded in 35 mm dishes. After overnight incubation, the cells were treated with YJI-7 and LPS (100 ng/mL), total cellular protein was extracted using RIPA lysis buffer containing Halt Protease and Phosphatase Inhibitor Single-Use Cocktail (Thermo Scientific, Rockford, IL, USA). For immunoblot analysis, 20–30 μg of protein was loaded in SDS-PAGE (10% or 12%) and transferred to PVDF membranes. After blocking with a 5% skim milk in PBS/Tween for 1 h, the membrane was incubated with the designated primary antibody in 3% BSA/PBST solution overnight at 4°C and further incubated with secondary antibody conjugated with horse radish peroxidase (HRP) for 1 h at room temperature. Finally, the chemiluminescent images of the blots were captured using a Fujifilm LAS-4000 mini imager (Fujifilm, Tokyo, Japan). The membranes were sripped and reprobed with β-actin as the loading control.
Enzyme-linked immunosorbent assay (ELISA) for IL-1β detection
RAW 264.7 macrophages were seeded into the 96-well plates at a density of 105 cells per well. After overnight culture, cells were pretreated with YJI-7 for 2 h followed by the stimulation with LPS for additional 8 h. The cell culture media were collected and used for the measurement of IL-1β using ELISA kit (BD Biosciences, San Diego, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
Values are presented as the mean ± SEM. Data were assessed by one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests using GraphPad prism software (GraphPad Software Inc. San Diego, CA, USA). Differences between groups were considered to be statistically significant at p<0.05.
RESULTS
YJI-7 suppresses LPS-stimulated ROS production via modulation of NADPH oxidase in RAW 264.7 macrophages
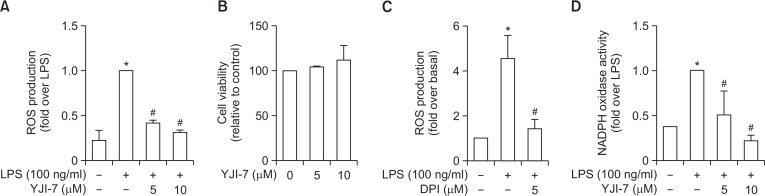
To investigate the anti-oxidant and anti-inflammatory actions of YJI-7, we first examined the effect of YJI-7 on ROS production in RAW 264.7 macrophages stimulated with LPS. As shown in Fig. 2A, pretreatment with YJI-7 caused a significant decrease in LPS-stimulated ROS production in a dose-dependent manner with an IC50 value of 1.34 μM. To verify that the suppression of ROS production was not due to cytotoxicity, we next examined the effect of YJI-7 on cell viability. As illustrated in Fig. 2B, cell viability was not affected by YJI-7 up to 10 μM determined by MTS assay, indicating that YJI-7 potently suppressed ROS production in macrophages without affecting cell viability.
Fig. 2.
Effects of YJI-7 on LPS-stimulated ROS production and NADPH oxidase activation in RAW 264.7 macrophages. (A) Cells were pre-treated with YJI-7 for 1 h followed by stimulation with LPS for additional 24 h. After washing with Hank’s balanced salt solution (HBSS), cells were incubated with 5-chloromethyl-2,7-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) for 30 min. ROS production was measured by the amount of CM-H2DCFDA converted into fluorescent DCF. Data are expressed as fold change in the amount of DCF relative to that observed in cells stimulated with LPS and are expressed as the mean ± SEM (n=3). Statistical significance is indicated as follows: *p<0.05 for comparison with values observed in control cells; #p<0.05 for comparisons with values observed in cells treated with LPS. (B) Effect of YJI-7 on cell viability was assessed by MTS assay. After treatment with indicated concentrations of YJI-7 for 24 h, the cells were incubated with MTS for 2 h and cell viability was determined as described in Materials and Methods. Values represent the fold change in the amount of formazan product relative to that of control cells. Data are presented as the mean ± SEM (n=3). (C) Cells were treated with YJI-7 for 24 h in the absence or presence of DPI, a pharmacological inhibitor of NADPH oxidase, and ROS production was determined as indicated previously. Data are expressed as fold change in the amount of fluorescent DCF compared to that of control cells and are expressed as the mean ± SEM (n=3). *p<0.05 for comparison with values observed in control cells; #p<0.05 for comparison with values observed in cells treated with LPS only. (D) RAW 264.7 macrophages were treated with YJI-7 for 2 h followed by the incubation with LPS for 6 h. Cellular lysates were prepared and incubated with lucigenin (100 μM) and NADPH for 30 min. NADPH oxidase activity was then assessed as described in Materials and Methods. Values represent fold change in comparison to the cells treated with LPS and are expressed as the mean ± SEM (n=3). *p<0.05 compared with the control cells; #p<0.05 compared with cells treated with LPS only.
ROS are produced from different cellular sources and this production is regulated by multiple mechanisms. To further elucidate the molecular mechanisms underlying modulation of ROS production by YJI-7, we investigated the effect of YJI-7 on NADPH oxidase. Treatment with diphenyleneiodonium (DPI), a pharmacological inhibitor of NADPH oxidase, almost completely blocked LPS-stimulated ROS production (Fig. 2C) consistent with previous reports, implying a critical role of NADPH oxidase in LPS-stimulated ROS production in our experimental condition. Furthermore, as indicated in Fig. 2D, pretreatment with YJI-7 markedly inhibited LPS-stimulated NADPH oxidase activation. Taken together, these results indicate that YJI-7 regulates ROS production via modulation of NADPH oxidase in macrophages.
YJI-7 inhibits ROS production and NADPH oxidase through suppression of the p38MAPK and JNK signaling pathways
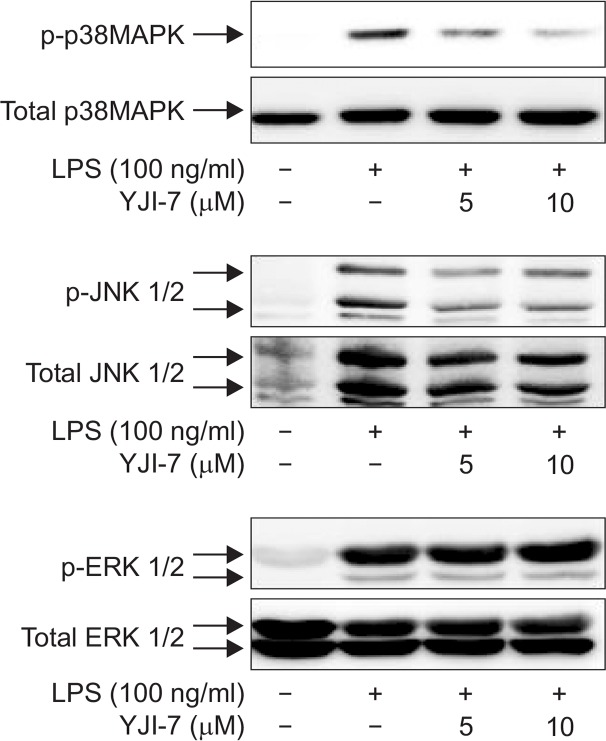
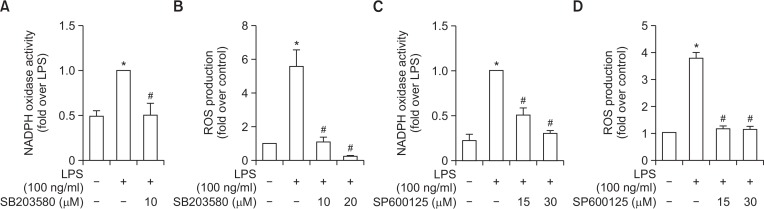
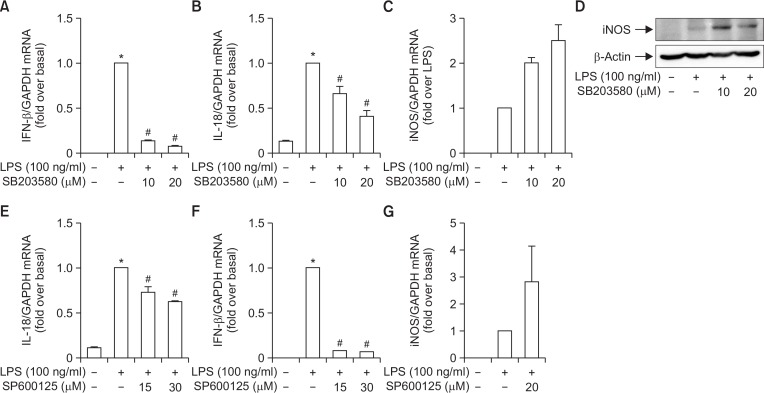
To further elucidate upstream signaling mechanisms that mediated inhibition of ROS production and NADPH oxidase activity by YJI-7, we examined whether these effects involve signaling cascades impinging on mitogen-activated protein kinases (MAPKs), since MAPKs cascades have been shown to regulate NADPH oxidase-dependent oxidative stress (Torres and Forman, 2003). For this, we first examined the effects of YJI-7 on the activation of MAPKs by LPS. As shown in Fig. 3, LPS treatment induced increase in phosphorylation of ERK, JNK and p38 MAPK in RAW 264.7 macrophages, which was consistent with previous reports. Pretreatment with YJI-7 substantially inhibited activation of p38MAPK and JNK, whereas no significant effect on ERK phosphorylation was observed. Next, we further verified the functional role of p38 MAPK and JNK signaling in LPS-stimulated ROS production and NADPH oxidase activation. Pretreatment of macrophages with the selective inhibitor of p38MAPK (SB203580) significantly suppressed LPS-induced NADPH oxidase activation (Fig. 4A). In addition, LPS-stimulated ROS production was also inhibited by pretreatment with the p38MAPK inhibitor (Fig. 4B), indicating a critical role of the p38MAPK cascade in NADPH oxidase activation and ROS production stimulated by LPS. Essentially similar effects on NADPH oxidase and ROS production were observed by following the treatment of macrophages with the inhibitor of JNK (SP600125) (Fig. 4C, 4D), suggesting the JNK signaling was also implicated in LPS-stimulated ROS production and NADPH oxidase activation in our experimental condition. All these results collectively indicate that YJI-7 suppresses NADPH oxidase activation and ROS production via regulation of p38MAPK and JNK signaling.
Fig. 3.
Effects of YJI-7 on LPS-stimulated activation of MAPKs in RAW 264.7 macrophages. Cells were pretreated with YJI-7 for 2 h and further stimulated with LPS for 30 min. The amounts of phosphor-and total forms of ERK, p38MAPK and JNK were measured by Western blot analysis. Images are representative of three independent experiments that showed similar results.
Fig. 4.
Role of p38MAPK and JNK signaling in the regulation of NADPH oxidase activity and ROS production stimulated by LPS in RAW 264.7 macrophages. (A, B) Cells were pretreated with SB203580, a pharmacological inhibitor of p38MAPK, followed by the incubation with LPS for 24 h. NADPH oxidase activity (A) and ROS production (B) were assessed as described previously. Values are presented as the fold change in respective parameters compared to the levels determined in cells stimulated with LPS and are expressed as the mean ± SEM (n=3). (C, D) Cells were treated with LPS for 30 min in the absence or presence of different concentration of SP600125, a selective inhibitor of JNK. NADPH oxidase activity (C) and ROS production (D) were determined as described previously. Data are presented as fold change compared to the LPS-treated cells and are expressed as mean ± SEM (n=3). *p<0.05 compared with control cells; #p<0.05 compared with cells treated with LPS only.
YJI-7 suppresses LPS-stimulated production of inflammatory mediators in RAW 264.7 macrophages
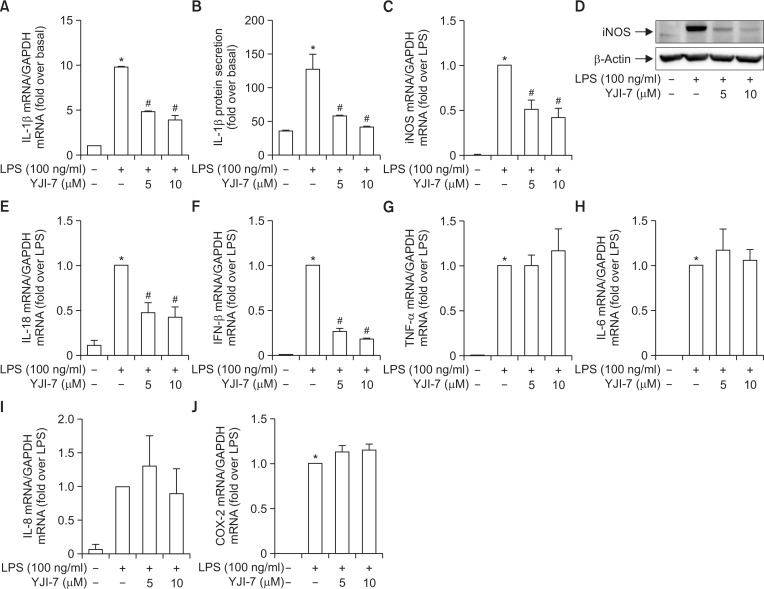
Having observed potent anti-oxidative effects of YJI-7, we further explore its potential anti-inflammatory properties. To this end, we examined the effects of YJI-7 on the expression of various inflammatory mediators stimulated by LPS treatment. As shown in Fig. 5A, pretreatment with YJI-7 dose-dependently suppressed LPS-induced mRNA expression of IL-1β (Left panel). Secretion of IL-1β was also decreased by treatment with YJI-7 (Right panel). In addition, treatment with YJI-7 substantially prevented LPS-stimulated expression of iNOS both mRNA and protein levels (Fig. 5B). YJI-7 treatment also suppressed LPS-induced IL-18 and IFN-β mRNA expression (Fig. 5C, 5D), indicating that YJI-7 diminishes expression of various inflammatory mediators in macrophages. However, YJI-7 treatment did not significantly affect LPS-induced mRNA expression of TNF-α, IL-6, IL-8 and COX-2 expression (Fig. 5E–5H), indicating that YJI-7 modulates production of inflammatory mediators in a gene selective manner.
Fig. 5.
Effects of YJI-7 on the expression of inflammatory mediators in RAW 264.7 macrophages. (A, B) RAW 264.7 macrophages were pretreated with indicated concentration of YJI-7 for 2 h prior to stimulation with LPS. (A) IL-1β mRNA expression was measured by qRT-PCR and normalized to that of GAPDH as described in Materials and Methods. (B) Secreted IL-1β was measured by ELISA. Values are presented as fold change compared to LPS-stimulated cells and are expressed as the mean ± SEM (n=3). *p<0.05 compared with control group, #p<0.05 compared with cells treated with LPS only. (C, D) Cells were pretreated with YJI-7 for 2 h followed by the incubation with LPS. (C) iNOS mRNA expression level was determined by qRT-PCR and normalized to that of GAPDH. Values are represented as fold change compared to LPS-stimulated cells and are expressed as mean ± SEM (n=3). *p<0.05 compared with control group, #p<0.05 compared with cells treated with LPS only. (D) Protein expression of iNOS was measured by Western blot analysis. (E-J) Cells were treated with YJI-7 for 2 h followed by the stimulation with LPS. Expression levels of IL-18 (E), IFN-β (F), TNF-α (G), IL-6 (H), IL-8 (I) and COX-2 (J) mRNA were determined by qRT-PCR. Values are presented as fold change compared to LPS-stimulated cells and are expressed as mean ± SEM (n=3). *p<0.05 compared with control group, #p<0.05 compared with cells treated with LPS only.
Suppression of inflammatory mediators production is mediated by modulation of p38MAPK and JNK signaling
Excessive ROS production acts as a signaling that induces inflammatory response. It has been shown that p38MAPK and JNK signaling cascades are molecular targets of YJI-7 that potentially mediates its effect on LPS-stimulated ROS production (Fig. 3, 4). We next elucidate whether p38MAPK and JNK signaling cascades are involved in the modulation of inflammatory mediators production. Pretreatment with the p38MAPK inhibitor (SB203580) almost completely blocked LPS-stimulated IFN-β expression (Fig. 6A). LPS-stimulated IL-18 expression was also substantially decreased by inhibition of p38MAPK (Fig. 6B), while LPS-stimulated iNOS expression was not affected by the incubation with SB203580 (Fig. 6C), implying that LPS induces IFN-β and IL-18 expression via p38MAPK-dependent manner, whereas iNOS induction by LPS would be mediated via p38MAPK-independent mechanism in our experimental conditions. Based on these data and the results from the previous study, we concluded that YJI-7 regulated expression of IL-18 and IFN-β through probably targeting p38MAPK. We also examined the involvement of JNK signaling in the modulation of the production inflammatory mediators by YJI-7. Pretreatment with the JNK inhibitor (SP600125) significantly decreased LPS-stimulated mRNA expression of IL-18 and IFN-β (Fig. 6D, 6E), while it did not significantly affect iNOS expression (Fig. 6F), which are essentially similar to the observations following treatment with the p38MAPK inhibitor. These results collectively indicate that p38MAPK and JNK signaling cascades are implicated in the regulation of IL-18 and IFN-β by YJI-7, whereas iNOS expression is likely to be regulated via different signaling mechanisms.
Fig. 6.
Role of p38MAPK and JNK signaling in the modulation of the expression of inflammatory mediators by YJI-7 in RAW 264.7 macrophages. (A, B) Cells were pretreated with SB203580, a pharmacological inhibitor of p38MAPK, followed by the incubation with LPS for 6 h. Expression levels of IFN-β (A) and IL-18 (B) mRNA were measured by qRT-PCR as described previously. Values represent the fold change in the mRNA expression level in relation to that detected in cells treated with LPS and are expressed as the mean ± SEM (n=4). Statistical significance is indicated as follows: *p<0.05 for comparisons with values observed in control cells, #p<0.05 for comparisons with values observed in cells treated with LPS alone. (C, D) Cells were treated with LPS for 6 h in the absence or presence of SB203580. Messenger RNA (C) and protein expression (D) levels of iNOS were determined by qRT-PCR and Western blot analyses, respectively. (E–G) RAW 264.7 macrophages were pretreated with SP600125, a selective inhibitor of JNK, for 1 h followed by the stimulation with LPS for additional 6 h. IL-18 (E), IFN-β (F) and iNOS (G) mRNA expression levels were determined by qRT-PCR as indicated previously. Values represent fold change compared to LPS-stimulated cells and are expressed as mean ± SEM (n=4). *p<0.05 compared with control cells, #p<0.05 compared with cells treated with LPS alone.
Effects of YJI-7 on the transcriptional activity of AP-1 and NF-κB in RAW 264.7 macrophages stimulated with LPS
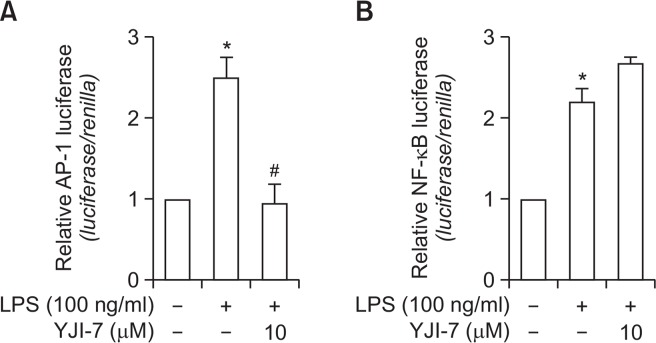
Activator protein (AP-1) and nuclear factor-κB (NF-κB) are well known for their critical roles in the transcriptional regulation of various inflammatory mediators (Roebuck, 1999). IL-18, IFN-β and iNOS have been also shown to contain binding sites for AP-1 and NF-κB. To identify the transcription factor(s) that could mediate the suppression of inflammatory mediators production and could be potentially targeted by YJI-7, we investigated the effects of YJI-7 on transcriptional activity of AP-1 and NF-κB using the luciferase reporter assay. Cells were transfected with the pAP-1-Luc plasmid containing repeated AP-1 recognition sequences or with the pNF-κB-Luc plasmid containing repeated NF-κB recognition sequences and stimulated with LPS in the absence or presence of YJI-7. As demonstrated in Fig. 7, pretreatment with YJI-7 led to a significant decrease in transcriptional activity of AP-1 (Fig. 7A). In contrast, LPS-induced NF-κB activation was not suppressed by pretreatment with YJI-7, rather slightly increased (Fig. 7B). These results suggested that YJI-7-suppression of the production of inflammatory mediators would be mediated via the inhibition of AP-1, rather than NF-κB.
Fig. 7.
Effects of YJI-7 on the transcriptional activity of AP-1 and NF-κB in RAW 264.7 macrophages. RAW 264.7 macrophages were transiently co-transfected with the pAP1-Luc plasmid (A) or pGL4/NF-κB plasmid (B), and Renilla reporter gene. After 24 h of incubation, cells were pretreated with an indicated concentration of YJI-7 for 2 h followed by the stimulation with LPS (100 ng/ml) for additional 6 h. AP-1 or NF-κB-dependent reporter gene expression was determined as indicated in the Materials and Methods. Values represent the fold change in the corresponding reporter expression level in relation to that detected in cells treated with LPS and are expressed as the mean ± SEM (n=3–4). Statistical significance is indicated as follows: *p<0.05 for comparisons with values observed in control cells; #p<0.05 for comparisons with values observed in cells treated with LPS alone.
DISCUSSION
Reactive oxygen species produced as byproducts of oxygen metabolism act as signaling molecules triggering various biological responses. Although ROS play important roles in the maintenance of cellular homeostasis and is required for the proper regulation of physiological processes, undue excessive production of ROS results in critical tissue damages collectively called oxidative stress (Hermes-Lima et al., 2015). Inflammation, a complicated biological process in response to the harmful stimuli, is initiated as a protective immune response. However, in addition to the clearance of harmful pathogens, chronic and excessive inflammation results in tissue injury and is closely associated with pathogenesis of various diseases (Coussens and Werb, 2002), which is similar to the consequences of excessive ROS production. It is widely accepted that ROS generation acts as a key signaling event in the progression of inflammatory disorders. Enhanced ROS production causes the expression of inflammatory mediators and contributes to the development and/or progression of inflammatory diseases (Nomura et al., 2013; Fischer and Maier, 2015). Therefore, modulation of ROS production can be a promising strategy for the prevention and treatment of diseases associated with inflammation. In the present study, we synthesized a chalcone derivative (YJI-7) and demonstrated that it significantly inhibited ROS production in macrophages stimulated with LPS. YJI-7 was also highly effective at suppressing the production of various anti-inflammatory mediators. In addition, YJI-7 did not generate any cytotoxic effects even at high concentration (up to 10 μM), indicating that its propensity to modulates ROS production could be potentially used for the treatment of inflammation-associated diseases.
Chalcones are well known for their pharmacological applications in various diseases. They were originally reported as to possess anti-tumor activity and have been extensively studied as promising scaffold molecules for anti-cancer agents (Mahapatra et al., 2015). Natural and synthetic chalcones generate cytotoxicity and induce apoptosis in various cancer cells. It has been reported that, in addition to their potent anti-tumor activity, chalcones and their various synthetic derivatives possess potent anti-inflammatory and anti-oxidant properties. Chalcones have been shown to suppress the expression of various inflammatory mediators, such as TNF-α, nitric oxide and interleukins (Kontogiorgis et al., 2008; Yadav et al., 2011; Zhang et al., 2014). In addition, they also potently regulate ROS generation in various experimental conditions. Since the initial observations of anti-inflammatory and anti-oxidant activity of chalcones, synthetic modifications of chalcones have been extensively studied in the attempts to develop promising therapeutic agents with enhanced biological activity and less profound side effects. We have previously shown that the synthetic chalcone derivative 3-(4-Hydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one inhibits ROS production and Akt signaling (Kim et al., 2015) and phenyl/hydroxyphenyl-3-thienylpropenones prevents nitric oxide production through HO-1 induction in macrophages (Kim et al., 2014). In this study, as part of our ongoing efforts to develop optimal anti-oxidant and anti-inflammatory agents, we prepared a series of chalcone derivatives incorporating hydroxyl and trifluormethyl groups in phenyl-3-thienylpropenones at different positions and evaluated their anti-oxidative properties. In a structure activity relationship (SAR) study of these chalcone derivatives, we found that a hydroxyl group at the meta or para position along with a trifluormethyl group at the ortho position is required for efficient suppression of ROS production (manuscript in preparation). Among the prepared synthetic chalcone derivatives, YJI-7 most potently suppressed ROS production. In the subsequent experiments, we therefore focused on YJI-7 to elucidate the molecular mechanisms underlying suppression of ROS production. While it is not sufficient for thorough understanding of the structure activity relationship (SAR) of the chalcones to demonstrate the anti-inflammatory and anti-oxidative properties at this stage, further studies seeking to SAR of the chalcone derivatives on suppressing ROS and inflammatory mediators production are worth considering.
Although ROS play vital roles in a number of physiological functions, their excessive production may result in damage various biological molecules ultimately leading to the tissue injuries. It is well established that ROS act as signaling molecules and activate many signaling cascades regulating the expression of inflammatory mediators. For example, ROS signaling is well known to activate the MAPK signaling cascade, which subsequently induces the expression of various inflammatory mediators (Millar et al., 2007; Reddy and Reddanna, 2009). In the present study, we demonstrated that YJI-7 treatment decreased LPS-stimulated ROS production and further prevented p38MAPK and JNK activation without a significant effect on ERK phosphorylation (Fig. 3). In addition to MAPK signaling, AP-1 and NF-κB are also activated in response to a variety of inflammatory signals. These transcription factors are responsible for the expression of genes encoding inflammatory mediators. In our experiments, we found that YJI-7 treatment inhibited LPS-stimulated transcriptional activation of AP-1, whereas no significant effect on NF-κB transcriptional activity was observed. These results collectively indicate that YJI-7 regulates LPS-stimulated production of inflammatory mediators via suppression of p38MAPK and JNK/AP-1 signaling pathways.
Due to the damaging effects of excessively produced ROS, intracellular ROS levels should be tightly regulated within a narrow range. ROS generation is mediated by a variety of cellular systems. Among the various mechanisms, NADPH oxidase has been considered as a major source for ROS generation in macrophages (Lam et al., 2010). NADPH oxidase is composed of multi-subunit components, including membrane-bound proteins (Nox2 and p22phox) and cytosolic components (p40phox, p47phox, p67phox) that translocate to the membrane upon stimulation. Herein, we demonstrated that YJI-7 significantly suppressed LPS-stimulated NADPH oxidase activity. To the best of our knowledge, this is the first report demonstrating the direct modulatory effect of chalcone derivative on NADPH oxidase in macrophages. To further elucidate the underlying molecular mechanisms, we examined the effects of LPS and YJI-7 on the expression of NADPH oxidase subunits and found that LPS treatment significantly enhanced the mRNA expression of p67phox and Nox2 without significant effect on the expression of other subunits in our experimental condition (data not shown). However, YJI-7 treatment did not significantly affect LPS-induced Nox2 and p67phox expression (data not shown), suggesting that the suppression of NADPH oxidase activity would not be mediated via regulating expression of NADPH oxidase subunits, but probably through different mechanisms.
Accumulating evidence suggests that chalcones regulate inflammatory responses via induction of anti-inflammatory genes. For example, synthetic chalcone derivatives suppressed LPS-stimulated nitric oxide production and down-regulated various inflammatory cytokines through heme oxygenase-1 (HO-1) induction (Kaufmann et al., 2016), indicating that HO-1 could partly underlie anti-inflammatory and anti-oxidant properties of chalcones. In the present study, to further elucidate the molecular mechanisms underlying the anti-inflammatory activity of YJI-7, we investigated its effect on HO-1 induction. As expected, YJI-7 treatment induced a significant increase in HO-1 expression. However, the blockade of HO-1 activity by tin-protoporphyrin IX (SnPP) did not restore suppression of ROS production by YJI-7. Moreover, SnPP did not restore diminished levels of IFN-β and IL-18 expression (data not shown), implying that HO-1 induction would not play a critical role in the modulation of inflammatory gene expression by YJI-7. In addition, proteins of the Forkhead Box O (FoxO) transcription factor family have been shown to play a role in the expression of the genes implicated in anti-oxidative stress (Salih and Brunet, 2008). In particular, FoxO3A is well known for its role in the transcriptional activation of diverse anti-oxidative genes, such as superoxide dismutase (Kops et al., 2002), catalase (Salih and Brunet, 2008) and thioredoxin (Li et al., 2009). Therefore, we also examined the involvement of FoxO3A in the regulation of ROS production by YJI-7. However, YJI-7 did not affect FoxO3A expression in our experimental condition (data not shown). At this stage, we could not identify the precise molecular mechanisms underlying the suppression of ROS production and expression of inflammatory genes by YJI-7. Further studies will provide the further insights into the molecular mechanisms that mediate underlying anti-inflammatory and anti-oxidant properties of YJI-7.
In conclusion, we demonstrated that YJI-7, a newly synthesized chalcone derivative, effectively suppressed LPS-stimulated expression of inflammatory genes and ROS production in murine macrophages. Anti-inflammatory and anti-oxidant effects by YJI-7 would be, at least partly, mediated by the suppression of p38MAPK and JNK signaling, and blockade of AP-1 activation.
Regardless of the strong biological activities of chalcone derivatives, previous studies have demonstrated that high concentrations of chalcones would generate cytotoxic effects in normal liver cells, as well as tumorous cells, demonstrating non-selectivity between normal and cancer cells. For example, chalcone derivatives cause excessive cell death in normal hepatocytes via potent suppression of mitochondrial membrane potential (Sabzevari et al., 2004) and induces apoptosis in hepatic stellate cells via enhancing Fas ligand expression (Lee et al., 2011). In addition, chalcones also exhibited cytotoxicity in liver epithelial cells (Forejtnikova et al., 2005), collectively implying that chalcone derivatives need to be carefully monitored before clinical application. In the present study, we found that YJI-7 generated potent anti-inflammatory and anti-oxidative responses without any significant cytotoxic effects even at relatively higher concentrations (up to 10 μM). Based on these results, YJI-7 may have a therapeutic potential for the treatment of diseases associated with inflammation.
Acknowledgments
This research was supported by the Yeungnam University research grant in 2015.
REFERENCES
- Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–11706. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Zhan SS, van Gool WA, Allsop D. Inflammatory mechanisms in Alzheimer’s disease. Trends Pharmacol Sci. 1994;15:447–450. doi: 10.1016/0165-6147(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev. 20152015:610813. doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forejtnikova H, Lunerova K, Kubinova R, Jankovska D, Marek R, Kares R, Suchy V, Vondracek J, Machala M. Chemoprotective and toxic potentials of synthetic and natural chalcones and dihydrochalcones in vitro. Toxicology. 2005;208:81–93. doi: 10.1016/j.tox.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Gordon S. The role of the macrophage in immune regulation. Res Immunol. 1998;149:685–688. doi: 10.1016/S0923-2494(99)80039-X. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Moreira DC, Rivera-Ingraham GA, Giraud-Billoud M, Genaro-Mattos TC, Campos EG. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic Biol Med. 2015;89:1122–1143. doi: 10.1016/j.freeradbiomed.2015.07.156. [DOI] [PubMed] [Google Scholar]
- Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- Kaufmann KB, Gothwal M, Schallner N, Ulbrich F, Rucker H, Amslinger S, Goebel U. The anti-inflammatory effects of E-alpha-(p-methoxyphenyl)-2′,3,4,4′-tetramethoxychalcone are mediated via HO-1 induction. Int Immunopharmacol. 2016;35:99–110. doi: 10.1016/j.intimp.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kadayat T, Kim DE, Lee ES, Park PH. TI-I-174, a synthetic chalcone derivative, suppresses nitric oxide production in murine macrophages via heme oxygenase-1 induction and inhibition of AP-1. Biomol Ther (Seoul) 2014;22:390–399. doi: 10.4062/biomolther.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Kadayat T, Um YJ, Jeong TC, Lee ES, Park PH. Inhibitory effect of 3-(4-Hydroxyphenyl)-1-(thiophen-2-yl) prop-2-en-1-one, a chalcone derivative on MCP-1 expression in macrophages via inhibition of ROS and Akt signaling. Biomol. Ther. (Seoul) 2015;23:119–127. doi: 10.4062/biomolther.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontogiorgis C, Mantzanidou M, Hadjipavlou-Litina D. Chalcones and their potential role in inflammation. Mini Rev Med Chem. 2008;8:1224–1242. doi: 10.2174/138955708786141034. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol. 2010;32:415–430. doi: 10.1007/s00281-010-0221-0. [DOI] [PubMed] [Google Scholar]
- Lee SH, Zhao YZ, Park EJ, Che XH, Seo GS, Sohn DH. 2′,4′,6′-Tris(methoxymethoxy) chalcone induces apoptosis by enhancing Fas-ligand in activated hepatic stellate cells. Eur J Pharmacol. 2011;658:9–15. doi: 10.1016/j.ejphar.2011.01.067. [DOI] [PubMed] [Google Scholar]
- Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, Coselli JS, Chen L, Wang XL, Zhang Y, Shen YH. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–2257. doi: 10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra DK, Bharti SK, Asati V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur J Med Chem. 2015;98:69–114. doi: 10.1016/j.ejmech.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Matos MJ, Vazquez-Rodriguez S, Uriarte E, Santana L. Potential pharmacological uses of chalcones: a patent review (from June 2011– 2014) Expert Opin Ther Pat. 2015;25:351–366. doi: 10.1517/13543776.2014.995627. [DOI] [PubMed] [Google Scholar]
- Millar TM, Phan V, Tibbles LA. ROS generation in endothelial hypoxia and reoxygenation stimulates MAP kinase signaling and kinase-dependent neutrophil recruitment. Free Radic Biol Med. 2007;42:1165–1177. doi: 10.1016/j.freeradbiomed.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepal S, Kim MJ, Hong JT, Kim SH, Sohn DH, Lee SH, Song K, Choi DY, Lee ES, Park PH. Autophagy induction by leptin contributes to suppression of apoptosis in cancer cells and xenograft model: involvement of 53/FoxO3A axis. Oncotarget. 2015;6:7166–7181. doi: 10.18632/oncotarget.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepal S, Park PH. Activation of autophagy by globular adiponectin attenuates ethanol-induced apoptosis in HepG2 cells: involvement of AMPK/FoxO3A axis. Biochim Biophys Acta. 2013;1833:2111–2125. doi: 10.1016/j.bbamcr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Nomura J, Busso N, Ives A, Tsujimoto S, Tamura M, So A, Yamanaka Y. Febuxostat, an inhibitor of xanthine oxidase, suppresses lipopolysaccharide-induced MCP-1 production via MAPK phosphatase-1-mediated inactivation of JNK. PLoS ONE. 2013;8:e75527. doi: 10.1371/journal.pone.0075527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DB, Reddanna P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res Commun. 2009;381:112–117. doi: 10.1016/j.bbrc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-κB (review) Int J Mol Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- Rojkind M, Dominguez-Rosales JA, Nieto N, Greenwel P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol Life Sci. 2002;59:1872–1891. doi: 10.1007/PL00012511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabzevari O, Galati G, Moridani MY, Siraki A, O’Brien PJ. Molecular cytotoxic mechanisms of anticancer hydroxychalcones. Chem Biol Interact. 2004;148:57–67. doi: 10.1016/j.cbi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. BioFactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- Tse G, Yan BP, Chan YW, Tian XY, Huang Y. Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: the link with cardiac arrhythmogenesis. Front Physiol. 2016;7:313. doi: 10.3389/fphys.2016.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Prasad S, Sung B, Aggarwal BB. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int Immunopharmacol. 2011;11:295–309. doi: 10.1016/j.intimp.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Yu LJ, Hui XC, Wu ZZ, Yin KL, Yang H, Xu Y. Hydroxy-safflor yellow A attenuates Aβ1–42-induced inflammation by modulating the JAK2/STAT3/NF-κB pathway. Brain Res. 2014;1563:72–80. doi: 10.1016/j.brainres.2014.03.036. [DOI] [PubMed] [Google Scholar]