SUMMARY
Telomerase maintains chromosome ends from humans to yeasts. Recruitment of yeast telomerase to telomeres occurs through its Ku and Est1 subunits, via independent interactions with telomerase RNA (TLC1) and telomeric proteins Sir4 and Cdc13, respectively. However, the structures of the molecules comprising these telomerase-recruiting pathways remain unknown. Here, we report crystal structures of the Ku heterodimer and Est1 complexed with their key binding partners. Two major findings are: (1) Ku specifically binds to telomerase RNA in a distinct, yet related, manner to how it binds DNA and (2) Est1 employs two separate pockets to bind distinct motifs of Cdc13. The N-terminal Cdc13-binding site of Est1 cooperates with the TLC1-Ku-Sir4 pathway for telomerase recruitment, whereas the C-terminal interface is dispensable for binding Est1 in vitro, yet is nevertheless essential for telomere maintenance in vivo. Overall, our results integrate previous models and provide fundamentally valuable structural information regarding telomere biology.
INTRODUCTION
Telomeres protect the chromosome ends from deleterious fusions and degradation, and allow complete replication of chromosome ends, thus promoting genome integrity and cell viability in eukaryotes (O’Sullivan and Karlseder, 2010). Telomeres are synthesized by telomerase, a multi-subunit RNA-protein (RNP) complex (Egan and Collins, 2012). In the budding yeast Saccharomyces cerevisiae, the telomerase core enzyme minimally consists of the catalytic subunit Est2 and the RNA subunit TLC1 Egan and Collins, 2012). TLC1 provides a template for reverse transcription by Est2 and also functions as a scaffold for the accessory subunits, Est1, the Ku heterodimer, the Sm7 heteroheptamer, and the Pop1/Pop6/Pop7 components of RNase P/MRP (Lemieux et al., 2016; Zappulla and Cech, 2004). Although Est2 and TLC1 are sufficient to reconstitute enzymatic activity in vitro (Zappulla et al., 2005), the additional accessory proteins Est1 and Est3 are required for telomerase function in vivo (Lendvay et al., 1996). Another key player at yeast telomeres is the telomeric single-stranded (ss) DNA-binding protein Cdc13, which acts as a platform for proteins that cap the ends and coordinate the replication of both the telomeric G- and C-rich strands (Nugent et al., 1996; Pennock et al., 2001).
To elongate telomeres, telomerase needs to be properly processed, assembled, and recruited to chromosome ends by interactions with telomeric proteins as well as accessory factors (Egan and Collins, 2012). Two pathways have been discovered that mediate the recruitment of budding-yeast telomerase to telomeres: one is mediated by Est1, and the other by Ku (Chan et al., 2008; Evans and Lundblad, 1999; Hass and Zappulla, 2015). During the G1 phase of cell cycle, the Ku heterodimer specifically recognizes a 48-nucleotide (nt) hairpin of TLC1 to regulate TLC1 nuclear retention and telomerase accumulation at telomeres (Fisher et al., 2004; Gallardo et al., 2008; Peterson et al., 2001). The Ku heterodimer is best known as a DNA-binding protein complex and for its function in the nonhomologous end-joining (NHEJ) pathway of double-stranded (ds) DNA break repair (Fell and Schild-Poulter, 2015). Notably, unlike the specific recognition of TLC1, Ku binds to DNA ends in a sequence-nonspecific manner through a preformed ring structure (Walker et al., 2001). It is still not clear how Ku discriminates between these two nucleic acids by different binding modes.
It was initially proposed that Ku could recruit telomerase to telomeres through simultaneous interactions with the telomerase RNA and the chromosome end (i.e., telomeric dsDNA) (Bertuch and Lundblad, 2003; Peterson et al., 2001). However, experiments using purified S. cerevisiae Ku heterodimer demonstrated that its binding to DNA ends and the TLC1 RNA are mutually exclusive (Pfingsten et al., 2012), strongly suggesting that Ku cannot directly bridge telomerase with telomeres. In addition to TLC1, Ku also interacts with Sir4, a subunit of the Silent Information Regulator (SIR) complex that is recruited to telomeres through the interaction with the telomeric dsDNA-binding protein Rap1 (Hass and Zappulla, 2015; Roy et al., 2004). Thus, Ku binding to Sir4 could contribute to recruit telomerase to telomeres (Figure 1A). Consistent with this hypothesis, Sir4, Ku and TLC1 have been shown to promote telomere lengthening by forming a telomerase-recruitment pathway (Hass and Zappulla, 2015). However, the structural explanation for how Ku simultaneously binds to TLC1 and Sir4 has yet to be elucidated.
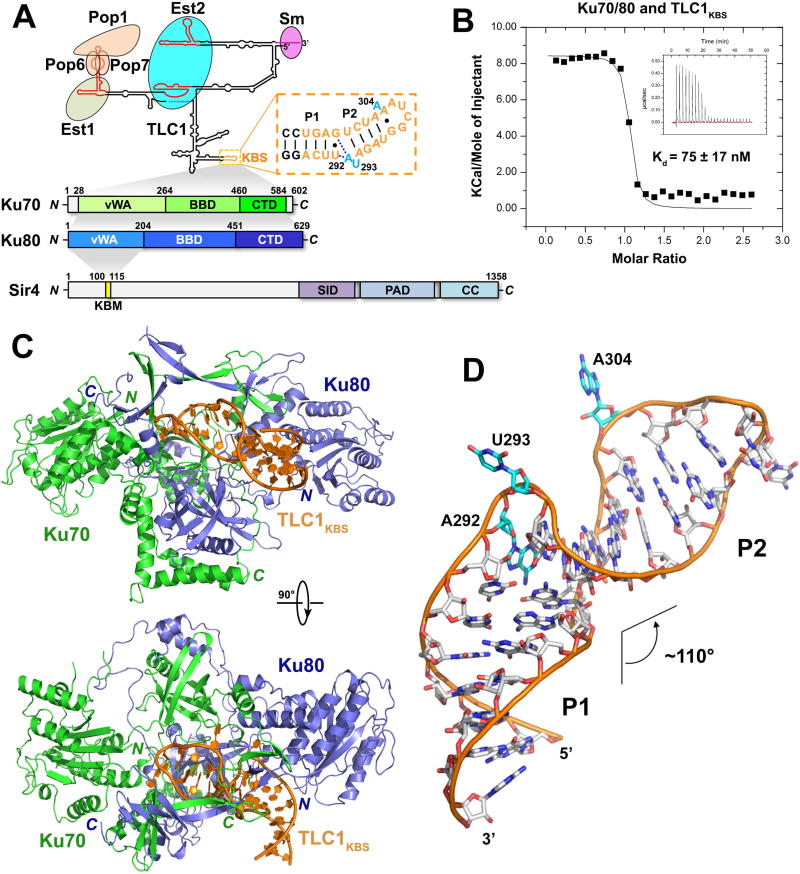
Figure 1. Overview of the Ku-Tlc1KBS Complex Structure.
(A) Domain organization of S. cerevisiae TLC1, Ku70/80, and Sir4. Top: predicted secondary structure of TLC1. Conserved domains, motifs and interacting proteins are designated. TLC1KBS is denoted with a dashed box. Bottom: domain organization of Ku70/80 and Sir4. The shaded areas indicate the interactions of Ku70/80-TLC1, Ku70-Ku80 and Ku80-Sir4, respectively. (SID: Sir2-interacting domain; PAD: partitioning and anchoring domain; CC: coiled coil). (B) ITC measurement of the interaction between Ku70/80 and TLC1KBS. Inset: ITC titration data. (C) Two orthogonal views of the overall structure of the Ku-TLC1KBS complex. Ku70, Ku80 and TLC1KBS are colored in green, slate blue and orange, respectively. (D) Overall conformation of TLC1KBS in the Ku-TLC1KBS complex. The A292U293 and A304 bulges are colored in cyan.
See also Figure S1 and Table S1.
While telomerase is targeted to the duplex region of telomeres by Ku, telomerase recruitment to the 3′ overhang of telomeres appears to be achieved by the interaction between Est1 and Cdc13 in late S phase (Evans and Lundblad, 1999; Pennock et al., 2001), when telomeric overhangs are extended in vivo (Wellinger et al., 1993). The interaction between Cdc13 and Est1 is favored by increased abundance of both proteins in late S/G2 phase and by CDK1-mediated phosphorylation of Cdc13 (Li et al., 2009; Osterhage et al., 2006; Wu and Zakian, 2011). Genetic and biochemical studies have demonstrated that Cdc13 interacts with Est1 via the recruitment domain (RD) of Cdc13 (Pennock et al., 2001).
An extensively studied mutant, cdc13–2 — a charge-swap (E252K) mutation within Cdc13RD — displays an ever-shorter-telomeres (est) phenotype and leads to a decrease, or even loss, of late S phase telomere association of Est1 and Est2 (Chan et al., 2008; Nugent et al., 1996). Notably, telomere shortening and senescence caused by cdc13–2 can be suppressed by another charge-swap (K444E) mutation in Est1 (est1–60), arguing that direct contact between Cdc13 and Est1 is required for telomerase recruitment to telomeres in late S phase (Pennock et al., 2001). However, inconsistent with this hypothesis, neither the Cdc13E252K nor the Est1K444E mutation affects the interaction between Est1 and Cdc13 in vitro (Wu and Zakian, 2011). These findings suggest that the Cdc13E252-Est1K444 salt bridge may not be required for the Cdc13-Est1 interaction per se, but for a subsequent step necessary for telomerase stabilization and/or activation. However, a high-resolution structure of the Est1-Cdc13 complex still has yet to be determined, hindering mechanistic understanding of its regulation.
Here we provide structural and functional insights into protein-protein and protein-RNA interfaces of key telomeric proteins involved in the control of telomerase recruitment. We provide an integrated model depicting how telomeric proteins cooperate to recruit yeast telomerase.
RESULTS
Overall structure of the Ku-TLC1 complex
Isothermal titration calorimetry (ITC) measurements revealed that a shorter 25-nt fragment of TLC1 (nt 288–312) is sufficient for binding to Ku70/80 with an equilibrium dissociation constant (Kd) of 75 nM (Figure 1A and 1B). TLC1288–312 was predicted to form a hairpin with a two-nucleotide bulge (A292U293) at the center (Figure 1A). Hereafter, we will refer to TLC1288–312 as the TLC1 Ku-binding-site or TLC1KBS (Figure 1A). To reveal the structural basis of the Ku heterodimer binding to TLC1, we determined the Ku70/80-TLC1KBS ternary complex structure at a resolution of 2.8 Å (Figure S1A and Table S1). The structure exhibits a 1:1:1 stoichiometry and buries a total of ~1,610 Å2 surface area between the Ku heterodimer and TLC1KBS (Figure 1C). The Ku heterodimer adopts a cradle-shaped closed-ring architecture (Figure 1C). Ku70 and Ku80 share a three-domain topology comprising an a/b von Willebrand factor type A (vWA) domain, a β-barrel domain (BBD), and an extended a-helical domain (CTD) (Figure S1B and S1C). The N-terminal vWA domains of Ku70 and Ku80 lie at the periphery of the cradle and make little contribution to heterodimerization. In contrast, the extended C-terminal a-helical motifs of both Ku70 and Ku80 embrace the β-barrels of the opposite subunits to form the flat base of the cradle, resulting in an extensive interface between Ku70 and Ku80 (Figure 1C). Within the β-barrel domains there is a marked insertion comprising an a helix and five or six β-strands that together form the arch of the cradle (Figure S1B and S1C).
The Ku70/80-TLC1KBS complex structure reveals that binding with the Ku heterodimer induces TLC1KBS to adopt a unique bulged stem-loop structure (Figure 1D). Regarding the AU bulge previously predicted in the secondary structure of free TLC1KBS, the crystal structure shows that only U293 flips out, while the base of A292 actually folds back into the RNA helix and stacks on the A294-U308 base pair (Figure 1D). Furthermore, there is a second bulge formed by A304 of TLC1KBS in the complex, which was not predicted in previous studies (Dalby et al., 2013; Peterson et al., 2001) (Figure 1D). The two base-paired stems of TLC1KBS before A292 (G286 to U291 paired with G309 to C314) and between U293 and A304 (A294 to U297 paired with A305 to U308) — hereafter referred to as Stems P1 and P2 — each adopt an A-form helix conformation (Figure 1D). Notably, binding with Ku70/80 induces a ~110° bend between the P1 and P2 helices of TLC1KBS (Figure 1D).
Structure of TLC1KBS and its interface with Ku
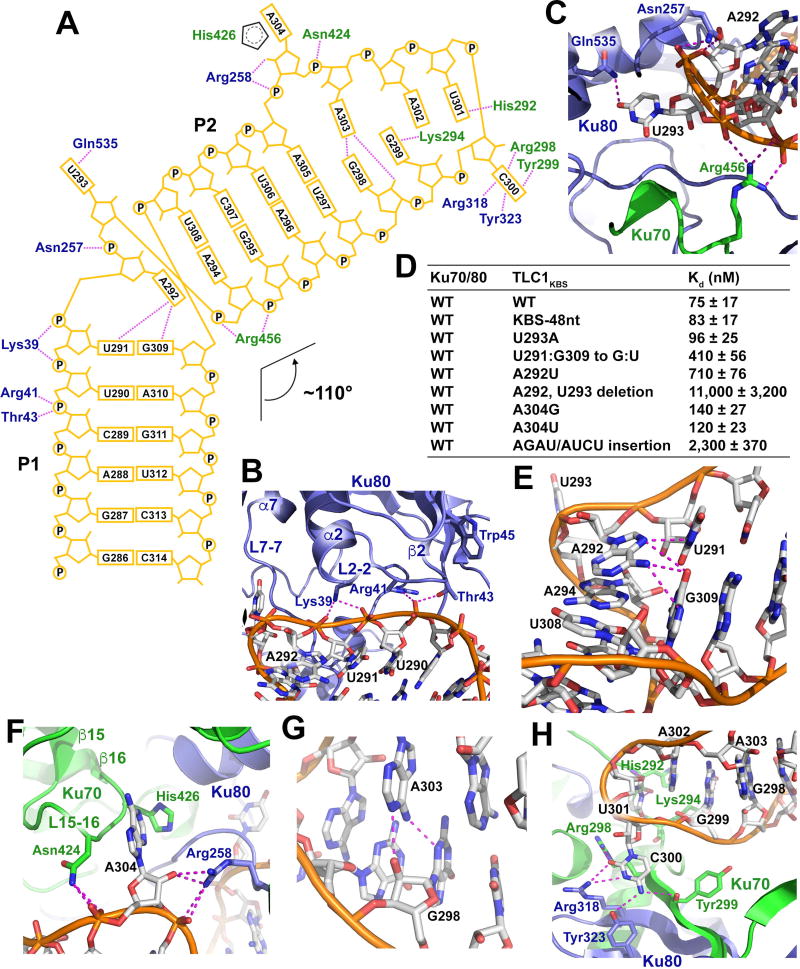
Close examination of the contacts between TLC1KBS and Ku70/80 suggests that the specificity of binding relies on both the conformation and the sequence of the RNA. The P1 stem of TLC1KBS skews towards the vWA domain of Ku80 (Figure 1C). Three consecutive nucleotides — U290, U291, and A292 — in Stem P1 are embedded into a concave groove formed by loops L2–2 (between helix α2 and strand β2) and L7–7 (between helix α7 and strand β7) of Ku80vWA (Figures 2A and 2B). This interface is stabilized by four hydrogen bonds between the RNA backbone and Ku80 residues Lys39, Arg41 and Thr43 (Figure 2A and 2B) mainly contributing to the binding affinity but not specificity. Notably, a previous study showed that a 5-amino-acid insertion at residue Trp45 in Ku80vWA (mutant yku80–135i) abolished the interaction between Ku and TLC1 (Stellwagen et al., 2003). This appears to be clearly explained by the crystal structure: the 5-residue insertion is right in loop L2–2 of Ku80 and would be expected to alter the conformation important for the recognition of Stem P1 of TLC1KBS by Ku80 (Figure 2B).
Figure 2. Structural and Mutational Analysis of the Ku-TLC1KBS Interaction.
(A) Schematic diagram of the Ku-TLC1KBS interactions. TLC1KBS is shown in orange. Ku70 and Ku80 residues are shown in green and slate blue, respectively. Hydrogen bonds are denoted as magenta dotted lines. (B) Detailed interactions between the P1 stem backbone of TLC1KBS with Ku80. (C) U293 of TLC1KBS flips out from the RNA into a pocket formed by both Ku70 and Ku80. (D) ITC data of the interactions between Ku70/80 and wild-type and mutant TLC1KBS(E) A292 of TLC1KBS mediates hydrogen-bonding interactions with the U291•G309 pair and stacking interaction with the A294-U308 pair. (F) Detailed interactions of the second bulge at A304 with its surrounding Ku70/80 residues. (G) Hydrogen-bonding interactions in the trans Sugar Edge/Watson-Crick G298•A303 pair. (H) Detailed interactions of the nucleotides G299C300U301A302 with their surrounding Ku residues.
See also Figure S2.
The most prominent features of TLC1KBS are the two bulges at the apical portion of the sharply bent P2 stem (Figure 1D). In the middle of TLC1KBS, the flipped-out U293 points into a pocket formed by both Ku70 and Ku80 (Figure 2C). Notably, the base of U293 in the pocket mediates only one hydrogen-bonding interaction with the side chain of Ku80Gln535 (Figure 2C). The structure shows that the cavity of the pocket should be large enough to accommodate bulky purine nucleotides (Figure 2C). Indeed, substituting A for U at position 293 still maintained a wild-type interaction with the Ku heterodimer (Figures 2D and S2A). The backbone phosphate groups at both sides of U293 form a panel of electrostatic interactions with Ku80Asn257 and Ku70Arg456, stabilizing the base of U293 in the pocket and helping define the sharp kink of TLC1KBS at the AU bulge (Figure 2C). In contrast to U293, A292 folds back into the ds stem of TLC1KBS, stacking on the A294-U308 pair, and making a ~70° angle relative to the plane of the U291•G309 pair (Figure 1D and 2E). Both the amino and imino groups of A292 mediate hydrogen-bonding interactions with the U291•G309 pair (Figure 2E). This extensive intramolecular interaction network provides a sequence constraint to TLC1KBS because a compensatory G291•U309 mutant of the U291•G309 pair and a uracil replacement of A292 led to ~6- and ~10-fold reductions of the binding affinity between TLC1KBS and Ku, respectively (Figures 2D and S2A). Collectively, the unusual configuration of the AU bulge induced by the binding of Ku defines the overall shape of TLC1KBS and creates a ~110° angle between the P1 and P2 stems (Figure 1D). Consistent with this observation, removal of the AU bulge greatly weakened the interaction between Ku and TLC1KBS (Figures 2D and S2A).
The second and previously unappreciated bulge in TLC1KBS, A304, is situated in a pocket formed by Ku70 (Figures 1D and 2F). The base of A304 packs against the aliphatic loop L15–16 (between strands β15 and β16) of Ku70 and makes no sequence-specific hydrogen-bonding interactions with the protein (Figure 2F). Substitution of A304 with any other nucleotide had marginal effect on the interaction between Ku and TLC1KBS (Figures 2D and S2A). Similar to the case of U293, the backbone phosphate groups at both sides of A304 form hydrogen bonds with residues of each Ku subunit (Figure 2F). In addition, the sugar-ring hydroxyl group of A304 also accepts a hydrogen bond from the side chain of Ku80Arg258 (Figure 2F). The bulged bases of U293 and A304 function as two anchor points to align the major groove of Stem P2 with the complementary binding surface on one side of the arch of the Ku heterodimer (Figure 1C). This structural feature suggests that the distance between the two anchor points can only accommodate four base pairs in TLC1KBS. Consistent with this observation, insertion of base pairs between U293 and A304 weakened the interaction between Ku and TLC1KBS by three orders of magnitude (Figures 2D and S2A).
At the very tip of the hairpin, the bases of G298 and A303 of TLC1KBS rotate away from their canonical Watson-Crick positions and form a trans Sugar Edge/Watson-Crick G•A pair (Figure 1D and Figure 2G) (Leontis and Westhof, 2001). The curved surface of the Ku aperture forces the terminal loop of TLC1KBS to adopt a compact conformation with the bases of G299, A301 and A302 stacking together (Figure 2H). Collectively, the structure of the Ku-TLC1KBS complex reveals that, except for Stems P1 and P2, the conformations of the two bulges and the terminal loop of TLC1KBS are largely determined by the interaction with the Ku heterodimer.
Structural comparison of yeast Ku-TLC1KBS and human Ku-dsDNA complexes
Comparison of the yeast Ku-TLC1KBS complex structure with that of human Ku bound to dsDNA reveals that the telomerase RNA utilizes a very different strategy to bind to the Ku heterodimer. First, in the Ku-dsDNA complex, the DNA adopts an orientation roughly perpendicular to the arch of Ku70/80, sitting right on top of the floor of the Ku cradle formed by the β-barrels of Ku70 and Ku80 (Figure S1D) (Walker et al., 2001). In contrast, in the Ku-TLC1KBS complex structure, the P1 stem of the RNA fits into a groove between the β-barrel and the vWA domain of Ku80 away from the central pseudo-dyad in the middle of the Ku heterodimer (Figure S1E). Distal to the AU bulge, the P2 stem alters the orientation to align almost parallel with the arch of the Ku complex (Figure S1E). Second, Ku binds to dsDNA with no sequence specificity, whereas in the Ku-TLC1KBS complex, there is a strong sequence constraint, based on the unique RNA double-bulge conformation essential for the interaction (Figures 2D and S2A). Despite these marked differences, the TLC1KBS RNA and dsDNA nevertheless occupy the same aperture of Ku70/80 (Figure S1D and S1E), suggesting that TLC1 RNA and dsDNA cannot bind to the Ku heterodimer simultaneously. It was previously hypothesized that Ku recruits telomerase by associating with telomerase through its interaction with TLC1 and concurrently binding chromosomal DNA ends (Peterson et al., 2001). Our structural data and a previous biochemical study clearly reveal that the Ku-TLC1 interaction is not compatible with an interaction between Ku and dsDNA (Figure S1D and S1E) (Pfingsten et al., 2012), and thus refute this hypothesis.
Characterization of the interaction between Ku80 and Sir4
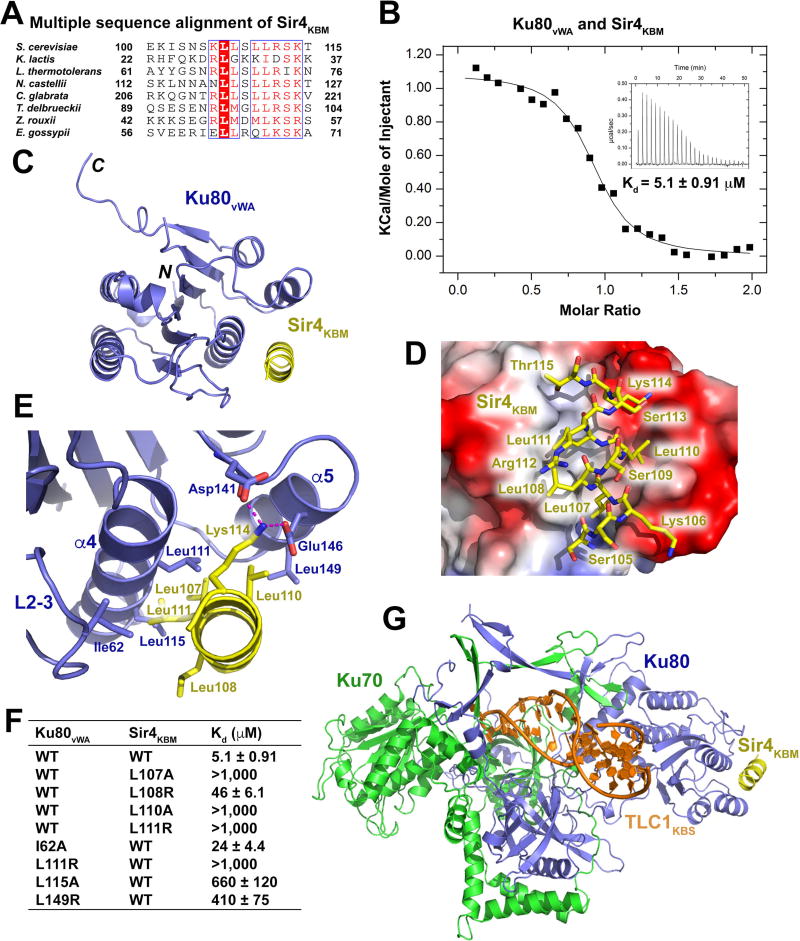
Another proposed mechanism of telomerase recruitment is through the interaction between Ku and Sir4 (Hass and Zappulla, 2015). The N-terminal region of Sir4 (residues 1–200) was reported to interact with Ku80 (Roy et al., 2004). Using yeast two-hybrid analysis, we found that a short conserved fragment of Sir4 consisting of only residues 100–115, hereafter referred to as Sir4KBM (Ku-binding motif), is necessary and sufficient for binding with Ku80 (Figure 1A, Figure 3A and Figure S3A). A previous study showed that mutations of residues in helix a5 in Ku80vWA disrupted the interaction with Sir4, suggesting that Ku80vWA might mediate the interaction with Sir4 (Ribes-Zamora et al., 2007). Yeast two-hybrid and ITC data confirmed that Ku80vWA itself was indeed sufficient to bind to Sir4KBM with an affinity of 5.1 µM (Figure 3B and Figure S3A).
Figure 3. Structural and Mutational Analyses of the Ku80vWA-Sir4KBM Interaction.
(A) Multiple sequence alignment of Sir4KBM. Conserved residues of Sir4KBM are boxed and highlighted in red. (B) ITC measurement of the Ku80vWA-Sir4KBM interaction. Inset, ITC titration data. (C) Overall structure of the Ku80vWA-Sir4KBM complex. Ku80vWA and Sir4KBM are colored in slate blue and yellow, respectively. (D) Electrostatic surface potential of the Sir4KBM binding site of Ku80vWA (positive potential, blue; negative potential, red). Sir4KBM is presented in stick model and colored in yellow. (E) Details of interactions between the Sir4KBM helix and helices α4 and α5 and loop2–3 of Ku80vWA. Hydrogen bonds are denoted as magenta dashed lines. (F) ITC data of wild-type and mutant Ku80vWA-Sir4KBM interactions. (G) Atomic model of the TLC1KBS-Ku70/80-SirKBM complex. TLC1KBS, Ku70, Ku80, and Sir4KBM are colored in orange, green, slate blue, and yellow, respectively.
See also Figure S3 and Table S2.
Structural basis for the Ku80vWA-Sir4KBM interaction
To reveal the structural basis of Sir4 recognition by Ku80, we determined the crystal structure of the Ku80vWA-Sir4KBM complex at a resolution of 2.4 Å (Figure S3C and Table S2). The Sir4KBM peptide is folded into a single amphipathic a-helix that fits into a hydrophobic groove of Ku80vWA (Figure 3D). The hydrophobic portion of the Sir4KBM helix packs against the floor of the groove formed by helices a4 and a5 and a portion of the loop L2–3 between strands β2 and β3 of Ku80vWA (Figure 3E). The intimate contacts among the hydrophobic side chains of residues at the interface define the specific recognition of Sir4KBM by Ku80vWA (Figure 3E). In addition, two salt-bridge connections between the side chains of Asp141 and Glu146 of Ku80 and Lys114 of Sir4 further strengthen the Ku80vWA-Sir4KBM interaction (Figure 3E).
Consistent with the structural data, ITC analysis showed that substitution of Leu107, Leu110, or Leu111 of Sir4KBM at the hydrophobic interface with a positively charged arginine residue completely disrupted the binding to Ku80vWA (Figures 3F and S3E). Notably, the Sir4 L108R single mutation weakened, but did not completely disrupt, the interaction, consistent with the fact that this residue protrudes away from the binding interface and only mediates a partial contact with Ku80 (Figures 3E, 3F and S3E). Similarly, mutations of Ku80 residues at the interface also either completely abolish or greatly weakened the Ku80-Sir4 interaction (Figures 3F and S3E). The effects of these mutants were also confirmed by yeast two-hybrid analysis (Figure S3B). Taken together, we conclude that the hydrophobic contacts observed in the crystal structure are the major driving force underlying the interaction between Ku80 and Sir4.
Superposition of the Ku80vWA-Sir4KBM complex structure with that of the Ku-TLC1KBS complex reveals that the Ku80-Sir4 interaction does not interfere with the interface between Ku and TLC1 (Figure 3G), raising the possibility that the Ku heterodimer is capable of binding with both TLC1 and Sir4 simultaneously. To test this idea, we performed GST pull-downs and discovered that Ku70/80, TLC1KBS and Sir4KBM indeed bind concurrently to form a single quaternary complex in solution (Figure S3D). Thus, the structural model of the TLC1KBS-Ku-Sir4KBM complex provides an atomic-level explanation of how telomerase is recruited to telomeres via binding between the Ku subunit and telomere-associated Sir4.
Ku Interactions with TLC1 and Sir4 are required for telomere-length maintenance
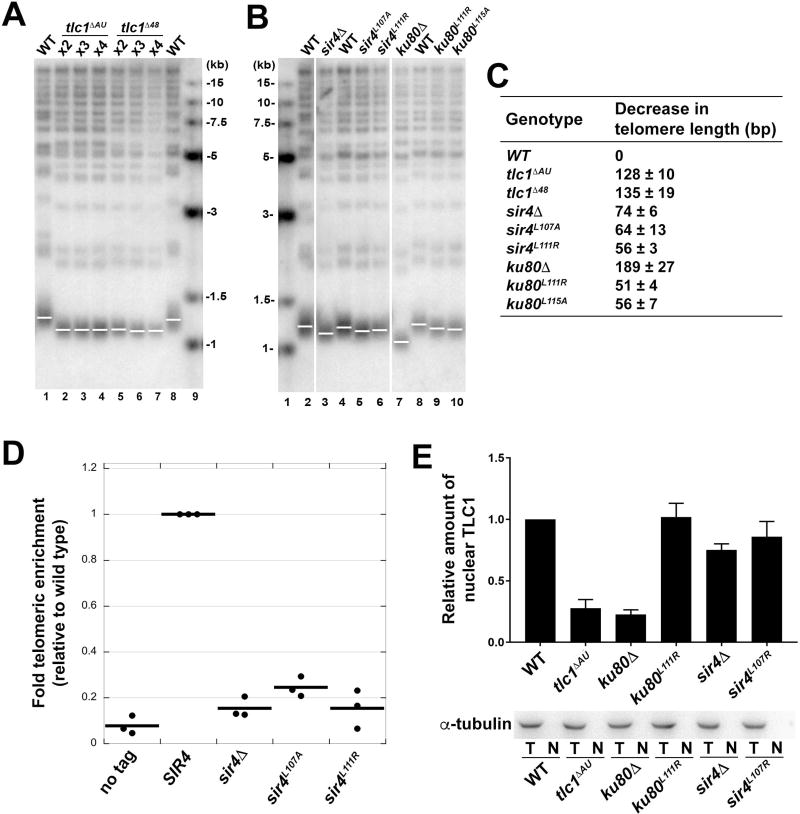
Based on the structural information, we assessed whether disruption of the Ku-TLC1 and the Ku-Sir4 interactions interferes with telomere length homeostasis. We replaced the wild-type TLC1 gene with deletion-mutant tlc1ΔAU lacking the A292U293 bulge in the Ku-binding site and measured the length of telomeres. We found that telomeres in tlc1ΔAU cells were ~128 bp shorter than wild type (Figures 4A and 4C). Notably, a deletion of the entire 48-nt Ku-binding stem-loop structure from TLC1 (tlc1Δ48) led to a ~135 bp reduction in telomere length, a defect very similar to that of the tlc1ΔAU mutant (Figures 4A and 4C). This finding that tlc1ΔAU phenocopies the tlc1Δ48 mutant suggests that the A292U293 bulge is essential for the in vivo interaction between TLC1 and Ku.
Figure 4. Mutations Disrupting the Ku-TLC1 or Sir4-Ku80 Interactions Reduce Telomere Length.
(A) Southern blot analysis of XhoI-digested yeast genomic DNA shows the length of Y’ telomeres and non-Y’ telomeres over the course of streaks 2–4 using TG(1–3) repeat telomeric probe. (B) Southern blot analysis of telomere lengths of wild-type, sir4 and ku80 mutant strains. Vertical white spaces between lanes 2 and 3, 6 and 7, indicate cropping of superfluous lanes. (C) Measurement of average telomere length of yeast strains shown in (A) and (B). Telomere length was calculated from three independent experiments (mean ± SEM). (D) ChIP analysis of Est2 enrichment at telomere VI-R in wild-type, sir4ΔA, sir4L107A , and sir4L111R strains. The thick horizontal lines on the graphs represent averages of three independent biological replicates indicated by black dots. (E) Upper panel: the quantification of relative TLC1 RNA in nuclei of wild-type, tlclΔAU, ku80A, ku80L111R, sir4Δ, sir4L107R strains. Results are from three independent experiments (mean ± SEM). Lower panel: Western blot of α-tubulin in total cell extracts (T) and nuclear extracts (N) from strains described above indicated the absence of cytoplasmic contaminants in the isolated nuclei.
See also Figure S4.
To further examine the functional significance of the Ku80-Sir4 interaction, we mutated two key Sir4-binding residues in Ku80 (Leu111 and Leu115) (Figure 3F) and examined their effects on yeast growth and telomere-length maintenance. We found that in both yku80 L111R and L115A strains, telomeres were 51–56 bp shorter than wild type (Figures 4B and 4C). Similarly, mutating two residues in Sir4, Leu107 and Leu111, on the other side of the interface also led to a 56–64 bp reduction in telomere length compared to wild type (Figures 3F, 4B, and 4C). Next, we directly examined the role of the Ku80-Sir4 interaction in recruiting telomerase to telomeres by performing chromatin immunoprecipitation (ChIP) on the telomerase catalytic subunit Est2 (Figure 4D). Telomerase enrichment at telomere VI-R in sir4 L107A and L111R cells was reduced to ~25% and ~15% the level of wild type, respectively, which is essentially the same as sir4Δ cells (Figure 4D). These ChIP results provide clear evidence that the Ku80-Sir4 interaction is required for Ku-mediated telomerase recruitment to telomeres.
Notably, yku80Δ cells maintained telomeres ~190 bp shorter than wild type, substantially shorter than telomere length of the Ku80-Sir4 binding-deficient mutant strains (Figures 4B and 4C). This was expected in part because, in addition to recruiting telomerase to telomeres, Ku70/80 is also important for the nuclear retention of the TLC1 RNA (Gallardo et al., 2008). Indeed, in both tlc1ΔAU and yku80Δ cells, nuclear TLC1 RNA was also reduced nearly 4-fold (Figure 4E). In contrast, in yeast cells where only the Ku80-Sir4 interaction was disrupted, Ku still maintained the wild-type interaction with TLC1, and thus retained the TLC1 RNA in the nucleus (Figure 4E). In addition, the DNA end-binding activity of Ku has been shown to play a role in telomere-length regulation and telomeric capping, independent of its role in TLC1 nuclear retention (Lopez et al., 2011; Williams et al., 2014), thus further explaining the shorter telomeres of yku80Δ cells relative to those expressing tlc1ΔAU (Figure 4C).
In all the aforementioned mutational studies, the mutant strains maintained shortened, but stable, telomere lengths with no senescence (Figure 4C, S4A and S4B). In addition, mutant strains showed no sign of telomere rearrangements, characteristic of the recombination-based “survivor” pathway for maintaining telomeres in the absence of telomerase (Figures 4A and 4B) (Lundblad and Blackburn, 1993). These results indicate that the TLC1-Ku-Sir4 interaction network contributes to telomerase recruitment, but it is not crucial for telomere maintenance as anticipated by the modest reduction of telomere length in the sir4Δ cells.
Crystal structure of the Est1-Cdc13EBM complex
In addition to the Ku-Sir4-mediated telomerase-recruitment pathway, yeast telomerase is also targeted to the 3′ overhang of telomeres through the direct interaction between Est1 and Cdc13 to avoid senescence (Pennock et al., 2001). Multiple-sequence alignment of yeast Est1 proteins revealed that the N-terminus of Est1 is well-conserved, whereas the C-terminus of Est1 is highly variable both in sequence and length and is likely structurally disordered (Figure S5A and S5B). To characterize Est1 and its interaction with Cdc13, we expressed and purified the conserved N-terminal domain of Est1 (NTD) from various yeast species. We found that Kluyveromyces lactis (Kl) Est1NTD (residues 1–600) could be expressed and purified in large quantities. Hereafter, for simplicity, we will refer to KlEst1NTD as KlEst1, unless stated otherwise.
The recruitment domain between the first and second Oligonucleotide/oligosaccharide binding (OB) folds of S. cerevisiae Cdc13 (ScCdc13RD, residues 232–334) was reported to interact with ScEst1 (Figure 5A) (Pennock et al., 2001). ITC analysis revealed that a short fragment of KlCdc13 (residues 213–240) within the corresponding KlCdc13RD (residues 213–356) was sufficient for binding to KlEst1, with an affinity of 5.9 µM (Figures 5B and S5C). Hereafter, we will refer to KlCdc13213–240 as KlCdc13EBM (Est1-binding motif). We determined the KlEst1-KlCdc13EBM complex crystal structure at a resolution of 2.2 Å (Figure 5C; Table S3). KlEst1 adopts a globular architecture with two subdomains — an N-terminal tetratricopeptide repeat (TPR)-containing subdomain and a downstream helical-hairpin subdomain — that pack against each other (Figure 5C). The helical-hairpin subdomain is built of multiple helix-turn-helix motifs with a β-hairpin at the C-terminus (Figure 5C). Notably, a large insert between helices α21 and α24 in the helical-hairpin subdomain forms an extended coil with three short helices that packs in the wedge between the TRP and helical hairpin subdomains (Figure 5C).
Figure 5. Structural and Mutational Analyses of the KlEst1-KlCdc13EBM Interaction.
(A) Domain organization of KlEst1 and KlC\Cdcl3. Domains of KlEst1 (TPR, tetratricopeptide repeat; HHD, helical hairpin domain; IM, insertion motif) are colored in green, cyan, and slate blue, respectively, and KlCdcl3EBM is in yellow. The shaded area indicates the interaction between KlEst1 and KlCdcl3EBM- (DBD: DNA-binding domain) (B) ITC measurements of interactions between KlEstl with different fragments of KlCdcl3 within KlCdcl3RD. Inset: ITC titration data. UD: undetectable. (C) Overall structure of the KlEstl-KlCdc13 complex. KlEstl and KlCdcl3EBM are colored as in (A). KlCdcl3EBM is shown as a stick model. (D) Multiple sequence alignment of Cdcl3EBM from different yeast species. The conserved residues are highlighted in red and KlCdcl3EBM-N and KlCdcl3EBM-c are highlighted in blue boxes. Glu233 in KlCdc13 (equivalent to Glu252 in ScCdcl3) is denoted with an arrow. (E) Electrostatic surface potential of the KlCdc13EBM-binding cavity of KlEst1 (positive potential, blue; negative potential, red). KlCdc13EBM is shown in a stick model and colored in yellow. (F) Details of interactions between KlCdc13EBM-N and its surrounding residues of KlEst1. Hydrogen bonds are denoted as dashed magenta lines. (G) ITC data of the wild-type and mutant KlEst1-KlCdc13EBM interactions. Counterpart residues in S. cerevisiae proteins are shown in parenthesis. (H) Details of interactions between KlCdc13EBM-C and its surrounding residues of KlEst1. Glu233 in KlCdc13 and Lys467 in KlEst1 are highlighted with boxes.
See also Figure S5, S6 and Table S3.
Multiple-sequence alignment reveals that Cdc13EBM contains two separate conserved motifs (Figure 5D). Accordingly, the KlCdc13EBM peptide adopts an extended conformation and folds into two separate binding sites of KlEst1 (Figure 5C). Notably, in the KlEst1-KlCdc13EBM complex, residues 213–222 and 232–238 of KlCdc13EBM assume well-defined conformations, whereas the middle portion is disordered (Figure S6A). Hereafter, we will refer to the two well-defined fragments as KlCdc13EBM-N and KlCdc13EBM-C, respectively (Figure 5D).
The Est1-Cdc13 interface
KlCdc13EBM-N adopts a slightly curved conformation and fits into the hydrophobic pocket formed by the TPR subdomain and the large insert in the helical hairpin subdomain of KlEst1 and contributes most of the interactions (Figures 5E and 5F). The 10-amino-acid motif of KlCdc13EBM-N is stabilized by three intramolecular hydrogen bonds formed by the side chains of Asn215 and Gln219 and the backbone of Asn215 and Leu217 (Figure 5F). This hydrogen-bonding network is further extended to KlEst1 through the side-chains of KlEst1 Arg266 and Glu523, both of which are highly conserved within the family of Est1 proteins (Figures 5F and S5A). KlCdc13 Pro216 is located at a critical position in KlCdc13EBM-N, where its pyrrolidine ring plugs into the narrow entrance of the KlCdc13EBM-N-binding pocket formed by the aliphatic side chains of Ile183, Met187, Arg266, and Leu267 of KlEst1 (Figure 5F). An alanine substitution of KlCdc13Pro216 completely abolished the KlEst1-KlCdc13EBM interaction (Figures 5G and S6B). The curved configuration of KlCdc13EBM-N positions the phenol ring of Phe218 into a deep hydrophobic pocket of KlEst1 (Figure 5F). Substitution of KlCdc13Phe218 with an alanine or even a tyrosine or histidine residue completely abolished the KlEst1-KlCdc13EBM interaction (Figures 5G and S6B), suggesting that the Phe218-binding pocket is highly specific for a phenylalanine side-chain. Similarly, previous studies suggested that mutation of the counterparts of Pro216 and Phe218 in ScCdc13 disrupted the interaction with ScEst1 (Gao et al., 2010). In addition to the highly conserved Pro216 and Phe218, Leu217 and Leu220 of KlCdc13EBM-N also contribute hydrophobic contacts with KlEst1 (Figures 5F, 5G and S6B).
In contrast to the hydrophobic nature of KlCdc13EBM-N, the prominent feature of KlCdc13EBM-C is that it contains two proximate acidic residues, Glu233 and Asp235 (Figure 5H). The KlCdc13EBM-C-binding cavity of KlEst1 has a highly basic potential and is surrounded by a group of basic residues (Figures 5E and 5H). The side-chain of KlCdc13Glu233 is directed toward KlEst1Lys467, indicating ionic interactions between the two residues (Figure 5H). Notably, previous genetic evidence showed that their equivalent residues in S. cerevisiae proteins, ScCdc13Glu252 and ScEst1Lys444, form an ion pair that is essential for telomere replication (Figure 5D) (Pennock et al., 2001). At the C-terminus of the KlEst1-KlCdc13EBM-C interface, the side chains of two conserved residues, KlCdc13Ser236 and KlEst1Arg478, are coordinated via a water molecule through hydrogen-bonding interactions (Figure 5H). In addition to these electrostatic contacts, the phenol ring of the conserved Phe234 in KlCdc13EBM-C fits into a conserved narrow pocket at the bottom of the KlCdc13EBM-C-binding cavity (Figure 5H). Strikingly, mutagenesis analysis demonstrated that none of the residues in KlCdc13EBM-C is required for the KlEst1-KlCdc13EBM interaction (Figures 5G and S6B). Furthermore, ITC measurement showed that KlCdc13EBM-N alone is capable of binding to KlEst1 with an affinity of 15.3 µM that is ~ 3-fold weaker than that of KlCdc13EBM (Figures 5B and S5C). In sharp contrast, KlCdc13EBM-C alone did not show any detectable interaction with KlEst1 (Figures 5B and S5C). Therefore, we concluded that binding of KlEst1 to KlCdc13 is mostly mediated by the interaction between KlEst1 and the N-terminal segment of the bipartite KlCdc13EBM, whereas the C-terminal motif is dispensable for the interaction.
Disruption of the Est1-Cdc13EBM-N interface compromises Est1 and Est2 association with telomeres in late S phase
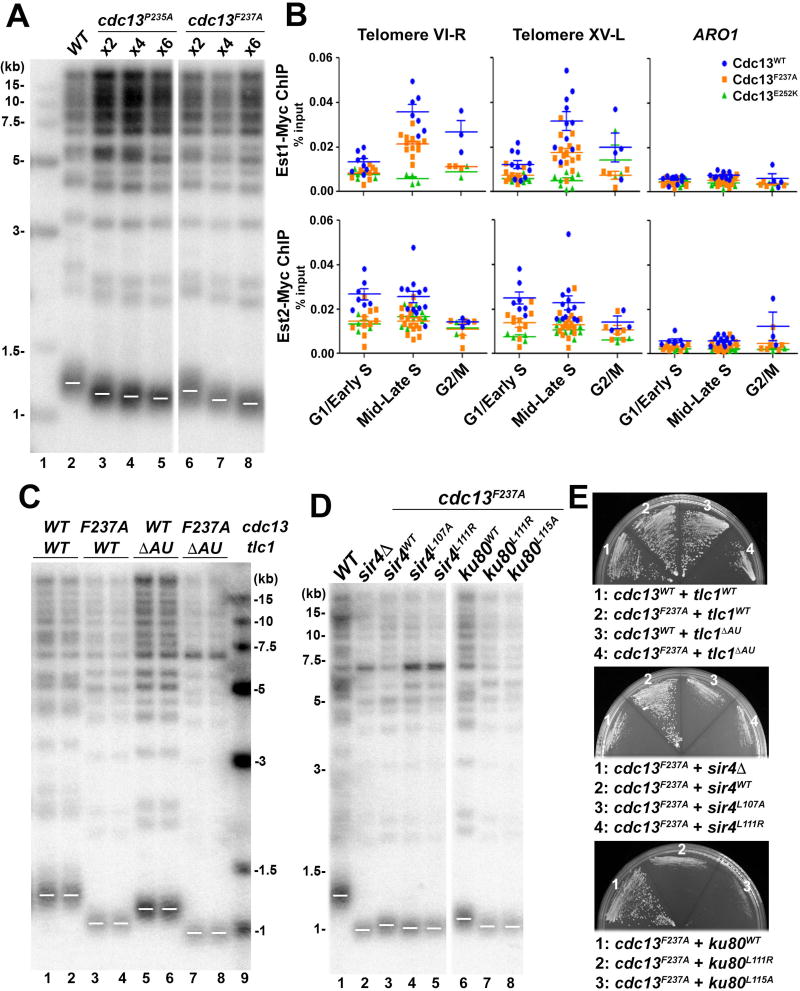
Residues important for the KlEst1-KlCdc13 interaction are highly conserved in ScEst1 and ScCdc13 (Figures 5D and S5A), suggesting that the bivalent interface observed in the KlEst1-KlCdc13EBM structure is also conserved in the interaction between ScEst1 and ScCdc13. To examine the functional significance of the Est1-Cdc13 interaction, we replaced two key residues in ScCdc13EBM-N, Pro235 and Phe237 (equivalent to Pro216 and Phe218 in KlCdc13EBM-N that are crucial for the KlEst1-KlCdc13 interaction) with alanine. Both the cdc13P235A and cdc13F237A strains displayed normal levels of Cdc13 and reduced but stable telomere lengths (~220 bp reduction) (Figures 6A, S7A and S7B) (Gao et al., 2010).
Figure 6. The TLC1-Ku70/80-Sir4 and the Est1-Cdc13 Pathways Have Additive Effect on Telomere Length Regulation.
(A) Mutations in Cdc13EBM-N (cdc13P235A and cdc13F237A) confer reduced but stable telomere length. Cells were taken from serial streaks 2, 4 and 6 after sporulation. Vertical white space between lanes 5 and 6 indicates cropping of superfluous lanes. (B) ChIP assay of Est1 and Est2 enrichment at telomeres VI-R and XV-L in wild-type, cdc13F237A, cdc13E252K strains. Recruitment to the internal ARO1 locus serves as a control of ChIP specificity. Each data point represents the outcome of individual ChIP reaction for at least three cell cycle synchronization experiments (biological replicates). The horizontal lines represent the means and the error bars are standard errors of the means. (C) Strains with both cdc13F237A and tlc1ΔAU mutations (lanes 7–8) exhibited further telomere shortening than single mutation of either cdc13F237A(lanes 3–4) or tlc1ΔAU (lanes 5–6). (D) Strains with both cdc13F237A and mutations that disrupt the Ku80-Sir4 interaction exhibited further telomere shortening. Vertical white space between lanes 5 and 6 indicates cropping of superfluous lanes. (E) Strains as described in (A), (C) and (D) were streaked onto plates to analyze senescence phenotype.
See also Figure S7.
We next investigated whether cdc13F237A would also affect the association of Est1 and Est2 with telomeres in vivo. ChIP analysis revealed that recruitment of Est1 to telomeres during S phase was decreased, although not completely abolished in the cdc13F237A strain compared to the CDC13 wild-type background (Figure 6B). Similarly, we found that Est2 association with telomeres in S phase was also compromised in the cdc13F237A strain (Figure 6B, lower panel), although it has been shown that Est2 telomere occupancy is less variable than that of Est1 during cell cycle (Chan et al., 2008; Schramke et al., 2004). Cdc13F237A binding to telomeres was unaffected (Figure S7C), consistent with the F237A mutation being outside of the Cdc13 DNA-binding domain (Figure 5A). Together, these in vivo observations support a model in which Phe218 in KlCdc13EBM-N that is crucial for interaction with KlEst1 in vitro (Figures 5G and S6B) also affects telomerase recruitment in vivo, in particular in S phase.
The TLC1-Ku-Sir4 and the Est1-Cdc13 telomerase-recruitment pathways have an additive effect on telomere-length regulation
Similar to the tlc1, ku80, and sir4 mutant strains studied above (Figures 4A and 4B), cdc13P235A and cdc13F237A cells displayed short but stable telomeres (Figure 6A) and grow without signs of senescence (Figures S4A, S4B and S7A). These results indicate that cells in which the interaction between Cdc13 and Est1 is compromised still maintain cell growth with stable but shortened telomeres. To examine the relationship between the two telomerase-recruitment pathways, we constructed double-mutant strains bearing both the cdc13F237A mutation as well as those that abolished either the Ku-TLC1 or the Ku80-Sir4 interaction. In sharp contrast to the single-mutant strains, all of the double-mutant strains showed a senescence phenotype (Figure 6E) and critically short telomeres (~300 bp shorter than wild type) (Figures 6C, 6D and S7D). Notably, the length of the double-mutant strains’ telomeres was shorter than that of any of the single-mutant strains (Figures 4A, 4B, 6C and 6D), suggestive of an additive effect of two telomerase-recruitment pathways. Collectively, we conclude that the TLC1-Ku-Sir4 and Est1-Cdc13 pathways represent two independent pathways that may backstop each other to recruit telomerase for telomere maintenance.
Functional analysis of the interface between Est1 and Cdc13EBM-C
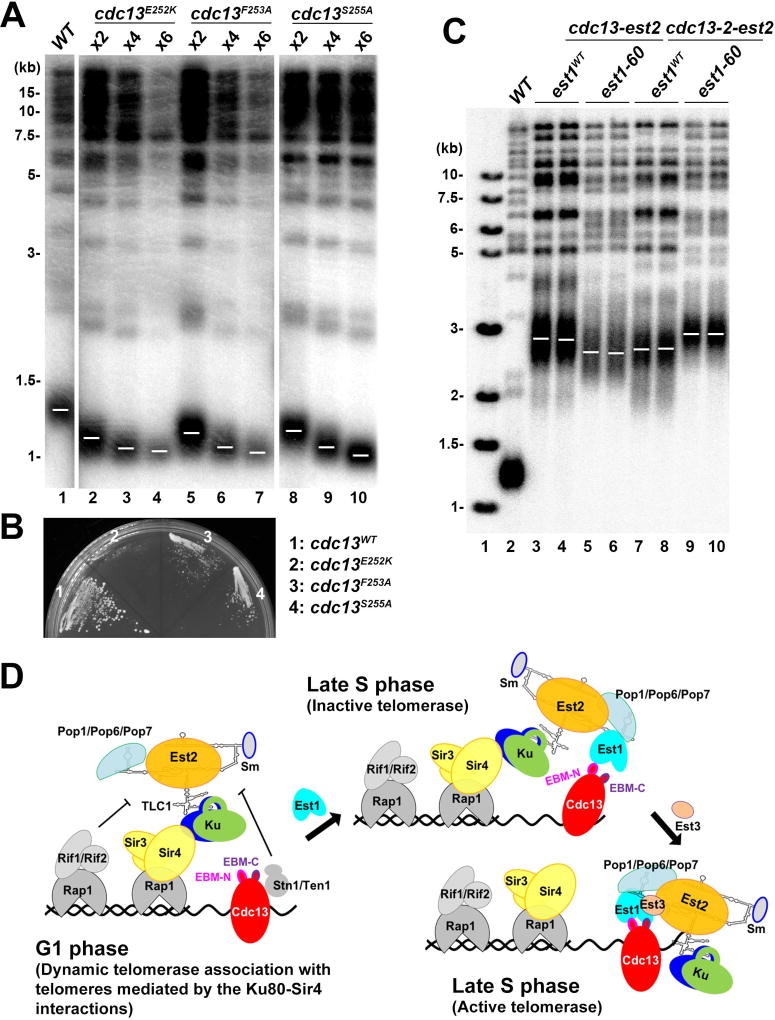
Previous genetic data suggested that Glu252 in ScCdc13EBM-C forms a salt bridge connection with Lys444 of ScEst1 such that single charge reversals at either of these positions (ScCdc13–2 allele, E252K; ScEst1–60 allele, K444E) result in critically short telomeres and a senescence phenotype (Figures 7A and 7B) (Nugent et al., 1996; Pennock et al., 2001). These two single mutations reciprocally suppress each other, leading to the hypothesis that physical contact between these two proteins has been restored by the charge-swap in the ScCdc13–2/ScEst1–60 double mutant allele (Pennock et al., 2001). Consistent with this idea, in the crystal structure of the KlEst1-KlCdc13EBM complex, KlCdc13Glu233 (equivalent to ScCdc13Glu252) and KlEst1Lys467 (equivalent to ScEst1Lys444 ) are adjacent to each other in three-dimensional space (Figure 5H). However, previous quantitative measurements using purified full-length ScCdc13 and ScEst1 proteins revealed that neither ScCdc13E252K nor ScEst1K444E had detectable impact on the ScCdc13-ScEst1 interaction (Wu and Zakian, 2011). Furthermore, our ITC experiments showed that both KlCdc13E233K and KlEst1K467E weakened the KlEst1- KlCdc13EBM interaction only 2- to 3-fold (Figures 5G and S6B). Similarly, mutations of other conserved residues of Cdc13 and Est1 (KlCdc13F234A/ScCdc13F253A, KlCdc13S236A/ScCdc13S255A, and KlEst1R478E/ScEst1R455E) that interfere with the Est1-Cdc13EBM-C interface caused very little or no defect in the in vitro Cdc13EBM-Est1 interaction, but nonetheless led to telomere loss and senescence (Figures 5G, 7A and 7B). Taken together, we conclude that the interface between Cdc13EBM-C and Est1 is not required for the Est1-Cdc13 interaction per se, but plays an essential role in telomere maintenance in yeast cells.
Figure 7. Cdc13EBM-C Has an Activation Effect on Telomerase.
(A) Mutations in Cdc13EBM-C (cdc13E252K, cdc13F253A and cdc13S255A) exhibited critically short telomeres. Cells were taken from serial streaks 2, 4 and 6 after sporulation. Vertical white spaces between lanes 1 and 2, 7 and 8, indicate cropping of superfluous lanes. (B) Strains as described in (A) were streaked onto plates to analyze senescence phenotype. (C) Fusion of Cdc13-Est2 cannot fully bypass the role of Est1. A diploid CDC13/cdc13Δ EST1/est1Δ strain was cotransformed with plasmids expressing Cdc13-Est2 (or Cdc13–2-Est2) and Est1 (or Est1–60). After sporulation, cells were taken from streak 6 and subjected to southern blot analysis. (D) A schematic model for telomerase recruitment to telomeres in budding yeast.
To examine the in vivo functional significance of the Est1-Cdc13EBM-C interface, we expressed a Cdc13-Est2 fusion protein in the absence of both Cdc13 and Est1 to bypass the requirement for the Est1-Cdc13 interaction to recruit telomerase to the telomere (Evans and Lundblad, 1999). Consistent with previous studies, supplementation of ectopically expressed Est1 resulted in further-elongated telomeres (Figure 7C, lanes 3 and 4), suggesting that, in addition to the Est1-Cdc13 interaction-mediated recruitment of telomerase, Est1 must play a role in a subsequent activation step important for telomerase action after telomerase recruitment to telomeres.
To examine whether the ScCdc13Glu252-ScEst1Lys444 salt bridge is involved in this telomerase-activating step, we ectopically expressed the Est1–60 mutant in the Cdc13-Est2 fusion strain. Notably, expression of Est1–60 led to a modest decline in telomere length compared with a wild-type Est1-expressing strain (Figure 7C). In a similar experiment, expression of mutant fusion protein (Cdc13–2)-Est2 and wild-type Est1 gave rise to the same degree of decrease in telomere length (Figure 7C). Remarkably, ectopic expression of both mutant Est1–60 and (Cdc13–2)-Est2 together restored the telomere length to the wild-type level (Figure 7C). These results confirmed that the interface between Cdc13EBM-C and Est1 involving the Cdc13Glu252-Est1Lys444 salt bridge indeed plays a role in the subsequent activation step after telomerase is recruited to telomeres.
To further address the functional significance of the Est1-Cdc13EBM-C interface, we investigated whether the cdc13–2 allele would affect association of Est1 and Est2 with telomeres in vivo. Interestingly, in contrast to the in-vitro interaction results between Est1 and Cdc13–2 (Figures 5G and S6B), recruitment of Est1 and, to a lesser extent, Est2 to telomeres during S phase was dramatically compromised by the cdc13E252K mutation (Figure 6B) (Chan et al., 2008). In fact, the extent of the reduction of the Est1 and Est2 association with telomeres in cdc13–2 cells was as — or even more — severe than that in the cdc13F237A mutant allele (Figure 6B). Since the Cdc13E252K protein is stable and had only modestly decreased telomere binding by ChIP (Figure S7B and S7C), this cannot account for the reduction in telomerase recruitment caused by this mutation. Thus, this observation suggests that although Glu252 in Cdc13EBM-C is largely dispensable for binding Est1 in vitro, it has a unique function in vivo.
DISCUSSION
Our structural and functional data reported here, when combined with previous studies, provide an integrated picture for yeast telomerase recruitment. In G1 phase of the cell cycle, Ku plays two important and non-redundant roles in facilitating telomerase accumulation at telomeres. First, Ku specifically recognizes a short bulged stem-loop structure of TLC1, and this interaction is crucial for the nuclear retention of TLC1 (Figure 4E) (Gallardo et al., 2008; Peterson et al., 2001). Second, through an interaction between Ku80 and Sir4, Ku brings telomerase to the duplex region of telomeres (Figure 7D) (Hass and Zappulla, 2015). Previous live-cell imaging analysis of yeast telomerase revealed diffusive behavior and transient association with telomeres in the G1 phase of the cell cycle (Gallardo et al., 2011). In concordance with this result, our in vitro analysis demonstrated that the binding affinity for the Ku80-Sir4 interaction was ~5 µM, which falls within the range of that for other transient interactions between yeast nuclear proteins (Shi et al., 2013). ChIP assays, which can capture this dynamic association of telomerase at telomeres, showed that the Ku80-Sir4 interaction is required for this Ku-mediated telomerase association with telomeres (Figure 4D) (Chan et al., 2008; Fisher et al., 2004; Hass and Zappulla, 2015). Our model is consistent with the idea that competitive binding between Sir4 and the Rif proteins to telomere proximal Rap1 regulates telomere length (Hass and Zappulla, 2015). In concordance with previous studies (Ribes-Zamora et al., 2007), we found that, in addition to regulating telomerase accumulation at telomeres, the Ku80-Sir4 binding interaction is also important for telomeric position-effect (TPE) transcriptional silencing, but not for Ku’s role in telomere end protection or NHEJ (Figure S4C and S4D). Although not studied here, we expect disruption of the Ku80-Sir4 to also affect the perinuclear localization of telomeres (Taddei and Gasser, 2012).
When the cell cycle progresses into late S phase, which is the time that coincides with the rising abundance of Est1 (Osterhage et al., 2006), Stn1-Ten1 dissociates from Cdc13, freeing Cdc13 for interaction with Est1 ( Li et al., 2009). Cdc13 may first utilize its N-terminal Est1-binding motif (Cdc13EBM-N) to interact with Est1 (Figure 5F). This Est1-Cdc13EBM-N interaction likely cooperates with the TLC1-Ku-Sir4 pathway to mediate the recruitment of telomerase to the 3′ ss overhang (Figure 7D). Notably, only simultaneous disruption of both the Ku and Cdc13 recruitment pathways can lead to critically short telomeres and senescence, underscoring the importance of both pathways in telomerase recruitment (Figures 6C–6E and S7D). We propose that the dynamic association of telomerase with telomeres mediated by the Ku80-Sir4 interaction may enhance the interaction between Est1 and Cdc13, and in the absence of the Est1-Cdc13EBM-N interaction, is able to supports the basal level of telomerase at telomeres for telomere synthesis in late S phase (Figures 6A and S7A).
After the initial contact between Est1 and Cdc13EBM-N, the adjacent C-terminal Est1 binding motif of Cdc13 (Cdc13EBM-C) may then contact the second surface of Est1, which is particularly important for the stable assembly of the telomerase holoenzyme at telomeres (Figure 7D). Strikingly, the extensively studied Cdc13Glu252-Est1Lys444 ion pair is right on this interface yet it is not important for the physical interaction between Est1 and Cdc13 per se (Figure 5G), but nonetheless is essential for the stable association of telomerase at telomeres (Figure 6B) (Chan et al., 2008). It is not yet clear what is happening between the Est1-Cdc13EBM-C interface and other critical components of telomerase after telomerase recruitment to the 3¢ end and the role of the Est1-Cdc13EBM-C interface in this process remains a fascinating open question.
In summary, the discovery of two separate Est1-binding motifs in Cdc13 provides a satisfactory answer to the long-lasting debate as to whether Cdc13 recruits telomerase to telomeres or activates it (Evans and Lundblad, 1999, 2002; Wu and Zakian, 2011). Our data support that Cdc13 is both a telomerase recruiter and an activator. Further structural and functional studies of the active yeast telomerase holoenzyme complex will be required to fully understand this important and sophisticated molecular machine at chromosome ends.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents may be directed to, and will be fulfilled by the Lead Contact, Ming Lei (leim@shsmu.edu.cn)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Expression plasmids contain cDNAs from Kluyveromyces lactis and Saccharomyces cerevisiae. Recombinant Ku70 and Ku80 proteins were overexpressed in Trichoplusia ni High Five™ cells (ThermoFisher) in ESF 921 medium (Expression systems) or ESF 921 Delta Series Methionine Deficient medium (Expression systems) for selenomethionine-derivatized proteins. Cells were grown at 27 °C at 110 rpm for 72 hr after infection. Other recombinant proteins were overexpressed in Escherichia coli BL21-CodonPlus(DE3)-RIL cells (Agilent) in LB broth (Sangon Biotech), or M9 medium for selenomethionine-derivatized proteins. Cells were grown at 37 °C at 225 rpm until OD600 reached 0.7 then immediately induced by addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) (Amresco) to a final concentration of 0.1 mM. After induction, cells were grown for 16 hr at 22 °C.
METHOD DETAILS
Protein expression and purification
S. cerevisiae Ku80vWA (residues 2–200), Sir4KBM (residues 100–115) and Sir4KBM-GGSGGS-Ku80vWA were cloned into a modified pET28a vector with a SUMO protein fused at the N terminus after the 6×His tag and were expressed in E. coli BL21(DE3) CodonPlus cells (Stratagene) (Wang et al., 2007). After induction for 18 hours with 0.1 mM IPTG at 22 °C, the cells were harvested by centrifugation and the pellets were resuspended in lysis buffer (50 mM Tris-HCl, pH8.0, 300 mM NaCl, 10% glycerol, 1mM PMSF, 5 mM benzamidine, 1 µg mL−1 leupeptin and 1 µg mL−1 pepstatin). The cells were then lysed by sonication and the cell debris was removed by ultracentrifugation. The supernatant was mixed with Ni-NTA agarose beads (Qiagen) and rocked for 2 hours at 4 °C before elution with 250 mM imidazole. The ULP1 protease was added to remove the His-SUMO tag. Ku80vWA and Sir4KBM-GGSGGS-Ku80vWA fusion protein were then further purified by Mono-Q and gel-filtration chromatography equilibrated with 25 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 5 mM dithiothreitol. The purified proteins were concentrated to 15 mg mL−1 and stored at −80 °C. Sir4KBM was further purified by gel-filtration chromatography equilibrated with 100 mM ammonium bicarbonate. The purified proteins were concentrated by Speed Vac system and then lyophilized. The lyophilized products were then resuspended in water at a concentration of 25 mg mL−1 and stored at −80 °C. Wild-type and mutant proteins used for ITC measurements were purified similarly.
S. cerevisiae full-length Ku70 and Ku80 were cloned into modified Bac-to-Bac vectors containing an N-terminal GST tag and an N-terminal MBP tag, respectively. For Ku70/80 heterodimer expression, High Five insect cells were infected at ~ 3×106 cells mL−1 with a multiplicity of infection of 10 plaque-forming unit mL−1 recombinant baculovirus. The cells were harvested after 72 hours by centrifugation. The pellets were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 10% glycerol, 1 mM PMSF, 5 mM benzamidine, 1 µg mL−1 leupeptin and 1 µg mL−1 pepstatin). The cells were then lysed by sonication, and the debris was removed by ultracentrifugation. The supernatant was mixed with glutathione Sepharose-4B beads (GE healthcare) and rocked for 2 hours at 4 °C before elution with 15 mM reduced glutathione (Sigma). PreScission protease (GE healthcare) was then added to remove the N-terminal GST and MBP tags. The proteins were further purified by Mono-Q and gel-filtration chromatography equilibrated with 25 mM Tris-HCl pH 8.0, 150 mM NaCl, and 5 mM dithiothreitol. The purified proteins were concentrated to 30 mg mL−1 and stored at −80 °C. For heavy atom-substituted Ku70/80 proteins expression, Met-free medium plus 60 mg mL−1 L-selenomethionine (SeMet) was used to replace standard insect-cell culture medium. SeMet-substituted Ku70/80 proteins were expressed and purified similar with the native proteins.
The N-terminal domain of K. lactis Est1 (KlEst1, residues 2–600) and K. lactis Cdc13EBM (KlCdc13EBM, residues 213–245) were cloned into a modified pET28a vector with a SUMO protein fused at the N terminus after the 6×His tag and were expressed in E. coli BL21(DE3) CodonPlus cells (Stratagene). After induction for 16 hours with 0.1 mM IPTG at 22 °C, the cells were harvested by centrifugation and the pellets were resuspended in lysis buffer (50 mM Tris-HCl, pH8.0, 300 mM NaCl, 10% glycerol, 2 mM 2-mercaptoethanol, 1mM PMSF, 5 mM benzamidine, 1 µg mL−1 leupeptin and 1 µg mL−1 pepstatin). The cells were then lysed by sonication and the cell debris was removed by ultracentrifugation. The supernatant was mixed with Ni-NTA agarose beads (Qiagen) and rocked for 2 hours at 4 °C before elution with 250 mM imidazole. The ULP1 protease was added to remove the His-SUMO tag. The proteins were then further purified by Mono-Q and by gel filtration chromatography equilibrated with 25 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 5 mM dithiothreitol. The purified proteins were concentrated to 15 mg mL−1 and stored at −80 °C. The purified KlEst1 and KlCdc13EBM were concentrated to 30 mg mL−1 and 20 mg mL−1, respectively, and stored at −80 °C. All the mutant and SeMet-substituted proteins were expressed and purified following the similar procedure as described above.
RNA preparation and purification
TLC1KBS (nucleotides 288–312) with the 5’ and 3’ ends stabilized by the addition of two G-C base pairs, fused with a glucosamine-6-phosphate-activated ribozyme (GlmS ribozyme) at the 3’ terminus, were generated by in vitro transcription with T7 RNA polymerase. TLC1KBS-GlmS RNAs were purified by gel-filtration chromatography with the buffer 50 mM HEPES-KOH, pH 7.5, 150 mM NaCl and 10 mM MgCl2 to remove the DNA template and NTPs. The purified RNAs were then treated with 1 mM glucosamine-6-phosphate at 25 °C for 30 min to cleave the GlmS ribozyme from the 3¢ site of TLC1KBS. Then the TLC1KBS RNAs were purified by gel-filtration chromatography on HiLoad Superdex200 and were concentrated to 7 mg mL−1.
Crystallization, data collection and structure determination
Crystals of the native Ku70/80-TLC1KBS complex were grown by sitting-drop vapor diffusion at 4 °C. The precipitant well solution consisted of 0.2 M calcium acetate, 0.1 M imidazole, pH8.0, 10% PEG8000. Crystals were gradually transferred into a harvesting solution containing 0.2 M calcium acetate, 0.1 M imidazole, pH8.0, 18% PEG8000, 25% glycerol, followed by flash-freezing in liquid nitrogen for storage. Crystals of SeMet-labeled Ku70/80-TLC1KBS were grown in the similar condition. Datasets were collected under cryogenic conditions (100K) at the Shanghai Synchrotron Radiation Facility (SSRF) beamlines BL18U1 and BL19U1. A 3.2-Å SeMet-SAD dataset of Ku70/80-TLC1KBS was collected at the Se peak wavelength (0.97853 Å) and was processed by HKL3000 (Minor et al., 2006). Fifty-five selenium atoms were located and refined, and the initial SAD electron density map was calculated using XDS (Kabsch, 2010). The initial SAD map was substantially improved by solvent flattening. The model was then refined using a native dataset with a 2.8-Å resolution using Phenix (Adams et al., 2010), together with manual building in Coot (Emsley et al., 2010). In the final Ramachandran plot, the favored and allowed residues are 97.3% and 100.0%, respectively.
Crystals of the native Ku80vWA-Sir4KBM complex were grown by sitting-drop vapor diffusion at 4 °C. The precipitant well solution consisted of 0.1 M HEPES, pH 7.5, 2.0 M Ammonium sulfate. Crystals were gradually transferred into a harvesting solution containing 0.1 M HEPES, pH 7.5, 2.5 M ammonium sulfate, 25% glycerol, followed by flash-freezing in liquid nitrogen for storage. Crystals of SeMet-labeled Ku80vWA-Sir4KBM were grown in the similar condition. Datasets were collected under cryogenic conditions (100K) at the Shanghai Synchrotron Radiation Facility (SSRF) beamlines BL18U1 and BL19U1. A 2.8-Å SeMet-SAD dataset of Ku80vWA-Sir4KBM was collected at the Se peak wavelength (0.97851 Å) and was processed by HKL3000 (Minor et al., 2006). Four selenium atoms were located and refined, and the initial SAD electron density map was calculated using Phenix (Adams et al., 2010). The initial SAD map was substantially improved by solvent flattening. The model was then refined using a native dataset with a 2.4-Å resolution using Phenix (Adams et al., 2010), together with manual building in Coot (Emsley et al., 2010). In the final Ramachandran plot, the favored and allowed residues are 98.4% and 100.0%, respectively.
Native KlEst1 and KlCdc13EBM were mixed together in a molecular ratio of 1:1.2 to a concentration of ~ 15 mg mL−1. Crystals of the native KlEst1-KlCdc13EBM complex were grown by sitting-drop vapor diffusion at 4 °C. The precipitant well solution consisted of 0.2 M potassium chloride, pH 7.0, and 20% PEG3350. Crystals were gradually transferred into a harvesting solution containing 0.2 M potassium chloride, pH 7.0, and 25% PEG3350, 25% glycerol, followed by flash-freezing in liquid nitrogen for storage. Crystals of SeMet-labeled KlEst1-KlCdc13EBM were grown in the similar condition. Datasets were collected under cryogenic conditions (100K) at the Shanghai Synchrotron Radiation Facility (SSRF) beamlines BL18U1 and BL19U1. A 2.5-Å SeMet-SAD dataset of KlEst1-KlCdc13EBM was collected at the Se peak wavelength (0.97860 Å) and was processed by HKL3000 (Minor et al., 2006). Eight selenium atoms were located and refined, and the initial SAD electron density map was calculated using XDS (Kabsch, 2010). The initial SAD map was substantially improved by solvent flattening. The model was then refined using a native dataset with a 2.2-Å resolution using Phenix (Adams et al., 2010), together with manual building in Coot (Emsley et al., 2010). In the final Ramachandran plot, the favored and allowed residues are 99.0% and 100.0%, respectively.
All of the crystal structural figures were generated using PyMOL software (Schrodinger, LLC).
Isothermal titration calorimetry
The equilibrium dissociation constants of the Ku-TLC1KBS, Ku80vWA-Sir4KBM, and KlEst1-KlCdc13EBM interactions were determined using a MicroCal iTC200 calorimeter (Malvern). The binding enthalpies were measured at 16 °C in 25 mM Tris-HCl, pH 8.0, 150 mM NaCl and 5 mM MgCl2. Two independent experiments were performed for every interaction described here. ITC data were subsequently analyzed and fitted using Origin 7 software (OriginLab).
Yeast two-hybrid assay
The yeast two-hybrid assays were performed as described previously (Sun et al., 2011). Briefly, the L40 strain was transformed with pBTM116 and pACT2 (Clontech) fusion plasmids, and colonies harboring both plasmids were selected on –Leu –Trp plates. The β-Galactosidase activities were measured by a liquid assay.
Plasmids and strains
The plasmids and yeast strains used in this study are shown in Tables S4 and S5, respectively. The BY4743 derived deletion strains (heterozygous diploid CDC13/cdc13Δ, haploid sir4Δ and haploid ku80Δ strains) are from EUROSCARF (SRD GmbH). Additional gene deletion was carried out by one-step gene disruption technique. All haploid strains used for assays of telomere length, senescence phenotype and RNA isolation were derived by either dissecting the relevant diploid strains or by introducing the relevant plasmids into the sir4Δ or ku80Δ haploid strain. As for cdc13F237A sir4 (or ku80) double mutations, the strains were derived from a cdc13F237A haploid strain harboring a covering CDC13 plasmid (pRS316-CDC13). After introducing SIR4 or KU80 point mutation, the cells were plated on 5-FOA plate to pop-out pRS316-CDC13. The haploid strains used for telomeric silencing and NHEJ assays were derived by introducing the relevant mutations into the endogenous YKU80 locus.
Southern blot for determining telomere length
Yeast genomic DNA was prepared, digested with XhoI (or ApaI), separated on 1% agarose gels, and transferred to Hybond-N+ membrane (GE Healthcare). The DNA fragments were hybridized with a ~300 bp TG1–3 telomere-specific probe as previously described (Sun et al., 2011).
Yeast nuclear RNA isolation
Yeast nuclei was prepared from ~3 × 108 cells harvested from cultures grown to an OD600 of ~0.5–1.0 using the Yeast Nuclei Isolation Kit (BioVision) according to the manufacturer’s instructions, except that 3 mM ribonucleoside-vanadyl complex (RVC) was added throughout the procedure to inhibit RNase activity. Then the isolated nuclei were processed for extracting yeast nuclear RNA according to the RNeasy Mini Kit protocol (Qiagen).
Reverse-transcription (RT) and quantitative real-time PCR reactions
RT and real-time PCR reactions were done as described previously except that PowerUp™ SYBR® Green Master Mix was used on a ViiA™ 7 Real-Time PCR System (Mozdy and Cech, 2006). Briefly, cDNAs were synthesized in 20 µL reactions containing 5 ng total nuclear RNA using the QuantiTect Reverse Transcription kit (Qiagen) according to the manufacture’s instruction. Then 4 µL of RT reaction was used as template in a 20 µL PCR reaction to measure the relative amount of cDNA of TLC1. Because of the RNase H activity of Quantiscript Reverse Transcriptase, the RNA:cDNA ratio is assumed to be 1:1. The TLC1-specific primers are: 5¢-CCATGGGAAGCCTACCAT and 5¢-GAACTCGTGCAAACTTTGCTAA. The cycling parameters for all assays were 95 °C denaturation for 10 min, followed by 45 cycles of 95 °C for 10 sec, 57 °C for 5 sec, and 72 °C for 10 sec, followed by melting curve measurement. Three independent experiments were performed for every strain, and the average value of nuclear TLC1 of wild-type strain was set to be 1.
GST pull-down assays
GST-fusion proteins and interacting partners were incubated with glutathione Sepharose 4B beads for 2 hr at 4 °C in 100 µl buffer (25 mM Tris-HCl, pH 8.0, 150 mM NaCl and 2 mM MgCl2). After extensive wash with the same buffer, the bound proteins were eluted in elution buffer (25 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM MgCl2 and 15 mM reduced glutathione). The protein and RNA of input samples and eluted samples were visualized on 12% SDS–PAGE by Coomassie blue staining and 8% denaturing PAGE (containing 8 M urea) by SYBR® Gold staining.
Cell cycle chromatin immunoprecipitation of Est1 and Est2
The Est1-myc13 and Est2-myc tagging cassettes were PCR-amplified using yEHB10280 and yEHB10282 strains, respectively (Blackburn lab) and integrated into the yCH001 diploid heterozygous for deletion of Cdc13. The resulting diploids were transformed with the CEN plasmids carrying either CDC13WT, cdc13F237A or cdc13E252K (Tables S4 and S5). The diploids were sporulated and the spores with desired genotypes were isolated. Freshly isolated spore clones were used in ChIP experiments to avoid senescence. Cell cycle synchronization was performed using the α-factor block and release. The samples were collected every 15 min after release (G1/Early S – 0 and 15 min; Mid-Late S – 30, 45, and 60 min; and G2/M – 75 min), crosslinked with 1% formaldehyde for 15 min, and subjected to standard ChIP protocol as described (Luciano et al., 2012) with one modification. After cell breakage, the whole cell lysate was centrifuged at 16,000 g for 30 min, and soluble chromatin fraction was collected for sonication. Chromatin samples were adjusted to a DNA concentration of 200 ng µl−1 and sonicated in 250-µl aliquots using Bioruptor Pico (Diagenode) for 4–5 cycles of 30 sec on/30 sec off. This procedure achieved consistent chromatin fragmentation to an average DNA fragment size of 0.3–0.5 kb. Chromatin associated with Est1- and Est2-myc was immunoprecipitated using c-Myc antibody (9E10) from Santa Cruz Biotechnology. To estimate the amount of telomeric DNA and of an internal control locus, real-time qPCR was performed using primers targeting VI-R and XV-L subtelomeric sequences immediately adjacent to telomeric tracts (Bianchi and Shore, 2007) and ARO1 primers (Sabourin et al., 2007). The results were expressed as percent of input DNA recovered after chromatin immunoprecipitation (% input).
Chromatin immunoprecipitation of Cdc13
The 18xMyc tag was introduced into the C terminus of Cdc13 proteins by swapping the BstAPI-NotI fragment between the pVL1042 (Lundblad lab) and the CEN plasmids carrying CDC13WT, cdc13F237A and cdc13E252K (Tables S4 and S5). The Cdc13-myc ChIP was performed using asynchronous cultures, but otherwise as described for Est1 and Est2.
Chromatin immunoprecipitation of Est2
The chromatin immunoprecipitation (ChIP) and quantitative real-time PCR (qPCR) assays in Figure 4D was performed as described previously (Fisher et al., 2004; Hass and Zappulla, 2015; Sabourin et al., 2007). Briefly, yeast cells expressing Est2 from its endogenous locus bearing a C-terminal 7×Myc tag and an 8-glycine linker were grown in 50-mL cultures to an optical density of 0.6–0.8, then crosslinked with formaldehyde. Crosslinked cells were pelleted, resuspended in lysis buffer, and flash-frozen in liquid nitrogen. Frozen cells were thawed and lysed with sterile glass beads, and chromatin was sheared by sonication in a Bioruptor (Diagenode). Sheared chromatin was subjected to immunoprecipitation using monoclonal mouse anti-myc antibodies (Clontech) followed by Protein G Dynabeads (Thermo Fisher Scientific). Formaldehyde-induced crosslinks were then reversed, and DNA samples were cleaned up using a modified PCR-purification kit (Qiagen). Telomeric enrichment was measured by qPCR using iQ SYBR Green Supermix (Bio-Rad) and using primer sets in the chromosome VI-R subtelomere and at the ARO1 locus described previously (Sabourin et al., 2007). For each sample, qPCR reactions were performed in technical triplicate with each primer set on both input DNA and immunoprecipitated DNA. The CT values from technical triplicate reactions were averaged, and fold telomeric enrichment was calculated as 2^[(CT(ARO IP)-CT(ARO Input))-(CT(TEL IP)-CT(TEL Input))].
Telomere silencing assay
Mutations in KU80 were integrated at the endogenous locus of the haploid strain UCC3505 (Singer and Gottschling, 1994), which bears the ADE2 and URA3 genes adjacent to telomeres V–R and VII-L, respectively. Cultures were grown to OD600 = 1.0 in YPD media. Five-fold serial dilutions were spotted onto -Ura (to monitor plating efficiency) and 5-fluorotic acid (5-FOA) plates to monitor expression of the URA3 gene. Growth was determined after 2 to 4 days at 28 °C.
NHEJ assay
Mutations in KU80 were integrated at the endogenous locus of the haploid strain JKM139 (Lee et al., 1998), which contains a single HO cleavage site and the HO endonuclease under the control of a galactose-inducible promoter. Cultures were grown to OD600 ~ 1.0 in YPD media. Five-fold serial dilutions were spotted onto YPD (to monitor plating efficiency) and YPGal to induce HO endonuclease expression. Growth was determined after 5 to 7 days at 28 °C.
QUANTIFICATION AND STATISTICAL ANALYSIS
For yeast two-hybrid, ChIP-qPCR and reverse-transcription-qPCR experiments, values were plotted using the Prism7 software as mean ± standard error from three independent experiments. The dissociation constants (Kd) in ITC experiments were calculated using the Origin 7 software as mean ± standard error from two independent experiments with a single site-specific binding model.
DATA AND SOFTWARE AVAILABILITY
The accession numbers for the Ku-TLC1KBS, Ku80vWA-Sir4KBM, and KlEst1-KlCdc13EBM structures reported in this paper are PDB 5Y58, 5Y59 and 5Y5A, respectively.
Supplementary Material
Acknowledgments
We thank Dr. Jin-Qiu Zhou for the plasmids and yeast strains. We thank L. Wu, D. Yao, and R. Zhang from BL18U1 and BL19U1 beamlines at NCPSS for help with crystal data collection, and thank protein production team at NCPSS for technical assistance. This work was supported by grants from the Ministry of Science and Technology of China (2013CB910402 to M.L.), the National Natural Science Foundation of China (31330040 and 31525007 to M.L., 31500625 to J.W.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB08010201) to M.L, the Youth Innovation Promotion Association of the Chinese Academy of Sciences to J.W. Research was also supported in part by the National Institute of General Medical Sciences of the National Institutes of Health (USA) under award number RO1GM118757 to D.C.Z. and RO1GM077509 to A.A.B. Work in V.G. laboratory is supported by the “Ligue contre le Cancer” (Equipe Labéllisée).
Footnotes
Supplemental information includes eight figures and five tables.
AUTHOR CONTRIBUTIONS
M.L. and J.W. conceived this study. J.X. and H.C. carried out the bulk of the experiments. S.S. carried out cloning and protein expression. J.W., J.X., and H.C. carried out structure determination, crystallographic analysis and interpreted the results. D.C. and P.L. performed experiments relative to Cdc13 and Cdc13 mutant ChIP and to Est1 and Est2 ChIP in Cdc13 mutant strains. E.P.H. performed the experiments of Est2 ChIP in Sir4 mutant strains. L.D.L. performed the telomere silencing assay and NHEJ assay. A.A.B., D.C.Z. and V.G. discussed the results and contributed to the writing of the manuscript. M.L. and J.W. wrote the manuscript.
None of the authors of this manuscript has a financial interest related to this work.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuch AA, Lundblad V. The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini. Mol Cell Biol. 2003;23:8202–8215. doi: 10.1128/MCB.23.22.8202-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A, Shore D. Early replication of short telomeres in budding yeast. Cell. 2007;128:1051–1062. doi: 10.1016/j.cell.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Chan A, Boule JB, Zakian VA. Two pathways recruit telomerase to Saccharomyces cerevisiae telomeres. PLoS Genet. 2008;4:e1000236. doi: 10.1371/journal.pgen.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby AB, Goodrich KJ, Pfingsten JS, Cech TR. RNA recognition by the DNA end-binding Ku heterodimer. RNA. 2013;19:841–851. doi: 10.1261/rna.038703.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan ED, Collins K. Biogenesis of telomerase ribonucleoproteins. RNA. 2012;18:1747–1759. doi: 10.1261/rna.034629.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SK, Lundblad V. Est1 and Cdc13 as comediators of telomerase access. Science. 1999;286:117–120. doi: 10.1126/science.286.5437.117. [DOI] [PubMed] [Google Scholar]
- Evans SK, Lundblad V. The Est1 subunit of Saccharomyces cerevisiae telomerase makes multiple contributions to telomere length maintenance. Genetics. 2002;162:1101–1115. doi: 10.1093/genetics/162.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell VL, Schild-Poulter C. The Ku heterodimer: function in DNA repair and beyond. Mutat Res Rev Mutat Res. 2015;763:15–29. doi: 10.1016/j.mrrev.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Fisher TS, Taggart AK, Zakian VA. Cell cycle-dependent regulation of yeast telomerase by Ku. Nature Struct Mol Biol. 2004;11:1198–1205. doi: 10.1038/nsmb854. [DOI] [PubMed] [Google Scholar]
- Gallardo F, Laterreur N, Cusanelli E, Ouenzar F, Querido E, Wellinger RJ, Chartrand P. Live cell imaging of telomerase RNA dynamics reveals cell cycle-dependent clustering of telomerase at elongating telomeres. Molecular cell. 2011;44:819–827. doi: 10.1016/j.molcel.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Gallardo F, Olivier C, Dandjinou AT, Wellinger RJ, Chartrand P. TLC1 RNA nucleo-cytoplasmic trafficking links telomerase biogenesis to its recruitment to telomeres. EMBO J. 2008;27:748–757. doi: 10.1038/emboj.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Toro TB, Paschini M, Braunstein-Ballew B, Cervantes RB, Lundblad V. Telomerase recruitment in Saccharomyces cerevisiae is not dependent on Tel1-mediated phosphorylation of Cdc13. Genetics. 2010;186:1147–1159. doi: 10.1534/genetics.110.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass EP, Zappulla DC. The Ku subunit of telomerase binds Sir4 to recruit telomerase to lengthen telomeres in S. cerevisiae. Elife. 2015;4:e07750. doi: 10.7554/eLife.07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr D Biol Crystallogr. 2010;66:133–144. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Moore JK, Holmes A, Umezu K, Kolodner RD, Haber JE. Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell. 1998;94:399–409. doi: 10.1016/s0092-8674(00)81482-8. [DOI] [PubMed] [Google Scholar]
- Lemieux B, Laterreur N, Perederina A, Noel JF, Dubois ML, Krasilnikov AS, Wellinger RJ. Active Yeast Telomerase Shares Subunits with Ribonucleoproteins RNase P and RNase MRP. Cell. 2016;165:1171–1181. doi: 10.1016/j.cell.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvay TS, Morris DK, Sah J, Balasubramanian B, Lundblad V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics. 1996;144:1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontis NB, Westhof E. Geometric nomenclature and classification of RNA base pairs. RNA. 2001;7:499–512. doi: 10.1017/s1355838201002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Makovets S, Matsuguchi T, Blethrow JD, Shokat KM, Blackburn EH. Cdk1-dependent phosphorylation of Cdc13 coordinates telomere elongation during cell-cycle progression. Cell. 2009;136:50–61. doi: 10.1016/j.cell.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CR, Ribes-Zamora A, Indiviglio SM, Williams CL, Haricharan S, Bertuch AA. Ku must load directly onto the chromosome end in order to mediate its telomeric functions. PLoS Genet. 2011;7:e1002233. doi: 10.1371/journal.pgen.1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano P, Coulon S, Faure V, Corda Y, Bos J, Brill SJ, Gilson E, Simon MN, Geli V. RPA facilitates telomerase activity at chromosome ends in budding and fission yeasts. The EMBO journal. 2012;31:2034–2046. doi: 10.1038/emboj.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad V, Blackburn EH. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993;73:347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- Mozdy AD, Cech TR. Low abundance of telomerase in yeast: implications for telomerase haploinsufficiency. RNA. 2006;12:1721–1737. doi: 10.1261/rna.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent CI, Hughes TR, Lue NF, Lundblad V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science. 1996;274:249–252. doi: 10.1126/science.274.5285.249. [DOI] [PubMed] [Google Scholar]
- O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhage JL, Talley JM, Friedman KL. Proteasome-dependent degradation of Est1p regulates the cell cycle-restricted assembly of telomerase in Saccharomyces cerevisiae. Nature structural & molecular biology. 2006;13:720–728. doi: 10.1038/nsmb1125. [DOI] [PubMed] [Google Scholar]
- Pennock E, Buckley K, Lundblad V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell. 2001;104:387–396. doi: 10.1016/s0092-8674(01)00226-4. [DOI] [PubMed] [Google Scholar]
- Peterson SE, Stellwagen AE, Diede SJ, Singer MS, Haimberger ZW, Johnson CO, Tzoneva M, Gottschling DE. The function of a stem-loop in telomerase RNA is linked to the DNA repair protein Ku. Nature genetics. 2001;27:64–67. doi: 10.1038/83778. [DOI] [PubMed] [Google Scholar]
- Pfingsten JS, Goodrich KJ, Taabazuing C, Ouenzar F, Chartrand P, Cech TR. Mutually exclusive binding of telomerase RNA and DNA by Ku alters telomerase recruitment model. Cell. 2012;148:922–932. doi: 10.1016/j.cell.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes-Zamora A, Mihalek I, Lichtarge O, Bertuch AA. Distinct faces of the Ku heterodimer mediate DNA repair and telomeric functions. Nature Struct Mol Biol. 2007;14:301–307. doi: 10.1038/nsmb1214. [DOI] [PubMed] [Google Scholar]
- Roy R, Meier B, McAinsh AD, Feldmann HM, Jackson SP. Separation-of-function mutants of yeast Ku80 reveal a Yku80p–Sir4p interaction involved in telomeric silencing. The Journal of biological chemistry. 2004;279:86–94. doi: 10.1074/jbc.M306841200. [DOI] [PubMed] [Google Scholar]
- Sabourin M, Tuzon CT, Zakian VA. Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Molecular cell. 2007;27:550–561. doi: 10.1016/j.molcel.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramke V, Luciano P, Brevet V, Guillot S, Corda Y, Longhese MP, Gilson E, Geli V. RPA regulates telomerase action by providing Est1p access to chromosome ends. Nature genetics. 2004;36:46–54. doi: 10.1038/ng1284. [DOI] [PubMed] [Google Scholar]
- Shi T, Bunker RD, Mattarocci S, Ribeyre C, Faty M, Gut H, Scrima A, Rass U, Rubin SM, Shore D, et al. Rif1 and Rif2 shape telomere function and architecture through multivalent Rap1 interactions. Cell. 2013;153:1340–1353. doi: 10.1016/j.cell.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Stellwagen AE, Haimberger ZW, Veatch JR, Gottschling DE. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 2003;17:2384–2395. doi: 10.1101/gad.1125903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Yang Y, Wan K, Mao N, Yu TY, Lin YC, DeZwaan DC, Freeman BC, Lin JJ, Lue NF, et al. Structural bases of dimerization of yeast telomere protein Cdc13 and its interaction with the catalytic subunit of DNA polymerase alpha. Cell research. 2011;21:258–274. doi: 10.1038/cr.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Gasser SM. Structure and function in the budding yeast nucleus. Genetics. 2012;192:107–129. doi: 10.1534/genetics.112.140608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- Wellinger RJ, Wolf AJ, Zakian VA. Saccharomyces telomeres acquire single-strand TG1–3 tails late in S phase. Cell. 1993;72:51–60. doi: 10.1016/0092-8674(93)90049-v. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ouenzar F, Lemon LD, Chartrand P, Bertuch AA. The principal role of Ku in telomere length maintenance is promotion of Est1 association with telomeres. Genetics. 2014;197:1123–1136. doi: 10.1534/genetics.114.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zakian VA. The telomeric Cdc13 protein interacts directly with the telomerase subunit Est1 to bring it to telomeric DNA ends in vitro. Proc Natl Acad Sci USA. 2011;108:20362–20369. doi: 10.1073/pnas.1100281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappulla DC, Cech TR. Yeast telomerase RNA: a flexible scaffold for protein subunits. Proc Natl Acad Sci USA. 2004;101:10024–10029. doi: 10.1073/pnas.0403641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappulla DC, Goodrich K, Cech TR. A miniature yeast telomerase RNA functions in vivo and reconstitutes activity in vitro. Nature Struct Mol Biol. 2005;12:1072–1077. doi: 10.1038/nsmb1019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.