Abstract
Brain-Computer Interfaces (BCIs) are real-time computer-based systems that translate brain signals into useful commands. To date most applications have been demonstrations of proof-of-principle; widespread use by people who could benefit from this technology requires further development. Improvements in current EEG recording technology are needed. Better sensors would be easier to apply, more confortable for the user, and produce higher quality and more stable signals. Although considerable effort has been devoted to evaluating classifiers using public datasets, more attention to real-time signal processing issues and to optimizing the mutually adaptive interaction between the brain and the BCI are essential for improving BCI performance. Further development of applications is also needed, particularly applications of BCI technology to rehabilitation. The design of rehabilitation applications hinges on the nature of BCI control and how it might be used to induce and guide beneficial plasticity in the brain.
Introduction
A Brain-Computer Interface (BCI) is a computer-based system that acquires, analyzes, and translates brain signals into output commands in real-time. The term BCI can be traced to Jacques Vidal who devised a BCI system in the 1970s that used visual evoked-potentials [1]. Since that time, the impressive advances in computer technology, machine learning, and neuroscience have enabled the development of a wide variety of BCI systems [2]. Many BCI systems use electroencephalographic (EEG) signals [2]; others use alternative recording modalities such as magnetoencephalography (MEG), electrocorticography (ECoG), intracortical microelectrode recording of single neuron action potentials or local field potentials, functional magnetic resonance imaging (fMRI), or functional near-infrared spectroscopy (fNIR) [3].
Most BCI studies have focused on using them to restore communication and control to people paralyzed by chronic neuromuscular disorders, such as amyotrophic lateral sclerosis (ALS), brainstem stroke, or high-level spinal cord injury. To date, these studies have been mainly demonstrations of proof-of-principle: actual long-term BCI use by individuals who need them has been limited to a handful of case studies [e.g., 4]. More recently, investigators have become interested in other applications of BCI technology, particularly the possibility that they might enhance neurorehabilitation for people with strokes and other chronic disorders [5].
Some issues relating to invasive methods will be considered. However this brief review focuses on key issues related to noninvasive EEG-based BCIs; they are the most widely researched due to their minimal risk and the relative convenience of conducting studies and recruiting participants, and they have the greatest immediate promise for rehabilitation applications. First, it reviews the major categories of EEGbased BCIs. Second, it addresses the current state of EEG recording methodologies. Third, it outlines the key issues involved in BCI-related signal analysis. Finally, it reviews the currently most exciting and promising area of BCI research and development: BCIs for neurorehabilitation.
EEG-Based BCIs
Farwell and Donchin [6] reported the first use of a P300-based BCI, in which a positive potential in the EEG about 300 msec after an attended target stimulus serves as the control signal. The P300 is elicited by a stimulus that has special significance; it is detected by averaging the EEG responses to relatively rare presentations of the target stimulus interspersed with many non-target stimuli [7]. Their subjects viewed a 6 × 6 matrix of items (letters and other symbols) and attended to a target item as the rows and columns of the matrix flashed repeatedly in random order. The average response to the flash of the target item differed from the average responses to the other items; the BCI detected this difference and thereby determined which item the subject wanted to select. With this BCI, a subject could spell words. The fact that the P300 potential reflects attention, rather than simply gaze direction, implies that this BCI could be used by people who lack eye-movement control [8]. Many research groups are further developing P300-based BCIs [9]. Several groups have explored BCIs that use auditory rather than visual stimuli; these would be useful for people with visual impairments [10].
Wolpaw et al. [11] reported the first use of sensorimotor rhythms (SMRs) for BCI control. SMRs are oscillations (i.e., mu (8-12 Hz) and beta (18-30 Hz)) recorded over sensorimotor cortices that change in amplitude with movement, imagined movement, or preparation for movement [12]. People can learn to control SMR amplitudes to move a cursor to hit targets on a video screen or perform other computer-based tasks. SMR amplitude is measured by spectral analysis; the subject learns to increase or decrease it as needed to move the cursor toward the target. The rapid bidirectional nature of this BCI control paradigm [11] distinguished it from prior studies that sought to produce long-term unidirectional changes in brain rhythms for therapeutic purpose [e.g., 13]. Subsequent studies by several groups have further developed this BCI method. Subjects can learn to use SMR amplitudes to control movement in multiple dimensions simultaneously and to support sequential mouse-like control [14-16].
Many efforts to develop SMR-based BCIs ask the user to generate specific mental states through motor imagery [16]. Different BCI commands are often linked to different imagery (e.g., imagine hand movement to move the cursor up and foot movement to move it down). However, as the user’s SMR control improves, and particularly when users advance to controlling multiple dimensions, imagery tends to disappear [14,15]. While motor imagery may provide a logical and effective starting point for user training, it becomes unnecessary and may even be an impediment as training progresses. Kober et al. [18] found that those subjects who reported using no specific mental strategy after ten SMR training sessions showed improved performance. In contrast, subjects reporting various mental strategies after ten training sessions showed no improvement. Such results suggest that successful SMR control after extended training involves implicit learning mechanisms. Thus, SMR-based BCI control after extended training resembles typical motor performance in that it tends to become automatic (i.e., implicit) with practice. Viewing SMR control as similar to other forms of motor control suggests using principles of motor learning for task design. Motor learning involves both explicit and implicit processes which have differing characteristics [19]. Explicit instructions can interfere with implicit processes, particularly in well-trained people [20].
Another type of BCI uses steady-state visual evoked potentials (SSVEPs) recorded over occipital cortex in response to lights that flash repeatedly [21]. In this approach, a subject views several lights that each flash at a different frequency. When the subject focuses attention on one particular light, EEG spectral analysis shows increased power at its frequency band; the BCI detects this and performs the action represented by that light. SSVEP-based BCIs can support high rates of information transfer [22].
In principle, an EEG-based BCI system comprises four modular subsystems; the first acquires the EEG signals; the second processes these signals to derive specific signal frequencies (e.g., SMR amplitudes) and translates these features into output commands that control an application; the third is the application itself (e.g., a spelling program or robotic arm); and the fourth is the protocol that specifies overall system operation (e.g., when stimuli occur) [23,3]. Each of these modular subsystems presents significant design problems. To be useful for a wide variety of users, EEG recording sensors and amplifiers need to provide reliable high quality signals and be comfortable and easy to apply and use for individuals who are not technically sophisticated [24]. Signal processing needs to extract the relevant signal features reliably and translate them accurately into output commands [2]. The next sections address these two critical areas of BCI development. Each presents engineering challenges, and at the same time provides opportunities for improving BCI performance.
Recording Methods
Effective BCI systems require reliable robust high-quality EEG recording. Standard recording uses wet electrodes; a conductive gel maintains good electrode contact with the scalp. While wet electrodes can provide excellent EEG recording, they are less than optimal, particularly for long-term daily use by people in their homes. Many find them inconvenient to use: they require careful application; the gel is sometimes messy and needs periodic replenishment; the cap or other apparatus the holds them in position on the head may be uncomfortable, awkward, or unattractive [25]. If the electrode density is too great, bridging can occur (i.e., two or more sensors are electrically coupled). In addition, surface electrodes are susceptible to a variety of artifacts due to non-brain activity (e.g., electromyographic (EMG) signals), bodily movements, or nearby electrical equipment, particularly if electrode impedance increases. While Ferree et al [26] suggest that high electrode impedance has little effect beyond powerline noise that can be easily filtered out, Kappenman & Luck [27] showed that it increases EEG noise primarily at lower frequencies and reduces the signal-to-noise ratio of the P300 response.
Several alternative wet electrode designs have appeared in recent years. The EPOC system (Emotive) uses moistened felt pads and a semi-rigid support that enables faster electrode placement but is less accurate than conventional placement methods and largely restricts placements to sites on the scalp perimeter, which are more susceptible to EMG contamination [28]. The g.SAHARA dry electrode (g.tec) consists of a set of 8 gold-plated pins; these electrodes are mounted in a conventional cap that does not limit electrode locations and is reported to provide P300-based BCI results similar to those provide by wet electrodes [29]. Both the EPOC and g.SAHARA electrodes rely on low impedance resistive contact with the scalp. In contrast, the dry electrode developed by QUASAR and Wearable Sensing uses a hybrid combination of high-impedance resistive and capacitive contact with the scalp [30].
The device that holds the recording electrodes on the scalp is extremely important, particularly for longterm home use. Ideally, this device allows electrodes to be accurately positioned anywhere on the scalp and keeps them firmly yet comfortably in place, readily accommodates differently sized and shaped heads, neither interferes with nor is disturbed by head positioning (e.g., on a headrest or pillow), and is reasonably inobtrusive and cosmetic. Insecure electrode placement can lead to noise due to sudden changes in impedance (“electrode pops”) and variable placement can increase day-to-day variations in the EEG features used by a BCI.
Nijboer et al [31] reported that a 32-channel Biosemi system produced higher P300-based BCI accuracy than an 8-channel g.Sahara or 14-channel EPOC systems; however, the BioSemi system and the g.Sahara system were comparable when their performances using the same eight electrode sites were compared. Hariston et al. [32] compared the EPOC, QUASAR, and B-Alert X-10 (Advanced Brain Monitoring) systems with the BioSemi system, which they considered the gold standard. While they did not evaluate signal quality or system performance, they found that only the BioSemi system accommodated variations in both head size and shape. They rated the B-Alert system next in terms of accommodation. The EPOC and QUASAR systems could produce uncomfortable pressure points and movement artifacts. Dry electrodes can be more difficult to secure to the scalp; this may create a trade-off between comfort and recording quality. At the same time, the recent advent of dry electrode systems (e.g., Wearable Sensing) that provide EEG signals comparable to those from wet-electrode systems is an exciting and important advance toward the realization of practical widely-used EEG-based BCI systems.
BCI methods that use epidural or subdural electrodes [33] or intracortical microelectrodes [34] offer more secure placement and better spatial resolution than EEG. On the other hand, it is not yet clear to what extent and for what purposes their invasive nature and increased cost is justified as non-invasive methods can produce comparable target acquisition times [15]. In addition, these invasive recording methods, particularly those using intracortical electrodes, have not yet demonstrated reliable longterm (i.e., years) recording stability [34]. Future advances in materials and techniques may achieve longterm stability and performance sufficient to justify implanted BCI systems [35].
EEG Analysis for BCIs
Communication and control applications depend on ongoing interaction between the user and the BCI system; the user observes the results of his or her intentions and adjusts the ongoing output in order to maintain good performance and correct mistakes. Thus, BCIs must operate in real time and provide feedback to their users. Many of the initial BCI studies satisfied this real-time requirement [2]. However, more recent studies are often based on offline analyses of pre-recorded data. For example, the Lotte et al. [36] review of studies evaluating BCI signal-classification algorithms found that most used offline analyses. Indeed, the current popularity of BCI research is doubtless due in part to the ease with which offline analyses can be performed on publicly available data sets. While such offline studies can help guide actual online BCI studies, there is no guarantee that offline results will generalize to online performance. When the algorithm that extracts EEG and translates them into outputs is changed, it changes the ongoing results that are fed back to the user; thus, it is likely to change the user’s subsequent EEG signals. The ultimate test of any new BCI design is comprehensive online testing; offline analysis is not in itself sufficient.
The two steps in EEG analysis for BCIs are feature extraction and feature translation [2]. A variety of spatial and temporal filtering methods have been applied to feature extraction. These include interest in currently popular algorithms such as convolutional neural networks [37]. Many recent studies have used data-driven spatial filtering methods such as common spatial patterns [38] and source imaging methods [39]. Recent interest in network models of CNS function has prompted studies exploring the use of phase information. Indeed, the success of the surface Laplacian for amplitude-based features may be due to inclusion of phase effects [40]. Phase effects may also be involved in amplitude-based common spatial patterns and other data-driven spatial filtering methods [41]. At the same time, the inclusion of phase complicates neurobiological interpretation of these complex multivariate models of scalp EEG activity.
Physiological and anatomical understanding of the features used by BCI systems is important for designing signal processing methods as well as for detecting and eliminating the impact of artifacts (e.g., non-brain activity such as EMG, EOG (electrooculographic activity)). In reviewing the literature on artifact detection and removal, Islam et al [42] concluded that none of the existing methods is a perfect solution. One problem is that it is often difficult to evaluate the effectiveness of the methods because completely uncontaminated data may not be available. Furthermore, the results of methods such as independent components analysis may be difficult to connect to specific underlying brain or non-brain (i.e., artifactual) events. They may also require some level of human intervention, which rules out their use in real-time systems unless generalization to novel data can be verified.
Perhaps the most common type of BCI study evaluates alternative feature translation algorithms [36], often using archival data from healthy subjects [e.g., 43]. Many of these data sets do not include data from more than one session; many were acquired during actual muscle-based control; and many did not provide online feedback to the user. Thus, many of these data sets do not closely approximate data from the individuals most likely to benefit from BCI-based communication and control. These individuals have severe neuromuscular disorders (e.g., ALS) that curtail their movements and/or may affect their EEG activity; and their data are often gathered under complicated and highly variable circumstances (e.g., home settings). Finally, when alternative translation algorithms are submitted to offline testing using the same data set, their differences in performance are often minimal [44].
Because effective BCIs must function reliably from moment to moment and day to day, and brain signals change continually on multiple time scales, EEG feature extraction and translation methods that require large amounts of training data are problematic. For example, Rasmussen et al [45] report that the directional tuning of neurons in the primary motor cortex of monkeys changed between two BCI tasks. They suggest that motor units show dynamic range adaptation in a manner analogous to that commonly seen in sensory neurons. Sussillo et al [46] suggest that robust BCI translation algorithms can be tuned by using data from a wide variety of recording conditions. An alternative approach would use continual adaption of parameters [47]. The importance of adaption may vary with the neural signal. For example, adaptive updating of feature weights improves SMR-based performance but not P300-based performance [47]. Thus, these two BCI signals have different characteristics that require alternative approaches to real-time signal processing. In general, the importance of ongoing adaptive changes by BCI algorithms favors use of simple algorithms that have relatively few parameters to adapt.
BCIs for Neurorehabilitation
Over the past decade, the possibility that BCIs might enhance rehabilitation for people with strokes or other CNS trauma or disease has generated steadily increasing interest for several reasons. Effective BCI-bsed rehabilitation could help many millions of people around the world. Furthermore, unlike BCIs for critical communication and control applications, BCIs for rehabilitation do not have to have near-perfect performance, they need only to be effective, that is, to enhance recovery of function beyond that achieved by standard rehabilitation therapies alone.
In theory, a BCI might contribute to functional recovery in several different ways [5]. Dobkin [48] suggested that practical BCI systems could be used as a tool to reinforce the use of spared neural representations or to insure that subjects were optimally prepared to execute a particular movement. Daly and Wolpaw [49] suggested two possible strategies. The first uses BCI-based feedback to normalize relevant brain activity with the expectation that this will be accompanied by improved motor function; the second strategy uses brain activity to enable practice of more normal neuromuscular control with the expectation that the more normal sensory input produced by better movement will induce plasticity that improves neuromuscular control. Prasad et al. [50] suggested that BCI technologies could be used to enhance motor imagery in individuals with stroke. Donati et al. [51] report that simply using a BCIcontroled exoskeleton over an extended period improves walking in patients with spinal cord injury. To date there have been a number of studies providing proof of principle, but only a few that provide clear evidence of efficacy (e.g., [52]).
The rationale for using BCI-facilitated motor imagery for rehabilitation is that it is likely to activate some of the same neural systems important in actual movement; thus it might be an effective therapy for stroke-related dysfunction. Given that brain lesions can impair imagery, methods to facilitate imagery might enhance recovery. In one such method, a BCI provides feedback based on sensorimotor rhythms (SMRs) while the patient imagines movement of the affected limbs [53]. SMR training is used to enhance motor imagery. Thus, these EEG features are employed as an index of a cognitive task, the rehearsal of which facilitates recovery from motor deficits following stroke.
Using BCI-based movement to reduce stroke-related motor deficits closes the sensorimotor loop [54]. With this paradigm, SMR desynchronization (i.e., decrease) is rewarded by the activation of an orthosis that moves the affected limb. This strategy assumes that the proprioceptive feedback produced by limb movement will activate motor cortex. Several alternative explanations have been provided for the effects of closing the sensorimotor loop, including Hebbian learning and priming of subsequent physical therapy [54].
BCI technology has also been used to train users to produce brain states that improve movement preparation [55,56]. In this paradigm, users learn to modulate SMRs in advance of the motor task to be practiced. This approach assumes that better preparation facilitates subsequent motor performance. Therapeutic benefit can then result from the correct performance of the facilitated motor behavior and also from the user’s learning of task-appropriate preparatory responses.
The goal of imagery enhancement is to reinforce weak imagery [51]. Closing the sensorimotor loop strives to associate intention with haptic feedback [53]. Improving preparation seeks to insure optimal preparation for the task [55,56]. These different approaches to BCI-based rehabilitation have the same basic goal – improved motor performance. The growing pace of BCI-based rehabilitation research ensures that in the coming years these approaches and others as well will be extensively tested. It is likely that BCI technology will soon complement other rehabilitation methods and enhance functional recovery for people with strokes and other chronic neuromuscular disorders.
Conclusions
Most EEG-based BCIs use the P300 evoked potential, sensorimotor rhythms (SMRs), or the steady-state visual evoked potential (SSVEP). All three BCI types can help to restore basic communication and control to people with severe neuromuscular disabilities. At present, their capabilities are limited. Improved EEG recording methods that can provide stable high-quality signals in all environments, are comfortable, and are easy to use are needed. New dry-electrode systems have considerable promise. Improved signal analysis algorithms that can consistently maintain accurate performance are also required. While much algorithmic development to date has relied on offline analyses of archival data, actual online testing of new algorithms is essential because it takes into account the crucial ongoing adaptive interactions between the user and the BCI. BCIs, particularly SMR-based BCIs, also show promise as new methods for enhancing functional recovery for people with strokes or other chronic disorders. Several strategies for using BCIs to induce beneficial plasticity are under study. Evidence that these methods can enhance recovery beyond that achieved by conventional methods alone is just beginning to emerge.
Figure.
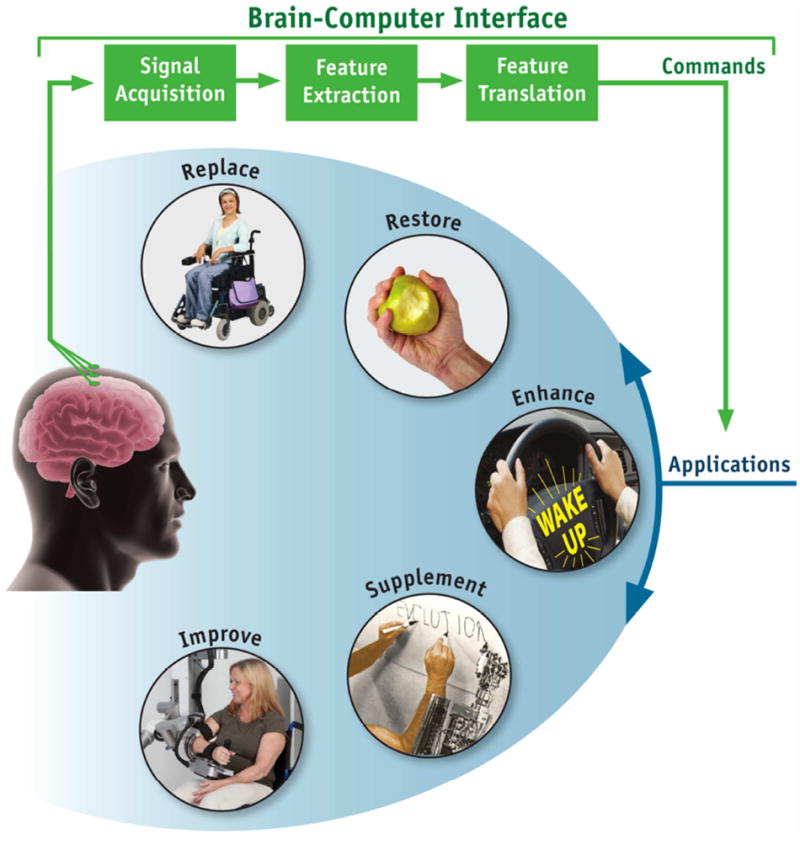
Basic design and operation of a brain-computer interface (BCI) system. The BCI is shown in green. Electrical signals produced by brain activity are recorded from the scalp, from the cortical surface, or from within the brain. They are analyzed to measure specific features (e.g., amplitudes of EEG rhythms or firing rates of single neurons) that reflect the BCI user’s intent. These features are translated into commands that operate applications that replace, restore, enhance, supplement, or improve natural (i.e., neuromuscular) CNS outputs. (From [3]).
HIGHLIGHTS.
Most EEG-based BCIs use P300 evoked potentials, sensorimotor rhythms (SMRs), or the steady-state visual evoked potential (SSVEP) to restore communication and control to people with severe disabilities.
Improved recording and signal processing are needed to increase BCI practicality.
Online evaluation of new algorithms is essential because it takes into account the crucial ongoing adaptive interactions between the user and the BCI.
BCIs, particularly SMR-based BCIs, also show promise as new methods for enhancing functional recovery for people with strokes or other chronic disorders.
Acknowledgments
The National Center for Adaptive Neurotechnologies is supported by the National Institute of Biomedical Imaging and Bioengineering of the NIH (Grant 1P41EB018783).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
-
*
of special interest
-
**
of outstanding interest
- 1.Vidal JJ. Towards direct brain-computer communication. Annu Rev Biophys Bioeng. 1973;2:157–180. doi: 10.1146/annurev.bb.02.060173.001105. [DOI] [PubMed] [Google Scholar]
- 2.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain–computer interfaces for communication and control. Clin Neurophysiol. 2002;113:767–91. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 3.Wolpaw JR, Wolpaw EW, editors. Brain–Computer Interfaces: Principles and Practice. New York, NY: Oxford University Press; 2012. Brain–computer interfaces: something new under the sun; pp. 3–12. ** This comprehensive textbook provides didactic coverage of the principles and practice of BCI research and development. [Google Scholar]
- 4.Sellers EW, Ryan DB, Hauser CK. Noninvasive brain-computer interface enables communication after brainstem stroke. Sci Transl Med. 2014;6:257re7. doi: 10.1126/scitranslmed.3007801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McFarland DJ, Daly J, Boulay C, Parvez MA. Therapeutic applications of BCI technologies. Brain Comput Interfaces. 2017;4:37–52. doi: 10.1080/2326263X.2017.1307625. * This article considers various alternative methods for application of BCI technologies for rehabilitation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farwell LA, Donchin E. Talking off the top of your head: Toward a mental prosthesis utilizing eventrelated brain potentials. EEG Clin Neurophysiol. 1988;70:510–523. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- 7.Sellers EW, Arbel Y, Donchin E. Brain–Computer Interfaces: Principles and Practice. New York, NY: Oxford University Press; 2012. BCIs that use P300 event-related potentials; pp. 215–226. [Google Scholar]
- 8.Aloise F, Arico P, Schettini F, Riccio A, Salinari S, Mattia D, Babiloni F, Cincotti F. A covert attention P300-based brain-computer interface: Geospell. Ergonomics. 2012;55:538–551. doi: 10.1080/00140139.2012.661084. [DOI] [PubMed] [Google Scholar]
- 9.Powers JC, Bielliaieva K, Wu S, Nam CS. The human factors and ergonomics of P300-based braincomputer interfaces. Brain Sci. 2015;5:318–356. doi: 10.3390/brainsci5030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klobassa DS, Vaughan TM, Brunner P, Schwartz NE, Wolpaw JR, Neuper C, Sellers EW. Toward a highthroughput auditory P300-based brain-computer interface. Clin Neurophysiol. 2009;120:1252–1261. doi: 10.1016/j.clinph.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. EEG Clin Neurophysiol. 1991;78:252–259. doi: 10.1016/0013-4694(91)90040-b. [DOI] [PubMed] [Google Scholar]
- 12.Pfurtscheller G, McFarland DJ. Brain–Computer Interfaces: Principles and Practice. New York, NY: Oxford University Press; 2012. BCIs that use sensorimotor rhythms; pp. 227–240. [Google Scholar]
- 13.Sterman MB, Egner T. Foundation and practice of neurofeedback for the treatment of epilepsy. Appl Psychophysiol Biofeedback. 2006;31:21–35. doi: 10.1007/s10484-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 14.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive braincomputer interface. PNAS. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic (EEG) control of threedimensional movement. J Neural Eng 2010. 2010;7:036007. doi: 10.1088/1741-2560/7/3/036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan H, He B. Brain-computer interfaces using sensorimotor rhythms: current state and future perspectives. IEEE Trans Biomed Eng. 2014;61:1425–35. doi: 10.1109/TBME.2014.2312397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotte F, Larrue F, Muhl C. Flaws in current human training protocols for spontaneous brain-computer interfaces: leasons learned from instructional design. Front Hum Neurosci. 2013;7:568. doi: 10.3389/fnhum.2013.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kober SE, Witte M, Ninaus M, Neuper C, Wood G. Learning to modulate one’s own brain activity: the effects of spontaneous mental strategies. Front Hum Neurosci. 2013;7:695. doi: 10.3389/fnhum.2013.00695. * This paper shows that explicit mental stratigies may be a sub-optimal approach to SMR-based BCI tasks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huberdeau DM, Krakauer JW, Haith AM. Dual-process decomposition in human sensorimotor adaptation. Curr Opin Neurobiol. 2015;33:71–77. doi: 10.1016/j.conb.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Lewthwaite R, Wulf G. Optimizing motivation and attention for motor performance and learning. Curr Opin Psychol. 2017;16:38–42. doi: 10.1016/j.copsyc.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Allison BZ, Faller J, Neuper C. Brain–Computer Interfaces: Principles and Practice. New York, NY: Oxford University Press; 2012. BCIs that use steady-state visual evoked potentials; pp. 241–250. [Google Scholar]
- 22.Chen X, Wang Y, Nakanishi M, Gao X, Jung TP, Gao S. High-speed spelling with a noninvasive brain-computer interface. PNAS. 2015;112:E6058–67. doi: 10.1073/pnas.1508080112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose braincomputer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 24.McFarland DJ, Vaughan TM. BCI in practice. Prog Brain Res. 2016;228:389–404. doi: 10.1016/bs.pbr.2016.06.005. * These authors describe problems limiting the use of BCI systems for individuals with disabilities. [DOI] [PubMed] [Google Scholar]
- 25.Peters B, Bieker G, Heckman SM, Huggins JE, Wolf C, Zeitlin D, Fried-Oken M. Brain-computer interface users speak up: the virtual users’ forum at the 2013 international brain-computer interface meeting. Arch Phys Med Rehabil. 2015;96:S33–S37. doi: 10.1016/j.apmr.2014.03.037. * This article considers BCI design from a users perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin Neurophysiol. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- 27.Mathewson KE, Harrison TJL, Kizuk SAD. High and dry? Comparing active dry EEG electrodes to active and passive wet electrodes. Psychophysiology. 2017;54:74–82. doi: 10.1111/psyp.12536. [DOI] [PubMed] [Google Scholar]
- 28.Mayaud L, Congedo M, Van Laghenhove A, Orlikowski D, Figere M, Azabou E, Cheliout-Heraut F. A comparison of recording modalities of P300 event-related potentials (ERP) for brain-computer interface (BCI) paradigm. Neurophysiol Clin. 2013;43:217–227. doi: 10.1016/j.neucli.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Guger C, Krausz G, Allison BZ, Edlinger G. Comparison of dry and gel based electrodes for P300 brain– computer interfaces. Front Neurosci. 2012;6:60. doi: 10.3389/fnins.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sellers EW, Turner P, Sarnacki WA, McManus T, Vaughan TM, Matthews R. A novel dry electrode for brain-computer interface. Lect Notes Comput Sci. 2009;5611:623–631. [Google Scholar]
- 31.Nijboer F, Van De Laar B, Gerritsen S, Nijholt A, Poel M. Usability of three electroencephalogram headsets for brain–computer interfaces: a within subject comparison. Interact Comput. 2015;27:500– 511. ** This study provides a systematic comparison of several commercial EEG recording systems. In particular it assesses the accuracy of online BCI performance. [Google Scholar]
- 32.Hairston WD, Whitaker KW, Ries AJ, Vettel JM, Bradford JC, Kerick SE, McDowell K. Usability of four commercially-oriented EEG systems. J Neural Eng. 2014;11:046018. doi: 10.1088/1741-2560/11/4/046018. [DOI] [PubMed] [Google Scholar]
- 33.Schalk G, Miller KJ, Anderson NR, Wilson JA, Smyth MD, Ojemann JG, Moran DW, Wolpaw JR, Leuthardt EC. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008;5:75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homer ML, Nurmikko AV, Donoghue JP, Hochberg LR. Sensors and decoding for intracortical brain computer interfaces. Annu Rev Biomed Eng. 2013;15:383–405. doi: 10.1146/annurev-bioeng-071910-124640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huggins JE, Guger C, Allison B, Anderson CW, Batista A, Brouwer AM, Brunner C, Chavarriaga R, Fried-Oken M, Gunduz A, Gupta D, Kubler A, Leeb R, Lotte F, Miller LE, Muller-Putz G, Rutkowski T, Tangermann M, Thompson DE. Workshops of the fifth international brain–computer interface meeting: defining the future. Brain Comput Interfaces. 2014;1:27–49. doi: 10.1080/2326263X.2013.876724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lotte F, Congedo M, Lecuyer A, Lamarche F, Arnaldi B. A review of classification algorithms for EEGbased brain-computer interfaces. J Neural Eng. 2007;4:R1–R13. doi: 10.1088/1741-2560/4/2/R01. [DOI] [PubMed] [Google Scholar]
- 37.Kwak N-S, Muller K-R, Lee S-W. A convolutional neural network for steady state visual evoked potential classification under ambulatory environment. PLoS ONE. 2017;12:e0172578. doi: 10.1371/journal.pone.0172578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sannelli C, Vidaurre C, Muller K-R, Blankertz B. Ensembles of adaptive spatial filters increase BCI performance: an online evaluation. J Neural Eng. 2016;13:046003. doi: 10.1088/1741-2560/13/4/046003. [DOI] [PubMed] [Google Scholar]
- 39.Edelman BJ, Baxter B, He B. EEG source imaging enhances the decoding of complex right-hand motor imagery tasks. IEEE Trans Biomed Eng. 2016;63:4–14. doi: 10.1109/TBME.2015.2467312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jian W, Chen M, McFarland DJ. EEG based zero-phase-locking value (PLV) and effects of spatial filtering during actual movement. Brain Res Bull. 2017;130:156–164. doi: 10.1016/j.brainresbull.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falzon O, Camilleri KP, Muscat J. The analytic common spatial patterns method for EEG-based BCI data. J Neural Eng. 2012;9:045009. doi: 10.1088/1741-2560/9/4/045009. [DOI] [PubMed] [Google Scholar]
- 42.Islam K, Rastegarnia A, Yang Z. Methods for artifact detection and removal from scalp EEG: a review. Neurophysiol Clin. 2016;46:287–305. doi: 10.1016/j.neucli.2016.07.002. * The authors provide a comprehensive review of many proposed methods for artifact reduction and note difficulties in assessing the results due to a lack of ground truth. [DOI] [PubMed] [Google Scholar]
- 43.Tangermann M, Muller K-R, Aertsen A, Birbaumer N, Braun C, Brunner C, Leeb R, Mehring C, Miller KJ, Muller-Putz GR, Nolte G, Pfurtscheller G, Preissl H, Schalk G, Schlogl A, Vidaurre C, Waldert S, Blankertz B. Review of BCI competition IV. Front Neurosci. 2012;6:55. doi: 10.3389/fnins.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krusienski DJ, Sellers EW, Cabestaing FC, Bayoudh S, McFarland DJ, Vaughan TM, Wolpaw JR. A comparison of classification techniques for the P300 speller. J Neural Eng. 2006;3:299–305. doi: 10.1088/1741-2560/3/4/007. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen RG, Schwartz A, Chase SM. Dynamic range adaptation in primary motor cortical populations. eLife. 2017;6:e21409. doi: 10.7554/eLife.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sussillo D, Stavisky SD, Kao JC, Ryu SI, Shenoy KV. Making brain-machine interfaces robust to future neural variability. Nat Commun. 2016;13:13749. doi: 10.1038/ncomms13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McFarland DJ, Sarnacki WA, Wolpaw JR. Should the parameters of a BCI translation algorithm be continually adapted? J Neurosci Meth. 2011;199:103–107. doi: 10.1016/j.jneumeth.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobkin BH. Brain-computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. J Physiol. 2007;579:637–642. doi: 10.1113/jphysiol.2006.123067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7:1032–1043. doi: 10.1016/S1474-4422(08)70223-0. * This review describes possible strategies for applying BCI technologies to rehabilitation of motor disorders. [DOI] [PubMed] [Google Scholar]
- 50.Prasad G, Herman P, Coyle D, McDonough S, Crosbie J. Applying a brain-computer interface to support motor imagery practice in people with stroke for upper limb recovery: a feasibility study. J Neuroeng Rehabil. 2010;7:60. doi: 10.1186/1743-0003-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donati ARC, Shokur S, Morya E, Campos DSF, Moioli RC, Gitti CM, Augusto PB, Tripodi S, Pires CG, Pereira GA, Brasil FL, Gallo S, Lin AA, Takigami AK, Aratanha MA, Joshi S, Bleuler H, Cheng G, Rudolph A. Nicolelis MAL Long-term training with a brain-machhine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep. 2016;6:30383. doi: 10.1038/srep30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos-Murguialday A, Broetz D, Rea M, et al. Brain-machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol. 2013;74:100–108. doi: 10.1002/ana.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pichiorri F, Morone G, Petti M, et al. Brain-computer interface boosts motor imagery practice during stroke recovery. Ann Neurol. 2015;77:851–865. doi: 10.1002/ana.24390. [DOI] [PubMed] [Google Scholar]
- 54.Curado MR, Cossio EG, Broetz D, et al. Residual upper arm motor function primes innervation of paretic forearm muscles in chronic stroke after brain-machine interface (BMI) training. PLoS ONE. 2015;10:e0140161. doi: 10.1371/journal.pone.0140161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulay CB, Sarnacki WA, Wolpaw JR, McFarland DJ. Trained modulation of sensorimotor rhythm can affect reaction time. Clin Neurophysiol. 2011;122:1820–1826. doi: 10.1016/j.clinph.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McFarland DJ, Sarnacki WA, Wolpaw JR. Effects of training pre-movement sensorimotor rhythms on behavioral performance. J Neural Eng. 2015;12:066021. doi: 10.1088/1741-2560/12/6/066021. [DOI] [PMC free article] [PubMed] [Google Scholar]


