Abstract
Our brains are constantly processing past events [1]. These off-line processes consolidate memories, leading in the case of motor skill memories to an enhancement in performance between training sessions. A similar magnitude of enhancement develops over a night of sleep following an implicit task, when a sequence of movements is acquired unintentionally, or following an explicit task, when the same sequence is acquired intentionally [2]. What remains poorly understood, however, is whether these similar offline improvements are supported by similar circuits, or through distinct circuits. We set out to distinguish between these possibilities by applying Transcranial Magnetic Stimulation (TMS), over the primary motor cortex (M1) or the inferior parietal lobule (IPL) immediately after learning in either the explicit or implicit task. These brain areas have both been implicated in encoding aspects of a motor sequence, and subsequently supporting offline improvements over sleep [3–5]. Here we show that offline improvements following the explicit task are dependent upon a circuit that includes M1 but not IPL. By contrast, offline improvements following the implicit task are dependent upon a circuit that includes IPL but not M1. Our work establishes the critical contribution made by M1 and IPL circuits to offline memory processing, and reveals that distinct circuits support similar offline improvements.
A memory continues to be processed after its formation [1, 6, 7]. These “offline” processes consolidate a memory, which can lead in the case of motor skill memories to their enhancement over sleep [1, 6, 8, 9]. Offline improvements of a similar magnitude develop following an implicit task, when a sequence of movements is acquired unintentionally, or following an explicit task, when the same sequence is acquired intentionally [2]. Similar circuits may support these similar improvements. Alternatively, distinct circuits may support these improvements because they are triggered by different learning tasks. Distinguishing between these possibilities will provide insight into the organization of offline processing.
During learning a memory for a skill is encoded across a network of brain areas [10]. Included within this network are the primary motor cortex (M1) and the inferior parietal lobule (IPL, [3, 10, 11]). Subsequent offline brain activity is perhaps, at least in part, due to the processing of the representations encoded during learning [12, 13]. The pattern of neuronal activity during the formation of a motor memory is replayed during sleep in the rat motor cortex, and this neuronal replay is correlated with the subsequent offline improvement in performance over sleep ([5]; for a review [14]). While in humans, sleep-spindle activity over the primary motor cortex (M1) is related to the subsequent motor skill improvements over a nap [15]. Similarly, slow-wave activity over the inferior parietal lobule (IPL) has been related to overnight motor skill improvements [4]. Thus, M1 and IPL circuits have both been implicated in the encoding and subsequent offline processing of memories.
Potentially, M1 and IPL may operate together as a unified circuit critical for all improvements over a night of sleep. In this scenario, there is no redundancy with only a single route to the offline enhancement of a memory. Alternatively, M1 and IPL may be part of functionally distinct circuits providing alternative routes to offline enhancements of a memory. Consolidation would then not be linked to a particular circuit, but would be a property that emerges from any circuit. This is a more complex organization than a single circuit supporting overnight improvements of all motor skill memories; yet, it might have the benefit of allowing multiple skills to be consolidated simultaneously over sleep. Despite showing the same magnitude of improvement over sleep, different memory tasks may trigger distinct offline processes. In sum, the circuits supporting consolidation may be determined solely by the behavioral expression of consolidation; for example, an improvement; alternatively, they may be determined by the learnt task and its properties.
To distinguish between these possibilities, we had participants learn and be tested on either an implicit or explicit task [2, 16–25]. The implicit task was introduced to participants as a test of reaction time while in the explicit version of the task, participants were made aware of the underlying sequence (Figure 1). Immediately after learning TMS was applied over M1 or IPL, when the encoded representation is sensitive to disruption [26]. Subsequent offline processing in M1 or IPL circuits may be critically dependent on this encoded information. As a consequence disrupting this information will impair offline improvements to reveal the importance of these circuits to offline processing. We applied stimulation at 1Hz for 10 minutes (i.e., 600 pulses), which commonly leads to a decrease in cortical excitability, and importantly has been widely used to disrupt a diverse array of cognitive processes in humans [27–30]. Following stimulation, participants remained awake for several hours, and then slept. After the night of sleep we retested participants’ performance. The difference between performance at testing and retesting provided a measure of off-line improvements (Figure 1).
Figure 1. Experimental Design.
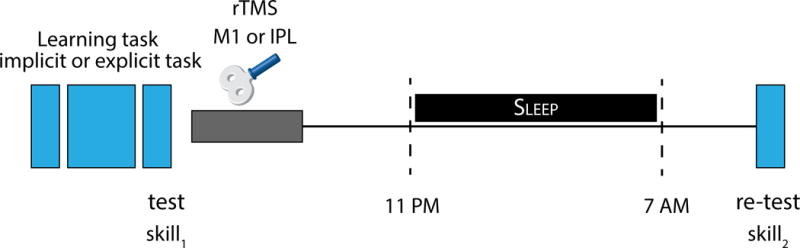
The implicit and explicit tasks required the acquisition of skill for the same sequence of movements. The implicit task, was introduced to participants as a test of reaction time; while, the explicit task was introduced to participants as a sequence learning task. After learning and testing in either the implicit or explicit task (8pm; skill1), repetitive TMS (rTMS) was applied to either M1 or IPL. Participants’ subsequent sleep was recorded (11pm to 7am), and after waking their skill on the learnt task was retested (8am; skill2). The difference between participants’ initial (skill1) and subsequent skill (skill2) provided a measure of off-line improvements over the night of sleep.
We found no significant difference in participants’ initial skill across the groups (ANOVA, F(3,47) = 0.226, p = 0.878). The number of errors made by participants during testing also did not differ significantly across the groups (ANOVA, F(3,47) = 0.557, p = 0.646). There was also no significant difference in time asleep, time awake, sleep efficiency or the proportion of time spent in the various sleep stages (N1, N2, SWS, REM) across the groups (Table 1; MANOVA, F(24,105) = 0.890, p = 0.614). Nonetheless, we found that off-line improvements differed significantly depending upon the site of stimulation and the type of learning task (i.e., site x task interaction; repeated measures ANOVA, F(1,47) = 9.945, p = 0.003; Figure 2). This interaction was not significant for the change in error between testing and retesting (ANOVA F(1,47) = 1.473, p = 0.231).
Table 1. Sleep time, efficiency and stages.
We found no significant difference in total sleep time, time spent awake, sleep efficiency or the proportion of time spent in the sleep stages across the four different groups (MANOVA, F(24,105) = 0.890, p = 0.614). The table shows the sleep measures (mean±sem) for the different tasks (implicit vs. explicit) and different sites of stimulation (M1 vs. IPL).
| Implicit task | Explicit task | |||
|---|---|---|---|---|
| M1 | IPL | M1 | IPL | |
| Sleep Time (minutes) | 431±51 | 435±38 | 450±38 | 432±36 |
| Wake Time (minutes) | 22±8 | 13±4 | 14±3 | 13±3 |
| Sleep Efficiency (%) | 95±6 | 97±3 | 97±2 | 97±2 |
| N1 (%) | 9±4 | 9±3 | 10±2 | 9±3 |
| N2 (%) | 52±7 | 52±8 | 53±8 | 50±8 |
| N3 (i.e. SWS) (%) | 22±10 | 19±7 | 17±5 | 21±7 |
| NREM (%) | 83±3 | 80±4 | 80±5 | 80±5 |
| REM (%) | 17±4 | 20±6 | 20±5 | 20±5 |
Figure 2. A double dissociation between site of stimulation, and subsequent offline improvements in different learning tasks.
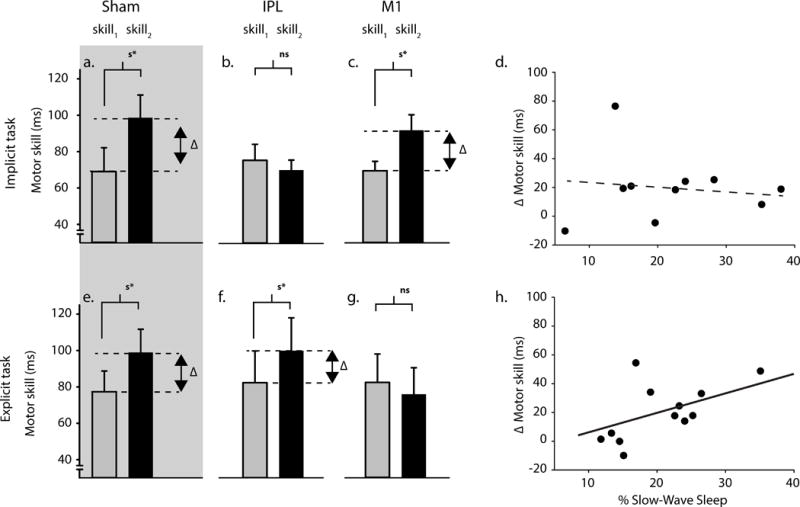
We found that off-line improvements differed significantly depending upon the learning task (implicit vs. explicit) and the site or type of stimulation (TMS to IPL vs. M1 or sham; ANOVA F(2,61) = 5.259, p = 0.008). But there was no significant difference across these groups at testing (i.e., skill1; ANOVA, F(5,61) = 0.204, p = 0.959; bar plots display, mean±sem). (a) Improvements developed over a night of sleep in the implicit task after sham stimulation (paired t-test, t(7) = 2.73, p = 0.03) (b) but no longer developed after applying TMS to IPL (paired t-test, t(13) = 0.521, p = 0.611). (c) By contrast, improvements continued to developed after applying TMS to M1 (paired t-test, t(11) = 3.188, p = 0.009). Recall for the sequence did not differ across the implicit task groups (i.e., sham vs. IPL vs. M1; ANOVA F(2,31) = 0.702, p = 0.503). (d) The improvements that did develop were not correlated with the percentage of sleep spent in slow-wave sleep in both a standard (R = 0.034, F(1,8) = 0.009, p = 0.925) and robust regression (p = 0.443), which is consistent with the improvements in the implicit task being time-dependent, rather than related to a brain state such as sleep. There continued to be no significant correlation even when the participant showing the greatest off-line improvement was removed from the analysis (R = 0.430, F(1,7) = 1.587, p = 0.248), and even without this participant there continued to be significant off-line improvements (paired t-test, t(10) = 3.31, p = 0.009). (e) Improvements developed over a night of sleep in the explicit task after sham stimulation (paired t-test, t(7) = 2.76, p = 0.027) (f) and also developed after applying TMS to IPL (paired t-test, t(13) = 3.222, p = 0.007). (g) By contrast, improvements no longer developed after applying TMS to M1 (paired t-test, t(11) = 0.832, p = 0.423). There was no significant difference in sequence recall across the explicit task groups (i.e., sham vs. IPL vs. M1; ANOVA F(2,30) = 0.660, p = 0.524). (h) The improvements following IPL stimulation were correlated with the percentage of total sleep that participants spent in slow-wave sleep in both a standard (R = 0.596, F(1,10) = 5.51, p = 0.04) and robust regression (p = 0.0038), which is consistent with the improvements in the explicit task being sleep-dependent. Only those participants with complete high-quality sleep recordings are shown in each of correlations (i.e., panels (d) and (h)).
Off-line improvements following learning in the implicit task were significantly less following IPL than M1 stimulation (repeated measures ANOVA, F(1,24) = 4.5, p = 0.044). We found no significant off-line improvements following IPL stimulation (mean±sem, 75±9ms vs. 70±6ms; paired t-test, t(13) = 0.521, p = 0.611); whereas, there were significant off-line improvements following M1 stimulation (70±6ms vs. 92±9ms; paired t-test, t(11) = 3.188, p = 0.009). These improvements were not correlated with the percentage of total sleep spent in either REM or slow-wave sleep, which is consistent with them developing independently of sleep (for further analysis; please see, Figure 2; REM, R = 0.289, F(1,8) = 0.728, p = 0.418; SWS, R = 0.034, F(1,8) = 0.009, p = 0.925; [1, 2, 18, 20, 21]). We also found significant off-line improvements following sham stimulation (69±14ms vs. 98±11ms; paired t-test, t(7) = 2.73, p = 0.03), and these were significantly greater than following IPL stimulation (unpaired t-test, t(20) = 2.16, p = 0.042). Thus, applying TMS to IPL but not M1 prevented the development of offline improvements in the implicit task.
By contrast, off-line improvements following learning in the explicit task were significantly less following M1 than IPL stimulation (repeated measures ANOVA; F(1,23) = 6.09, p = 0.021). We found no significant off-line improvements following M1 stimulation (82±16ms vs. 75±15ms; paired t-test, t(11) = 0.832, p = 0.423); whereas, there were significant off-line improvements following IPL stimulation (82±17ms vs. 99±18ms; paired t-test, t(12) = 2.979, p = 0.011). These improvements were correlated with the percentage of total sleep spent in slow-wave sleep, which is consistent with them being dependent upon sleep (for further analysis; please see, Figure 2; R = 0.578, F(1,10) = 5.51, p = 0.04; [1, 2, 18, 20, 21]). Off-line improvements also developed following sham stimulation (77±11ms vs. 99±13ms;paired t-test, t(7) = 2.78, p = 0.027), and these were significantly greater than following M1 stimulation (unpaired t-test, t(18) = 2.42, p = 0.026). Thus, applying TMS to M1 but not IPL prevented the development of off-line improvements in the explicit task. Overall, different mechanistic routes, one IPL-dependent and at the other M1-dependent, lead to the development of off-line improvements over sleep.
We applied TMS after learning for three important reasons. Firstly, by applying TMS immediately after learning, we prevented stimulation from affecting the initial learning and formation of a memory. Secondly, a memory is frequently most sensitive to disruptive interventions such as TMS or protein synthesis inhibitors immediately after learning [26]. Finally, applying TMS after learning minimized the potential for stimulation to affect subsequent sleep. Brain stimulation can affect sleep when it is applied immediately before or during sleep [31–33]. By contrast, in the current study several hours passed between applying TMS and the onset of sleep. We found no difference in the length of sleep or the proportion of time in each sleep stage across the groups. Thus, there was no site or task specific effect of stimulation on sleep, and so the current pattern of results cannot be explained by TMS affecting sleep. Overall, we applied stimulation at a time when it would affect memory consolidation and not formation, when the memory is highly sensitive to disruption, and when there was a minimal chance of stimulation affecting subsequent sleep.
Our results reveal a double dissociation. Following learning in the explicit task, a circuit containing M1, but not IPL, is critical for the development of improvements over sleep. By contrast, following learning in an implicit task, a circuit containing IPL, but not M1, is critical for the development of improvements over sleep. In both these tasks, improvements over sleep are of a similar magnitude. Thus, these results establish a degenerate organization for human memory processing, with distinct networks responsible for the same magnitude of enhancement.
The different tasks may trigger different behavioral patterns of consolidation. Improvements typically only develop over sleep, but following learning in the implicit task, improvements have also been reported to develop over wakefulness [2, 19]. We examined the development of offline improvements following learning in the implicit and explicit tasks. In an additional group, participants’ skill was tested, and then retested, at 8pm, after a 12-hr interval of wakefulness. We found no significant difference in initial skill at testing following learning in the implicit and explicit tasks (unpaired t-test, t(20) = 0.181, p = 0.858). Yet, offline improvements differed significant between the two tasks (repeated measures ANOVA, F(1,20) = 6.08, p = 0.023). Following learning in the implicit task there were significant improvements between testing and subsequent retesting over wakefulness (72±9ms vs. 100±9ms paired t-test, t(11) = 2.97, p = 0.013); whereas, following learning in the explicit task there were no significant improvements (74±11ms vs. 62±13ms paired t-test, t(9) = 0.887, p = 0.398). Overall, improvements following the implicit task develop over wakefulness, and as shown in the earlier experiments they also develop over sleep, which is a time-dependent pattern. By contrast, improvements in the explicit task do not develop over wakefulness, and instead only develop over a night of sleep, which is a sleep-dependent pattern.
Different networks may support the different behavioral patterns of consolidation. The implicit task shows a time-dependent pattern with improvements developing not only over sleep but also over wakefulness. At least 4–5 hours between testing and retesting is required in the implicit task for the development of offline improvements [2, 19]. By contrast, the explicit task shows a sleep-dependent pattern with improvements developing exclusively over sleep. Other similar tasks in which participants also intentionally learn a sequence of movements; such as the finger tapping task, show improvements over sleep ([16, 17]; please also see [1]). Improvements can develop rapidly within 5–30 minutes of rest after learning a finger tapping task, yet, these improvements are transient, while sustained offline improvements develop only over sleep [34, 35]. In this explanation, time and sleep-dependent improvements, are not merely descriptions of when improvements develop, but are mechanistically distinct, describing different processing routes. One route is dependent upon an IPL circuit, and the other dependent upon an M1 circuit.
Sleep and time-dependent consolidation are triggered by different tasks. As a consequence any explanation for the different circuits supporting offline improvements may lie with the tasks. Information is encoded during learning of these tasks. Applying TMS immediately after learning disrupts that information, which reveals its importance, and that of the targeted circuits (i.e., M1 and IPL) to subsequent offline processing. Thus, by understanding the information encoded during learning it may be possible to explain the differential contribution of M1 and IPL circuits to subsequent consolidation of the implicit and explicit tasks.
The implicit task has multiple components [18]. One of these components, the goal of the movement is enhanced over sleep, and this aspect of the memory is encoded during learning in a circuit that includes IPL [1, 3, 18]. As a consequence, applying TMS to IPL immediately after learning prevents improvements from developing over a night of sleep. Consistent with the importance of IPL for improvements over sleep, an increase in slow-wave power occurs over the parietal cortex after learning a visuomotor learning task [4]. The perturbation that participants adapt to in this task is introduced slowly over a number of trials, and so, like the sequence in the implicit task, participants are not likely to be completely aware of what they are learning. Subsequent improvements in this type of task, like the implicit task in the current study, develop over both wakefulness and sleep [36]. Thus, converging evidence shows that an IPL circuit is critical for improvements over sleep, particularly in tasks that show time-dependent improvements, potentially because of the aspect of the task encoded within the IPL circuit.
Another component of the implicit task is the actual sequence of movements themselves (as opposed to goal of those movements). This component is enhanced exclusively over wakefulness, and is encoded during learning in a circuit that includes M1 [1, 3, 18]. As a consequence, disrupting this circuit does not prevent improvements from developing over sleep. The component encoded within the M1 circuit is not necessary for improvements to develop over sleep, and so even with its disruption; these enhancements can still occur. By contrast, applying TMS over M1 prevents the development of improvements over wakefulness [18]. In sum, different components of the implicit task are encoded within M1 and IPL circuits. Only the goal component, encoded within the IPL circuit, is enhanced over sleep, and so the IPL circuit, and not the M1 circuit, is critical for improvements over sleep.
The explicit task, unlike the implicit task, only shows improvements over sleep [2, 37]. An inhibition of M1 prevents off-line improvements from developing over wakefulness [38]. Preventing inhibition of the M1 circuits is sufficient to induce improvements over wakefulness, and likewise, all that may be necessary for improvements over sleep, is the information within M1 circuits [38, 39]. Consistent with this idea, we show that applying TMS over M1, but not over IPL, prevents improvements from developing over sleep.
Other studies have also linked M1 to the development of offline improvements over sleep. For example, patterns of M1 functional activation change following overnight improvements in a task, which like the explicit task in the current study, involved participants intentionally learning a sequence of finger movements, which only showed improvements over sleep [7, 16, 17, 40]. Thus, an M1 circuit appears to be critical for supporting improvements over sleep, which is consistent with the physiological changes associated with other sleep-dependent tasks. Information encoded within, and only within the M1 circuit is critical for subsequent offline improvements, and so when this is disrupted (e.g., by applying TMS), improvements no longer develop.
Different circuits are critical for the offline processing of different tasks, despite those tasks showing similar offline improvements. Offline processing is related to the tasks learnt, and in turn the consolidation processes triggered (i.e., sleep vs. time-dependent), as opposed to being linked simply to the end result, in this case the performance enhancement of consolidation. Different circuits are supporting the same expression of consolidation, in this case enhancement, suggesting that, at least in principle, any circuit can support consolidation. With this degenerate organization there are alternative routes to consolidation, which may explain the diminished interference between memories over a night of sleep [1, 21]. A degenerate organization is in contrast to the perhaps more familiar redundant organization when multiple circuits are engaged simultaneously to support the same behavior (please see; for further discussion of redundancy vs. degeneracy [41]). Overall, offline enhancement during consolidation arises through different circuits, which are selected by features of the task during learning.
Methods
Experimental Design
A motor sequence learning task was introduced to participants as either a test of reaction time, the implicit task, or as a sequence learning task, the explicit task (please see, Motor sequence learning tasks). Both of these tasks show improvements over a night of sleep ([2, 16–18, 22]; for reviews [1, 9, 42]). Participants practiced, and were tested on one of these motor skill tasks, at 8pm (skill1). After learning TMS was immediately applied to either the primary motor cortex (M1) or inferior parietal lobule (IPL). Participants’ subsequently slept from 11pm to 7am, which was recorded using polysomongraphy (PSG), and participants’ motor skill was retested the next day at 8am (skill2). Their prior night sleep had also been recorded in the same sleep laboratory to allow them to adapt to sleeping in a novel environment. The change in motor skill between testing and subsequent retesting provided a measure of off-line change in motor skill. In sum, we used a 2(task; implicit vs. explicit) x2(site;M1 vs. IPL) between-subject design to understand how applying stimulation to different sites (i.e., site, IPL vs. M1) affected subsequent changes in skill between testing and retesting across different tasks (i.e., task, implicit vs. explicit) over sleep.
In another set of experiments, we applied sham rather than real stimulation. As in the earlier experiments, participants learnt either the implicit or the explicit task, had their skill tested (at 8pm; skill1) but immediately after learning; sham rather than real stimulation was applied. Subsequently participants slept over night, and had their skill retested the next day at 8am (skill2).
In the final set of experiments, we examined the development of offline improvements over wakefulness, as opposed to over a night of sleep. Following learning of either the implicit or explicit task, participants had their skill tested (at 8am; skill1), and subsequently retested, 12-hrs later (at 8pm; skill2). Examining improvements over wakefulness complemented the other experiments examining improvements over sleep. It allowed us to determine whether tasks show improvements over any interval, or, in contrast, one or both of the tasks showed improvements restricted to a particular interval.
Participants
We recruited only right-handed participants (defined by the Edinburgh handedness questionnaire [43]), 18–35 years of age, with no medical, neurological or psychiatric history, and either normal or corrected to normal vision were recruited to the study. All participants provided informed consent for the study, which was approved by the local institutional review board.
We randomly allocated fifty-five participants across the 4 groups of the 2(M1 vs. IPL) ×2(implicit vs. explicit) between subject design. Four participants allocated to the implicit task groups were able to recall 4 or more of the 12-item of the sequence, which modifies a task so that it comes to resemble the explicit task. Specifically, the four participants have a higher recall, which is associated with the explicit task. This higher recall may also alter the properties of offline improvements preventing them from developing over wake, and instead they develop exclusively over sleep [1, 2]. Disrupting recall to approximately 4-items allows improvements to develop over wake, and, in other earlier studies, excluding participants with a higher recall has allowed the implicit task to retain its capacity to develop improvements over both wake and sleep [2, 18, 21, 37]. A recall of more than four items can modify the implicit task, the properties of the offline improvements, and potentially the circuits supporting those improvements. Thus, those four participants with a recall of 4 or more items were removed from further analysis. Of the remaining 51 participants (24 male, 20.9±2.4 years; mean ± std) 12 were allocated to each of the M1 groups, 14 allocated to learn the implicit task in an IPL group, and 13 to learn the explicit task in the final IPL group.
A further sixteen participants (8 male, 21.6±0.8 years; mean ± std) were recruited, equally distributed and randomly allocated between the two groups that received sham stimulation.
For the final set of experiments, twenty five participants were recruited, of those, three allocated to implicit group were able to recall 4 or more of the 12-item of the sequence, and so were removed from subsequent analysis. Of the remaining 22 participants (9 male, 20.4±2.1 years; mean ± std) 10 were allocated to explicit task group, while the remaining 12 were allocated to the implicit task group. In both groups, participants were trained, tested, and following a 12-hr interval of wakefulness retested on the respective task.
For each group, we recruited approximately the same, and at times, more participants than used in earlier studies to detect offline improvements (please see; for example, [2, 37, 44]). Participants did not know the group they had been assigned to. Nonetheless, it was necessary for the individual running the study to know the assigned group for the appropriate task and site of stimulation to be used. Final analysis was conducted with knowledge of group allocation.
Motor sequence learning tasks
We used a modified version of the serial reaction time task (SRTT; [45, 46]). A solid circular visual cue (diameter 20mm, viewed from approximately 800mm) could appear at any one of four possible positions, designated 1 to 4, and arranged horizontally on a computer screen. Each of the four possible positions corresponded to one of the four buttons on a response pad (Cedrus, RB-410), upon which the fingers of the participant’s right hand rested. When a target appeared, participants were instructed to respond as quickly and accurately as possible by pressing the appropriate button on the pad. If the participant made an incorrect response, the stimulus remained until the correct button was selected. Once the correct response was made, the cue on the screen disappeared and was replaced by the next cue after a delay of 400ms. Response time was defined as the interval between presentation of a stimulus and selection of the correct response.
We used two versions of the SRTT. In one version, the explicit task, participants were introduced to the task as a sequence learning task. Participants were instructed that a change in the color of the stimuli from black to blue heralded the beginning of a repeating sequence (2-3-1-4-3-2-4-1-3-4-2-1). The color change was exclusively used to mark the introduction of the sequence, not its removal. The color change was used to mark the introduction of the sequence during all the training, test and retest blocks. Participants were neither told the sequence itself nor its length. By contrast, in the implicit task participants are introduced to the task as a test of reaction time. So, in this task participants were not told about the sequence, and there were no cues marking the introduction of the sequence. The stimuli did not change color during this task instead they remained black. The same regular and repeating 12-item sequence was used in both tasks. Each item within the sequence appeared at the same frequency, there were no item repeats, and it was a higher-order sequence with no item (n) being determined exclusively by the preceding item ((n-1); [46]).
To compare between the offline improvements that develop following learning in the implicit and explicit tasks, we used a design that has been successfully applied in earlier work [2, 18, 44]. Sequence learning can occur more quickly in the explicit than in the implicit task [47, 48]. Acquiring different amounts of skill could alter the subsequent pattern of offline improvements. It is the skill achieved, as opposed to the number of repetitions during training, which has been identified as being important for triggering consolidation [49]. Acquiring only a small amount of skill during learning may be insufficient to trigger subsequent offline improvements [49]. Conversely, substantial learning, at least in principle, may prevent or impair further improvements, as the maximum, or close to the maximum, skill has already been achieved. Thus, it was important to ensure that similar amounts of skill were acquired in both tasks. As in earlier work, we achieved this by using a different number of trials in each task [2, 38].
There was an initial short training block of either fifteen (implicit task) or nine (explicit task) repetitions of the sequence, the main training block had either twenty-five (implicit task) or fifteen (explicit task) repetitions, and then the test block had the same number of repetitions as the initial short training block. Participants were tested and subsequently retested the next morning (approximately 12 hrs. later, at 8am) on the same task (i.e., implicit or explicit task). The retest block has the same number of repetitions as the earlier test block. The difference between skill at testing (skill1) and retesting (skill2) provided a measure of off-line motor skill (skill2-skill1).
Fifty random trials preceded and followed the sequential trials in the training and test blocks, of both tasks. Within these random trials there were no item repeats (for example, -1-1- was illegal), and each item had approximately the same frequency of appearance. Each set of random trials in the training and test blocks were unique. This minimised the chance that participants might become familiar with the random trials. Nonetheless, the same random trials used within both tasks, which allowed performance of motor sequence that was common to both tasks to be compared to a common set of random trials.
We administered a free recall test when participants had completed the SRTT, following retesting. For the explicit task, participants had already been told about the sequence and so were simply asked to recall as many items of the sequence as possible. For the implicit task, participants were asked if they had noticed anything about the visual cues, and to describe that property. If participants had realised that there was a sequence, they were asked to recall as many items of the sequence as possible. Participants accurately recalling 4 or more items from the 12-item sequence were removed from further analysis. Declarative knowledge for the sequence modifies the task so that off-line improvements are no longer able to develop over wakefulness but only develop over a night of sleep (i.e., off-line improvements become sleep-dependent; [2, 37]).
Transcranial Magnetic Stimulation
Using a Magstim 2 Super Rapid (Magstim Inc), we applied TMS at 1Hz for 10-minutes (i.e., 600 pluses) at an intensity of 90% of motor threshold (MT). These same parameters have been widely adopted to disrupt a diverse array of cognitive processes in humans [27–30]. Using a stimulation intensity beneath MT minimized the movements elicited at each site of stimulation, which makes it easier to compare the effects of stimulation between these sites. Stimulation was applied immediately after one of the motor sequence learning tasks to either the left M1 or left IPL. The left M1 was identified as the optimal location for inducing contractions in the right abductor pollicus brevis muscle, and the lowest intensity of stimulation that was capable of inducing visible muscle contractions in at least 6 out of 10 trials was defined as the motor threshold (MT; [50]). For all the participants, we determined the MT before they practiced the motor sequence learning task. To identify the position of the IPL, we used frameless stereotaxy (Polaris, Northern Digital Inc). T1 weighted MR images were transformed into MNI/Talairach coordinates (Brainsight, Rogue Research, Montreal, Canada) and used to guide the position of the coil. The left IPL was defined as the MNI coordinates x=−48, y=−56, z=46. This site has been implicated in representing the goal of a movement sequence, which is the aspect of a motor memory that is enhanced over a night of sleep [3, 18]. For all participants, a commercially available 70 mm figure-of-eight coil (Magstim Inc) was positioned tangentially to the scalp with the handle of the coil pointing posteriorly at 45 degrees from the midsagittal axis. Based upon this coil position and orientation, we expect that the current induced in the brain during the first part of each biphasic pulse to have flowed in a posterior to anterior direction, and in the reverse, anterior to posterior direction, during the second and final part of the pulse [51].
Sham Stimulation
Using a commercially available figure-of-eight 70 mm sham coil, we applied sham stimulation at 1Hz, at 80% of stimulator output for ten minutes. Sham stimulation was applied either with the center of the coil placed at the C3 or P3 electrode position. The orientation of the coil was identical to that of real stimulation (i.e., tangentially to the scalp with coil handle at 45 degrees). The intensity of stimulator output (i.e., 80%) was selected to give auditory and cutaneous stimulation similar to that of real TMS. All participants recruited to the sham groups were naïve to TMS, and so assumed that the effects they experienced were due to receiving real TMS.
Polysomnography (PSG) recording
We recorded PSG with standardized techniques using digital EEG, EMG, and EOG signals acquired with the Embla system (sampling rate: 256 Hz, high- and low-pass filter 0.3 and 35 Hz, respectively, notch filter 60 Hz). A referenced PSG electrode montage was used, including sites C3, C4, O1, O2 of the Internal 10–20 system, referenced to the mastoids. We also used two electrooculogram (EOG) and two electromyogram (EMG) channels, which were also placed in a standard manner with the EOG electrodes offset (one above and one below the eye) and the EMG electrodes placed under the chin. We recorded the PSG from before lights out at (11pm) until just after the lights came on in the morning (7pm).
Data Analysis
Polysomnography Data
We scored the PSG record using standard American Academy of Sleep Medicine (AASM) criteria, with manual scoring of 30s epochs, while blinded to participants’ behavioural performance [52, 53]. The signals were displayed on a computer monitor and rated visually, epoch by epoch, as awake, movement time, N1, N2, slow-wave sleep (SWS), or Rapid Eye Movement (REM) sleep. The AASM manual for scoring of sleep and associated events was followed. We used a MANOVA to compare the total sleep time, time awake, and the proportion of time spent within each sleep stage across the groups.
Behavioural Data
Response times (RT) were defined as the time to make a correct response. We explored graphically all of the data in MATLAB. Specifically, we examined the distribution of the data using histograms, and normal probability plots. Any response time in the top one percentile (i.e., α = 0.01) of a participant’s data was identified using a Grubbs’ Test and removed.
We quantified the amount of sequence learning in the tasks by subtracting the average response time of the final fifty sequential trials from the average response time of the subsequent fifty random trials [45, 54]. The difference between sequential and subsequent random RT is a widely used learning measure [46]. In part, it has proven popular because it is a specific measure of sequence learning. Comparing between sequential and subsequent random RT factors out other influences upon RT to give a measure specific to sequence learning. For example, during practice fatigue may accumulate impairing RT. Providing 10–15 minutes rest after practice gives an opportunity for this fatigue to dissipate, and performance to improve [55]. These rapid boosts in performance are observed in tasks such as the finger tapping task, and the rotary pursuit task, which use a simple performance measure such as the number of correctly completed sequences in 30s interval [34, 55]. By contrast, an improved performance is not detected after a 10–15 minute rest when using the difference between sequential and subsequent random RTs as a performance measure [2, 19]. Instead, improvements in performance only become detectable with 4–6 hours after initial practice [19]. Thus, using the difference between sequential and subsequent RT factors out influences such as fatigue that can lead to rapid boosts in performance after only 10–15 minutes of rest, and instead allows a focus upon improvements developing over longer time intervals (>6hrs).
The difference between sequential and subsequent random RT also provides a sensitive measure of sequence learning. During learning sequential RT would be expected to decrease. Subsequently introducing random trials would increase RT. An important component of this increase is because participants inappropriately expect the visual cues to continue to follow the sequence. As a consequence the response times during random trials can be higher than those prior to the sequence because they are inflated by participants’ expecting a cue at one location but finding it another. Thus, both the decrease in RT during sequential trials and the increase in RT during the subsequent random trials contain a component that is due to sequence learning, and so the difference between the sequential and random RT provides a sensitive measure of sequence learning. Overall, the difference between sequential RT and subsequent random RT provides both a specific and sensitive measure of motor sequence learning (for example; [45, 54, 56]; for review [46]).
We also calculated the number of incorrect responses (i.e., errors) during those final fifty sequential trials. The free recall of the motor sequence was scored as the longest, continuous and accurate verbally recalled segment of the sequence that was at least 3-items long (i.e., a triplet or more).
We calculated skill (skill1) during the test block of the first session, and after a night of sleep during the retest block (skill2). Similarly, we calculated the number of incorrect responses (i.e., errors) during the final fifty sequential trials at both testing and retesting. Using a Shapiro-Wilk test, we verified that these data followed a normal distribution. The difference between skill at testing and retesting provide a measure of off-line improvements developing during consolidation (skill2-skill1; [2, 6, 16, 23, 42]). We used a mixed repeated measures ANOVA to compare the changes in skill or error between testing and retesting across the different tasks (i.e., task, implicit vs. explicit) and sites of stimulation (i.e., site, IPL vs. M1). For each task, we compared the change in skill between testing and retesting following stimulation at the different sites. Subsequently, for each site of stimulation, we then used paired t-tests to examine the change in skill between initial testing and subsequent retesting. Using unpaired t-tests, we compared the effect of real stimulation against sham stimulation, in the additional control experiments, on offline changes following the same task type (i.e., implicit or explicit task). All the t-tests used in the analysis of this study were two-tailed. An ANOVA was used to compare initial skill at testing across the different groups. Using a mixed repeated measures ANOVA, we also explored changes in the response time during the sequential trials across the different tasks and sites of stimulation (please see Figure 3). The sphericity of the data was examined using a Mauchy’s test. If sphericity was violated, we used a Greenhouse-Geisser correction, which is shown in the main text as a correction to the degrees-of-freedom. Finally, we performed planned regressions between off-line improvements and REM and SWS sleep stages. Converging evidence links REM and particularly SWS to motor memory consolidation over a night of sleep (for a review see; [9]). We used a traditional regression using a least squares fit, and a complementary analysis with a robust regression that minimized the influence of outliers using a bisquare weighted function.
Figure 3. Response time changes during learning in the different tasks.
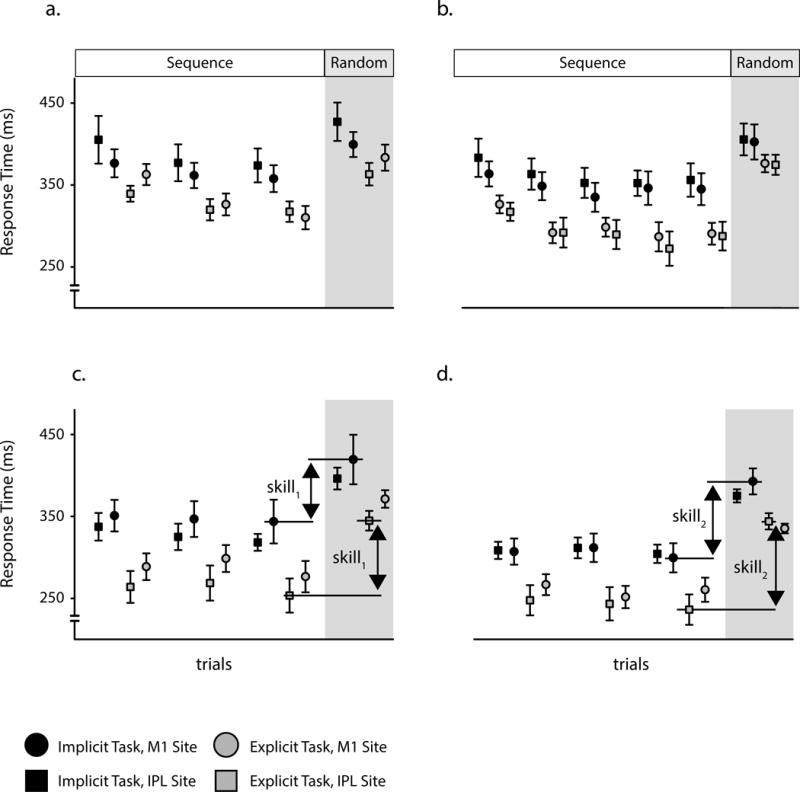
The task was introduced to participants as either a reaction time task (i.e., implicit task), or as a sequence learning task (i.e., explicit task). Despite these different instructions, both tasks required participants to acquire skill at performing a sequence of movements. Following learning in these tasks TMS was applied to either the primary motor cortex (M1) or the inferior parietal lobule (IPL). We explored for response time changes during the sequential trials and how these depended upon the site of stimulation and the type of learning task (i.e., site x task interaction). (a) During the initial training block, we found a significant change in response time during the sequential trials (F(1.4,68.9) = 25.53, p<0.001), which did not differ significantly across the site of stimulation and learning tasks (F(1.4,68.9) = 2.9, p = 0.093). (b) We found a similar pattern during the subsequent training block. There was a significant change in response time during the sequential trials (F(2.92,139.2) = 14.1, p<0.001), and this again did not differ significantly across the sites of stimulation and learning tasks (F(2.92,139.2) = 0.229, p = 0.79). (c) Similarly, during the test block there was a significant change in response time (F(2,94) = 3.52, p = 0.033), and once again this was not significantly different across the stimulation sites and learning tasks (F(2,94) = 0.252, p = 0.778). We also found that the difference in response time between the final epoch of sequential trials and the subsequent random trials, which is a widely used measure of sequence learning in these tasks, did not differ significantly across the different stimulation sites and tasks at the initial block (F(1,47) = 2.02, p = 0.162), at the training block (F(1,47) = 0.117, p = 0.734), or the test block (F(1,47) = 0.039, p = 0.844; [46, 54]). Together these different measures of learning converge to suggest that similar learning took place across the groups (i.e., the task X site interaction was not significant). (d) We found no significant change in response time during the sequential trials of the retest block (F(2,94) = 2.5, p = 0.09), and this did not differ significantly across the sites of stimulation and tasks (F(2,94) = 1.337, p = 0.249). Yet, when the random trials were introduced at the end of the block, the change in response time differed significantly across the different sites of stimulation and tasks (F(1,47) = 4.595, p = 0.037). The change in response time factors out general aspects of task performance to provide a measure of skill specifically for the sequence [46]. Overall, there was no difference in skill across the groups at testing, little evidence of differences in learning during retesting, and so the difference in skill across the groups at retesting is most likely attributable to the offline interval. The double vertical arrow shows the skill at testing (skill1; see c) and retesting (skill2; see d) in those groups that show offline improvements (see Figure 2). At each block there was a significant difference in sequential response time between the two tasks (for all four blocks; F(1,49)>7, p<0.01). The colour of each symbol shows the task (implicit vs. explicit), its shape the site of stimulation (IPL vs. M1), and its value is an epoch of trials (mean ± sem). For the random trials (highlighted in grey) each epoch is 50. To divide each set of sequential trials equally required a different epoch size for the implicit (60 trials) and explicit tasks (36 trials). These epoch sizes are used in the analysis provided in this legend; while, in the main text, the change in response time between sequential and random trials was based upon uniform epoch sizes of 50 trials. Regardless, of the epoch size (50 vs. 60 or 36 sequential trials) our results followed a similar pattern.
Data availability
The data that support the findings of this study are available on request from the corresponding author (EMR).
Acknowledgments
We are grateful to the National Science Foundation (Division of Behavioral and Cognitive Sciences, BCS, 0921177; E.M.R.), the National Institutes of Health (R01 NS051446-03S1; E.M.R.) who funded this work. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We would also like to thank Sanjin Tunovic for his assistance with many of the experiments, to Jared Saletin for helping with some aspects of the sleep analysis, to Janet Mullington and Monika Haack for helping with both the recording and scoring of participants’ sleep.
Footnotes
Contributions
J.B. performed the experiment, and analyzed the data. E.M.R designed the study, performed the experiments, analyzed the data, interpreted the results, and wrote the manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Robertson EM. From creation to consolidation: a novel framework for memory processing. PLoS biology. 2009;7:e19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Current Biology. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Grafton ST, Hazeltine E, Ivry RB. Abstract and Effector-Specific Representations of Motor Sequences Identified with PET. Journal of Neuroscience. 1998;18:9420–9428. doi: 10.1523/JNEUROSCI.18-22-09420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 5.Ramanathan DS, Gulati T, Ganguly K. Sleep-Dependent Reactivation of Ensembles in Motor Cortex Promotes Skill Consolidation. PLoS biology. 2015;13:e1002263. doi: 10.1371/journal.pbio.1002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133:911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Walker MP. A refined model of sleep and the time course of memory formation. The Behavioral and brain sciences. 2005;28:51–64. doi: 10.1017/s0140525x05000026. discussion 64–104. [DOI] [PubMed] [Google Scholar]
- 9.Diekelmann S, Born J. The memory function of sleep. Nature reviews. Neuroscience. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 10.Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Current opinion in neurobiology. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 11.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Current biology : CB. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genzel L, Robertson EM. To Replay, Perchance to Consolidate. PLoS biology. 2015;13:e1002285. doi: 10.1371/journal.pbio.1002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PloS one. 2007;2:e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 17.Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Off-line learning of motor skill memory: A double dissociation of goal and movement. PNAS. 2005;102:18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Press DZ, Casement MD, Pascual-Leone A, Robertson EM. The time course of off-line motor sequence learning. Brain research. Cognitive brain research. 2005;25:375–378. doi: 10.1016/j.cogbrainres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Cohen DA, Robertson EM. Motor sequence consolidation: constrained by critical time windows or competing components. Experimental brain research. 2007;177:440–446. doi: 10.1007/s00221-006-0701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown RM, Robertson EM. Off-Line Processing: Reciprocal Interactions between Declarative and Procedural Memories. J Neurosci. 2007;27:10468–10475. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer RM, Sunm M, Ivry RB. Sleep-dependent consolidation of contextual learning. Current biology : CB. 2006;16:1001–1005. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 23.Galea JM, Albert NB, Ditye T, Miall RC. Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. Journal of cognitive neuroscience. 2010;22:1158–1164. doi: 10.1162/jocn.2009.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen DA, Robertson EM. Preventing interference between different memory tasks. Nat Neurosci. 2011;14:953–955. doi: 10.1038/nn.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosha N, Robertson EM. Unstable Memories Create a High-Level Representation that Enables Learning Transfer. Current biology : CB. 2016;26:100–105. doi: 10.1016/j.cub.2015.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson EM. New insights in human memory interference and consolidation. Current biology : CB. 2012;22:R66–71. doi: 10.1016/j.cub.2011.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 28.Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM. The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science. 1999;284:167–170. doi: 10.1126/science.284.5411.167. [DOI] [PubMed] [Google Scholar]
- 29.Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cerebral cortex. 2002;12:369–375. doi: 10.1093/cercor/12.4.369. [DOI] [PubMed] [Google Scholar]
- 30.Robertson EM, Theoret H, Pascual-Leone A. Studies in cognition: the problems solved and created by transcranial magnetic stimulation. Journal of cognitive neuroscience. 2003;15:948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- 31.Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 33.Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PloS one. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotermans C, Peigneux P, Maertens de Noordhout A, Moonen G, Maquet P. Early boost and slow consolidation in motor skill learning. Learning & memory. 2006;13:580–583. doi: 10.1101/lm.239406. [DOI] [PubMed] [Google Scholar]
- 35.Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. The European journal of neuroscience. 2008;28:1216–1221. doi: 10.1111/j.1460-9568.2008.06421.x. [DOI] [PubMed] [Google Scholar]
- 36.Doyon J, Korman M, Morin A, Dostie V, Hadj Tahar A, Benali H, Karni A, Ungerleider LG, Carrier J. Contribution of night and day sleep vs. simple passage of time to the consolidation of motor sequence and visuomotor adaptation learning. Experimental brain research. 2009;195:15–26. doi: 10.1007/s00221-009-1748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nat Neurosci. 2007;10:148–149. doi: 10.1038/nn1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunovic S, Press DZ, Robertson EM. A physiological signal that prevents motor skill improvements during consolidation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5302–5310. doi: 10.1523/JNEUROSCI.3497-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breton J, Robertson EM. Flipping the switch: mechanisms that regulate memory consolidation. Trends in cognitive sciences. 2014;18:629–634. doi: 10.1016/j.tics.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:11248–11255. doi: 10.1523/JNEUROSCI.1743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friston KJ, Price CJ. Degeneracy and redundancy in cognitive anatomy. Trends in cognitive sciences. 2003;7:151–152. doi: 10.1016/s1364-6613(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 42.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 44.Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- 46.Robertson EM. The Serial Reaction Time Task: Implicit Motor Skill Learning? J Neurosci. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominey PF, Lelekov T, Ventre-Dominey J, Jeannerod M. Dissociable processes for learning the surface structure and abstract structure of sensorimotor sequences. Journal of cognitive neuroscience. 1998;10:734–751. doi: 10.1162/089892998563130. [DOI] [PubMed] [Google Scholar]
- 48.Willingham DB, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. Journal of neurophysiology. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- 49.Hauptmann B, Reinhart E, Brandt SA, Karni A. The predictive value of the leveling off of within session performance for procedural memory consolidation. Brain research. Cognitive brain research. 2005;24:181–189. doi: 10.1016/j.cogbrainres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Wassermann EM, Wang B, Zeffiro TA, Sadato N, Pascual-Leone A, Toro C, Hallett M. Locating the motor cortex on the MRI with transcranial magnetic stimulation and PET. Neuroimage. 1996;3:1–9. doi: 10.1006/nimg.1996.0001. [DOI] [PubMed] [Google Scholar]
- 51.Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2001;112:250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- 52.Silber MH, Ancoli-Israel S, Bonnet MH, Chokroverty S, Grigg-Damberger MM, Hirshkowitz M, Kapen S, Keenan SA, Kryger MH, Penzel T, et al. The visual scoring of sleep in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007;3:121–131. [PubMed] [Google Scholar]
- 53.Rechtschaffen A, Kales A. Manual of standardized terminology, techniques and scoring systems for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service; 1968. [Google Scholar]
- 54.Willingham DB, Nissen MJ, Bullemer P. On the development of procedural knowledge. J Exp Psychol Learn Mem Cogn. 1989;15:1047–1060. doi: 10.1037//0278-7393.15.6.1047. [DOI] [PubMed] [Google Scholar]
- 55.Eysenck HJ, Frith CD. Reminiscence, motivation, and personality: A case study in experimental psychology. New York: Plenum Press; 1977. [Google Scholar]
- 56.Boyd LA, Winstein CJ. Implicit motor-sequence learning in humans following unilateral stroke: the impact of practice and explicit knowledge. Neuroscience letters. 2001;298:65–69. doi: 10.1016/s0304-3940(00)01734-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (EMR).


