Motivation is critical for survival, and involves multiple behavioural functions mediated by a number of interacting neural circuits. Salamone et al . review basic neuroscience research, animal models, and clinical studies focused on brain mechanisms of effort-based decision making, and also consider the origins and treatment of motivational impairments in psychopathology.
Keywords: dopamine, reward, depression, fatigue, anergia
Motivation is critical for survival, and involves multiple behavioural functions mediated by a number of interacting neural circuits. Salamone et al . review basic neuroscience research, animal models, and clinical studies focused on brain mechanisms of effort-based decision making, and also consider the origins and treatment of motivational impairments in psychopathology.
Abstract
Motivation has been defined as the process that allows organisms to regulate their internal and external environment, and control the probability, proximity and availability of stimuli. As such, motivation is a complex process that is critical for survival, which involves multiple behavioural functions mediated by a number of interacting neural circuits. Classical theories of motivation suggest that there are both directional and activational aspects of motivation, and activational aspects (i.e. speed and vigour of both the instigation and persistence of behaviour) are critical for enabling organisms to overcome work-related obstacles or constraints that separate them from significant stimuli. The present review discusses the role of brain dopamine and related circuits in behavioural activation, exertion of effort in instrumental behaviour, and effort-related decision-making, based upon both animal and human studies. Impairments in behavioural activation and effort-related aspects of motivation are associated with psychiatric symptoms such as anergia, fatigue, lassitude and psychomotor retardation, which cross multiple pathologies, including depression, schizophrenia, and Parkinson’s disease. Therefore, this review also attempts to provide an interdisciplinary approach that integrates findings from basic behavioural neuroscience, behavioural economics, clinical neuropsychology, psychiatry, and neurology, to provide a coherent framework for future research and theory in this critical field. Although dopamine systems are a critical part of the brain circuitry regulating behavioural activation, exertion of effort, and effort-related decision-making, mesolimbic dopamine is only one part of a distributed circuitry that includes multiple neurotransmitters and brain areas. Overall, there is a striking similarity between the brain areas involved in behavioural activation and effort-related processes in rodents and in humans. Animal models of effort-related decision-making are highly translatable to humans, and an emerging body of evidence indicates that alterations in effort-based decision-making are evident in several psychiatric and neurological disorders. People with major depression, schizophrenia, and Parkinson’s disease show evidence of decision-making biases towards a lower exertion of effort. Translational studies linking research with animal models, human volunteers, and clinical populations are greatly expanding our knowledge about the neural basis of effort-related motivational dysfunction, and it is hoped that this research will ultimately lead to improved treatment for motivational and psychomotor symptoms in psychiatry and neurology.
Motivation is critical for survival, and involves multiple behavioural functions mediated by a number of interacting neural circuits. Salamone et al. review basic neuroscience research, animal models, and clinical studies focused on brain mechanisms of effort-based decision making, and also consider the origins and treatment of motivational impairments in psychopathology.
On the neural regulation of motivated behaviour: conceptual overview
Motivation has been defined as the process that allows organisms to regulate their internal and external environment, and control the probability, proximity, and availability of stimuli ( Salamone, 1992 , 2010 ). Clearly, motivation is a complex process that is critical for survival, and involves multiple behavioural functions mediated by an array of interacting neural circuits. Classical motivation theory has emphasized that there are distinct facets of motivation; for example directional and activational aspects ( Duffy, 1963 ; Cofer and Apley, 1964 ; Salamone, 1987 , 1988, 2010 ). Thus, behaviour can be directed towards some stimuli (e.g. food, water, sex) and away from others (e.g. painful conditions, predators, stressors). Furthermore, it is generally recognized that motivation has an activational or energetic component. Motivated behaviour is characterized by a high degree of behavioural activation, as demonstrated by the speed, vigour or persistence seen in the instigation and maintenance of instrumental responding ( Salamone, 1988 , 1992 ; Salamone and Correa, 2002 , 2012 ; Robbins and Everitt, 2007 ; Croxson et al. , 2009 ; Kurniawan et al. , 2010 ; Nicola, 2010 ; McGinty et al. , 2013 ; Floresco, 2015 ), as well as the induction of a wide variety of activities by the presentation of motivational stimuli ( Robbins and Koob, 1980 ; Salamone, 1988 ; McCullough and Salamone, 1992 ). The ability to generate rapid or vigorous responses, and maintain them over time, is a fundamental and highly adaptive feature of motivational processes. Such neurobehavioural mechanisms allow organisms to forage over wide areas, quickly pounce on prey, work with vigour towards a selected goal, and exert effort to overcome obstacles that block access to significant stimuli.
The neural mechanisms mediating directional aspects of motivation, such as the selection of particular foods, water, sodium, or sexual activity, can be quite specific. For example, the brain circuits that instigate the selection of specific foods have neural components that are distinct from those that instigate thirst motivation ( Carlson, 2014 ). Indeed, even thirst motivation is not a unitary construct, because there are different thirst-related signals that impinge upon distinct neural mechanisms (i.e. osmotic versus volemic thirst). However, there is considerable evidence indicating that the brain circuitry regulating activational aspects of motivation can be shared across different classes of stimuli and conditions. One of the key components of the neural circuitry mediating behavioural activation and effort-related processes is the mesolimbic dopamine system ( Salamone et al. , 1997 , 2007 , 2012 ; Salamone and Correa, 2002 , 2012 ; Robbins and Everitt, 2007 ). Of course, this system, with its target in nucleus accumbens/ventral striatum, is only one part of a broader forebrain circuitry that includes multiple neurotransmitters, brain areas, and pathways ( Salamone and Correa, 2012 ).
Impairments in behavioural activation and effort-related aspects of motivation can result in psychiatric symptoms that span multiple pathologies ( Salamone et al. , 2006 ). According to Demyttenaere et al. (2005) , fatigue/loss of energy is one of the most common of all psychiatric symptoms in general medicine. Motivational/psychomotor symptoms such as retardation, fatigue, lassitude, loss of energy and reduced exertion of effort are critical and debilitating features of major depressive disorder ( Stahl, 2002 ; Demyttenaere et al. , 2005 ; Salamone et al. , 2006 ; Treadway and Zald, 2011 ; Fava et al. , 2014 ). The severity of such effort-related symptoms in depression is highly correlated with problems in social function, employment, and treatment outcomes ( Tylee et al. , 1999 ; Stahl, 2002 ), and these motivational symptoms are highly resistant to treatment ( Stahl, 2002 ; Nutt et al. , 2007 ; Fava et al. , 2014 ). Many common antidepressants, such as serotonin (5-HT) uptake inhibitors (e.g. fluoxetine and citalopram), are relatively limited in their ability to treat motivational dysfunction, and in some people can induce or exacerbate these symptoms ( Padala et al. , 2012 ; Stenman and Lilja, 2013 ; Fava et al. , 2014 ). Moreover, effort-related motivational symptoms are present in diverse psychiatric and neurological disorders, including bipolar disorder, schizophrenia, parkinsonism, chronic fatigue syndrome and multiple sclerosis ( Caligiuri and Ellwanger, 2000 ; Salamone et al. , 2006 , 2010 ; Friedman et al. , 2007 ; Tellez et al. , 2008 ; Chong et al. , 2015 ).
The present review discusses the role of brain dopamine and related circuits in behavioural activation, exertion of effort, and effort-related decision-making, based upon both animal and human studies. Moreover, the clinical significance and neural underpinnings of motivational/psychomotor pathologies such as anergia, fatigue, lassitude and psychomotor retardation are reviewed, and the contribution of animal models to the development of treatments for these symptoms is discussed. This review is intended to provide an interdisciplinary approach that integrates findings from basic behavioural neuroscience, behavioural economics, clinical neuropsychology, psychiatry, and neurology, to provide a coherent framework for future research and theory in this critical field.
Background on the role of dopamine in activational/effort-related aspects of motivation: animal studies
Considerable evidence from the animal literature indicates that nucleus accumbens dopamine is involved in behavioural activation and energy expenditure ( Salamone, 1988 , 1992 ; Salamone and Correa, 2002 , 2012 ; Robbins and Everitt, 2007 ; Beeler et al. , 2012 , 2015 ). Microinjections of stimulants that enhance dopamine transmission into nucleus accumbens can increase locomotor activity ( Delfs et al. , 1990 ). Accumbens dopamine depletions or antagonism suppress stimulant-induced and novelty-induced locomotion ( Koob et al. , 1978 ; Cousins et al. , 1993 ; Baldo et al. , 2002 ; Correa et al. , 2002 ), and accumbens dopamine participates in sensorimotor gating functions ( Koob and Swerdlow, 1988 ; Swerdlow et al. , 1990 ). Accumbens dopamine is also involved in schedule-induced activity, which is thought to be a model of compulsive behaviour. Periodic non-contingent presentation of small food pellets to food-restricted rats can induce vigorous motor activities, including excessive drinking, wheel running, and locomotion ( Staddon and Simmelhag, 1971 ; Killeen, 1975 ; Killeen et al. , 1978 ; López-Crespo et al. , 2004 ). Such schedule-induced activities are marked by concomitant increases in accumbens dopamine release ( McCullough and Salamone, 1992 ), and are suppressed by dopamine antagonists and accumbens dopamine depletions ( Robbins and Koob, 1980 ; Wallace et al. , 1983 ; Salamone, 1988 ; McCullough and Salamone, 1992 ). Locomotor activity also can be instigated by the presentation of cues associated with sucrose, an effect that is blocked by dopamine antagonism ( Salamone et al. , 2015 b ).
Accumbens dopamine also regulates instrumental response output. Stimulants that enhance dopamine transmission increase operant responding on schedules that generate low baseline rates of responding, an effect that is diminished by neurotoxic depletion of accumbens dopamine ( Robbins et al. , 1983 ). De Jong et al. (2015) reported that knockdown of ventral tegmental dopamine D2 autoreceptors, which enhance accumbens dopamine transmission, selectively increased incentive motivation for food and cocaine as measured by progressive ratio responding. Moreover, the effects of dopamine antagonism or depletion interact powerfully with the response requirements of the task ( Salamone, 1986 ; Salamone et al. , 2003 ). One way of varying the response requirements of instrumental behaviour is to vary the ratio requirements of operant schedules (i.e. the number of lever presses required). Caul and Brindle (2001) reported that the dopamine antagonist haloperidol had a substantial effect on progressive ratio performance (i.e. a schedule in which the ratio lever pressing requirement gradually increments) at low doses that had no effect on fixed ratio 1 (FR1) responding. Accumbens dopamine depletions suppress lever pressing on ratio schedules in a manner that is related to the size of the ratio requirement. FR1 responding is only marginally and transiently affected by dopamine depletion, while rats responding on moderate size ratio schedules (FR5, 16, 20) showed modest reductions in response rates, and animals tested on schedules with high ratios (e.g. FR16, 64, 300) were severely impaired ( McCullough et al. , 1993 a ; Aberman et al. , 1998 ; Aberman and Salamone, 1999 ; Salamone et al. , 2001 ; Ishiwari et al. , 2004 ). Thus, as described by Salamone and Correa (2002) , accumbens dopamine depletions blunt the response-enhancing effects of moderate sized ratio requirements, and also enhance the response-suppressing effects of very large ratio requirements (i.e. they induce ‘ratio strain’, or ‘breaking’).
It is reasonable to ask if the dopaminergic manipulations that decrease instrumental behaviour are doing so because they mimic extinction (i.e. non-delivery of reward), impair appetite or generally disrupt primary motivation, alter hedonic reactivity to the primary reward, or make animals particularly sensitive to time requirements such as delays. For many years it was suggested that dopamine is the ‘reward transmitter’ or the ‘pleasure chemical’, but as discussed in previous papers, this view has many conceptual and empirical problems ( Salamone et al. , 1997 , 2005, 2007 ; Salamone and Correa, 2002 , 2012 ; Floresco, 2015 ). Although it was suggested several decades ago that dopamine antagonism or depletion produced an extinction-like effect, numerous studies have shown a lack of similarity between dopamine antagonism or depletion and the effects of extinction ( Tombaugh et al. , 1980 ; Faustman and Fowler, 1981 ; Salamone, 1986 , 1988 ; McCullough et al. , 1993 a ; Salamone et al. , 1995 , 1997 , 2007 ; Rick et al. , 2006 ; see review by Salamone and Correa, 2002 ). Across a number of behavioural conditions, the effects of low doses of dopamine antagonists or accumbens dopamine depletions on operant behaviour do not generally resemble the effects of devaluation of food reinforcement or appetite suppressant drugs ( Salamone et al. , 1991 , 2002 ; Aberman and Salamone, 1999 ; Sink et al. , 2008 ; Randall et al. , 2012 , 2014 ). Although striatal mechanisms are known to be involved in mediating the action/outcome associations that underlie reinforcement learning, this effect is more generally attributed to neostriatal (i.e. dorsal striatal) mechanisms rather than nucleus accumbens (i.e. ventral striatum; Corbit et al. , 2001 ; Yin et al. , 2008 ; Belin et al. , 2009 ; Corbit and Janak, 2010 ; Lex and Hauber, 2010 ; Salamone and Correa, 2012 ). Furthermore, alteration of dopamine transmission with drugs, dopamine depletions, or genetic manipulations does not alter hedonic reactivity to sucrose ( Berridge and Robinson, 1998 , 2003 ; Sederholm et al. , 2002 ; Peciña et al. , 2003 ; Berridge, 2007 ; Smith et al. , 2011 ; Berridge and Kringelbach, 2015 ; Pardo et al. , 2015 ; see the distinction between ‘liking’ and ‘wanting’)
Thus, although low doses of dopamine antagonists and nucleus accumbens dopamine depletions impair many features of behavioural activation and instrumental responding (e.g. response rate, responding on high ratio schedules, see also studies of Pavlovian-to-Instrumental transfer, including Wyvell and Berridge, 2000 ; Parkinson et al. , 2002 ; Dalley et al. , 2005 ; Lex and Hauber, 2008 , 2010 ; Yin et al. , 2008 ; Corbit and Balleine, 2011 ), there also are many fundamental features of appetitive motivation that are left intact after these manipulations. Such findings are not unique to food reinforcement; they also are seen when water ( Horvitz et al. , 1993 ), sex ( Hull et al. , 1991 ), social play behaviour ( Achterberg et al. , 2016 ), and maternal behaviour ( Pereira and Ferreira, 2006 , 2015 ) are used as the motivational stimulus. Moreover, mesolimbic dopamine is known to be involved in aversive motivation and responsiveness to stress ( McCullough et al. , 1993 b ; Salamone, 1994 ; Tidey and Miczek, 1996 ; Anstrom and Woodward, 2005 ; Anstrom et al. , 2009 ; Fernando et al. , 2014 ). Thus, the effects of dopamine antagonists and accumbens dopamine depletions are not accurately described as being broad or general effects on ‘reward’, hedonia, reinforcement, or motivation; instead, they are selective and dissociative in nature, substantially affecting some aspects of appetitive and aversive motivation, while leaving others intact ( Salamone et al. , 2005 ; Floresco, 2015 ). Behavioural responses that are most sensitive to interference with accumbens dopamine transmission tend to be vigorous activities, including instrumental behaviours, which are elicited and supported by conditioned stimuli ( Salamone and Correa, 2012 ). Thus, mesolimbic dopamine participates in functions akin to the ‘anticipation-invigoration’ mechanism proposed by Cofer and Apley (1964) in their treatise on incentive motivation. In a sense, the integrity of mesolimbic dopamine transmission enables organisms to transcend the psychological distance that separates them from motivationally relevant stimuli ( Salamone and Correa, 2012 ).
Consistent with these ideas, prolonged dopamine signalling in response to distal cues during maze learning has been suggested to provide a sustained motivational drive that maintains instrumental behaviour ( Howe et al. , 2013 ). More recently, Hamid et al. (2016) studied fast cyclic voltammetry responses of rats responding on distinct phases of a flexible decision-making task. They reported that phasic dopamine responses increased as animals progressed towards the increasing likelihood of reinforcement, and thus represented a temporally discounted estimate of future reinforcement. These dopamine signals were correlated with important features of behavioural output, such as response latencies, and it was suggested that mesolimbic dopamine helps to translate estimates of reinforcer availability into decisions to work for reward. Thus, mesolimbic dopamine release could be used as a motivational signal, which regulates motivational excitement and the decision of whether or not to engage in effortful activity ( Hamid et al. , 2016 ).
Nucleus accumbens dopamine and the forebrain circuitry regulating effort-related decision-making in animals
It was suggested years ago that studies of cost/benefit decision-making involving work-related response costs could shed light on the behavioural functions of mesolimbic dopamine ( Salamone, 1987 , 1991, 1992 ). Organisms in their natural environment make effort-based decisions and allocate behavioural resources into goal-directed actions based on assessments of work-related response costs and motivational value or preference. This type of function is critical for animals foraging in the wild (see ‘Optimal foraging theory’ section; Krebs, 1977 ), but also can be adapted to experimental procedures in laboratories. Thus, ideas about how animals allocate their behavioural resources in choice situations eventually led to the development of procedures that study effort-related choice behaviour (also known as effort-related or effort-based decision-making). Effort-related decision-making is typically studied using tasks that offer a choice between high effort instrumental actions leading to more highly valued reinforcers versus an alternative low effort/low reward option.
One such procedure involves an operant task that offers a choice between FR5 lever pressing to obtain a relatively preferred food (high carbohydrate pellets), versus approaching and consuming a concurrently available but less preferred food (standard laboratory chow; Salamone et al. , 1991 ). Under baseline or control conditions, rats typically eat only small amounts of chow, and get most of their food by lever pressing on the FR5 schedule. However, low doses of dopamine antagonists and depletions or antagonism of accumbens dopamine dramatically shift choice behaviour, decreasing the tendency to work for food by lever pressing, but substantially increasing chow intake ( Salamone et al. , 1991 , 2002 ; Koch et al. , 2000 ; Nowend et al. , 2001 ; Sink et al. , 2008 ; Farrar et al. , 2010 ). Thus, despite the reduced lever pressing produced by impaired dopamine transmission, rats show a compensatory reallocation of behaviour and select a new path to an alternative food source. The use of this task as a measure of effort-related choice behaviour has been validated in several ways. In contrast to the effects of impaired dopamine transmission, pre-feeding to devalue food reinforcement reduced both lever pressing and chow intake ( Salamone et al. , 1991 ). Drug treatments that produced the shift in choice behaviour did not alter food intake or preference in free-feeding choice tests ( Salamone et al. , 1991 ; Koch et al. , 2000 ; Farrar et al. , 2008 ; Nunes et al. , 2013 a , b ; Pardo et al. , 2015 ). Increasing the lever pressing work requirement with larger ratios shifts response allocation from lever pressing to chow intake, indicating that these procedures are sensitive to work requirements ( Salamone et al. , 1997 ; Randall et al. , 2012 ). Unlike the effects of dopamine antagonism or depletion, appetite suppressants such as fenfluramine and cannabinoid CB1 antagonists do not increase chow intake at doses that suppress lever pressing ( Salamone et al. , 2002 ; Sink et al. , 2008 ; Randall et al. , 2012 , 2014 ). Thus, dopamine antagonism or depletion do not simply reduce appetite for food or primary food motivation ( Salamone and Correa, 2002 , 2009, 2012 ).
Another task that has been used to assess effort-related decision-making is the T-maze barrier choice procedure ( Salamone et al. , 1994 a ). There are two choice arms of the maze, which can have different reinforcement densities (e.g. four versus two food pellets, or four versus zero), and to provide an effort-related challenge, a vertical barrier is placed in the arm with the higher density of reinforcement. Low doses of dopamine antagonists and accumbens dopamine depletions shift choice behaviour and bias animals towards the low effort alternative, decreasing selection of the high reward/high cost arm with the barrier, but increasing selection of the low reward with no barrier ( Salamone et al. , 1994 ; Cousins et al. , 1996 ; Denk et al. , 2005 ; Mott et al. , 2009 ; Mai et al. , 2012 ; Pardo et al. , 2012 ). When there is no barrier in the arm with the high reward density, or when there is a barrier in both arms of the maze, dopamine antagonism and accumbens dopamine depletions do not alter response choice ( Salamone et al. , 1994 ; Pardo et al. , 2012 ). Also, when the barrier arm contains four reinforcement pellets but the other arm contains none, rats with impaired accumbens dopamine transmission still choose the high density arm, climb the barrier, and consume all the food pellets ( Cousins et al. , 1996 ; Yohn et al. , 2015 b ).
Discounting tasks are often used in decision-making research. With these tasks, conditions related to reinforcement such as delay and probability are systematically varied within a test session, and the animal is offered a variety of choices with different trade-offs. Effort discounting tasks have been developed in the last few years. Bardgett et al. (2009) showed that D1 or D2 antagonism reduced selection of the high effort arm with the barrier using a discounting procedure based upon the T-maze barrier task. In contrast, administration of amphetamine, which increases dopamine transmission, biased rats towards selection of the high effort arm. Floresco and colleagues ( Floresco et al. , 2008 ; Hosking et al. , 2015 ) have developed effort-discounting procedures based on alterations in the ratio requirements (number of lever presses) to obtain reinforcements. Dopamine antagonism has been shown to bias selection towards the lower ratio option. In addition, inactivation of nucleus accumbens core neurons by local blockade of GABA A/B receptors also reduced selection of the higher effort alternative ( Ghods-Sharifi and Floresco, 2010 ).
A test that combines features of the FR5/chow feeding choice task and effort discounting procedures is the progressive ratio (PROG)/chow feeding concurrent choice task. With this task, rats can lever press on a PROG schedule reinforced by preferred high-carbohydrate food pellets, or alternatively approach and consume the less-preferred lab chow that is concurrently available. If a rat receives no reinforcer for 2 min, the lever pressing component becomes inactivated, and eventually all rats reach a break point and switch to chow. The dopamine antagonists haloperidol, eticlopride and ecopipam decreased PROG lever pressing, but did not decrease levels of chow intake ( Randall et al. , 2012 , 2014 a ). Moreover, the effects of dopamine antagonism or depletion differed markedly from those of appetite-related manipulations such as pre-feeding and cannabinoid CB1 receptor antagonists or inverse agonists, all of which decreased both PROG lever pressing and chow intake. Performance on the PROG/chow feeding choice task is highly variable; some rats lever press very little and have high levels of chow intake (low responders), while others lever press much more and consume only small amounts of chow (high responders). Immunocytochemical analysis of the signal transduction protein pDARPP-32(Thr34) (i.e. DARPP-32 phosphorylated at the threonine 34 residue), which is involved in dopamine-related signalling, revealed that there was significantly higher expression of pDARPP-32(Thr34) in accumbens core in high responders compared to low responders ( Randall et al. , 2012 ).
It is clear that dopamine antagonism and accumbens dopamine depletions cause animals to reallocate their instrumental response selection based on the response requirements of the task, and select lower cost alternatives ( Salamone et al. , 2007 , 2012 ; Salamone and Correa, 2012 ). Furthermore, dopamine transmission appears to exert a bidirectional influence over response output in tasks involving effort-related choice behaviour ( Bardgett et al. , 2009 ). Dopamine transporter (DAT, encoded by Slc6a3 ) knockdown mice showed increased lever pressing and decreased chow intake compared to wild-type mice ( Cagniard et al. , 2006 ). Trifilieff et al. (2013) reported that selective overexpression of D2 receptors in the nucleus accumbens of adult mice also led to an increase in selection of high effort alternatives in choice tasks.
It is important to consider that there appear to be multiple dimensions of effort ( Westbrook and Braver, 2015 ). Hosking et al. (2015) compared the effects of the dopamine antagonists on a ratio discounting task that assesses physical effort versus a cognitive effort discounting task. While dopamine antagonism altered decision-making based upon physical effort, it had no effect on discounting based upon cognitive effort (difficulty of a discrimination task). Also, effort-related challenges presented by different force requirements may not be regulated in the same way as those presented by tasks involving repeated responding, such as that seen in ratio schedules. Ishiwari et al. (2004) studied the effect of accumbens dopamine depletions on lever pressing tasks that involved different force or ratio requirements, and reported that the effects of dopamine depletions interacted strongly with the ratio requirements, but not with the force requirements. This is consistent with studies from Fowler et al. (1986) , who observed that dopamine antagonism affected the temporal aspects of responding more than the ‘force domain’. Another important consideration is that selection of high-effort alternatives on decision-making tasks appears to be somewhat dissociable from other measures of behavioural activation, such as response speed ( Wardle et al. , 2011 ; Yohn et al. , 2015 a , b ).
Considerable evidence indicates that the effects of dopamine antagonism and accumbens dopamine depletions on ratio lever pressing output and effort-based decision-making are not simply due to an interaction with the effects of reinforcement intermittency or delay. Rats responding on conventional variable interval (VI) schedules (e.g. VI 30, 60 or 120 s) were not affected by accumbens dopamine depletions that substantially suppressed responding when a ratio requirement (FR5 or 10) was attached to the same interval requirements ( Correa et al. , 2002 ; Mingote et al. , 2005 ). Although interference with dopamine transmission alters performance on progressive ratio schedules, it did not affect performance on a progressive interval schedule ( Wakabayashi et al. , 2004 ). These observations are consistent with reports indicating that accumbens dopamine depletions failed to disrupt delay discounting ( Winstanley et al. , 2005 ). Moreover, the effects of systemic dopamine antagonism on ratio discounting do not depend simply on delay-related actions; Floresco et al. (2008) demonstrated that dopamine antagonism produced a bias towards the low ratio option in rats tested on a ratio discounting task even when an ‘equivalent delay’ procedure was used that controlled for the time to complete the ratio components. Although inactivation of nucleus accumbens core by blockade of GABA receptors reduced selection of the higher effort alternative ( Ghods-Sharifi and Floresco, 2010 ), this same manipulation was actually reported to increase delay discounting (i.e. increase selection of the long delay option; Moschak and Mitchell, 2014 ).
In addition to the empirical studies reviewed above, various computational approaches have been developed to account for the role of mesolimbic dopamine in effort-related processes. Niv et al. (2007) developed a model that focused upon the role of mesolimbic dopamine in regulating instrumental response vigour. Phillips et al. (2007) provided a simple mathematical framework for how dopamine modulates cost/benefit decisions and provides an opportunistic drive that regulates the threshold cost expenditure for obtaining rewards. Collins and Frank (2014) also described a model that is useful for characterizing the effects of dopamine depletions on ratio lever press performance and choice incentives involved in effort-based choice.
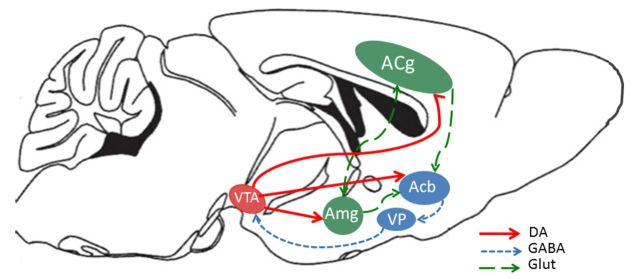
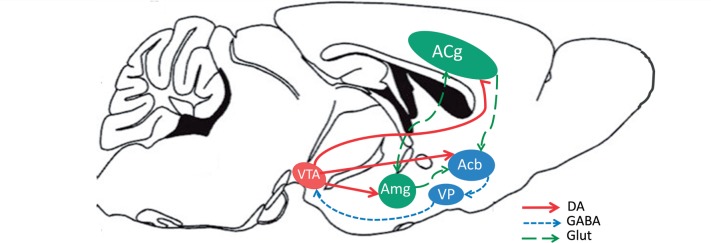
Several other transmitters and neuromodulators in addition to dopamine, across multiple brain areas, interact to regulate effort-related functions. Dopamine D2 and adenosine A 2A receptors are co-localized on striatal medium spiny neurons and interact with each other, and systemic or local intra-accumbens core injections of adenosine A 2A antagonists can reverse the effort-related effects of dopamine antagonists and restore near-normal patterns of behaviour ( Farrar et al. , 2007 , 2010 ; Mott et al. , 2009 ; Salamone et al. , 2009 ; Worden et al. , 2009 ; Nunes et al. , 2010 ; Pardo et al. , 2012 ; Santerre et al. , 2012 ). Conversely, intra-accumbens injections of adenosine A 2A agonists can induce effects on effort-related choice that resemble those resulting from dopamine antagonism or depletion ( Font et al. , 2008 ; Mingote et al. , 2008 ). Systemic administration of the adenosine A 2A antagonist MSX-3 to rats increased work output on the lever pressing component of the PROG/chow feeding choice procedure ( Randall et al. , 2012 ). Intra-accumbens injections of the muscarinic agonist pilocarpine altered effort-related choice, decreasing selection of the high effort option, which is consistent with previous studies indicating that striatal dopamine and acetylcholine interact ( Nunes et al. , 2013 a ). Activity of locus coeruleus norepinephrine neurons is correlated with exertion of effort in monkeys responding on a force grip task ( Varazzani et al. , 2015 ). A number of papers have shown that there is a distributed neural circuitry that regulates effort-based decision-making, which includes basolateral amygdala, prefrontal/anterior cingulate cortex, and ventral pallidal GABA in addition to nucleus accumbens; this has been confirmed in studies using ‘disconnection methods’ that involve combined contralateral manipulation of two different parts of the circuit ( Salamone et al. , 1994 , 1997 , 2007 ; Walton et al. , 2003 ; Floresco and Ghods-Sharifi, 2007 ; Farrar et al. , 2008 ; Mingote et al. , 2008 ; Hauber and Sommer, 2009 ; see Fig. 1 ).
Figure 1.
Schematic showing anatomical connections in the rodent brain between structures involved in effort-related choice behaviour. Acb = nucleus accumbens; ACg = anterior cingulate gyrus; Amg = amygdala; DA = dopamine; GABA = gamma aminobutyric acid; Glut = glutamate; VP = ventral pallidum; VTA = ventral tegmental area.
Animal models of effort-related motivational impairments in psychopathology, and implications for treatment
Because of the importance of effort-related dysfunctions in psychopathology (see discussion in the sections below), animal tests of effort-based decision-making have recently been used to develop formal models of motivational symptoms. The rodent tasks described above have been studied for their sensitivity to some of the conditions associated with depression, and also for assessment of potential and well-known therapeutic agents. Reduced selection of high effort choices in rodents can be induced by several conditions associated with depression, including stress ( Shafiei et al. , 2012 ), injections of the proinflammatory cytokine interleukin 1β (IL1β, encoded by Il1b ; Nunes et al. , 2014 ), and administration of tetrabenazine (TBZ). In rats tested on the FR5/chow feeding choice task, injections of low doses of IL1β shifted effort-related choice, decreasing lever pressing and increasing chow intake at doses that did not change food preference or induce fever. These effects of IL1β were reversed by co-administration of the adenosine A 2A receptor antagonist MSX-3 ( Nunes et al. , 2014 ).
Several recent studies have focused on the effort-related effects of TBZ. TBZ inhibits VMAT-2 (i.e. vesicular monoamine transporter type 2, encoded by Slc18a2 ), which results in reduced vesicular storage and depletion of monoamines. The greatest effects of TBZ at low doses have been reported to be on dopamine in the striatal complex, which is substantially depleted relative to norepinephrine and 5-HT ( Pettibone et al. , 1984 ; Tanra et al. , 1995 ). Originally developed as a reserpine-type antipsychotic, TBZ has been approved for use as a treatment for Huntington’s disease and other movement disorders, but its major side effects include depressive symptoms ( Frank, 2009 , 2010 ; Guay, 2010 ; Chen et al. , 2012 ). Like reserpine, TBZ has been used in studies involving classical animal models of depression ( Preskorn et al. , 1984 ; Kent et al. , 1986 ; Wang et al. , 2010 ). Low doses of TBZ that decreased accumbens dopamine release and dopamine-related signal transduction altered effort-related choice behaviour as assessed by concurrent lever pressing/chow feeding choice procedures ( Nunes et al. , 2013 b ; Randall et al. , 2014 ). The doses of TBZ that decreased selection of FR or PROG lever pressing did not alter preference for high carbohydrate pellets (the reinforcer for the high effort option) versus chow intake ( Nunes et al. , 2013 b ), and did not produce effects similar to reinforcer devaluation by prefeeding, or appetite suppressant drugs ( Randall et al. , 2012 , 2014 ). The shift from lever pressing to chow intake was also produced by local infusions of the TBZ into nucleus accumbens core, but not overlying medial dorsal striatum ( Nunes et al. , 2013 b ). A version of the concurrent lever pressing/chow intake task was recently developed, in which different sucrose concentrations were used as the reinforcer ( Pardo et al. , 2015 ). TBZ shifted choice behaviour by reducing lever pressing for the strongly preferred higher concentration of sucrose, and increasing selection of the low concentration of sucrose that was obtained with low effort (i.e. drinking with no lever pressing requirement). The same doses of TBZ that produced this shift had no effect on sucrose preference or hedonic taste reactivity ( Pardo et al. , 2015 ). Low doses of TBZ (0.25–0.75 mg/kg) altered effort-related decision-making in rats tested on the T-maze barrier task, but did not affect arm selection when there was no barrier in the maze, or when the arm with the barrier had four reinforcement pellets but the other arm had no pellets (Yohn et al. , 2015 a , b ). This pattern of results demonstrates that TBZ did not reduce selection of the high effort alternative because it was impairing sensitivity to reinforcement density, preference for four pellets versus two, discrimination of left versus right, or reference memory, or because of an inability to climb the barrier or an absolute ceiling level of barrier crossings ( Yohn et al. , 2015 a ).
An important feature of animal models is their utility for studies of drug development, which includes validation with known therapeutic agents as well as the assessment of novel compounds or strategies. Recently, several drugs have been tested for their ability to reverse deficits in effort-related decision-making. Adenosine A 2A antagonists produce antiparkinsonian effects in animal models and human clinical studies, and one of them, istradefylline, is used clinically in Japan. Recent case reports indicate that fatigue in some parkinsonian patients can be alleviated by treatment with istradefylline ( Nomoto et al. , 2014 ). Furthermore, A 2A antagonists have been shown to induce antidepressant-like effects in rodents, as assessed by classical behavioural models ( Hodgson et al. , 2009 ; Hanff et al. , 2010 ; Yamada et al. , 2013 , 2014 ). The adenosine A 2A antagonist MSX-3 has been shown to reverse the effort-related effects of TBZ in rats tested on several different procedures ( Nunes et al. , 2013 b ; Randall et al. , 2014 ; Yohn et al. , 2015 a ). Adenosine A 2A receptors are co-localized with dopamine D2 family receptors on enkephalin-positive medium spiny neurons in both neostriatum and accumbens ( Rosin et al. , 1998 ; Svenningson et al. , 1999 ). Adenosine A 2A and dopamine D2 receptors can form heteromeric complexes, and they converge onto the same c-AMP/protein kinase A signal transduction cascade ( Ferré et al. , 2008 ; Santerre et al. , 2012 ). A dose of 0.75 mg/kg TBZ reduced dopamine-related signal transduction mediated by D1 and D2 receptors (e.g. changes in cFos and expression of multiple forms of pDARPP-32), and MSX-3 significantly attenuated the cellular effects of TBZ related to D2 signalling ( Nunes et al. , 2013 b ). Thus, adenosine A 2A antagonists appear to be acting on enkephalin-positive neurons that contain D2 receptors and form part of the ventral striatopallidal pathway ( Mingote et al. , 2008 ; Farrar et al. , 2010 ; Santerre et al. , 2012 ; Nunes et al. , 2013 b ).
Catecholamine uptake inhibitors are reported to be moderately efficacious for treating psychomotor retardation and fatigue symptoms of depression ( Fabre et al. , 1983 ; Rampello et al. , 1991 ; Pae et al. , 2007 ; Cooper et al. , 2014 ), and can be more effective than 5-HT uptake blockers for treating motivational dysfunction in depressed patients ( Papakostas et al. , 2006 ; Cooper et al. , 2014 ). Bupropion (Wellbutrin®) is a catecholamine uptake inhibitor, which has been shown to occupy dopamine transporters in humans at doses that are clinically useful for treating depression ( Learned-Coughlin et al. , 2003 ), and to elevate extracellular dopamine and norepinephrine in rats as measured by microdialysis ( Hudson et al. , 2012 ; Randall et al. , 2015 ). In rats tested on the T-maze barrier choice task, bupropion fully reversed the effects of TBZ, increasing selection of the barrier arm in TBZ-treated rats ( Yohn et al. , 2015 a ). Bupropion also reversed the effects of TBZ in rats tested on the FR5/chow feeding choice ( Nunes et al. , 2013 b ) and PROG/chow feeding choice tasks ( Randall et al. , 2014 ). In the absence of TBZ, bupropion increased PROG output in rats responding on the PROG/chow feeding choice task, at doses that increased extracellular dopamine and DARPP-32 expression in nucleus accumbens core ( Randall et al. , 2015 ). Because bupropion is known to act as an antidepressant in humans, these results serve to validate the hypothesis that tests of effort-related choice behaviour can be used to assess the effort-related motivational effects of well-known or putative therapeutic agents. Moreover, these results are consistent with studies showing that PROG choice lever pressing output was increased by the novel dopamine uptake inhibitor MRZ-9547 ( Sommer et al. , 2014 ), and that amphetamine increased selection of the high effort alternative in humans responding on an effort-related decision-making task ( Wardle et al. , 2011 ).
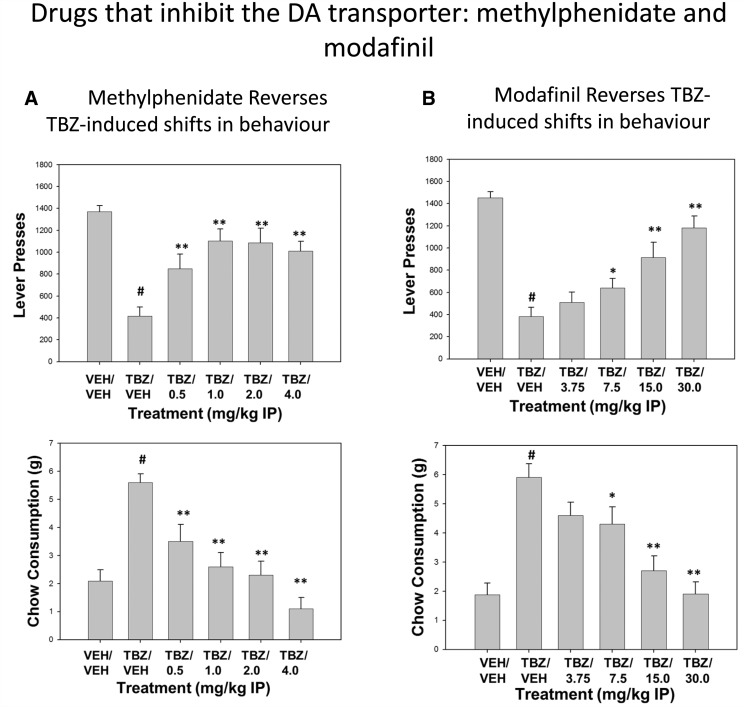
Recent studies have focused on the effects of monoamine uptake inhibitors with different patterns of selectivity for their ability to reverse the effects of TBZ ( Yohn et al. , 2016 b ). The selective dopamine transport inhibitor GBR12909 also reversed the effects of TBZ of FR5/chow feeding choice performance. However, the norepinephrine uptake inhibitor desipramine and the 5-HT uptake inhibitor fluoxetine both failed to reverse the effects of TBZ. Moreover, higher doses of fluoxetine and desipramine, when administered alone or in combination with TBZ, led to further behavioural impairments ( Yohn et al. , 2016 b ). These studies demonstrate that drugs acting on dopamine transmission appear to be relatively effective at reversing the effort-related effects of TBZ and enhancing work-related behavioural output, which is consistent with recent studies demonstrating that the amphetamine pro-drug lisdexamfetamine reverses the effort-related effects of TBZ, while the 5-HT uptake blocker s-citalopram did not ( Yohn et al. , 2016 a ). Recent research from our laboratory has shown that methylphenidate and modifinil, which both block dopamine uptake and have been used to treat motivational dysfunction in humans ( Stotz et al. , 1999 ; Lam et al. , 2007 ), also can reverse the effects of TBZ in rats tested on the FR5/chow feeding choice task ( Fig. 2 ). These findings are consistent with the hypothesis that augmentation of dopamine transmission may be an effective treatment strategy for the amelioration of effort-related psychiatric symptoms in humans.
Figure 2.
Ability of methylphenidate and modafinil to reverse the effects of TBZ in rats responding on the concurrent FR5/chow choice task. All rats (adult male, Sprague-Dawley rats, Harlan Sprague-Dawley) were trained as described in Yohn et al. (2016 a ), and tested in 30-min sessions. Rats were tested 5 days/week, and drug testing was conducted 1 day each week, with a randomized order of drug treatments. ( A ) Methylphenidate. Rats ( n = 12) received intraperitoneal (IP) injections of vehicle or 0.75 mg/kg of TBZ 90 min prior to testing, and also received intraperitoneal injections of vehicle or methylphenidate 45 min prior to testing. Top : Mean [± standard error of the mean (SEM)] number of lever presses. There was an overall significant effect of drug treatment on lever pressing [ F (5,55) = 14.7, P < 0.001]. Planned comparisons showed that TBZ significantly decreased lever pressing compared to vehicle ( #P < 0.05), and that all doses of methylphenidate plus TBZ significantly increased lever pressing relative to TBZ plus vehicle ( **P < 0.01). Bottom : Mean (±SEM) gram quantity of chow intake. There was an overall significant effect of drug treatment on chow intake [ F (5,55) = 19.6, P < 0.001]. Planned comparisons showed that TBZ significantly increased chow consumption relative to vehicle ( #P < 0.05), and that all doses of methylphenidate plus TBZ significantly decreased chow intake relative to TBZ plus vehicle ( **P < 0.01). ( B ) Modafinil. Rats ( n = 12) received intraperitoneal injections of vehicle or 0.75 mg/kg of TBZ 90 min prior to testing, and intraperitoneal injections of either vehicle or modafinil 30 min prior to testing. Top : Mean (±SEM) number of lever presses. There was an overall significant effect of drug treatment on lever pressing [ F (5,55) = 21.0, P < 0.001]. Planned comparisons showed that TBZ significantly decreased lever pressing compared to vehicle ( #P < 0.05), and that the 7.5–30.0 mg/kg doses of modafinil plus TBZ significantly increased lever pressing relative to TBZ plus vehicle (* P < 0.05; **P < 0.01). Bottom : Mean (±SEM) gram quantity of chow intake. There was an overall significant effect of drug treatment on chow intake [ F (5,55) = 14.1, P < 0.001]. Planned comparisons showed that TBZ significantly increased chow consumption relative to vehicle ( #P < 0.05), and that the 7.5–30.0 mg/kg doses of modafinil plus TBZ significantly increased lever pressing relative to TBZ plus vehicle (* P < 0.05; **P < 0.01). Results are from the unpublished thesis of Augustyna Gojol, University of Connecticut, 2015.
Tasks involving effort-related decision-making also have been used to model negative symptoms of schizophrenia. Although local overexpression of dopamine D2 receptors in adult rodents leads to increased behavioural activation and effort expenditure ( Trifilieff et al. , 2013 ), several studies have shown that overexpression of D2 receptors in striatal medium spiny neurons throughout development leads to the opposite effect (i.e. a reduction of behavioural activation and exertion of effort in motivated behaviour; Ward et al. , 2012 ). D2 overexpressing mice show attenuated PROG responding ( Drew et al. , 2007 ; Simpson et al. , 2011 ), and reduced selection of the high effort alternative in a test of effort-based choice ( Ward et al. , 2012 ). Nevertheless, they do not show alterations in hedonic reactivity to food rewards, or changes in food preference or intake. Thus, it has been hypothesized that the effort-related impairments in D2 receptor overexpressing mice could be useful for modelling some of the negative symptoms of schizophrenia ( Simpson et al. , 2011 ; Markou et al. , 2013 ).
Translational studies of effort-related decision-making in non-pathological human subjects
In 2009, Treadway, Zald and colleagues ( Treadway et al. , 2009 ) developed the Effort-Expenditure for Rewards Task (EEfRT) to extend work on effort-related decision-making to humans. People are given a choice on each trial between a difficult (high effort) choice and an easy (low effort) option. The more difficult choice required the subject to make 100 button presses using the non-dominant little finger within 21 s, while the easy choice required 30 button presses with the index finger of the dominant hand within 7 s. Monetary reward was kept constant for the easy task ($1.00), while for the hard task, people could earn more money ($1.24–$4.30). Moreover, reward probability can be varied across trials. This task has been used in several studies over the last few years, including research involving both non-pathological subjects and people with various psychiatric disorders. An initial study examined the effects of d-amphetamine, which enhances dopamine transmission by stimulating release and blocking uptake of dopamine ( Wardle et al. , 2011 ). Healthy human volunteers were assessed using the EEfRT task. Over three sessions, subjects received treatments with either placebo, 10 mg or 20 mg d-amphetamine under counterbalanced double-blind conditions. As predicted, amphetamine enhanced willingness to exert effort, increasing selection of the high-effort option. This effect was particularly strong when reward probability was relatively low. However, amphetamine did not alter the effect of reward magnitude on willingness to exert effort.
In another study ( Treadway et al. , 2012 a ), healthy human volunteers went through a dual-scan PET imaging protocol with 18 F-fallypride and d-amphetamine to measure dopamine transmission, and were separately tested on the EEfRT task. Individual differences in dopamine transmission in the left striatum and ventromedial prefrontal cortex were correlated with the willingness to expend greater effort for larger rewards, especially when reward probability was low. Furthermore, variability in dopamine responses in the bilateral insula was negatively correlated with willingness to expend effort for rewards, which is consistent with evidence indicating that this brain area is involved in the processing of response costs. These results emphasize the role of dopamine signalling in striatal and prefrontal areas in humans as a key neurochemical component of the mechanisms underlying individual differences in cost/benefit decision-making, and are consistent with animal research on individual differences in effort-related processes ( Randall et al. , 2012 ).
Several other imaging papers have focused on the relation between neural activity and mental or physical effort in humans. Some evidence indicates that ventral striatal functional MRI activity can reflect responsiveness to reward discounted by the amount of effort that is required. Botvinick et al. (2009) found that nucleus accumbens functional MRI activity was less strongly activated following a high-demand mental effort task compared to a low demand one. Croxson et al. (2009) tested subjects who were scanned while they performed a series of effortful actions to obtain access to secondary reinforcement. Subjects were presented with one of eight different visual cues at the beginning of each trial, which they had previously learned would signal how much effort the course of action would require, and how much reward could be expected upon completion. Cue-evoked functional MRI activity in the ventral striatum and midbrain signalled the expected amount of reward discounted by the amount of effort to be invested. Activity in dorsal anterior cingulate cortex also reflected the interaction between expected reward and effort costs. However, ventral striatal functional MRI responses also seem to be somewhat context-dependent, and increased activity seen during some tasks can reflect increases in response to the effort that is involved in performing the task. Evidence also indicates that fast phasic dopamine signals as measured by voltammetry and electrophysiology also are context-dependent, and signal different things depending upon the context of the behavioural conditions being studied ( Hollon et al. , 2014 ; Marinelli and McCutcheon, 2014 ; Hamid et al. , 2016 ). Schmidt et al. (2012) observed that ventral striatum functional MRI blood oxygen level-dependent (BOLD) activity was related to anticipation of reward and exertion of mental and physical effort, but not to receipt of the monetary reward. Another functional MRI study by Kurniawan et al. (2013) reported that the supplementary motor cortex, anterior cingulate cortex, and striatum showed higher BOLD responses during anticipation of high effort, and that striatal signals during anticipation were more directly related to anticipated effort rather than expected valence. Furthermore, functional MRI activity in nucleus accumbens was shown to predict high exertion of effort in people performing an instrumental motivation task ( Kroemer et al. , 2014 ).
Psychopathological symptoms related to impairments in activational and effort-related aspects of motivation
Impairments in behavioural activation and effort-related processes can manifest themselves as pathological symptoms that are seen across multiple psychiatric and neurological disorders. Fatigue/loss of energy is one of the most common of all psychiatric symptoms in general medicine ( Demyttenaere et al. , 2005 ). Effort-related motivational/psychomotor symptoms are present in diverse conditions, including major depression, bipolar disorder, schizophrenia, parkinsonism, organic brain disease, immune or inflammatory challenge, chronic fatigue syndrome and multiple sclerosis ( Caligiuri and Ellwanger, 2000 ; Salamone et al. , 2006 , 2010 ; Friedman et al. , 2007 ; Tellez et al. , 2008 ; Clarke et al. , 2011 ; Barch et al. , 2014 , 2015 ; Wolf et al. , 2014 ; Chong et al. , 2015 ; Johnson et al. , 2015 ). The neural bases of effort-related dysfunctions in humans are still being characterized, nevertheless, considerable evidence implicates central dopamine, basal ganglia, and related corticolimbic circuitry ( Rogers et al. , 1987 ; Brown and Gershon, 1993 ; Hickie et al. , 1999 ; Caligiuri and Ellwanger, 2000 ; Brody et al. , 2001 ; Schmidt et al. , 2001 ; Volkow et al. , 2001 ; Salamone et al. , 2006 , 2007 ; Tellez et al. , 2008 ; Treadway and Zald, 2011 ).
One of the disorders that is commonly accompanied by motivational dysfunction is major depression. In addition to being marked by emotional and cognitive symptoms, the majority of depressed patients demonstrate effort-related motivational symptoms, including psychomotor retardation, anergia, lassitude, and fatigue ( Stahl, 2002 ; Demyttenaere et al. , 2005 ; Salamone et al. , 2006 ; Treadway and Zald, 2011 ; Fava et al. , 2014 ; Gorwood et al. , 2014 ). Lack of self-reported energy is related to low mood in people with bipolar disorder ( Johnson et al. , 2015 ), and in depressed people is the symptom that is most strongly correlated with the impairments in social function and work-related factors such as days in bed, days of lost work, and low work productivity ( Tylee et al. , 1999 ; Stahl, 2002 ). Depressed people show reductions in locomotor activity that are related to signs of clinical improvement ( Todder et al. , 2009 ). In a factor analytic study of patients with major depression, Gullion and Rush (1998) identified a ‘lack of energy’ factor (i.e. problems with energy/fatigability, psychomotor retardation, inability to work), which was the factor that loaded most strongly onto a second order general depression factor.
Treatment of motivational dysfunction in depressed people is more problematic than treatment of mood or anxiety symptoms. Many common antidepressants, including 5-HT transport (SERT) inhibitors such as fluoxetine, are relatively ineffective for treating motivational dysfunction, and in fact have been reported to induce or exacerbate these symptoms in some patients ( Nutt et al. , 2007 ; Targum and Fava, 2011 ; Padala et al. , 2012 ; Stenman and Lilja, 2013 ; Fava et al. , 2014 ; Rothschild et al. , 2014 ). SERT inhibitors appear to be better at treating anxiety-related symptoms as opposed to motivational symptoms ( Papakostas et al. , 2008 ). Bell et al. (2013) reported that individual differences in behavioural traits can differentiate between depressed patients that are more responsive to fluoxetine (people with mood problems, irritability, and rumination) versus the catecholamine uptake blocker bupropion (i.e. motivated, achievement-oriented, active, exercise-oriented people). Catecholamine uptake inhibitors such as bupropion have been reported to improve fatigue or anergic symptoms ( Rampello et al. , 1991 ; Papakostas et al. , 2006 ; Pae et al. , 2007 ; Cooper et al. , 2014 ).
Although motivational/psychomotor symptoms are most often measured by rating scales or subscales on various tests, recent studies with objective measures have shown that depressed patients also show reduced effort exertion and alterations in effort-based decision-making ( Clery-Melin et al. , 2011 ; Treadway et al. , 2012 b ; Yang et al. , 2014 ). Clery-Melin et al. (2011) reported that patients with depression exerted less effort (i.e. lower force output involving hand grip) compared to control subjects on a task in which monetary incentives were used. Interestingly, the depressed patients had increased ratings of their perceived effort when the high monetary incentive was used, whereas control subjects showed a decrease. Treadway et al. (2012 b ) reported that patients with major depression showed reduced selection of the high effort choice on the EEfRT task compared to control subjects, particularly when reward probability was high, and therefore control-level performance was at its highest. Yang et al. (2014) used the same task, and also observed that patients diagnosed with major depression, as well as those with subsyndromal depression, showed reduced selection of the high effort alternative. In a subsequent paper, these authors observed diminished caudate responsiveness after presentation of high reward magnitudes in depressed patients who showed reduced high effort selection on the EEfRT task ( Yang et al. , 2015 ).
Motivational symptoms also are widely reported in schizophrenic patients. Although schizophrenia is typically considered to be a ‘thought disorder’, with cardinal positive symptoms such as hallucinations and delusions, schizophrenics also display a host of other impairments, including memory dysfunctions and negative symptoms such as avolition and amotivation ( Gard et al. , 2009 ; Barch and Dowd, 2010 ; Fervaha et al. , 2013 , 2015 ; Markou et al. , 2013 ; Davis et al. , 2014 ; Foussias et al. , 2015 ; Tsapakis et al. , 2015 ). Research by Gard et al. (2014) indicated that schizophrenic patients have fundamental problems with engagement in effortful behaviour that are not dependent upon difficulties with experiencing pleasure or setting pleasure-based goals. Gold et al. (2013) reported that schizophrenics showed reduced selection of high-effort alternatives on a novel decision-making task, and this initial observation has been followed by a recent wave of papers demonstrating that people with schizophrenia show ‘effort shyness’; i.e. reduced selection of high-effort options in objective tests of effort-based decision-making ( Barch et al. , 2014 , 2015 ; Hartmann et al. , 2015; Gold et al. , 2015 ; Green et al. , 2015 ; Horan et al. , 2015 ; Reddy et al. , 2015 a , b ; Treadway et al. , 2015 ). In a recent review, Reddy et al. (2015 a ) surveyed a group of tasks that assessed cognitive, perceptual and physical effort for their suitability in assessing motivational impairments in schizophrenics. The EEfRT task showed good reliability and utility with repeated measures, while a force-grip task yielded large differences between patient and control groups ( Reddy et al. , 2015 a ). Horan et al. (2015) assessed the same tasks for their external validity and correlates, and reported that performance on the effort-based tasks generally showed small/medium correlations with clinical ratings of life functions, negative symptoms, and motivation.
Parkinson’s disease is a movement disorder characterized by degeneration of nigrostriatal dopamine neurons, additional neuropathologies, and motor symptoms such as akinesia, bradykinesia, rigidity and tremor. Nevertheless, patients with Parkinson’s disease also demonstrate depressive symptoms and motivational dysfunctions that typically are labelled as fatigue in the literature ( Friedman et al. , 2007 ). Fatigue symptoms in parkinsonian patients are characterized by subjective reports of a lack of energy (i.e. ‘my battery runs down’ or ‘my energy bubble just bursts’; Friedman et al. , 2007 ), and reduced selection of high-effort activities ( Elbers et al. , 2009 ). Shore et al. (2011) studied appetitive motivation in parkinsonian patients using food reinforcement and presentation of food-related cues. While control subjects show marked behavioural activation in response to food-associated cues, parkinsonian patients showed the opposite effect (i.e. reduced response rates). In addition, Aarts et al. (2012) reported that parkinsonian patients showed a reduced capacity to repeat performance of the current task-set under conditions of high reinforcement.
Recent reports also have examined the exertion of effort in parkinsonian patients. Porat et al. (2014) studied patients with asymmetrical dopamine loss for their ability to exert effort to maximize monetary gains and minimize losses, using a progressive ratio schedule. Patients with relatively greater dopamine impairments in the left hemisphere, when tested OFF medication, showed greater approach deficits (i.e. less effort to increase gain than to avoid loss). In contrast, the opposite pattern of effort expenditure was demonstrated by patients with greater right hemisphere dopamine deficits. If patients performed the same task while medicated, there was increased willingness to expend effort. Chong et al. (2015) studied effort-related decision-making in patients with idiopathic Parkinson’s disease. They developed a novel paradigm in which subjects decided whether or not they were willing to squeeze a hand-held dynamometer at varying levels of force for different magnitudes of reward. For each subject, the effort level at which the probability of accepting a reward was 50% (i.e. the effort ‘indifference point’) was determined. Parkinsonian patients were tested during both the ON and OFF phases of their dopaminergic medication effect, and their performance on the task was compared to that of age-matched controls. None of the patients was clinically apathetic as defined by the Lille Apathy Rating Scale. Regardless of medication status, parkinsonian patients chose to engage in less effort than controls for the lowest level of reward. Interestingly, dopamine transmission had a motivating effect on the choice behaviour of the patients; more effort was exerted by patients when they were in the ON medication state relative to the OFF state. Importantly, the effort-related effects of medication were not related to general improvements in motor function. Thus, Chong et al. (2015) suggested that deficits in motivational decision-making are present in patients with Parkinson’s disease, and that enhancement of dopamine transmission acts to eliminate motivational deficits by promoting the allocation of effortful responding.
Pro-inflammatory cytokines also have been implicated in the fatigue-related symptoms seen in patients with infectious or inflammatory disease ( Dantzer et al. , 2008 ; Harboe et al. , 2009 ; Miller and Norman Cousins Lecture, 2009 ), multiple sclerosis ( Lapierre and Hum, 2007 ), Parkinson’s disease ( Katsarou et al. , 2007 ), and major depression ( Dantzer et al. , 2008 ; Dantzer, 2009 ; Miller and Norman Cousins Lecture, 2009 ; Piser, 2010 ). Cytokines such as interleukin-1 (IL1) mediate a set of behavioural signs known as ‘sickness behaviour’ ( Kent et al. , 1992 ); these include depressed activity, loss of interest or motivation, and lack of body-care activities. Initially linked to infectious diseases, research on cytokines has been extended to studies of neurological and psychiatric disorders, including investigations related to fatigue, anergia, and depression ( Smith, 1991 ; Dantzer et al. , 2008 ; Miller and Norman Cousins Lecture, 2009 ). Peripheral cytokines can act on macrophage-like cells in the choroid plexus and circumventricular organs, which induces synthesis of cytokines that diffuse into brain tissue ( Dantzer, 2009 ). Also, peripheral cytokines act on afferent branches of cranial nerves, instigating the central production of cytokines by microglia ( Dantzer, 2009 ). Cytokines are involved in the fatigue-related symptoms seen in patients with infectious or inflammatory disease ( Dantzer et al. , 2008 ; Harboe et al. , 2009 ; Miller and Norman Cousins Lecture, 2009 ), multiple sclerosis ( Lapierre and Hum, 2007 ), and Parkinson’s disease ( Katsarou et al. , 2007 ). Considerable evidence indicates that cytokines are involved in effort-related symptoms such as psychomotor slowing, anergia and fatigue in patients with major depression ( Raison et al. , 2006 ; Dantzer et al. , 2008 ; Miller and Norman Cousins Lecture, 2009 ). Patients with depression have been reported to have increased levels of pro-inflammatory cytokines, including IL6, and IL1β ( Smith, 1991 ; Raison et al. , 2006 ; van den Biggelaar et al. , 2007 ; Dantzer et al. , 2008 ; Dowlati et al. , 2010 ; Hiles et al. , 2012 ). High levels of IL1β in depressed patients were predictive of a lack of therapeutic response to the antidepressants nortriptyline and escitalopram ( Cattaneo et al. , 2013 ). Cytokines such as IL2 and interferon-α (IFNα) have been shown to induce depression with associated psychomotor slowing and fatigue in patients who were given this treatment to boost their immune system ( Dantzer et al. , 2008 , 2012 ; Majer et al. , 2008 ; Lotrich, 2009 ). Fatigue and loss of energy was reported to be the most common symptom induced by IFNα although depressed mood was reported by some patients (30–60%), fatigue and loss of energy occurred in 80% of patients receiving treatment with IFNα ( Miller and Norman Cousins Lecture, 2009 ). Moreover, patients that received IFNα treatment, when compared to healthy people with major depression, showed less agitation and suicidal ideation, but significantly greater psychomotor slowing ( Capuron et al. , 2009 ). Emerging evidence has implicated prefrontal cortex, basal ganglia and limbic system in mediating the impact of cytokines on depressive symptoms ( Capuron et al. , 2005 ; Dantzer et al. , 2008 ; Majer et al. , 2008 ; Miller and Norman Cousins Lecture, 2009 ; Felger et al. , 2013 ). IFNα-induced increases in glucose metabolism in basal ganglia areas, including nucleus accumbens, were correlated with the development of psychomotor slowing and fatigue ( Capuron et al. , 2005 , 2007 ; Miller and Norman Cousins Lecture, 2009 ).
Despite the fact that depression, schizophrenia and Parkinson’s disease are distinct disorders with unique sets of behavioural and neural pathologies, it is important to consider that there may be some overlap in terms of the neural mechanisms involved in the motivational dysfunctions that are seen. The research domain criterion (RDoC) initiative offered by the US National Institute of Mental Health ( Cuthbert and Insel, 2013 ) has promoted the idea that scientists and clinicians should study the neural circuits that mediate specific symptoms in addition to focusing on traditional diagnostic categories. This idea is potentially important for understanding the circuitry underlying motivational/psychomotor pathologies, such as effort-related dysfunction ( Salamone and Correa, 2012 ; Barch et al. , 2015 ). For several years, it has been suggested that there is overlap between some of the psychomotor symptoms of depression and parkinsonism ( Caligiuri and Ellwanger, 2000 ; Rogers et al. , 2000 ). It has been suggested that dopamine systems and related frontostriatal circuits could be involved in psychomotor and motivational symptoms that are seen across multiple neurological and psychiatric disorders ( Salamone et al. , 2006 , 2015 a ; Winograd-Gurvich et al. , 2006 ). Moreover, drugs that augment dopamine transmission have been reported to have positive effects on motivational or psychomotor functions. Brown and Gershon (1993) reported that l -DOPA was not an effective antidepressant in a broad sense, but it did improve psychomotor function in depressed patients. Beierholm et al. (2013) reported that l -DOPA enhanced response vigour in healthy human volunteers. Methylphenidate can attenuate fatigue and apathy in patients with Parkinson’s disease ( Friedman et al. , 2007 ; Devos et al. , 2013 ) and major depression ( Rizvi et al. , 2014 ). Stotz et al. (1999) reported that amphetamine and methylphenidate increased self-reported energy and psychomotor activity in depressed patients within hours after administration. Although level or type of antipsychotic medication has not been found to be a strong correlate of impairments in effort-related decision-making in schizophrenics ( Green et al. , 2015 ), administration of antipsychotic dopamine antagonists to normal subjects has been shown to induce negative symptoms ( Artaloytia et al. , 2006 ). A PET study of raclopride binding potential in striatum reported that lower baseline dopamine D2 receptor transmission in ventral striatum of unmedicated schizophrenic patients was associated with more severe negative symptoms such as apathy and social withdrawal ( Kegeles et al. , 2010 ).
Complications in assessing effort-related dysfunctions in psychopathology
As is the case with the animal research reviewed above, interpretation of the significance of effort-related symptoms associated with pathological states should be considered in the context of other possible dysfunctions in motivational or affective processes ( Gold et al. , 2015 ). For example, it is reasonable to ask if the bias towards low effort options seen in people with depression, schizophrenia or Parkinson’s disease is simply dependent upon reduced in-the-moment hedonic reactivity to primary rewards. Sienkiewicz-Jarosz et al. (2013) reported that patients with Parkinson’s disease did not differ from control subjects in terms of pleasantness ratings of gustatory and olfactory stimuli, or intensity ratings of higher concentrations of sucrose. Gard et al. (2007) developed a self-report trait measure of anticipatory versus consummatory pleasure, and observed that schizophrenics showed impairments in anticipatory but not consummatory aspects of pleasure. However, Hartmann et al. (2015) reported that anticipatory pleasure for monetary reward could not totally explain the alterations in effort-related decision-making seen in schizophrenic patients. In a recent review by Barch et al. (2015) , it was noted that there are consistent reports in the literature of intact in-the-moment hedonic (i.e. ‘liking’) in schizophrenics. Surprisingly, hedonic ratings of sweet tastes and odour stimuli in depressed patients generally do not differ from those of control subjects ( Amsterdam et al. , 1987 ; Berlin et al. , 1998 ; Clepce et al. , 2010 ; Dichter et al. , 2010 ; Treadway and Zald, 2011 ; Pizzagalli, 2014 ; Barch et al. , 2015 ) (this observation suggests a lack of validity in the use of sucrose intake or preference as an animal model of anhedonia in depression; see also Pardo et al. , 2015 ). Moreover, the severity of depressive symptoms in a non-clinical sample was not correlated with hedonic ratings of various types of tastes ( Scinska et al. , 2004 ). Nevertheless, there is considerable evidence of impairments in positively reinforced instrumental behaviour and anticipatory aspects of motivation and affect in depressed patients and schizophrenics ( Dichter, 2010 ; Treadway and Zald, 2011 ; Pizzagalli, 2014 ; Barch et al. , 2015 ). At this point, it is not clear which specific psychological processes underlie the alterations in effort-related aspects of motivation that are observed in various patient populations. These effects could reflect changes in behavioural activation or aspects of reward processing, as well as cognitive or affective functions related to reward anticipation or the impact of delayed reinforcement, and in fact could differ across disorders, and between specific individuals.
Part of the difficulty in interpreting the functional significance of effort-related dysfunction in psychopathology lies in the imprecise nature of the concepts and vocabulary that are used. The term ‘reward’, when used to denote a neurobehavioural process, is so ill-defined, and so variably used, as to be almost meaningless ( Salamone et al. , 2005 ; Salamone and Correa, 2012 , 2013 ). Moreover, it is clear that mesolimbic dopamine is involved in aversive motivation and stress ( Salamone, 1994 ; Salamone et al. , 1997 , 2007 , 2015 b ). For these reasons, as well as the evident complexity of the actual behavioural functions of mesolimbic dopamine ( Salamone and Correa, 2012 ; Salamone et al. , 2015 b ), it is problematic to label mesolimbic dopamine as the ‘reward system’ or nucleus accumbens as the ‘reward centre’ or ‘pleasure centre’ of the brain. In that case, one cannot label an imaging response from the ventral midbrain or ventral striatum induced by presentation of a primary reinforcer as representing a hedonic response, simply because that response is occurring in the ‘reward system’.
There are similar problems with the clinical use of the term ‘anhedonia’ ( Treadway and Zald, 2011 ; Markou et al. , 2013 ). According to its original psychiatric definition, anhedonia referred to an inability to experience pleasure ( Ribot, 1896 ). However, through many years, the use of the term has evolved to include a heterogeneous and at times ambiguous mixture of functions that are dissociable from each other ( Dichter, 2010 ; Treadway and Zald, 2011 ). Thus, it now has to be explained in detail that there are types of anhedonia that are ‘motivational’ in nature, and not necessarily marked by reduced hedonic response to primary rewards ( Treadway and Zald, 2011 ). Furthermore, labelling effort-related dysfunctions as a type of anhedonia implies the primacy of an emotional component in the impairment, while diminishing and obscuring the role of processes involving aspects of motivation such as behavioural activation and action instigation, as well as cognitive functions involved in estimating or predicting future events. Though it can be suggested that effort-based impairments are related to trait measures of anticipatory pleasure, it is not clear that anticipatory pleasure self-reported on an inventory is precisely the same thing as ‘pleasure during anticipation’ (i.e. the affective state that is directly experienced during anticipation or instrumental responding). Moreover, years of study of affective processes have emphasized the important distinction between emotional valence and arousal ( Gerber et al. , 2008 ). As a result, it is not clear if people suffering from problems with anticipatory pleasure are basing their self-report on blunted positive valence or their perception of their own diminished arousal or intensity. Also, while it is often suggested that affect causes motivation, why cannot the opposite also be true? Perhaps affective states in part reflect the experience of being motivated. Another consideration is that the relation between terms such as anergia, fatigue, lassitude, amotivation, apathy, and psychomotor retardation remains uncertain ( Clarke et al. , 2011 ), and it is unclear how these are related to empirically measured alterations in effort-based choice. These terminological points are not trivial; scientific and clinical terms and definitions are tools that are every bit as important as any device or technique ( Salamone and Correa, 2012 ). Thus, despite the great progress that has been made, continued research will be necessary to develop and refine the measures and concepts that are used to characterize different aspects of appetitive motivation in clinical populations.
Importance of behavioural economic concepts for understanding effort-based choice
One of the most powerful ways of characterizing instrumental behaviour has been the use of terms and concepts from behavioural economics. For example, the use of the term ‘value’ has skyrocketed in both the basic neuroscience and clinical literatures, and investigators widely use terms such as reinforcement value, valuation, devaluation, discounted value, etc. Other economic concepts, such as cost/benefit analysis, preference, utility, substitution, demand, and elasticity also are frequently used. Much of the initial impetus for this came from researchers studying the experimental analysis of behaviour, and economic concepts have been particularly important in this area ( Bickel et al. , 1995 ; Hursh and Winger, 1995 ; Madden et al. , 2000 , 2007 ). However, economic terms and concepts are also now readily seen in the basic and clinical neuroscience literatures. Economic terms are so readily applicable to the study of complex behaviour because economics is not really about money; it is about choice. The main subject of the present review, effort-related choice behaviour, thus lends itself to this type of analysis.
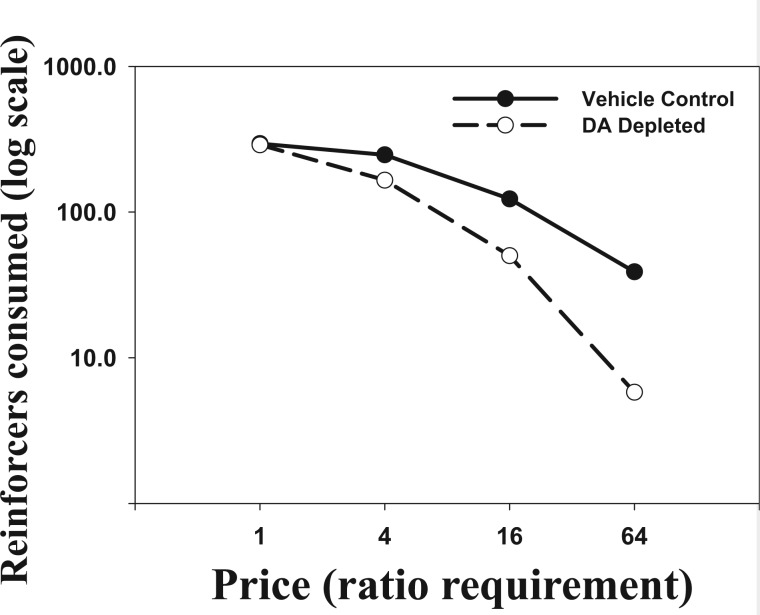
A full review of behavioural economic concepts is beyond the scope of the present paper. Nevertheless, there are some fundamental points that can be raised, which shed light on the basic and clinical research described above. Based on an economic analysis, a reinforcer is a good or commodity, and the instrumental behaviour is essentially labour that is bartered for access to the reinforcer. The response requirement is therefore the price that needs to be paid, in terms of the labour performed. Thus, the results of some behavioural experiments can be analysed as a demand curve ( Hursh and Winger, 1995 ), which plots response output as a function of price (response costs; x -axis) versus the amount of reinforcer obtained ( y -axis). As accumbens dopamine depletions have little effect when the response cost is low, but substantial effects with increasing response requirement, it can be said that accumbens dopamine depletions increase elasticity of demand ( Fig. 3 ; Aberman and Salamone, 1999 ; Salamone et al. , 2009 , 2012 ). In other words, dopamine depletions decrease the willingness to pay higher prices, in terms of response costs (i.e. costs involving physical effort), for food reinforcement. Demand analysis also has been an effective tool for characterizing the role that various neural systems play in regulating drug seeking and taking ( Hursh and Winger, 1995 ; Heyman, 2000 ; Madden and Kalman, 2010 ; Heinz et al. , 2012 ; Bentzley et al. , 2013 , 2014 ; Bentzley and Aston-Jones, 2015 ).
Figure 3.
The effect of increasing price, shown as ratio requirement, on the number of operant pellets consumed in rats with accumbens dopamine depletions compared to rats in the vehicle control group. These results are based on data from Aberman and Salamone (1999) . The data are represented as a demand curve, calculated from the mean number of reinforcement pellets consumed (shown on a log scale) as a function of price (ratio requirement). Although comparable levels of consumption in dopamine (DA)-depleted and control groups were seen with the FR1 schedule, dopamine-depleted rats showed markedly reduced consumption relative to the control group at higher ratio levels.
In the lever pressing/chow feeding procedures described above, an additional factor is added, because animals are given a low-cost substitute, in the form of the concurrently available chow. Economic decisions can be powerfully affected by the availability of substitutes; a person who cannot afford or is unwilling to pay for an expensive car can purchase a cheaper alternative. In the case of the lever pressing/chow feeding choice procedures described above, the presence of the concurrent chow acts to pull animals away from lever pressing, and as higher costs are applied (i.e. higher FR or PROG requirements), the animals decrease lever pressing and increase chow intake ( Salamone et al. , 1997 ; Randall et al. , 2012 ). This effect is accentuated by interference with accumbens dopamine transmission, and animals shift from lever pressing to chow intake, as described above.
Given these findings, the question of whether or not a manipulation such as interference with dopamine transmission affects the reinforcement value of the primary reinforcer (e.g. food) is a complex one. For example, research on response/reinforcement matching offers various methods for measuring reinforcement value ( Heyman et al. , 1987 ). In these experiments animals match relative responding to the relative value (e.g. magnitude, density or rate) of reinforcement across multiple alternatives. However, as described in detail previously ( Williams, 1988 ; Salamone et al. , 1997 , 2012 ), reinforcement value as measured by the matching equations is not strictly speaking a measure of the reinforcement value of food per se ; rather, it is a measure of the relative value of the whole activity of lever pressing for, obtaining, and consuming the food. In a matching analysis, Aparicio (2007) reported that the dopamine antagonist haloperidol did not alter the discrimination between reinforcement rich versus reinforcement poor levers, but did affect response bias. According to work by Baum and Rachlin (1969) , the relative reinforcement value of activities can be measured by the relative allocation of time between alternatives. In that case, studies using the FR5/chow feeding choice procedure indicate that dopamine antagonism or depletion would be decreasing the relative value of lever pressing for the preferred food, but actually increasing the relative value of chow intake. These findings hardly yield a clear picture of the effect of dopaminergic manipulations on the value of the food itself. This picture is further complicated by papers demonstrating that the doses of dopamine antagonists or the mesolimbic dopamine depletions that produce alterations in effort-related choice do not change hedonic reactivity to the primary reinforcer, consumption of the reinforcers, or preference between the different sources of food reinforcement in free-feeding tests ( Salamone et al. , 1991 ; Berridge et al. , 2007 ; Nunes et al. , 2013 b ; Pardo et al. , 2015 ; Yohn et al. , 2015 a ). Thus, even if reinforcement value is empirically defined as how much an organism will pay for a commodity (i.e. ‘essential value’, Hursh and Silberberg, 2008 ), it appears that interference with dopamine transmission dissociates ‘willingness to pay’ from preference and utility (i.e. the total satisfaction or benefit received from consuming a good). What types of conditions could generate this dissociation? Although behavioural processes may appear to be associated with each other under baseline or control conditions, they can be dissociated by brain manipulations or pathologies ( Salamone and Correa, 2002 ; Berridge and Robinson, 2003 ; Salamone et al. , 2007 ). Thus, there is something about the processes that are measured by willingness to pay that appear to be particularly sensitive to dopaminergic manipulations. One possible economic concept related to the role of dopamine could be ‘purchasing power’, which in economic terms refers to the available monetary resources that enable a person to make cost-related choices. It is possible that interference with dopamine transmission, by reducing the activating effects of motivational stimuli, constrains the behavioural resources available for investing time and effort in lever pressing for a reinforcer such as food, which leads to a re-allocation of behavioural resources when the response costs are high ( Salamone et al. , 2016 ). Such an effect is nevertheless dissociable from an action on preference or hedonic reactivity when costs are low.
Although the discussion in this section has focused on the effects of dopaminergic manipulations in animals, the principles being highlighted are of equal importance in assessing human studies, including the types of imaging experiments and clinical research described above ( Shenhav et al. , 2013 ). Under pathological conditions, one cannot assume that empirically derived measures of reinforcement value equal preference, hedonia or satisfaction. Brain manipulations and pathological states are capable of parsing complex processes into dissociable components, and this provides another reason why caution should be exercised in the use of language to describe those impairments.
Conclusions
In summary, converging lines of evidence ranging from classical studies of motivation to contemporary research in basic and clinical neuroscience emphasize the importance of behavioural activation and effort-related aspects of motivation. Dopamine systems are a critical part of the brain circuitry regulating behavioural activation, exertion of effort, and effort-related decision-making. Interference with mesolimbic dopamine transmission biases animals towards selection of low effort options, but these effects are not dependent upon interference with the primary or unconditioned reinforcing effects of stimuli such as food. Doses of dopamine antagonists or mesolimbic dopamine depletions that reduce selection of high effort alternatives do not change hedonic reactivity to the primary reinforcer, consumption of the reinforcers, or preference between the different sources of food reinforcement in free-feeding tests ( Salamone et al. , 1991 , 2007 ; Berridge et al. , 2007 ; Salamone and Correa, 2012 ; Nunes et al. , 2013 b ; Pardo et al. , 2015 ; Yohn et al. , 2015 a ). In addition to mesolimbic dopamine, effort-related functions engage a distributed neural circuitry that includes multiple neurotransmitters across basal ganglia, limbic and cortical areas. Moreover, there is a striking similarity between the brain areas involved in behavioural activation and effort-related processes in rodents and in humans. An emerging body of evidence indicates that alterations in effort-based decision-making are evident in several psychiatric and neurological disorders. While depression, schizophrenia and parkinsonism are marked by reduced selection of high effort alternatives, autistic adults show the opposite effect ( Damiano et al. , 2012 ), and it will be important to conduct further investigations of effort-related functions in people with bipolar disorder ( Johnson et al. , 2012 ; Whitton et al. , 2015 ). Formal animal models of effort-related dysfunction have been developed, which are not necessarily functioning as models of a particular disorder, but rather are focused upon specific symptom dimensions and circuits that span multiple disorders, in line with the RDoC initiative described above. Translational studies linking research with animal models, human volunteers, and clinical populations is already beginning to revolutionize the understanding of the neural basis of effort-related motivational dysfunction, and it is hoped that this research will ultimately lead to improved treatment for motivational and psychomotor symptoms in psychiatry and neurology.
Funding
This work was supported by a grant to J.S. from the National Institute of Mental Health (MH094966), and to Mercè Correa from U.J.I. P1.1A2013-01.
Conflict of interest
J. S has received grants from Merck-Serrono, Pfizer, Roche, Shire, and Prexa. M.C. has received a grant from Servier.
Glossary
Abbreviations
- EEfRT
Effort-Expenditure for Rewards Task
- FR =
fixed ratio
- PROG
progressive ratio
- TBZ
tetrabenazine
References
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive ratio performance . Pharmacol Biochem Behav 1998. ; 61 : 341 – 8 . [DOI] [PubMed] [Google Scholar]
- Aarts E, Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Cools R. Aberrant reward processing in Parkinson's disease is associated with dopamine cell loss . Neuroimage 2012. ; 59 : 3339 – 46 . [DOI] [PubMed] [Google Scholar]
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make animals more sensitive to high ratio requirements but do not impair primary food reinforcement . Neuroscience 1999. ; 92 : 545 – 55 . [DOI] [PubMed] [Google Scholar]
- Achterberg EJ, van Kerkhof LW, Servadio M, van Swieten MM, Houwing DJ, Aalderink M , et al. . Contrasting roles of dopamine and noradrenaline in the motivational properties of social play behavior in rats . Neuropsychopharmacology 2016. ; 41 : 858 – 68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression . Biol Psychiatry 1987. ; 22 : 1481 – 5 . [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats . Neuropsychopharmacology 2005. ; 30 : 1832 – 40 . [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats . Neuroscience 2009. ; 161 : 3 – 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio CF. Haloperidol, dynamics of choice, and the parameters of the matching law . Behav Process 2007. ; 75 : 206 – 12 . [DOI] [PubMed] [Google Scholar]
- Artaloytia JF, Arango C, Lahti A, Sanz J, Pascual A, Cubero P , et al. . Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers . Am J Psychiatry 2006. ; 163 : 488 – 93 . [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity . Behav Brain Res 2002. ; 137 : 165 – 77 . [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. [Review] . Schizophr Bull 2010. ; 36 : 919 – 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment . J Abnorm Psychol 2014. ; 123 : 387 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Pagliaccio D, Luking K. Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia . Curr Top Behav Neurosci 2015[Epub ahead of print] . [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision-making in rats . Behav Neurosci 2009. ; 123 : 242 – 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum WM, Rachlin HC. Choice as time allocation . J Exp Anal Behav 1969. ; 12 : 861 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Frazier CR, Zhuang X. Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources . Front Integr Neurosci 2012. ; 6 : 49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Faust RP, Turkson S, Ye H, Zhuang X. Low dopamine D2 receptor increases vulnerability to obesity via reduced physical activity not increased appetitive motivation . Biol Psychiatry 2015[Epub ahead of print] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierholm U, Guitart-Masip M, Economides M, Chowdhury R, Düzel E, Dolan R , et al. . Dopamine modulates reward-related vigor . Neuropsychopharmacology 2013. ; 38 : 1495 – 503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction . Behav Brain Res 2009. ; 199 : 89 – 102 . [DOI] [PubMed] [Google Scholar]
- Bell DS, Shipman WM, Cleves MA, Siegelman J. Which drug for which patient? Is there a fluoxetine responding versus a bupropion responding personality profile? Clin Pract Epidemiol Ment Health 2013. ; 9 : 142 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures . Psychopharmacology 2013. ; 226 : 113 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat . Proc Natl Acad Sci USA 2014. ; 111 : 11822 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues . Eur J Neurosci 2015. ; 41 : 1149 – 56 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects . Eur Psychiatry 1998. ; 13 : 303 – 9 . [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience . Psychopharmacology (Berl) 2007. ; 191 : 391 – 431 . [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 1998. ; 28 : 309 – 69 . [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward . Trends Neurosci 2003. ; 26 : 507 – 13 . Review. Erratum in: Trends Neurosci. 2003; 26: 581. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. [Review] . Neuron 2015. ; 86 : 646 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Green L, Vuchinich RE. Behavioral economics . J Exp Anal Behav 1995. ; 64 : 257 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Barsom MW, Bota RG, Saxena S. Prefrontal-subcortical and limbic circuit mediation of major depressive disorder . Semin Clin Neuropsychiatry 2001. ; 6 : 102 – 12 . [DOI] [PubMed] [Google Scholar]
- Brown AS, Gershon S. Dopamine and depression . J Neural Transm Gen Sect 1993. ; 91 : 75 – 109 . [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens . Cogn Affect Behav Neurosci 2009. ; 9 : 16 – 27 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward . Neuropsychopharmacology 2006. ; 31 : 1362 – 70 . [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Ellwanger J. Motor and cognitive aspects of motor retardation in depression . J Affect Disord 2000. ; 57 : 83 – 93 . [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS , et al. . Anterior cingulate activation and error processing during interferon-alpha treatment . Biol Psychiatry 2005. ; 58 : 190 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B , et al. . Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy . Neuropsychopharmacology 2007. ; 32 : 2384 – 92 . [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord 2009. ; 119 : 181 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson NR. Physiology of behavior . Harlow, Essex, UK: : Pearson Education Ltd; .; 2014. . p. 401 – 42 . [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ , et al. . Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets' . Neuropsychopharmacology 2013. ; 38 : 377 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caul WF, Brindle NA. Schedule-dependent effects of haloperidol and amphetamine: multiple-schedule task shows within-subject effects . Pharmacol Biochem Behav 2001. ; 68 : 53 – 63 . [DOI] [PubMed] [Google Scholar]
- Chen JJ, Ondo WG, Dashtipour K, Swope DM. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. [Review] . Clin Ther 2012. ; 34 : 1487 – 504 . [DOI] [PubMed] [Google Scholar]
- Chong TT, Bonnelle V, Manohar S, Veromann KR, Muhammed K, Tofaris GK , et al. . Dopamine enhances willingness to exert effort for reward in Parkinson's disease . Cortex 2015. ; 69 : 40 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? A review of the psychometric evidence . J Psychosom Res 2011. ; 70 : 73 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clepce M, Gossler A, Reich K, Kornhuber J, Thuerauf N. The relation between depression, anhedonia and olfactory hedonic estimates–a pilot study in major depression . Neurosci Lett 2010. ; 471 : 139 – 43 . [DOI] [PubMed] [Google Scholar]
- Cléry-Melin ML, Schmidt L, Lafargue G, Baup N, Fossati P, Pessiglione M. Why don't you try harder? An investigation of effort production in major depression . PLoS One 2011. ; 6 : e23178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofer CN, Appley MH. Motivation: theory and research . New York: : John Wiley and Sons; ; 1964. . [Google Scholar]
- Collins AG, Frank MJ. Opponent actor learning (OpAL): modeling interactive effects of striatal dopamine on reinforcement learning and choice incentive . Psychol Rev 2014. ; 121 : 337 – 66 . [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell . J Neurosci 2011. ; 31 : 11786 – 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning . Eur J Neurosci 2010. ; 31 : 1312 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell . J Neurosci 2001. ; 21 : 3251 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa M, Carlson BB, Wisniecki A, Salamone JD. Nucleus accumbens dopamine and work requirements on interval schedules . Behav Brain Res 2002. ; 137 : 179 – 87 . [DOI] [PubMed] [Google Scholar]
- Cooper JA, Tucker VL, Papakostas GI. Resolution of sleepiness and fatigue: a comparison of bupropion and selective serotonin reuptake inhibitors in subjects with major depressive disorder achieving remission at doses approved in the European Union . J Psychopharmacol 2014. ; 28 : 118 – 24 . [DOI] [PubMed] [Google Scholar]
- Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal (DA) depletions on instrumental response selection in the rat . Pharmacol Biochem Behav 1993. ; 46 : 943 – 51 . [DOI] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens (DA) depletions alter relative response allocation in a T-maze cost/benefit task . Behav Brain Res 1996. ; 74 : 189 – 97 . [DOI] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TE, Rushworth MF. Effort-based cost-benefit valuation and the human brain . J Neurosci 2009. ; 29 : 4531 – 41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC . BMC Med 2013. ; 11 : 126 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y , et al. . Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens . Proc Natl Acad Sci USA 2005. ; 102 : 6189 – 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Treadway M, Bodfish JW, Dichter GS. Adults with autism spectrum disorders exhibit decreased sensitivity to reward parameters when making effort-based decisions . J Neurodev Disord 2012. ; 4 : 13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression . Immunol Allergy Clin North Am 2009. ; 29 : 247 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Perry VH , et al. . Identification and treatment of symptoms associated with inflammation in medically ill patients . Psychoneuroendocrinology 2008. ; 33 : 18 – 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. [Review] . Nat Rev Clin Oncol 2012. ; 9 : 414 – 26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MC, Horan WP, Marder SR. Psychopharmacology of the negative symptoms: current status and prospects for progress . Eur Neuropsychopharmacol 2014. ; 24 : 788 – 99 . [DOI] [PubMed] [Google Scholar]
- de Jong JW, Roelofs TJ, Mol FM, Hillen AE, Meijboom KE, Luijendijk MC , et al. . Reducing ventral tegmental dopamine D2 receptor expression selectively boosts incentive motivation . Neuropsychopharmacology 2015. ; 40 : 2085 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat . J Neurosci 1990. ; 10 : 303 – 10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder . Int J Neuropsychopharmacol 2005. ; 8 : 93 – 105 . [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort . Psychopharmacology (Berl) 2005. ; 179 : 587 – 96 . [DOI] [PubMed] [Google Scholar]
- Devos D, Moreau C, Delval A, Dujardin K, Defebvre L, Bordet R. Methylphenidate: a treatment for Parkinson's disease? CNS Drugs 2013. ; 27 : 1 – 14 . [DOI] [PubMed] [Google Scholar]
- Dichter GS. Anhedonia in unipolar major depressive disorder: a review . Open Psychiatry J 2010. ; 4 : 1 – 9 . [Google Scholar]
- Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test . Depress Anxiety 2010. ; 27 : 859 – 63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK , et al. . A meta-analysis of cytokines in major depression . Biol Psychiatry 2010. ; 67 : 446 – 57 . [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S , et al. . Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing . J Neurosci 2007. ; 27 : 7731 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy E. Activation and behavior . New York: : John Wiley and Sons; ; 1963. . [Google Scholar]
- Elbers R, van Wegen EE, Rochester L, Hetherington V, Nieuwboer A, Willems AM , et al. . Is impact of fatigue an independent factor associated with physical activity in patients with idiopathic Parkinson's disease? Mov Disord 2009. ; 24 : 1512 – 8 . [DOI] [PubMed] [Google Scholar]
- Fabre LF, Brodie HK, Garver D, Zung WW. A multicenter evaluation of bupropion versus placebo in hospitalized depressed patients . J Clin Psychiatry 1983. ; 44 : 88 – 94 . [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of (DA) receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing . Psychopharmacology 2007. ; 191 : 579 – 86 . [DOI] [PubMed] [Google Scholar]
- Farrar AM, Font L, Pereira M, Mingote SM, Bunce JG, Chrobak JJ , et al. . Forebrain circuitry involved in effort-related choice: injections of the GABAA agonist muscimol into ventral pallidum alters response allocation in food-seeking behavior . Neuroscience 2008. ; 152 : 321 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar AM, Segovia KN, Randall PA, Nunes EJ, Collins LE, Stopper CM , et al. . Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and (DA) D2 receptors . Neuroscience 2010. ; 166 : 1056 – 67 . [DOI] [PubMed] [Google Scholar]
- Faustman WO, Fowler SC. Use of operant response duration to distinguish the effects of haloperidol from nonreward . Pharmacol Biochem Behav 1981. ; 15 : 327 – 9 . [DOI] [PubMed] [Google Scholar]
- Fava M, Ball S, Nelson JC, Sparks J, Konechnik T, Classi P , et al. . Clinical relevance of fatigue as a residual symptom in major depressive disorder . [Review] Depress Anxiety 2014. ; 31 : 250 – 7 . [DOI] [PubMed] [Google Scholar]
- Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL , et al. . Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations . Brain Behav Immun 2013. ; 153 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F , et al. . An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors [Review] . Curr Pharm Des 2008. ; 14 : 1468 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, Remington G. Neural substrates underlying effort computation in schizophrenia . Neurosci Biobehav Rev 2013. ; 37 : 2649 – 65 . [DOI] [PubMed] [Google Scholar]
- Fernando AB, Urcelay GP, Mar AC, Dickinson TA, Robbins TW. The role of the nucleus accumbens shell in the mediation of the reinforcing properties of a safety signal in free-operant avoidance: dopamine-dependent inhibitory effects of d-amphetamine . Neuropsychopharmacology 2014. ; 39 : 1420 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Duncan M, Foussias G, Agid O, Faulkner GE, Remington G. Effort-based decision-making as an objective paradigm for the assessment of motivational deficits in schizophrenia . Schizophr Res 2015. ; 168 : 483 – 90 . pii: S0920-9964(15)00388-6. [DOI] [PubMed] [Google Scholar]
- Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action . Annu Rev Psychol 2015. ; 66 : 25 – 52 . [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making . Cereb Cortex 2007. ; 17 : 251 – 60 . [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay - based decision-making . Neuropsychopharmacology 2008. ; 33 : 1966 – 79 . [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar AM, Pereira M, Worden L, Stopper C , et al. . Intra-accumbens injections of the adenosine A2A agonist CGS 21680 affect effort-related choice behavior in rats . Psychopharmacology 2008. ; 199 : 515 – 26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foussias G, Siddiqui I, Fervaha G, Mann S, McDonald K, Agid O , et al. . Motivated to do well: an examination of the relationships between motivation, effort, and cognitive performance in schizophrenia . Schizophr Res 2015. ; 166 : 276 – 82 . [DOI] [PubMed] [Google Scholar]
- Fowler SC, LaCerra MM, Ettenberg A. Effects of haloperidol on the biophysical characteristics of operant responding: implications for motor and reinforcement processes . Pharmacol Biochem Behav 1986. ; 25 : 791 – 6 . [DOI] [PubMed] [Google Scholar]
- Frank S. Tetrabenazine as anti-chorea therapy in Huntington disease: an open-label continuation study. Huntington Study Group/TETRA-HD Investigators . BMC Neurol 2009. ; 9 : 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. Tetrabenazine: the first approved drug for the treatment of chorea in US patients with Huntington disease . Neuropsychiatr Dis Treat 2010. ; 6 : 657 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JH, Brown RG, Comella C, Garber CE, Krupp LB, Lou JS , et al. . Fatigue in Parkinson’s disease: a review. [Review] . Mov Disord 2007. ; 22 : 297 – 308 . [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure . Schizophr Res 2007. ; 93 : 253 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia . Schizophr Res 2009. ; 115 : 74 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S. Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? J Abnorm Psychol 2014. ; 123 : 771 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber AJ, Posner J, Gorman D, Colibazzi T, Yu S, Wang Z , et al. . An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces . Neuropsychologia 2008. ; 46 : 2129 – 39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Floresco SB. Differential effects on effort discounting induced by inactivations of the nucleus accumbens core or shell . Behav Neurosci 2010. ; 124 : 179 – 91 . [DOI] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations . Biol Psychiatry 2013. ; 74 : 130 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Frank MJ. Effort cost computation in schizophrenia: a commentary on the recent literature. [Review] . Biol Psychiatry 2015. ; 78 : 747 – 53 . pii: S0006-3223(15)00402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P, Richard-Devantoy S, Baylé F, Cléry-Melun ML. Psychomotor retardation is a scar of past depressive episodes, revealed by simple cognitive tests . Eur Neuropsychopharmacol 2014. ; 24 : 1630 – 40 . [DOI] [PubMed] [Google Scholar]
- Green MF, Horan WP, Barch DM, Gold JM. Effort-based decision-making: a novel approach for assessing motivation in schizophrenia . Schizophr Bull 2015. ; 41 : 1035 – 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay DR. Tetrabenazine, a monoamine-depleting drug used in the treatment of hyperkinetic movement disorders . Am J Geriatr Pharmacother 2010. ; 8 : 331 – 73 . [DOI] [PubMed] [Google Scholar]
- Gullion CM, Rush AJ. Toward a generalizable model of symptoms in major depressive disorder . Biol Psychiatry 1998. ; 44 : 959 – 72 . [DOI] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM , et al. . Mesolimbic dopamine signals the value of work . Nat Neurosci 2016. ; 19 : 117 – 26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff TC, Furst SJ, Minor TR. Biochemical and anatomical substrates of depression and sickness behavior. [Review] . Isr J Psychiatry Relat Sci 2010. ; 47 : 64 – 71 . [PubMed] [Google Scholar]
- Harboe E, Tjensvoll AB, Vefring HK, Gøransson LG, Kvaløy JT, Omdal R. Fatigue in primary Sjögren's syndrome–a link to sickness behaviour in animals? Brain Behav Immun 2009. ; 23 : 1104 – 8 . [DOI] [PubMed] [Google Scholar]
- Hartmann MN, Hager OM, Reimann AV, Chumbley JR, Kirschner M, Seifritz E , et al. . Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort . Schizophr Bull 2015. ; 41 : 503 – 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision-making . Cereb Cortex 2009. ; 19 : 2240 – 7 . [DOI] [PubMed] [Google Scholar]
- Heinz AJ, Lilje TC, Kassel JD, de Wit H. Quantifying reinforcement value and demand for psychoactive substances in humans . Curr Drug Abuse Rev 2012. ; 5 : 257 – 72 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman GM. An economic approach to animal models of alcoholism . Alcohol Res Health 2000. ; 24 : 132 – 9 . [PMC free article] [PubMed] [Google Scholar]
- Heyman GM, Monaghan MM, Clody DE. Low doses of cis-flupentixol attenuate motor performance . Psychopharmacology 1987. ; 93 : 477 – 82 . [DOI] [PubMed] [Google Scholar]
- Hickie I, Ward P, Scott E, Haindl W, Walker B, Dixon J , et al. . Neo-striatal rCBF correlates of psychomotor slowing in patients with major depression . Psychiatry Res 1999. ; 92 : 75 – 81 . [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity . Brain Behav Immun 2012. ; 26 : 1180 – 8 . [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, Fredduzzi S , et al. . Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2,4-difluorophenyl]-1-piperazinyl]ethyl]-2-(2-furanyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] in rodent models of movement disorders and depression . J Pharmacol Exp Ther 2009. ; 330 : 294 – 303 . [DOI] [PubMed] [Google Scholar]
- Hollon NG, Arnold MM, Gan JO, Walton ME, Phillips PE. Dopamine-associated cached values are not sufficient as the basis for action selection . Proc Natl Acad Sci USA 2014. ; 111 : 18357 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Reddy LF, Barch DM, Buchanan RW, Dunayevich E, Gold JM , et al. . Effort-based decision-making paradigms for clinical trials in schizophrenia: part 2-external validity and correlates . Schizophr Bull 2015. ; 41 : 1055 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC, Richardson WB, Ettenberg A. Dopamine receptor blockade and reductions in thirst produce different effects on drinking behavior . Pharmacol Biochem Behav 1993. ; 45 : 725 – 8 . [DOI] [PubMed] [Google Scholar]
- Hosking JG, Floresco SB, Winstanley CA. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: a comparison of two rodent cost/benefit decision-making tasks . Neuropsychopharmacology 2015. ; 40 : 1005 – 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe MW, Tierney PL, Sandberg SG, Phillips PE, Graybiel AM. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards . Nature 2013. ; 500 : 575 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AL, Lalies MD, Silverstone P. Venlafaxine enhances the effect of bupropion on extracellular dopamine in rat frontal cortex . Can J Physiol Pharmacol 2012. ; 90 : 803 – 9 . [DOI] [PubMed] [Google Scholar]
- Hull EM, Weber MS, Eaton RC, Dua R, Markowski VP, Lumley L , et al. . Dopamine receptors in the ventral tegmental area affect motor, but not motivational or reflexive, components of copulation in male rats . Brain Res 1991. ; 554 : 72 – 6 . [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers . J Exp Anal Behav 1995. ; 64 : 373 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh HR, Silberberg A. Economic demand and essential value . Psychol Rev 2008. ; 115 : 186 – 98 . [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements . Behav Brain Res 2004. ; 151 : 83 – 91 . [DOI] [PubMed] [Google Scholar]
- Johnson SL, Fulford D, Carver CS. The double-edged sword of goal engagement: consequences of goal pursuit in bipolar disorder . Clin Psychol Psychother 2012. ; 19 : 352 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Gershon A, Starov V. Is energy a stronger indicator of mood for those with bipolar disorder compared to those without bipolar disorder? Psychiatry Res 2015. ; 230 : 1 – 4 . [DOI] [PubMed] [Google Scholar]
- Katsarou Z, Bostantjopoulou S, Hatzizisi O, Giza E, Soler-Cardona A, Kyriazis G. Immune factors or depression? Fatigue correlates in Parkinson's disease [in Spanish] . Rev Neurol 2007. ; 45 : 725 – 8 . [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M , et al. . Increased synaptic dopamine function in associative regions of the striatum in schizophrenia . Arch Gen Psychiatry 2010. ; 67 : 231 – 9 . [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development . Trends Pharmacol Sci 1992. ; 13 : 24 – 8 . [DOI] [PubMed] [Google Scholar]
- Kent TA, Preskorn SH, Glotzbach RK, Irwin GH. Amitriptyline normalizes tetrabenazine-induced changes in cerebral microcirculation . Biol Psychiatry 1986. ; 21 : 483 – 91 . [DOI] [PubMed] [Google Scholar]
- Killeen P. On the temporal control of behavior . Psychol Rev 1975. ; 82 : 89 – 115 . [Google Scholar]
- Killeen P, Hanson S, Osbourne S. Arousal: its genesis and manifestation as response rate . Psychol Rev 1978. ; 85 : 571 – 81 . [PubMed] [Google Scholar]
- Koob GF, Swerdlow NR. The functional output of the mesolimbic dopamine system . [Review] Ann N Y Acad Sci 1988. ; 537 : 216 – 27 . [DOI] [PubMed] [Google Scholar]
- Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat . J Comp Physiol Psychol 1978. ; 92 : 917 – 27 . [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens (DA) D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward . Psychopharmacology 2000. ; 152 : 67 – 73 . [DOI] [PubMed] [Google Scholar]
- Krebs JR. Optimal foraging: theory and experiment . Nature 1977. ; 268 : 583 – 4 . [Google Scholar]
- Kroemer NB, Guevara A, Ciocanea Teodorescu I, Wuttig F, Kobiella A, Smolka MN. Balancing reward and work: anticipatory brain activation in NAcc and VTA predict effort differentially . Neuroimage 2014. ; 102 (Pt 2): 510 – 9 . [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Seymour B, Talmi D, Yoshida W, Chater N, Dolan RJ. Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action . J Neurophysiol 2010. ; 104 : 313 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan IT, Guitart-Masip M, Dayan P, Dolan RJ. Effort and valuation in the brain: the effects of anticipation and execution . J Neurosci 2013. ; 33 : 6160 – 9 . 3; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JY, Freeman MK, Cates ME. Modafinil augmentation for residual symptoms of fatigue in patients with a partial response to antidepressants . Ann Pharmacothere 2007. ; 41 : 1005 – 12 . [DOI] [PubMed] [Google Scholar]
- Lapierre Y, Hum S. Treating fatigue. [Review] . Int MS J 2007. ; 14 : 64 – 71 . [PubMed] [Google Scholar]
- Learned-Coughlin SM, Bergström M, Savitcheva I, Ascher J, Schmith VD, Långstrom B. In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography . Biol Psychiat 2003. ; 54 : 800 – 5 . [DOI] [PubMed] [Google Scholar]
- Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer . Learn Mem 2008. ; 15 : 483 – 91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex B, Hauber W. The role of nucleus accumbens dopamine in outcome encoding in instrumental and Pavlovian conditioning . Neurobiol Learn Mem 2010. ; 93 : 283 – 90 . [DOI] [PubMed] [Google Scholar]
- López-Crespo G, Rodriguez M, Pellon R, Flores P. Acquisition of schedule-induced polydipsia by rats in proximity to upcoming food delivery . Learn Behav 2004. ; 32 : 491 – 9 . [DOI] [PubMed] [Google Scholar]
- Lotrich FE. Major depression during interferon-alpha treatment: vulnerability and prevention . Dialogues Clin Neurosci 2009. ; 11 : 417 – 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Kalman D. Effects of bupropion on simulated demand for cigarettes and the subjective effects of smoking . Nicotine Tobacco Res 2010. ; 12 : 416 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK, Jacobs EA. Three predictions of the economic concept of unit price in a choice context . J Exp Anal Behav 2000. ; 73 : 45 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Smethells JR, Ewan EE, Hursh SR. Tests of behavioral-economic assessments of relative reinforcer efficacy II: economic complements . J Exp Anal Behav 2007. ; 88 : 355 – 67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai B, Sommer S, Hauber W. Motivational states influence effort-based decision-making in rats: the role of dopamine in the nucleus accumbens . Cogn Affect Behav Neurosci 2012. ; 12 : 74 – 84 . [DOI] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C . Brain Behav Immun 2008. ; 22 : 870 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, McCutcheon JE. Heterogeneity of dopamine neuron activity across traits and states . Neuroscience 2014. ; 282C : 176 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Salamone JD, Bussey TJ, Mar AC, Brunner D, Gilmour G , et al. . Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. [Review] . Neurosci Biobehav Rev 2013. ; 37 : 2149 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens . Neuron 2013. ; 78 : 910 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Salamone JD. Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: a microdialysis and behavioral study . Brain Res 1992. ; 592 : 29 – 36 . [DOI] [PubMed] [Google Scholar]
- McCullough LD, Cousins MS, Salamone JD. The role of nucleus accumbens dopamine in responding on a continuous reinforcement operant schedule: a neurochemical and Behavioral study . Pharmacol Biochem Behav 1993a. ; 46 : 581 – 6 . [DOI] [PubMed] [Google Scholar]
- McCullough LD, Sokolowsi JD, Salamone JD. A neurochemical and behavioral investigation of the involvement of nucleus accumbens dopamine in instrumental avoidance . Neuroscience 1993b. ; 52 : 919 – 25 . [DOI] [PubMed] [Google Scholar]
- Miller AH , Norman Cousins Lecture . Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface . Brain Behav Immun 2009. ; 23 : 149 – 58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD. Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions . Europ J Neurosci 2005. ; 21 : 1749 – 57 . [DOI] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM , et al. . Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway . J Neurosci 2008. ; 28 : 9037 – 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschak TM, Mitchell SH. Partial inactivation of nucleus accumbens core decreases delay discounting in rats without affecting sensitivity to delay or magnitude . Behav Brain Res 2014. ; 268 : 159 – 68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J , et al. . The adenosine A2A antagonist MSX-3 reverses the effects of the (DA) antagonist haloperidol on effort-related decision-making in a T-maze cost/benefit procedure . Psychopharmacology 2009. ; 204 : 103 – 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior . J Neurosci 2010. ; 30 : 16585 – 600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor . Psychopharmacology 2007. ; 191 : 507 – 20 . [DOI] [PubMed] [Google Scholar]
- Nomoto M, Nagai M, Nishikawa N. Clinical nonmotor aspect of A2A antagonist in PD treatment . Int Rev Neurobiol 2014. ; 119 : 191 – 4 . [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption . Pharmacol Biochem Behav 2001. ; 69 : 373 – 82 . [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Estrada A, Epling B, Hart EE, Lee CA , et al. . Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/chow feeding choice task . Psychopharmacology (Berl) 2014. ; 23 : 727 – 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Santerre JL, Given AB, Sager TN, Correa M , et al. . Differential effects of selective adenosine antagonists on the effort-related impairments induced by (DA) D1 and D2 antagonism . Neuroscience 2010. ; 170 : 268 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors . Neurosci Biobehav Rev 2013a. ; 37 : 2015 – 25 . [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Hart EE, Freeland C, Yohn SE, Baqi Y , et al. . Effort-related motivational effects of the VMAT-2 inhibitor tetrabenazine: implications for animal models of the motivational symptoms of depression . J Neurosci 2013b. ; 33 : 19120 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL , et al. . The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. [Review] . J Psychopharmacol 2007. ; 21 : 461 – 71 . [DOI] [PubMed] [Google Scholar]
- Pae CU, Lim HK, Han C, Patkar AA, Steffens DC, Masand PS , et al. . Fatigue as a core symptom in major depressive disorder: overview and the role of bupropion. [Review] . Expert Rev Neurother 2007. ; 7 : 1251 – 63 . [DOI] [PubMed] [Google Scholar]
- Padala PR, Padala KP, Monga V, Ramirez DA, Sullivan DH. Reversal of SSRI associated apathy syndrome by discontinuation of therapy . Ann Pharmacother 2012. ; 46 : e8 . [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors . Biol Psychiatry 2006. ; 60 : 1350 – 5 . [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Stahl SM, Krishen A, Seifert CA, Tucker VL, Goodale EP , et al. . Efficacy of bupropion and the selective serotonin reuptake inhibitors in the treatment of major depressive disorder with high levels of anxiety (anxious depression): a pooled analysis of 10 studies . J Clin Psychiatry 2008. ; 69 : 1287 – 92 . [DOI] [PubMed] [Google Scholar]
- Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Muller CE , et al. . Adenosine A2A receptor antagonism and genetic deletion attenuate the effects of dopamine D2 antagonism on effort-related decision-making in mice . Neuropharmacology 2012. ; 62 : 2068 – 77 . [DOI] [PubMed] [Google Scholar]
- Pardo M, López-Cruz L, Miguel NS, Salamone JD, Correa M. Selection of sucrose concentration depends on the effort required to obtain it: studies using tetrabenazine, D1, D2, and D3 receptor antagonists . Psychopharmacology 2015. ; 232 : 2377 – 91 . [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G , et al. . Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function . Behav Brain Res 2002. ; 137 : 149 – 63 . [DOI] [PubMed] [Google Scholar]
- Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards . J Neurosci 2003. ; 23 : 9395 – 402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats . Behav Brain Res 2006. ; 175 : 139 – 48 . [DOI] [PubMed] [Google Scholar]
- Pereira M, Ferreira A. Neuroanatomical and neurochemical basis of parenting: dynamic coordination of motivational, affective and cognitive processes . Horm Behav 2015. ; 77 : 72 – 85 . [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Totaro JA, Pflueger AB. Tetrabenazine-induced depletion of brain monoamines: characterization and interaction with selected antidepressants . Eur J Pharmacol 1984. ; 102 : 425 – 30 . [DOI] [PubMed] [Google Scholar]
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine . Psychopharmacology 2007. ; 191 : 483 – 95 . [DOI] [PubMed] [Google Scholar]
- Piser TM. Linking the cytokine and neurocircuitry hypotheses of depression: a translational framework for discovery and development of novel anti-depressants . Brain Behav Immun 2010. ; 24 : 515 – 24 . [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model . Annu Rev Clin Psychol 2014. ; 10 : 393 – 423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Kent TA, Glotzbach RK, Irwin GH, Solnick JV. Cerebromicrocirculatory defects in animal model of depression . Psychopharmacology (Berl) 1984. ; 84 : 196 – 9 . [DOI] [PubMed] [Google Scholar]
- Porat O, Hassin-Baer S, Cohen OS, Markus A, Tomer R. Asymmetric dopamine loss differentially affects effort to maximize gain or minimize loss . Cortex 2014. ; 51 : 82 – 91 . [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. [Review] . Trends Immunol 2006. ; 27 : 24 – 31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampello L, Nicoletti G, Raffaele R. Dopaminergic hypothesis for retarded depression: a symptom profile for predicting therapeutical responses . Acta Psychiatr Scand 1991. ; 84 : 552 – 4 . [DOI] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, López Cruz L, Vemuri VK, Makriyannis A , et al. . Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow task: pharmacological studies and role of individual differences . PLoS One 2012. ; 7 : e47934 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Nunes EJ, Yohn SE, Nowak V, Khan B , et al. . The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: reversal with antidepressant drugs . PLoS One 2014. ; 9 : e99320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Podurgiel SJ, Hart E, Yohn SE, Jones M , et al. . Bupropion increases selection of high effort activity in rats tested on progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms . Int J Neuropsychopharmacol 2015. ; 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Barch DM, Buchanan RW, Dunayevich E, Gold JM , et al. . Effort-based decision-making paradigms for clinical trials in schizophrenia: part 1-psychometric characteristics of 5 paradigms . Schizophr Bull 2015a. ; 41 : 1045 – 54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Green MF. Motivational deficits and negative symptoms in schizophrenia: concepts and assessments . Curr Top Behav Neurosci 2015b[Epub ahead of print] . [DOI] [PubMed] [Google Scholar]
- Ribot T. , 1896. . La psychologie des sentiment (The Psychology of Feelings) . Felix Alcan; , Paris: . [Google Scholar]
- Rick JH, Horvitz JC, Balsam PD. Dopamine receptor blockade and extinction differentially affect behavioral variability . Behav Neurosci 2006. ; 120 : 488 – 92 . [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Geraci J, Ravindran A, Kennedy SH. Predictors of response to adjunctive osmotic-release methylphenidate or placebo in patients with major depressive disorder: effects of apathy/anhedonia and fatigue . J Clin Psychopharmacol 2014. ; 34 : 755 – 9 . [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006). [Review] . Psychopharmacology 2007. ; 191 : 433 – 7 . [DOI] [PubMed] [Google Scholar]
- Robbins TW, Koob GF. Selective disruption of displacement behaviour by lesions of the mesolimbic dopamine system . Nature 1980. ; 285 : 409 – 12 . [DOI] [PubMed] [Google Scholar]
- Robbins TW, Roberts DC, Koob GF. Effects of d-amphetamine and apomorphine upon operant behavior and schedule-induced licking in rats with 6-hydroxydopamine-induced lesions of the nucleus accumbens . J Pharmacol Exp Ther 1983. ; 224 : 662 – 73 . [PubMed] [Google Scholar]
- Rogers D, Lees AJ, Smith E, Trimble M, Stern GM. Bradyphrenia in Parkinson's disease and psychomotor retardation in depressive illness. An experimental study . Brain 1987. ; 110 : 761 – 76 . [DOI] [PubMed] [Google Scholar]
- Rogers DC, Costall B, Domeney AM, Gerrard PA, Greener M, Kelly ME , et al. . Anxiolytic profile of ropinirole in the rat, mouse and common marmoset . Psychopharmacology (Berl) 2000. ; 151 : 91 – 7 . [DOI] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system . J Comp Neurol 1998. ; 401 : 163 – 86 . [PubMed] [Google Scholar]
- Rothschild AJ, Raskin J, Wang CN, Marangell LB, Fava M. The relationship between change in apathy and changes in cognition and functional outcomes in currently non-depressed SSRI-treated patients with major depressive disorder . Compr Psychiatry 2014. ; 55 : 1 – 10 . [DOI] [PubMed] [Google Scholar]
- Salamone JD. Different effects of haloperidol and extinction on instrumental behaviours . Psychopharmacology (Berl) 1986. ; 88 : 18 – 23 . [DOI] [PubMed] [Google Scholar]
- Salamone JD. The actions of neuroleptic drugs on appetitive instrumental behaviors . In: Iversen LL, Iversen SD, Snyder SH , editors. Handbook of psychopharmacology . New York: : Plenum Press; ; 1987. . p. 575 – 608 . [Google Scholar]
- Salamone JD. Dopaminergic involvement in activational aspects of motivation: effects of haloperidol on schedule induced activity, feeding and foraging in rats . Psychobiology 1988. ; 16 : 196 – 206 . [Google Scholar]
- Salamone JD. Behavioral pharmacology of dopamine systems: a new synthesis . In: Willner P, Scheel-Kruger J , editors. The mesolimbic dopamine system: from motivation to action . Cambridge, England: : Cambridge University Press; ; 1991. . p. 599 – 613 . [Google Scholar]
- Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes . Psychopharmacology (Berl) 1992. ; 107 : 160 – 74 . [DOI] [PubMed] [Google Scholar]
- Salamone JD. Involvement of nucleus accumbens dopamine in appetitive and aversive motivation . Behav Brain Res 1994. ; 61 : 117 – 33 . [DOI] [PubMed] [Google Scholar]
- Salamone JD. Preladenant, a novel adenosine A(2A) receptor antagonist for the potential treatment of parkinsonism and other disorders . IDrugs 2010. ; 13 : 723 – 31 . [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. [Review] . Behav Brain Res 2002. ; 137 : 3 – 25 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Dopamine/adenosine interactions involved in effort-related aspects of food motivation. [Review] . Appetite 2009. ; 53 : 422 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine . Neuron 2012. ; 76 : 470 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food-choice procedure . Psychopharmacology 1991. ; 104 : 515 – 21 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens (DA) depletion on instrumental response selection in a T-maze cost/benefit procedure . Behav Brain Res 1994a. ; 65 : 221 – 9 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption . Pharmacol Biochem Behav 1994b. ; 49 : 25 – 31 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Kurth P, McCullough LD, Sokolowski JD. The effects of nucleus accumbens dopamine depletions on continuously reinforced operant responding: contrasts with the effects of extinction . Pharmacol Biochem Behav 1995. ; 50 : 437 – 43 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens DA: empirical and conceptual problems with the anhedonia hypothesis . Neurosci Biobehav Rev 1997. ; 21 : 341 – 59 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food: contrasts between the effects of SKF 83566, raclopride and fenfluramine on a concurrent lever pressing/chow consumption task . Psychopharmacology 2002. ; 160 : 371 – 80 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. [Review] . J Pharmacol Exp Ther 2003. ; 305 : 1 – 8 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Insulin and ventral tegmental dopamine: what's impaired and what's intact? . Cell Metab 2013. ; 17 : 469 – 70 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine . Curr Opin Pharmacol 2005. ; 5 : 34 – 41 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus Accumbens (DA) and the forebrain circuitry involved in behavioral activation and effort-related decision-making: implications for understanding anergia and psychomotor slowing in depression . Curr Psychiatry Rev 2006. ; 178 : 267 – 80 . [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens (DA) and associated forebrain circuits . Psychopharmacology 2007. ; 191 : 461 – 82 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yohn S, Lopez Cruz L., San Miguel N, Alatorre L. The pharmacology of effort-related choice behavior: Dopamine, depression, and individual differences . Behav Processes 2016. ; [Epub ahead of print] . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Farrar AM, Font L, Patel V, Schlar DE, Nunes EJ , et al. . Differential actions of adenosine A1 and A2A antagonists on the effort-related effects of (DA) D2 antagonism . Behav Brain Res 2009. ; 201 : 216 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Collins LE. The role of (DA)/adenosine interactions in the brain circuitry regulating effort-related decision-making: insights into pathological aspects of motivation . Future Neurology 2010. ; 5 : 377 – 92 . [Google Scholar]
- Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M. The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine and beyond . J Exp Anal Behav 2012. ; 97 : 125 – 46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Koychev I, Correa M, McGuire P. Neurobiological basis of motivational deficits in psychopathology . Eur Neuropsychopharmacol 2015a. ; 25 : 1225 – 38 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Pardo M, Yohn SE, López-Cruz L, San Miguel N, Correa M. Mesolimbic Dopamine and the Regulation of Motivated Behavior . In: Current Topics in Behavioral Neurosciences . Springer: : Heidelberg; ; 1987. . p. 1 – 27 . [DOI] [PubMed] [Google Scholar]
- Salamone JD, Wisniecki A, Carlson BB, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement . Neuroscience 2001. ; 105 : 863 – 70 . [DOI] [PubMed] [Google Scholar]
- Santerre JL, Nunes EJ, Kovner R, Leser CE, Randall PA, Collins-Praino LE , et al. . The novel adenosine A(2A) antagonist prodrug MSX-4 is effective in animal models related to motivational and motor functions . Pharmacol Biochem Behav 2012. ; 102 : 477 – 87 . [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nolte-Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence . Pharmacopsychiatry 2001. ; 34 : 66 – 72 . [DOI] [PubMed] [Google Scholar]
- Schmidt L, Lebreton M, Cléry-Melin ML, Daunizeau J, Pessiglione M. Neural mechanisms underlying motivation of mental versus physical effort . PLoS Biol 2012. ; 10 : e1001266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scinska A, Sienkiewicz-Jarosz H, Kuran W, Ryglewicz D, Rogowski A, Wrobel E , et al. . Depressive symptoms and taste reactivity in humans . Physiol Behav 2004. ; 82 : 899 – 904 . [DOI] [PubMed] [Google Scholar]
- Sederholm F, Johnson AE, Brodin U, Södersten P. Dopamine D(2) receptors and ingestive behavior: brainstem mediates inhibition of intraoral intake and accumbens mediates aversive taste behavior in male rats . Psychopharmacology 2002. ; 160 : 161 – 9 . [DOI] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB. Acute stress induces selective alterations in cost/benefit decision-making . Neuropsychopharmacology 2012. ; 37 : 2194 – 209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function . Neuron 2013. ; 79 : 217 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore DM, Rafal R, Parkinson JA. Appetitive motivational deficits in individuals with Parkinson's disease . Mov Disord 2011. ; 26 : 1887 – 92 . [DOI] [PubMed] [Google Scholar]
- Sienkiewicz-Jarosz H, Scinska A, Swiecicki L, Lipczynska-Lojkowska W, Kuran W, Ryglewicz D , et al. . Sweet liking in patients with Parkinson's disease . J Neurol Sci 2013. ; 329 : 17 – 22 . [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S , et al. . Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia . Biol Psychiatry 2011. ; 69 : 928 – 35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior . Psychopharmacology 2008. ; 196 : 565 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression . Med Hypotheses 1991. ; 35 : 298 – 306 . Erratum in: Med Hypotheses 1991; 36(2):178. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry . Proc Natl Acad Sci USA 2011. ; 108 : E255 – 64 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S, Danysz W, Russ H, Valastro B, Flik G, Hauber W. The dopamine reuptake inhibitor MRZ-9547 increases progressive ratio responding in rats . Int J Neuropsychopharmacol 2014. ; 17 : 2045 – 56 . [DOI] [PubMed] [Google Scholar]
- Staddon JER, Simmelhag VL. The “superstition” experiment: a reexamination of its implications for the principles of adaptive behavior . Psychol Rev 1971. ; 78 : 3 – 43 . [Google Scholar]
- Stahl SM. The psychopharmacology of energy and fatigue . J Clin Psychiat 2002. ; 63 : 7 – 8 . [DOI] [PubMed] [Google Scholar]
- Stenman E, Lilja A. Increased monoaminergic neurotransmission improves compliance with physical activity recommendations in depressed patients with fatigue . Med Hypotheses 2013. ; 80 : 47 – 9 . [DOI] [PubMed] [Google Scholar]
- Stotz G, Woggon B, Angst J. Psychostimulants in the therapy of treatment-resistant depression Review of the literature and findings from a retrospective study in 65 depressed patients . Dialogues Clin Neurosci 1999. ; 1 : 165 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. [Review] . Prog Neurobiol 1999. ; 59 : 355 – 96 . [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine . Psychopharmacology (Berl) 1990. ; 100 : 413 – 6 . [DOI] [PubMed] [Google Scholar]
- Tanra AJ, Kagaya A, Okamoto Y, Muraoka M, Motohashi N, Yamawaki S. TJS-010, a new prescription of oriental medicine, antagonizes tetrabenazine-induced suppression of spontaneous locomotor activity in rats . Prog Neuropsychopharmacol Biol Psychiatry 1995. ; 19 : 963 – 71 . [DOI] [PubMed] [Google Scholar]
- Targum SD, Fava M. Fatigue as a residual symptom of depression . Innov Clin Neurosci 2011. ; 8 : 40 – 3 . [PMC free article] [PubMed] [Google Scholar]
- Tellez N, Alonso J, Rio M, Tintore M, Nos C, Montalban X , et al. . The basal ganglia: a substrate for fatigue in multiple sclerosis . Neuroradiology 2008. ; 50 : 17 – 23 . [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study . Brain Res 1996. ; 721 : 140 – 9 . [DOI] [PubMed] [Google Scholar]
- Todder D, Caliskan S, Baune BT. Longitudinal changes of day-time and night-time gross motor activity in clinical responders and non-responders of major depression . World J Biol Psychiat 2009. ; 10 : 276 – 84 . [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Anisman H, Tombaugh J. Extinction and dopamine receptor blockade after intermittent reinforcement training: failure to observe functional equivalence . Psychopharmacology 1980. ; 70 : 19 – 28 . [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience . Neurosci Biobehav Rev 2011. ; 35 : 537 – 55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the 'EEfRT'? The effort expenditure for rewards task as an objective measure of motivation and anhedonia . PLoS One 2009. ; 4 : e6598 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia . J Abnorm Psychol 2012a. ; 121 : 553 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS , et al. . Dopaminergic mechanisms of individual differences in human effort-based decision-making . J Neurosci 2012b. ; 32 : 6170 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia . Schizophr Res 2015. ; 161 : 382 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM , et al. . Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation . Mol Psychiatry 2013. ; 18 : 1025 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapakis EM, Dimopoulou T, Tarazi FI. Clinical management of negative symptoms of schizophrenia: an update. [Review] . Pharmacol Ther 2015. ; 153 : 135 – 47 . [DOI] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community . Int Clin Psychopharmacol 1999. ; 14 : 139 – 51 . [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Gussekloo J, de Craen AJ, Frölich M, Stek ML, van der Mast RC , et al. . Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age . Exp Gerontol 2007. ; 42 : 693 – 701 . [DOI] [PubMed] [Google Scholar]
- Varazzani C, San-Galli A, Gilardeau S, Bouret S. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys . J Neurosci 2015. ; 35 : 7866 – 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D , et al. . Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers . Am J Psychiatry 2001. ; 158 : 377 – 82 . [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Fields HL, Nicola SM. Dissociation of the role of nucleus accumbens dopamine in responding to reward-predictive cues and waiting for reward . Behav Brain Res 2004. ; 154 : 19 – 30 . [DOI] [PubMed] [Google Scholar]
- Wallace M, Singer G, Finlay J, Gibson S. The effect of 6-OHDA lesions of the nucleus accumbens septum on schedule-induced drinking, wheel running and corticosterone levels in the rat . Pharmacol Biochem Behav 1983. ; 18 : 129 – 36 . [DOI] [PubMed] [Google Scholar]
- Wang H, Chen X, Li Y, Tang TS, Bezprozvanny I. Tetrabenazine is neuroprotective in Huntington's disease mice . Mol Neurodegener 2010. ; 5 : 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI , et al. . Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia . Neuropsychopharmacology 2012. ; 37 : 1699 – 707 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, Bradshaw JL, White OB. Negative symptoms: a review of schizophrenia, melancholic depression and Parkinson's disease. [Review] . Brain Res Bull 2006. ; 70 : 312 – 21 . [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders . Neuropsychopharmacology 2005. ; 30 : 669 – 82 . [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MFS. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions . J Neurosci 2003. ; 23 : 6475 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making . J Neurosci 2011. ; 31 : 16597 – 602 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, Braver TS. Cognitive effort: a neuroeconomic approach . Cogn Affect Behav Neurosci 2015. ; 15 : 395 – 415 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA. Reinforcement, choice, and response strength . In Atkinson RC, Herrnstein RJ, Lindsey G, Luce RD , editors. Stevens’ handbook of experimental psychology , Vol. 2 . New York: : John Wiley and Sons; ; 1988. ; p. 167 – 74 . [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia . Curr Opin Psychiatry 2015. ; 28 : 7 – 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA , et al. . Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures . Schizophr Bull 2014. ; 40 : 1328 – 37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden LT, Shahriari M, Farrar AM, Sink KS, Hockemeyer J, Muller CE , et al. . The adenosine A2A antagonist MSX-3 reverses the effort-related effects of (DA) blockade: differential interaction with D1 and D2 family antagonists . Psychopharmacology 2009. ; 203 : 489 – 99 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement . J Neurosci 2000. ; 20 : 8122 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Kobayashi M, Kanda T. Involvement of adenosine A2A receptors in depression and anxiety . Int Rev Neurobiol 2014. ; 119 : 373 – 93 . [DOI] [PubMed] [Google Scholar]
- Yamada K, Kobayashi M, Mori A, Jenner P, Kanda T. Antidepressant-like activity of the adenosine A(2A) receptor antagonist, istradefylline (KW-6002), in the forced swim test and the tail suspension test in rodents . Pharmacol Biochem Behav 2013. ; 114-115 : 23 – 30 . [DOI] [PubMed] [Google Scholar]
- Yang XH, Huang J, Zhu CY, Wang YF, Cheung EF, Chan RC , et al. . Motivational deficits in effort-based decision-making in individuals with subsyndromal depression, first-episode and remitted depression patients . Psychiatry Res 2014. ; 220 : 874 – 82 . [DOI] [PubMed] [Google Scholar]
- Yang XH, Huang J, Lan Y, Zhu CY, Liu XQ, Wang YF , et al. . Diminished caudate and superior temporal gyrus responses to effort-based decision-making in patients with first-episode major depressive disorder . Prog Neuropsychopharmacol Biol Psychiatry 2015. ; 64 : 52 – 9 . [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks . Eur J Neurosci 2008. ; 28 : 1437 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn SE, Thompson C, Randall PA, Lee CA, Müller CE, Baqi Y , et al. . The VMAT-2 inhibitor tetrabenazine alters effort-related decision-making as measured by the T-maze barrier choice task: reversal with the adenosine A2A antagonist MSX-3 and the catecholamine uptake blocker bupropion . Psychopharmacology 2015a. ; 232 : 1313 – 23 . [DOI] [PubMed] [Google Scholar]
- Yohn SE, Santerre JL, Nunes EJ, Kozak R, Podurgiel SJ, Correa M , et al. . The role of dopamine D1 receptor transmission in effort-related choice behavior: effects of D1 agonists . Pharmacol Biochem Behav 2015b. ; 135 : 217 – 26 . [DOI] [PubMed] [Google Scholar]
- Yohn SE, Lopez-Cruz L, Hutson PH, Correa M, Salamone JD. Effects of lisdexamfetamine and s-citalopram, alone and in combination, on effort-related choice behavior in the rat . Psychopharmacology 2016a. ; 233 : 949 – 60 . [DOI] [PubMed] [Google Scholar]
- Yohn SE, Collins SL, Contreras-Mora HM, Errante EL, Rowland MA, Correa M , et al. . Not all antidepressants are created equal: differential effects of monoamine uptake inhibitors on effort-related choice behavior . Neuropsychopharmacology 2016b. ; 41 : 686 – 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]