The nervous system’s capacity to repair itself declines with age. Rawji et al. discuss the effects of ageing on neurological diseases and propose that some of these effects may reflect differential senescence of macrophages and microglia. Therapeutic strategies to reverse macrophage/microglia senescence may promote regeneration in the ageing nervous system.
Keywords: microglia, macrophage, white matter injury, ageing, rejuvenation
The nervous system’s capacity to repair itself declines with age. Rawji et al. discuss the effects of ageing on neurological diseases and propose that some of these effects may reflect differential senescence of macrophages and microglia. Therapeutic strategies to reverse macrophage/microglia senescence may promote regeneration in the ageing nervous system.
Abstract
Ageing of the central nervous system results in a loss of both grey and white matter, leading to cognitive decline. Additional injury to both the grey and white matter is documented in many neurological disorders with ageing, including Alzheimer’s disease, traumatic brain and spinal cord injury, stroke, and multiple sclerosis. Accompanying neuronal and glial damage is an inflammatory response consisting of activated macrophages and microglia, innate immune cells demonstrated to be both beneficial and detrimental in neurological repair. This article will propose the following: (i) infiltrating macrophages age differently from central nervous system-intrinsic microglia; (ii) several mechanisms underlie the differential ageing process of these two distinct cell types; and (iii) therapeutic strategies that selectively target these diverse mechanisms may rejuvenate macrophages and microglia for repair in the ageing central nervous system. Most responses of macrophages are diminished with senescence, but activated microglia increase their expression of pro-inflammatory cytokines while diminishing chemotactic and phagocytic activities. The senescence of macrophages and microglia has a negative impact on several neurological diseases, and the mechanisms underlying their age-dependent phenotypic changes vary from extrinsic microenvironmental changes to intrinsic changes in genomic integrity. We discuss the negative effects of age on neurological diseases, examine the response of senescent macrophages and microglia in these conditions, and propose a theoretical framework of therapeutic strategies that target the different mechanisms contributing to the ageing phenotype in these two distinct cell types. Rejuvenation of ageing macrophage/microglia may preserve neurological integrity and promote regeneration in the ageing central nervous system.
Introduction
Grey and white matter injury is common in many neurological diseases and numerous studies have demonstrated a decreased capacity for neurological repair with ageing ( Sacco, 1997 ; Dai, 2001 ; Blasko et al. , 2004 ; Marquez de la Plata et al. , 2008 ; Goldschmidt et al. , 2009 ). Major components of the inflammatory response accompanying neurological disorders are microglia and macrophages, innate immune cells important for CNS regeneration. These cells undergo senescence in distinct ways, negatively impacting the degenerative and repair response in the ageing CNS ( Shaw et al. , 2013 ). Studies using transcriptome profiling and genetic strategies have demonstrated important differences in gene expression and function between CNS-resident microglia and peripheral macrophage populations during health as well as injury ( Butovsky et al. , 2014 ; Gosselin et al. , 2014 ; Greenhalgh and David, 2014 ; Lavin et al. , 2014 ; Yamasaki et al. , 2014 ; Shemer et al. , 2015 ). Understanding how the ageing process affects these different cell types should reveal important insights into potential mechanistic targets that can be harnessed for therapeutic attenuation of neurodegenerative processes as well as enhancement of reparative activities in the ageing CNS. Furthermore, as many potential pharmacological agents may not be able to penetrate the CNS, and monocytes in different inflammatory states may have divergent routes of entry into the CNS, understanding how ageing affects peripherally-derived monocytes differently from CNS-resident microglia should help direct systemic therapeutics for rejuvenation of ageing monocytes to repair the ageing CNS ( Shechter et al. , 2013 ). This article will discuss the evidence that CNS-intrinsic microglia age differently from peripherally-derived macrophages. Mechanisms potentially explaining the divergent effects of ageing on these cell types will be presented. Finally, a theoretical framework is proposed on how best to rejuvenate microglia and macrophages for repair of the ageing CNS.
Microglia and macrophages
Genesis of cells
Microglia and macrophages are two distinct myeloid populations with separate developmental origins ( Ginhoux et al. , 2010 ; Schulz et al. , 2012 ; Kierdorf et al. , 2013 ). In mice, microglia derive from erythromyeloid progenitors in the foetal yolk sac prior to embryonic Day 8 and then migrate to the developing CNS by embryonic Day 9.5. Macrophages derive from extravasated monocytes that are produced from erythromyeloid progenitors initially in the aorta-gonad-mesonephros at embryonic Day 10.5 and then in the foetal liver at embryonic Day 12.5 ( Perdiguero et al. , 2015 ; Prinz and Priller, 2014 ). Postnatally, monocytes are produced from hematopoietic stem cells (HSCs) in the bone marrow, which then circulate in the blood and differentiate into macrophages following extravasation into tissues ( Prinz and Priller, 2014 ).
Activation of microglia and macrophages
Microglia are the resident immune cells of the CNS and are thought to be self-sustaining throughout adulthood ( Ajami et al. , 2007 ; Bruttger et al. , 2015 ). In the healthy, uninjured CNS, microglia have a ramified morphology and are maintained in a quiescent state through transforming growth factor-β and inhibitory ligand-receptor interactions with neurons, astrocytes, and oligodendrocytes ( Perry and Holmes, 2014 ). Despite this relatively calm state, microglia are constantly extending and retracting their processes, surveying the surrounding parenchyma for any abnormalities ( Nimmerjahn et al. , 2005 ). Microglia and infiltrating monocytes express pattern recognition receptors which detect molecules that are released by injured CNS cells or that are inherent on the surfaces of invading pathogens. Detection of these molecules induces pro-inflammatory signalling cascades, stimulating microglia and infiltrating monocytes to adopt an activated phenotype that displays an amoeboid morphology with an enlarged cytoplasm. The activated microglia and differentiated monocyte-derived macrophages upregulate many pro-inflammatory markers, secrete a variety of pro-inflammatory cytokines, and express many molecules important for antigen presentation ( Prinz and Priller, 2014 ).
Upon injury to the CNS, activated microglia and infiltrating monocyte-derived macrophages appear phenotypically similar, possessing many of the same cell surface receptors and appearing amoeboid in morphology. Due to this phenotypic similarity after injury, many researchers have grouped these distinct cell types as macrophages/microglia ( Rawji and Yong, 2013 ). However, these two cell types have different genetic and transcriptomic signatures, suggesting different functions of these cells within the CNS injury site ( Butovsky et al. , 2014 ; Gosselin et al. , 2014 ; Lavin et al. , 2014 ). Furthermore, attempts using bone marrow and parabiosis chimeras, as well as novel transgenic mice, have uncovered different roles that these two cell types perform following CNS injury ( Ajami et al. , 2011 ; Greenhalgh and David, 2014 ; Yamasaki et al. , 2014 ). Despite these significant advances, this review will refer to these two cell types as macrophages/microglia as methodological limitations of previous studies have not permitted their separate identification.
Microglia and macrophage subsets
The activation status of macrophages/microglia have been classified under two broad phenotypic states, termed M1 and M2, where M1 cells are generally associated with the secretion of pro-inflammatory cytokines, and M2 cells with the production of regulatory or anti-inflammatory cytokines ( Martinez et al. , 2008 ). It is now appreciated, however, that the M1/M2 classification is insufficient to address the spectrum of macrophage programmes that arise upon activation by different stimuli ( Martinez and Gordon, 2014 ); remarkably, at least nine distinct subsets of activated macrophages have been proposed based on transcriptomic signatures ( Xue et al. , 2014 ). Thus, while some of the reports below address senescent macrophages/microglia in terms of so-called M1 or M2 polarization, we will refer to these cells as pro-inflammatory and regulatory, respectively.
Epigenetic regulation of global gene expression, in addition to a complex array of transcription factors, signalling pathways, and post-transcriptional regulators, are thought to underlie the diversity of macrophage plasticity not only after infection or tissue injury, but also within different tissue environments ( Lawrence and Natoli, 2011 ; Butovsky et al. , 2014 ; Gosselin et al. , 2014 ; Lavin et al. , 2014 ). It was recently shown that in response to different tissue environments, the macrophage lineage-determining factor PU.1 interacts with diverse secondary transcription factors to induce a tissue-specific enhancer landscape ( Gosselin et al. , 2014 ). In addition, microglial function is regulated by the host microbiota ( Erny et al. , 2015 ), introducing further complexity to the diversity of macrophages/microglia.
Phenotype accompanying macrophage and microglia senescence
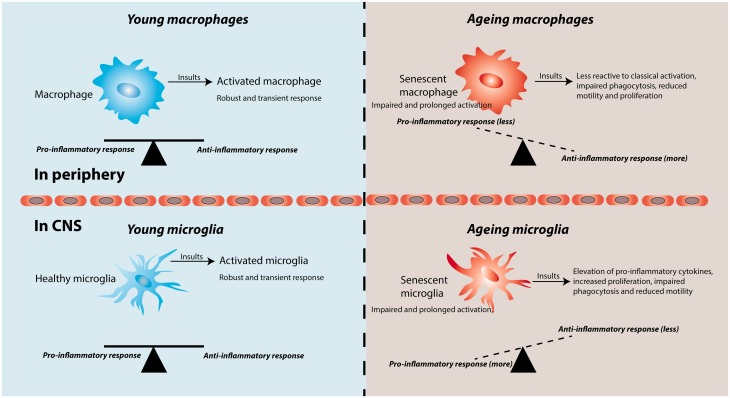
While both macrophages and microglia display diminished phagocytosis and chemotaxis with ageing, the effects of age manifest differently with regards to their pro-inflammatory status. With ageing, macrophages are less able to produce a functional pro-inflammatory response ( Shaw et al. , 2013 ). Microglia, on the other hand, exhibit an exaggerated pro-inflammatory response, a phenomenon referred to as microglia priming ( Perry and Holmes, 2014 ; Michell-Robinson et al. , 2015 ). This section will discuss the phenotypic changes that occur in macrophages and microglia with ageing ( Fig. 1 ).
Figure 1.
Phenotypic changes associated with macrophages and microglia during normal ageing. During ageing, senescent macrophages have decreased pro-inflammatory cytokine secretion, impaired phagocytosis and chemotaxis, and decreased proliferation. In contrast, senescent microglia display an elevation in pro-inflammatory cytokines and proliferative capacity, while showing a deficit in phagocytosis and motility.
Both ageing microglia and macrophages exhibit deficits in phagocytic and chemotactic functions. In studies examining the capability for microglia to engulf amyloid-β fibrils, microglia isolated from ageing but not young mice lost the ability for phagocytosis ( Floden and Combs, 2011 ; Njie et al. , 2012 ). In a model of focal white matter demyelination, the age-associated delay in remyelination is associated with impaired clearance of inhibitory myelin debris, accompanied by alteration of the retinoid X receptor pathway in macrophages ( Kotter et al. , 2006 ; Ruckh et al. , 2012 ; Natrajan et al. , 2015 ). When young macrophages are introduced to the ageing demyelinated CNS, clearance of myelin debris and remyelination is enhanced. In addition to reduced phagocytic activity, both ageing microglia and macrophages have deficits in chemotaxis. Ageing microglia display decreased process motility and cellular migration in response to laser-induced injury and extracellular ATP, when compared to young microglia ( Damani et al. , 2011 ). In a demyelinating model, the age-related decrease in remyelination is associated with a reduction in macrophage/microglia recruitment ( Zhao et al. , 2006 ).
Though ageing microglia and macrophages both have deficits in phagocytosis and chemotaxis, ageing macrophages seem to be less able to produce a functional pro-inflammatory response, whereas ageing microglia exhibit an exaggerated pro-inflammatory response. When stimulated with strong activating agents such as the TLR4 agonist, lipopolysaccharide, or the TLR2 agonist, zymosan, ageing macrophages significantly decreased their production of the pro-inflammatory cytokines TNF-α and IL-6 when compared to young macrophages ( Boehmer et al. , 2004 , 2005 ). This group, in addition to other groups, associated these observations with decreased TLR4 expression and reduced activation of NF-κB ( Boehmer et al. , 2005 ; Chelvarajan et al. , 2005 ). In human monocytes isolated from elderly patients, diminished pro-inflammatory cytokine production was observed when cells were stimulated with Toll-like receptor (TLR) ligands, correlating with decreased TLR1 surface expression ( Gon et al. , 1996 ; van Duin et al. , 2007 ). In contrast, several studies found an increased propensity for ageing microglia to initiate a stronger response to an inflammatory stimulus. Upon peripheral injection of lipopolysaccharide, ageing mice had a pronounced increase in pro-inflammatory cytokines and microglial activity in the CNS compared to young mice ( Godbout et al. , 2005 ; Sierra et al. , 2007 ). In addition, ex vivo cultures of microglia from ageing mice increased their basal and stimulated secretion of TNF-α and IL-6 relative to microglia from young mice ( Njie et al. , 2012 ). Morphologically, ageing microglia have enlarged processes, cytoplasmic hypertrophy, and a less ramified appearance ( Sheng et al. , 1998 ; Damani et al. , 2011 ; Tremblay et al. , 2012 ). Moreover, there is elevated immunoreactivity for markers of activation, including IL-1 and major histocompatibility complex II ( Sheng et al. , 1998 ; Conde and Streit, 2006 ).
Though difficult to distinguish between peripherally-derived macrophages and CNS-resident microglia, in vivo studies show that the capacity for ageing macrophages and microglia to adopt a regulatory phenotype is compromised with ageing. In a model of spinal cord injury, ageing macrophages and microglia are impaired in the induction of IL-4 receptor α, an important receptor in stimulating a regulatory phenotype ( Fenn et al. , 2014 ). This observation was associated with impaired recovery. In a model of cortical traumatic brain injury, ageing mice display an exaggerated pro-inflammatory macrophage/microglia response along with increased lesion size ( Kumar et al. , 2013 ). Another study found that the impaired remyelination in ageing mice was associated with a decrease in regulatory macrophages and microglia ( Miron et al. , 2013 ). Rejuvenation of remyelination in ageing mice correlated with an increase in regulatory macrophages and microglia.
Mechanisms underlying microglial and macrophage senescence
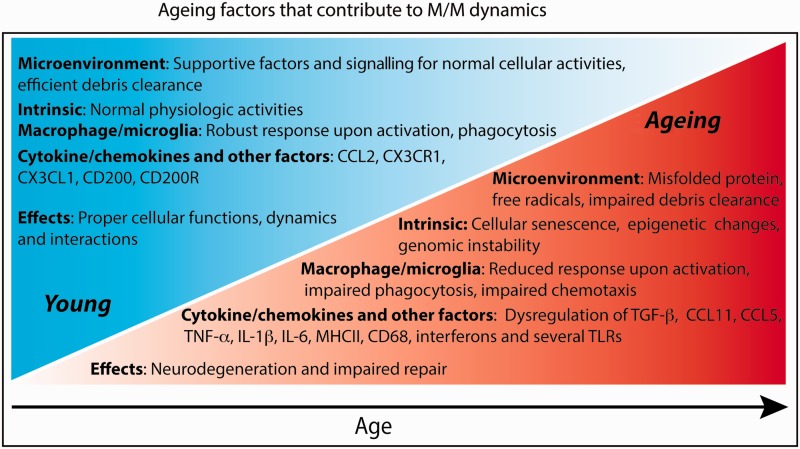
As discussed, ageing microglia show a propensity to increase pro-inflammatory cytokines, but are deficient in phagocytosis and chemotaxis. There are several possible mechanisms that may underlie this ageing phenotype ( Fig. 2 ). First, as neurons become damaged with age, there is a loss of inhibitory ligand-receptor interactions with microglia ( Perry and Holmes, 2014 ). Second, misfolded proteins such as amyloid-β accumulate during normal ageing; amyloid-β has been found to elevate pro-inflammatory cytokines in microglia ( Flanary et al. , 2007 ; Perry and Holmes, 2014 ). Third, the increase of transforming growth factor-β expression with age, and chronic exposure of microglia to this cytokine, may impair the capacity of microglia to secrete anti-inflammatory cytokines ( Doyle et al. , 2010 ; Cohen et al. , 2014 ). This transforming growth factor-β-mediated impairment is attributed to a downregulation of interferon regulatory factor-7, a transcription factor important in switching microglia from a pro-inflammatory to an anti-inflammatory phenotype ( Cohen et al. , 2014 ). In addition, it was found that the anti-inflammatory cytokine IL-4 was elevated at the choroid plexus with ageing ( Baruch et al. , 2013 ); IL-4 is thought to induce the choroid plexus epithelial cells to produce high levels of CCL11, which may skew microglia to a more pro-inflammatory state ( Villeda et al. , 2011 ; Baruch and Schwartz, 2013 ; Schwartz et al. , 2013 ). The same group further demonstrated that the choroid plexus of ageing mice have an increased type I interferon response, and that neutralization of this response not only restored CCL11 levels, but also decreased age-related chronic neuroinflammation ( Baruch et al. , 2014 ). In addition to these changes in the ageing CNS microenvironment, differences in the localization of injury may also influence the ageing microglia response. Microglia responding to injuries in the white matter may differ from injuries predominantly present in the grey matter. In studies in which controlled cortical impact injuries were inflicted to the hippocampus and thalamus, ageing rodents have an increased macrophage/microglia response compared to young rodents ( Sandhir et al. , 2008 ; Kumar et al. , 2013 ). In contrast, in a study in which focal demyelination was induced in a white matter tract, ageing rodents have a decreased macrophage/microglia response ( Zhao et al. , 2006 ).
Figure 2.
Factors associated with ageing that negatively impact CNS regeneration. During normal ageing, changes in HSCs, macrophages, microglia, systemic peripheral and central factors, as well as shifts in the microenvironment composition may all contribute to the increased neurodegeneration and decreased repair observed in CNS injury in ageing subjects. CX3CR1 = chemokine (C-X3-C motif) receptor 1; CX3CL1 = chemokine (C-X3-C motif) ligand 1; CD200 = cluster of differentiation 200; CD200R = cluster of differentiation 200 receptor; TGF-β = transforming growth factor β; TNF-α = tumour necrosis factor α; MHCII = major histocompatibility complex II; CD68 = cluster of differentiation 68.
In leukocortical multiple sclerosis lesions, there is an enhanced density of macrophages/microglia in the white matter compared to the adjacent grey matter ( Peterson et al. , 2001 ). Reasons for these differences are unknown, but may be a function of the microenvironment, such as the presence of the extracellular matrix molecules versican and hyaluronan, that are predominantly present in white matter lesions ( Chang et al. , 2012 ) and which may affect the functions of myeloid antigen-presenting cells. This is an interesting area requiring further study.
In contrast to microglia, ageing monocyte-derived macrophages appear deficient in elevating pro-inflammatory cytokines, and are impaired in phagocytic and chemotactic responses. As circulating monocytes have a half-life of ∼71 h in humans, it is conceivable that the mechanisms underlying the senescent phenotype arise at early stages in monocyte development ( Whitelaw, 1972 ). Indeed, HSCs undergo senescence and have decreased regenerative potential ( Florian et al. , 2012 ; Geiger et al. , 2013 ). Studies suggest that senescence of HSCs, and thus by extension, monocytes, is influenced mainly by intrinsic factors and partially by extrinsic factors ( Geiger et al. , 2013 ). Examples of intrinsic features include telomere shortening, epigenetic changes, and accumulation of DNA damage ( Rube et al. , 2011 ; Beerman et al. , 2013 ). When ageing HSCs are transplanted into young recipient mice, there is retention of the senescent phenotype, indicating the influence intrinsic changes have on the HSCs ( Geiger et al. , 2013 ). Genes important in maintaining the integrity of the genome are among 1600 genes that are downregulated in ageing HSCs, suggesting that loss of genomic preservation underlies HSC ageing ( Chambers et al. , 2007 ). Furthermore, numerous cell cycle regulatory proteins become dysregulated in ageing HSCs, providing a possible explanation underlying the decreased self-renewal and the loss of cell polarity witnessed in ageing HSCs ( Janzen et al. , 2006 ; Florian et al. , 2012 ; Geiger et al. , 2013 ).
Though HSC ageing is thought to be mainly influenced by intrinsic factors, there is evidence emerging to suggest that extrinsic factors in the microenvironment also play a role. For example, the ageing microenvironment contains high levels of CCL5, which stimulate an increase in myeloid progenitors and a decrease in lymphoid progenitors, classical hallmarks of HSC senescence ( Ergen et al. , 2012 ). Another study using heterochronic parabiosis demonstrated the capability of a youthful circulation to reverse characteristics of ageing HSCs, emphasizing the influence of systemic factors on HSC ageing ( Mayack et al. , 2010 ). Altogether, it appears that the many phenotypic changes of monocytes with ageing are likely initiated at earlier stages of hematopoiesis ( Fig. 2 ).
Strategies to rejuvenate macrophages/microglia for CNS regeneration
Promoting ageing microglial phagocytosis and chemotaxis whilst reducing pro-inflammatory cytokine levels
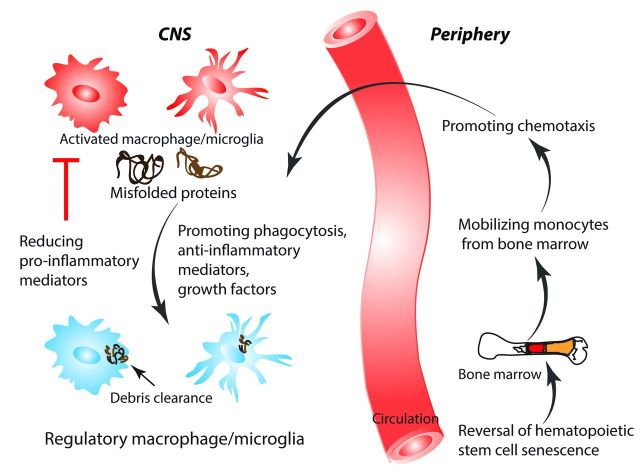
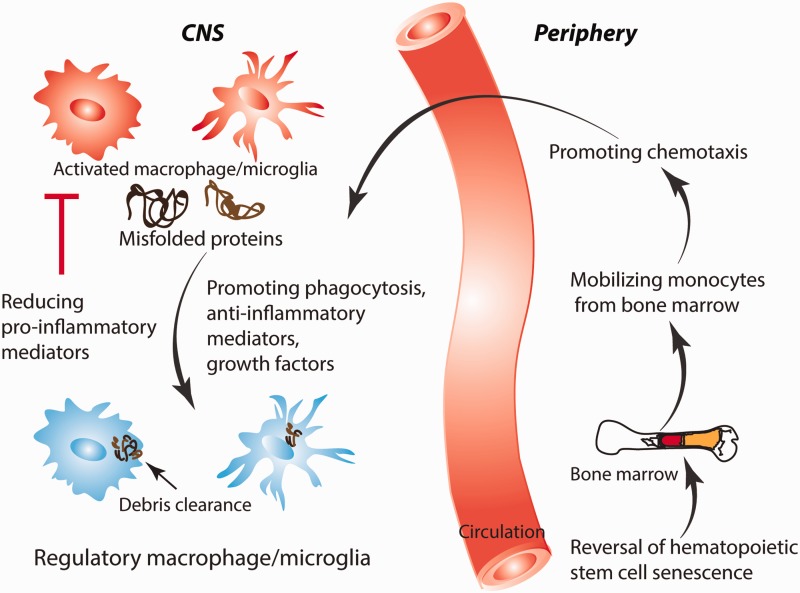
As ageing microglia produce more pro-inflammatory cytokines, agents that penetrate into the CNS to inhibit microglia, such as minocycline or laquinimod, may be beneficial in tempering an excessive pro-inflammatory response ( Fan et al. , 2007 ; Kobayashi et al. , 2013 ; Samanani et al. , 2013 ; Mishra et al. , 2014 ). This approach may only be partially effective, however, as beneficial effects of microglia such as amyloid-β phagocytosis may also be hindered. Thus, a more desired therapy is one that preferentially enhances phagocytosis and chemotaxis, but not excessive pro-inflammatory cytokine secretion. Such an agent could be monophosphoryl lipid A, a modified form of lipopolysaccharide that stimulates mainly the TRIF (TIR-domain-containing adapter-inducing interferon-β) and not the more pro-inflammatory MyD88 pathway downstream of TLR4 ( Mata-Haro et al. , 2007 ). Our group administered monophosphoryl lipid A in an Alzheimer’s disease mouse model and found increased microglial phagocytosis, reduced amyloid-β load and improved clinical outcome ( Michaud et al. , 2013 ). Another group pharmacologically upregulated class A1 scavenger receptors on mononuclear phagocytes, resulting in increased amyloid-β clearance in an Alzheimer’s disease mouse model ( Frenkel et al. , 2013 ). This approach of stimulating microglial phagocytosis but not pro-inflammatory cytokine secretion would conceivably be cytoprotective in many injuries in the ageing CNS. Moreover, stimulating microglia to phagocytose inhibitory myelin debris without an excessive pro-inflammatory cytokine response could enhance axon regeneration and remyelination. Several groups have also examined strategies to enhance a more regulatory microglial phenotype ( Yamanaka et al. , 2012 ; Cohen et al. , 2014 ; Wang et al. , 2015 ). Such treatments target transcriptional regulators important in promoting a regulatory microglia phenotype, such as upregulation of interferon regulatory factor-7 or activation of peroxisome proliferator-activated receptor γ ( Yamanaka et al. , 2012 ; Cohen et al. , 2014 ). Strategies in which a regulatory phenotype is promoted is promising as beneficial functions such as phagocytosis would be enhanced, while detrimental functions such as production of toxic pro-inflammatory molecules would be reduced ( Fig. 3 and Table 1 ).
Figure 3.
Therapeutic manipulation of ageing macrophages and microglia. As monocyte-derived macrophages age differently from CNS-intrinsic microglia, different therapeutic strategies are required to harness the benefits of these cells for CNS repair. Therapies that reverse HSC senescence, increase monocyte chemotaxis, enhance microglia and macrophage phagocytosis, generate a regulatory macrophage/microglia phenotype, and reduce toxic pro-inflammatory mediators are promising for repair of the injured and ageing CNS.
Table 1.
Pharmacological approaches to rejuvenate macrophages and microglia in CNS disorders with ageing
| Pharmacological approach | Proposed mode of action | Reference |
|---|---|---|
| Enhancement of regulatory phenotype | ||
| Interferon-β1 | Upregulation of interferon regulatory factor-7 | Cohen et al. (2014) |
| Scriptaid | Promotion of GSK3β/PTEN/Akt through inhibition of class I/II histone deacetylases | Wang et al. (2015) |
| DSP-8658 | Activation of PPARγ, promoting a regulatory phenotype, and enhancing amyloid-β phagocytosis | Yamanaka et al. (2012) |
| Promotion of chemotaxis and phagocytosis | ||
| Monophosphoryl lipid A | TRIF-mediated TLR4 agonist | Michaud et al. (2013) |
| Protollin | Upregulation of class A1 scavenger receptors and CCL2 | Frenkel et al. (2013) |
| Macrophage colony stimulating factor | Increased mobilization of bone marrow-derived monocytes | Boissonneault et al. (2009) ; Lampron et al. (2013) ; Doring et al. (2015) |
| Amphotericin B | MyD88/TRIF-dependent upregulation of pro-inflammatory cytokines | Sarkar et al. (2014) ; Doring et al. (2015) |
| Reversal of HSC senescence | ||
| Rapamycin | Inhibition of mTOR-dependent HSC senescence | Chen et al. (2009 ) |
| CASIN | Inhibition of cell division control protein 42-mediated HSC senescence | Florian et al. (2012) |
| Inhibition of excess microglial pro-inflammatory cytokine secretion | ||
| Minocycline | Inhibition of NF-κB | Fan et al. (2007) ; Kobayashi et al. (2013) |
| Laquinimod | Reduction of several pro-inflammatory signalling pathways | Mishra et al. (2014) |
GSK3β =glycogen synthase kinase 3 beta; PTEN = phosphatase and tensin homologue; Akt = protein kinase B; PPARγ = peroxisome proliferator-activated receptor γ; TRIF = TIR-domain-containing adapter-inducing interferon-β; TLR4 = Toll-like receptor 4; CCL2 = chemokine (C-C motif) ligand 2; MyD88 = myeloid differentiation primary response protein 88; mTOR = mechanistic target of rapamycin; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells.
Several heterochronic parabiosis studies have now demonstrated the negative impact that the ageing systemic circulation has on muscle regeneration, remyelination, and neurogenesis ( Conboy et al. , 2005 ; Villeda et al. , 2011 ; Ruckh et al. , 2012 ). Therefore, supplementing the ageing CNS microenvironment with rejuvenating factors present in the young circulation may correct the dysregulated microglial phenotype.
Stimulating ageing monocytes and macrophages
As monocytes age differently from microglia, agents that mobilize monocytes from the bone marrow and enhance functional aspects of ageing circulating monocytes, in addition to strategies that reverse HSC senescence, are promising avenues to rejuvenate the monocyte-derived macrophage population for neurological repair. To mobilize monocytes from the bone marrow, macrophage colony stimulating factor may be considered. In models of Alzheimer’s disease or stroke, macrophage colony stimulating factor promoted the mobilization and migration of bone marrow-derived macrophages into the CNS and reduced injury ( Boissonneault et al. , 2009 ; Lampron et al. , 2013 ). In a model of demyelination in mice, macrophage colony stimulating factor facilitated the increased representation of macrophages in the CNS ( Doring et al. , 2015 ).
Pharmacological agents may also be considered for direct stimulation of monocytes and macrophages, so as to aid in the secretion of beneficial factors and in the promotion of phagocytosis and the clearance of inhibitory debris in CNS lesions to allow repair to occur. One such medication is amphotericin B, an anti-fungal agent that stimulates monocytes and macrophages to produce molecules such as TNF-α, IL-10, and CCL2, in addition to increasing their phagocytic activity ( Doring et al. , 2015 ). Indeed, we found that amphotericin B stimulation of monocytes, macrophages and microglia reprogrammed the compromised cells around brain tumours to curb cancer growth ( Sarkar et al. , 2014 ). Amphotericin B and macrophage colony stimulating factor combinational treatment of mice with demyelination of the spinal cord improved subsequent remyelination ( Doring et al. , 2015 ). The severe hepato- and nephrotoxicity of amphotericin B in humans would preclude its widespread use, however.
Many studies have derived proof-of-principle evidence for the capability to reverse HSC senescence. As ageing HSCs have increased mTOR levels, pharmacological inhibition of this kinase using rapamycin has reversed many characteristics associated with HSC ageing ( Chen et al. , 2009 ). As the young systemic circulation has been shown to rejuvenate different stem and progenitor cells through heterochronic parabiosis, identifying specific rejuvenating factors are potential therapeutic strategies to rejuvenate HSCs ( Oh et al. , 2014 ). Another therapeutic strategy is HSC transplantation, in which an ageing, dysregulated HSC population is replaced by a more functional population ( Prinz and Priller, 2014 ).
Conclusion
The effects of ageing on CNS regeneration culminate in an exacerbated lesion accompanied by impaired repair. This article proposes that, during ageing, monocyte-derived macrophages manifest differently from CNS-intrinsic microglia. Ageing macrophages show decreased pro-inflammatory cytokine secretion, phagocytosis, and chemotaxis. In contrast, senescent microglia display a primed, more pro-inflammatory phenotype, but also deficient phagocytosis and chemotaxis. Differential mechanisms, such as extrinsic changes in the microenvironment as well as intrinsic changes including genomic instability, underlie the divergent phenotypes manifested in these two cell types with age. We propose that therapeutic strategies that selectively target these distinct cell types through the underlying mechanisms are promising approaches for rejuvenation ( Fig. 3 and Table 1 ). One has to be mindful, however, not to worsen detrimental aspects, when attempting to stimulate beneficial features such as chemotaxis. Restoring the dysregulated macrophage/microglia phenotype that occurs with ageing is a promising strategy for regeneration in the ageing CNS.
Funding
We thank the Canadian Institutes of Health Research, the Multiple Sclerosis Scientific Research Foundation, the Multiple Sclerosis Society of Canada and Alberta Innovates – Health Solutions CRIO Team program for support of operating funds. K.S.R. is supported by a Vanier Canada Graduate Scholarship and a studentship from the University of Calgary Faculty of Medicine. M.K.M. was supported by a fellowship from Alberta Innovates Health Solutions. N.J.M. is supported by a Multiple Sclerosis Society of Canada Studentship and the Eyes High Doctoral Scholarship from the University of Calgary. V.W.Y., P.K.S. and S.R. acknowledge salary support from the Canada Research Chair program.
Glossary
Abbreviations
- HSC
hematopoietic stem cell
- IL
interleukin
- TLR
Toll-like receptor
References
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM . Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool . Nat Neurosci 2011. ; 14 : 1142 – 9 . [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM . Local self-renewal can sustain CNS microglia maintenance and function throughout adult life . Nat Neurosci 2007. ; 10 : 1538 – 43 . [DOI] [PubMed] [Google Scholar]
- Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, et al. . Aging-induced type I interferon response at the choroid plexus negatively affects brain function . Science 2014. ; 346 : 89 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, et al. . CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging . Proc Natl Acad Sci USA 2013. ; 110 : 2264 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Schwartz M . CNS-specific T cells shape brain function via the choroid plexus . Brain Behav Immun 2013. ; 34 : 11 – 16 . [DOI] [PubMed] [Google Scholar]
- Beerman I, Bock C, Garrison BS, Smith ZD, Gu H, Meissner A, et al. . Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging . Cell Stem Cell 2013. ; 12 : 413 – 25 . [DOI] [PubMed] [Google Scholar]
- Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B . How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes . Aging Cell 2004. ; 3 : 169 – 76 . [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ . Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression . J Leukoc Biol 2004. ; 75 : 342 – 9 . [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ . Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway . Mech Ageing Dev 2005. ; 126 : 1305 – 13 . [DOI] [PubMed] [Google Scholar]
- Boissonneault V, Filali M, Lessard M, Relton J, Wong G, Rivest S . Powerful beneficial effects of macrophage colony-stimulating factor on beta-amyloid deposition and cognitive impairment in Alzheimer’s disease . Brain 2009. ; 132 ( Pt 4 ): 1078 – 92 . [DOI] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, et al. . Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system . Immunity 2015. ; 43 : 92 – 106 . [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. . Identification of a unique TGF-beta-dependent molecular and functional signature in microglia . Nat Neurosci 2014. ; 17 : 131 – 43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA . Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation . PLoS Biol 2007. ; 5 : e201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Staugaitis SM, Dutta R, Batt CE, Easley KE, Chomyk AM, et al. . Cortical remyelination: a new target for repair therapies in multiple sclerosis . Ann Neurol 2012. ; 72 : 918 – 26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelvarajan RL, Collins SM, Van Willigen JM, Bondada S . The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function . J Leukoc Biol 2005. ; 77 : 503 – 12 . [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu Y, Zheng P . mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells . Sci Signal 2009. ; 2 : ra75 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Matcovitch O, David E, Barnett-Itzhaki Z, Keren-Shaul H, Blecher-Gonen R, et al. . Chronic exposure to TGFbeta1 regulates myeloid cell inflammatory response in an IRF7-dependent manner . EMBO J 2014. ; 33 : 2906 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA . Rejuvenation of aged progenitor cells by exposure to a young systemic environment . Nature 2005. ; 433 : 760 – 4 . [DOI] [PubMed] [Google Scholar]
- Conde JR, Streit WJ . Microglia in the aging brain . J Neuropathol Exp Neurol 2006. ; 65 : 199 – 203 . [DOI] [PubMed] [Google Scholar]
- Dai LY . Acute central cervical cord injury: the effect of age upon prognosis . Injury 2001. ; 32 : 195 – 9 . [DOI] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT . Age-related alterations in the dynamic behavior of microglia . Aging Cell 2011. ; 10 : 263 – 76 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring A, Sloka S, Lau L, Mishra M, van Minnen J, Zhang X, et al. . Stimulation of monocytes, macrophages, and microglia by amphotericin B and macrophage colony-stimulating factor promotes remyelination . J Neurosci 2015. ; 35 : 1136 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS . TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke . J Neuroinflammation 2010. ; 7 : 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergen AV, Boles NC, Goodell MA . Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing . Blood 2012. ; 119 : 2500 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. . Host microbiota constantly control maturation and function of microglia in the CNS . Nat Neurosci 2015. ; 18 : 965 – 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, et al. . Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid . J Neurosci 2007. ; 27 : 3057 – 63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP . IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury . J Neurosci 2014. ; 34 : 8904 – 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ . Evidence that aging and amyloid promote microglial cell senescence . Rejuvenation Res 2007. ; 10 : 61 – 74 . [DOI] [PubMed] [Google Scholar]
- Floden AM, Combs CK . Microglia demonstrate age-dependent interaction with amyloid-beta fibrils . J Alzheimers Dis 2011. ; 25 : 279 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian MC, Dorr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. . Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation . Cell Stem Cell 2012. ; 10 : 520 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Wilkinson K, Zhao L, Hickman SE, Means TK, Puckett L, et al. . Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression . Nat Commun 2013. ; 4 : 2030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger H, de Haan G, Florian MC . The ageing haematopoietic stem cell compartment . Nat Rev Immunol 2013. ; 13 : 376 – 89 . [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. . Fate mapping analysis reveals that adult microglia derive from primitive macrophages . Science 2010. ; 330 : 841 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, et al. . Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system . FASEB J 2005. ; 19 : 1329 – 31 . [DOI] [PubMed] [Google Scholar]
- Goldschmidt T, Antel J, Konig FB, Bruck W, Kuhlmann T . Remyelination capacity of the MS brain decreases with disease chronicity . Neurology 2009. ; 72 : 1914 – 21 . [DOI] [PubMed] [Google Scholar]
- Gon Y, Hashimoto S, Hayashi S, Koura T, Matsumoto K, Horie T . Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly . Clin Exp Immunol 1996. ; 106 : 120 – 6 . [PubMed] [Google Scholar]
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, et al. . Environment drives selection and function of enhancers controlling tissue-specific macrophage identities . Cell 2014. ; 159 : 1327 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, David S . Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death . J Neurosci 2014. ; 34 : 6316 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, et al. . Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a . Nature 2006. ; 443 : 421 – 6 . [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. . Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways . Nat Neurosci 2013. ; 16 : 273 – 80 . [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, et al. . Minocycline selectively inhibits M1 polarization of microglia . Cell Death Dis 2013. ; 4 : e525 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJ . Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation . J Neurosci 2006. ; 26 : 328 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ . Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states . Neurobiol Aging 2013. ; 34 : 1397 – 411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampron A, Pimentel-Coelho PM, Rivest S . Migration of bone marrow-derived cells into the central nervous system in models of neurodegeneration . J Comp Neurol 2013. ; 521 : 3863 – 76 . [DOI] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. . Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment . Cell 2014. ; 159 : 1312 – 26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Natoli G . Transcriptional regulation of macrophage polarization: enabling diversity with identity . Nat Rev Immunol 2011. ; 11 : 750 – 61 . [DOI] [PubMed] [Google Scholar]
- Marquez de la Plata CD, Hart T, Hammond FM, Frol AB, Hudak A, Harper CR, et al. . Impact of age on long-term recovery from traumatic brain injury . Arch Phys Med Rehabil 2008. ; 89 : 896 – 903 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S . The M1 and M2 paradigm of macrophage activation: time for reassessment . F1000Prime Rep 2014. ; 6 : 13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M . Macrophage activation and polarization . Front Biosci 2008. ; 13 : 453 – 61 . [DOI] [PubMed] [Google Scholar]
- Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC . The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4 . Science 2007. ; 316 : 1628 – 32 . [DOI] [PubMed] [Google Scholar]
- Mayack SR, Shadrach JL, Kim FS, Wagers AJ . Systemic signals regulate ageing and rejuvenation of blood stem cell niches . Nature 2010. ; 463 : 495 – 500 . [DOI] [PubMed] [Google Scholar]
- Michaud JP, Halle M, Lampron A, Theriault P, Prefontaine P, Filali M, et al. . Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology . Proc Natl Acad Sci USA 2013. ; 110 : 1941 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell-Robinson MA, Touil H, Healy LM, Owen DR, Durafourt BA, Bar-Or A, et al. . Roles of microglia in brain development, tissue maintenance and repair . Brain 2015. ; 138 ( Pt 5 ): 1138 – 59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. . M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination . Nat Neurosci 2013. ; 16 : 1211 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra MK, Wang J, Keough MB, Fan Y, Silva C, Sloka S, et al. . Laquinimod reduces neuroaxonal injury through inhibiting microglial activation . Ann Clin Transl Neurol 2014. ; 1 : 409 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nunez V, Johnson KR, et al. . Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination . Brain 2015. ; 138 : 3581 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F . Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo . Science 2005. ; 308 : 1314 – 18 . [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ . Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function . Neurobiol Aging 2012. ; 33 : 195.e1–12. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Lee YD, Wagers AJ . Stem cell aging: mechanisms, regulators and therapeutic opportunities . Nat Med 2014. ; 20 : 870 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero EG, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. . Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors . Nature 2015. ; 518 : 547 – 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Holmes C . Microglial priming in neurodegenerative disease . Nat Rev Neurol 2014. ; 10 : 217 – 24 . [DOI] [PubMed] [Google Scholar]
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD . Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions . Ann Neurol 2001. ; 50 : 389 – 400 . [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J . Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease . Nat Rev Neurosci 2014. ; 15 : 300 – 12 . [DOI] [PubMed] [Google Scholar]
- Rawji KS, Yong VW . The benefits and detriments of macrophages/microglia in models of multiple sclerosis . Clin Dev Immunol 2013. ; 2013 : 948976 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, et al. . Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging . PLoS One 2011. ; 6 : e17487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, et al. . Rejuvenation of regeneration in the aging central nervous system . Cell Stem Cell 2012. ; 10 : 96 – 103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL . Risk factors, outcomes, and stroke subtypes for ischemic stroke . Neurology 1997. ; 49 ( Suppl 4 ): S39 – 44 . [DOI] [PubMed] [Google Scholar]
- Samanani S, Mishra M, Silva C, Verhaeghe B, Wang J, Tong J, et al. . Screening for inhibitors of microglia to reduce neuroinflammation . CNS Neurol Disord Drug Targets 2013. ; 12 : 741 – 9 . [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE . Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury . Exp Neurol 2008. ; 213 : 372 – 80 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Doring A, Zemp FJ, Silva C, Lun X, Wang X, et al. . Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells . Nat Neurosci 2014. ; 17 : 46 – 55 . [DOI] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. . A lineage of myeloid cells independent of Myb and hematopoietic stem cells . Science 2012. ; 336 : 86 – 90 . [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A . How do immune cells support and shape the brain in health, disease, and aging? . J Neurosci 2013. ; 33 : 17587 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AC, Goldstein DR, Montgomery RR . Age-dependent dysregulation of innate immunity . Nat Rev Immunol 2013. ; 13 : 875 – 87 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, et al. . Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus . Immunity 2013. ; 38 : 555 – 69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer A, Erny D, Jung S, Prinz M . Microglia plasticity during health and disease: an immunological perspective . Trends Immunol 2015. ; 36 : 614 – 24 . [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS . Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain . Acta Neuropathol 1998. ; 95 : 229 – 34 . [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K . Microglia derived from aging mice exhibit an altered inflammatory profile . Glia 2007. ; 55 : 412 – 24 . [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Zettel ML, Ison JR, Allen PD, Majewska AK . Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices . Glia 2012. ; 60 : 541 – 58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, et al. . Age-associated defect in human TLR-1/2 function . J Immunol 2007. ; 178 : 970 – 5 . [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. . The ageing systemic milieu negatively regulates neurogenesis and cognitive function . Nature 2011. ; 477 : 90 – 4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, et al. . HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis . Proc Natl Acad Sci USA 2015. ; 112 : 2853 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw DM . Observations on human monocyte kinetics after pulse labeling . Cell Tissue Kinet 1972. ; 5 : 311 – 17 . [DOI] [PubMed] [Google Scholar]
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. . Transcriptome-based network analysis reveals a spectrum model of human macrophage activation . Immunity 2014. ; 40 : 274 – 88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT . PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice . J Neurosci 2012. ; 32 : 17321 – 31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, et al. . Differential roles of microglia and monocytes in the inflamed central nervous system . J Exp Med 2014. ; 211 : 1533 – 49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Li WW, Franklin RJ . Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination . Neurobiol Aging 2006. ; 27 : 1298 – 307 . [DOI] [PubMed] [Google Scholar]