Abstract
Background
Thrombotic effects are possible complications of red blood cell transfusion. The generation and accumulation of procoagulant red blood cell extracellular vesicles during storage may play an important role in these thrombotic effects. The objective of this study was to assess the value of a simple phospholipid-dependent clot-based assay (STA®-Procoag-PPL) to estimate the procoagulant activity of stored red blood cells and changes in this activity during storage of the blood component.
Materials and methods
Extracellular vesicles from 12 red blood cell concentrates were isolated at 13 storage time-points and characterised by quantitative and functional methods: the degree of haemolysis (direct spectrophotometry), the quantification and determination of cellular origin (flow cytometry) and the procoagulant activity (thrombin generation and STA®-Procoag-PPL assays) were assessed.
Results
The mean clotting time of extracellular vesicles isolated from red blood cell concentrates decreased from 117.2±3.6 sec on the day of collection to 33.8±1.3 sec at the end of the storage period. This illustrates the phospholipid-dependent procoagulant activity of these extracellular vesicles, as confirmed by thrombin generation. Results of the peak of thrombin and the STA®-Procoag-PPL were well correlated (partial r=−0.41. p<0.001). In parallel, an exponential increase of the number of red blood cell-derived extracellular vesicles from 1,779/μL to 218,451/μL was observed.
Discussion
The STA®-Procoag-PPL is a potentially useful technique for assessing the procoagulant activity of a red blood cell concentrate.
Keywords: extracellular vesicles, red blood cell concentrates, coagulation, storage
Introduction
Observations suggest that red blood cell (RBC) transfusion is associated with increased thrombotic events and mortality. Blood transfusion in the setting of acute coronary syndromes was associated with higher mortality (odds ratio [OR] 3.94)1. Khorana et al. observed that RBC transfusion was independently associated with an increased risk of venous (OR 1.60) and arterial (OR 1.53) thrombotic events and mortality (OR 1.34) in hospitalised cancer patients2. Similarly, Abu-Rustum et al. demonstrated an association between transfusion and increased risk of thrombotic events in women during adnexal or peritoneal cancer surgery (OR 4.80)3. Nilsson et al. also observed that peri-operative blood transfusion was associated with an increased risk of venous thromboembolism in women undergoing colorectal cancer resection (OR 1.80)4. More recently, an increase of peri-operative graft thrombosis was seen in patients transfused during lower extremity bypass surgery (OR 2.10 to 4.80, depending on the number of units transfused)5. Finally, Kumar and collaborators also reported that red blood cell transfusion was associated with an increased risk of thrombotic events in patients with subarachnoid haemorrhage (OR 2.4)6.
The mechanisms underlying these findings remain unclear. It is known that RBC transfusions alter blood viscosity both by raising the haematocrit and by introducing erythrocytes with altered viscoelastic properties into the circulation, increasing the thrombotic risk4. It has also been shown that increasing the circulating red cell mass may improve haemostasis2 while the depletion of nitric oxide, which is observed in all stored RBC units, may promote vasoconstriction and platelet aggregation1. The delivery of redox-active iron and pro-inflammatory mediators by transfusion may also have consequences2. Finally, the release of procoagulant extracellular vesicles (EV) contained in the transfused product may promote haemostasis and increase the risk of thrombosis. Indeed, it has been demonstrated that the number of RBC-derived EV (REV) detected in packed RBC by flow cytometry increases during storage7 and that REV isolated from blood units are able to initiate and propagate thrombin generation8,9.
The techniques currently available to study EV (including enzyme-linked immunosorbent assays, flow cytometry and thrombin generation assays) have several limitations including their costs, the technical expertise required, the turnaround time and their availability10. The STA®-Procoag-PPL assay (Diagnostica Stago SAS, Asnières-sur-Seine, France) is a clot-based assay sensitive to the presence of EV. This assay is cheap, simple, rapid, reproducible and could be widely available in clinical laboratories11. In addition, the STA®-Procoag-PPL assay showed good performances in comparison to flow cytometry and thrombin generation in clinical studies11–15.
The objective of this study was to assess the value of the STA®-Procoag-PPL assay for estimating the procoagulant potential of RBC concentrates before transfusion.
Materials and methods
Preparation of red blood cell concentrates
This study was approved by our local ethics committee (Comité d‘Ethique Medicale Mont-Godinne, Namur, Belgium). After providing informed, written consent, 12 volunteer adult donors each gave 450±50 mL of whole blood. The mean age of the donors was 38 years (minimum: 24, maximum: 53) and their mean body mass index was 25.3 (minimum: 19.7, maximum: 33.5). The male:female sex ratio was 1:3. The blood was collected by venipuncture, in quadruple blood bags (Fenwall Inc., Lake Zurich, IL, USA) containing citrate-phosphate-dextrose (CPD) and processed in the Blood Transfusion Centre of Mont-Godinne (CHU UCL Namur, Belgium) following the standard procedure. Whole blood units were stored between 18 and 22 °C for a maximum of 18 hours before centrifugation at 3,500 g for 13 minutes at room temperature to separate RBC from plasma and buffy coat. The RBC were then separated in a bag containing 100 mL of sodium-adenine-glucose-mannitol (SAG-M) using an automated blood component extractor (Optipress, Fenwall Inc). Before RBC storage, white blood cells were removed by filtration. RBC concentrates were anonymised and labelled from “RC01” to “RC12”. They were then stored up to 42 days at 4±2 °C.
Isolation of extracellular vesicles
A sampling port (Beldico, Aye, Belgium) was introduced into the blood bag and left in place during the total storage time. Samples were collected every 82±2 hours from each bag, from the day of collection (D0) to day 42 (D42).
After gentle homogenisation of the bag, a sample of 15 mL was collected in a Falcon tube. Each tube was manually presented to the automated haematology analyser (Sysmex XN-9000, Kobe, Japan) to acquire the complete blood count.
The remaining samples were simultaneously centrifuged at 1,850 g for 20 minutes at 4 °C. After this, the supernatant was pipetted and placed in a second tube, then re-centrifuged under the same conditions7. The second supernatant was then pipetted, distributed in aliquots of 700 μL in low temperature freezer vials (VWR International bvba, Leuven, Belgium), and frozen without delay at −80 °C, for further use for the STA®-Procoag-PPL assay, haemolysis measurement, flow cytometry and thrombin generation assay.
It is important to note that a further concentration step of EV by ultracentrifugation was later applied for the thrombin generation assay (see below) but not for the STA®-Procoag-PPL, haemolysis measurement or flow cytometry analyses. This ultracentrifugation was performed to overcome the lack of sensitivity of the thrombin generation assay16.
STA®-Procoag-PPL
The STA®-Procoag-PPL (STAPPL) kit is designed for the detection of procoagulant phospholipids (PPL) in plasma samples by a chronometric method. This assay measures clotting time, in the presence of factor Xa and CaCl2, of a system in which all the factors are present at physiological levels (supplied by PPL-depleted plasma) except the PPL supplied by the plasma sample being tested17. In our method, plasma samples were replaced by isolated EV (see above).
The frozen second supernatants of the 12 RBC concentrates during storage were tested and constituted our samples. After thawing in a water bath at 37 °C for 4 minutes, 500 μL of each sample were placed in a cuvette and introduced in a STA-R Evolution® coagulometer (Diagnostica Stago SAS).
Harboe’s direct spectrophotometric method to measure haemolysis
After thawing in a water bath at 37 °C for 4 minutes, 200 μL of each sample were placed in a cuvette (Sarstedt AG & Co., Nümbrecht, Germany) and then 1,800 μL of distilled water were added, giving a 10-fold dilution of the sample. A pipette was used for gentle homogenisation followed by resting for 10 minutes. The absorbance of each sample was read at three different wavelengths (415, 380 and 450 nm) against a distilled water blank in the spectrophotometer (Genesys 10S UV-Vis, Thermo Fisher Scientific Inc., Waltham, MA, USA). The absorbance was converted into haemoglobin according to Han et al.18 before calculating the degree of haemolysis. This last value was calculated by taking into account the haematocrit and total haemoglobin level.
Flow cytometry
Samples were analysed on a FACS Aria flow cytometer with FACSDiva V6.1.3 software (BD Biosciences, San Jose, CA, USA). The cytometer was optimised and programmed following the standardised procedures for measurement of EV provided by the International Society on Thrombosis and Haemostasis19–21.
The cytometer was calibrated with Megamix-Plus SSC beads (BioCytex, Marseille, France). These beads are optimised for use on a FACS Aria cytometer21. The separation index was used to evaluate resolution on the side scatter (SSC) parameter22.
The monoclonal antibodies used were: CD235a-PE (IOTest, clone 11E4B-7-6, Beckman Coulter, Marseille, France) for RBC origin, CD31-V450 (clone WM59, BD Biosciences, Erembodegem, Belgium), CD41a-PerCP-Cy5.5 (clone HIP8, BD Biosciences) for platelet origin.
Annexin V-FITC (BD Pharmingen, Erembodegem, Belgium) was used to tag phosphatidylserine present on the surface of EV. Phosphatidylserine, usually present on the inner membrane of intact cells, is exposed on the outer side of the membrane during the process of microvesiculation that is observed during apoptosis or cell activation23. Combined with the forward scatter (FSC) (size) and SSC (granularity) of events detected in flow cytometry, the expression of phosphatidylserine can help to separate the population of EV from cell fragments or intact RBC7,24. EV tagged by annexin V were defined as annexin V-positive (V+). The ones not tagged by annexin V were defined as annexin V-negative (V−).
Prior to use, each monoclonal antibody was centrifuged at 15,000 g for 10 minutes at 4 °C. They were stored at 4–6 °C in the dark until use (within 4 hours). Samples were thawed in a water bath at 37 °C for 4 minutes, just before incubation with antibodies for 30 minutes at room temperature in the dark.
The samples were then introduced into the flow cytometer. The number of events during the acquisition period of 120 seconds was recorded. With an identical volume of sample and a constant flow during each analysis (5.4 μL/minute), the absolute concentration of EV could be calculated.
Calibrated automated thrombography
The thrombin generation tests were performed on a Fluoroskan Ascent (Thermo Scientific) in triplicate.
Concentration of extracellular vesicles, reconstitution of the conservation solution and collection of the normal pooled plasma
Samples of EV were thawed six by six in a water bath at 37 °C for 4 minutes. To separate EV, 700 μL of each sample were placed in a microcentrifuge polyallomer tube (Beckman Coulter) and ultracentrifuged at 100,000 g for 90 minutes at 4 °C. The supernatant was pipetted and stored at −20 °C before running 70 μL into the cuvette, to concentrate EV ten times more than in the frozen sample. This further concentration step of EV was needed to trigger the thrombin generation assay without adding tissue factor or phospholipids. The cuvettes were spun for 1 minute and were stored on ice until being plated for calibrated automated thrombogram (CAT) analysis.
To exclude an effect of the conservation solution used in the RBC concentrates on the amount of thrombin generated, we reconstituted this solution at the identical concentration by adding 315 μL of CPD to 500 μL of SAG-M.
The use of normal pooled plasma (NPP) was required to assess the impact of EV from samples on thrombin generation. The NPP was constituted from the whole blood of 47 healthy subjects, collected on the same day by venipuncture. These samples were centrifuged twice at 2,500 g for 15 minutes at 20 °C. After pooling, the NPP was frozen quickly in liquid nitrogen and stored at 80 °C.
Thrombin generation assay
Ten microlitres of each sample (concentrated 10 times) were placed in six wells. Twenty microlitres of thrombin calibrator were added to the first three wells and 20 μL of filtered phosphate-buffered saline (PBS) were added to the other three wells. No tissue factor or phospholipids were added to the wells.
For inter-plate quality control, three wells contained 10 μL of filtered PBS and 20 μL of PPP reagent low (Thrombinoscope BV, Maastricht, the Netherlands).
The plate was incubated for 10 minutes in the CAT at 37 °C. During this time, the NPP was thawed in a water bath at 37 °C for 8 minutes: 70 μL of NPP were then added to each well and 20 μL of FLUCA were added to the dispenser.
Statistical analysis
To study the changes over time of different parameters, we computed the mean of the twelve RBC concentrates at each time-point. Linear, exponential and logarithmic models were then applied to determine which model best described the temporal evolution of each variable during the storage.
The strength of an association between two variables (X, Y) was assessed from the partial correlation coefficient25. The principle is to compute a Pearson’s correlation coefficient on X and Y after removing the potential confounding effect of covariables, namely the storage time and the RBC concentrate. Given the high number of variable combinations (105) and thus statistical tests, p-values were corrected using Bonferroni’s method. Adjusted p-values less than 0.05 (which correspond to an unadjusted p-value of 0.00048) were considered statistically significant.
All analyses were performed with R 3.1.2 (R Foundation for Statistical Computing, Wien, Austria).
Results
Clotting time depending on phospholipids decreases with storage
Samples were collected every 82±2 hours from the 12 RBC concentrates although it was not possible to obtain samples of RC04 and RC12 on D0 because these two concentrates were collected too late in the afternoon.
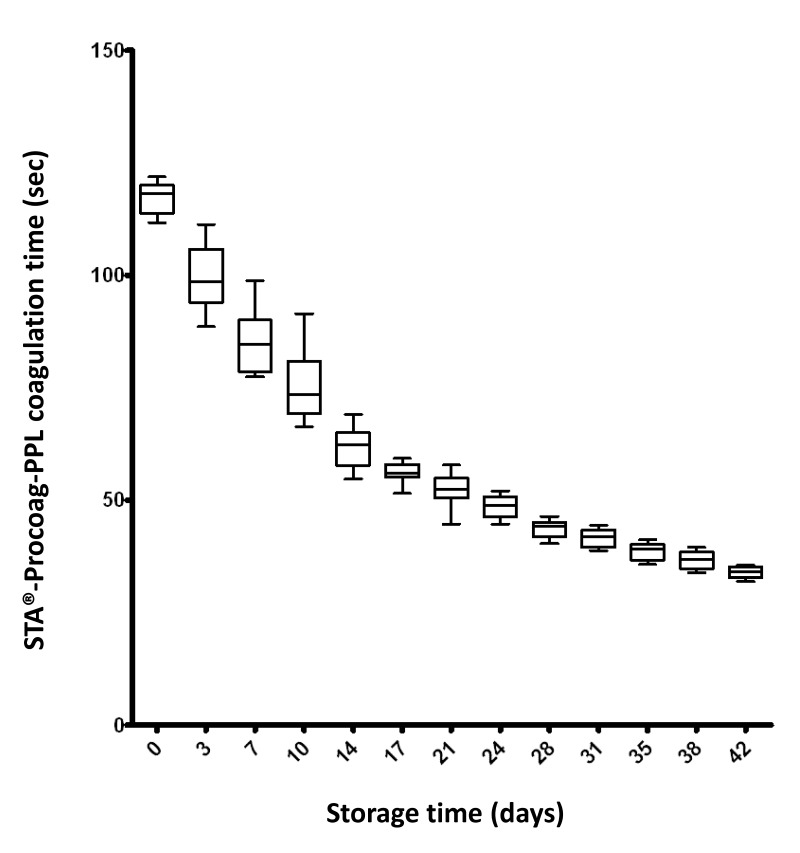
With the STA®-Procoag-PPL assay, we observed a steady decrease in the clotting time during the storage of the RBC concentrates (Figure 1). The mean clotting time fell from 117.2±3.6 (minimum: 111.7, maximum: 121.9) seconds on D0 to 33.9±1.3 (minimum: 31.9, maximum: 35.5) seconds on D42. This corresponds to a 3.5-fold (minimum: 3.1-fold, maximum: 3.6-fold) decrease. A mixed model linear regression confirmed that this decrease did not simply occur by chance (data not shown). These observations reflect the phospholipid-dependent procoagulant effect of the supernatant increasing with storage.
Figure 1.
Changes in clotting time in relation to storage duration of 12 red blood cell (RBC) concentrates in the STA®-Procoag-PPL assay.
Twelve RBC concentrates were analysed at 13 time-points during storage for a total of 42 days. There was a steady decrease of the clotting time during storage. A shorter clotting time reflects more important procoagulant activity. In the figure, boxes include from the first to the third quartile and whiskers indicate maximum and minimum values.
The clotting time was inversely correlated with the number of annexin V− REV which strongly expressed glycophorin A (partial r=−0.44. p<0.001) (see below).
Increase of free haemoglobin concentration in red blood cell concentrates during storage
The mean supernatant haemoglobin concentration on the day of collection (D0) was 0.01 g/dL. During storage, we observed a steady increase in this value until D24–D32, with a mean peak of 0.4 g/dL, followed by a decrease until D42.
The degree of haemolysis, which was very low on D0 (0.02±0.004 %), changed in a similar manner to the supernatant haemoglobin concentration during storage, with a mean peak of 0.7±0.1%.
There was a strong correlation between supernatant haemoglobin concentration and the number of REV, both for annexin V+ REV (partial r=0.66) and annexin V− REV (partial r=0.60). However, the change in mean supernatant haemoglobin concentration and the degree of haemolysis during storage were not correlated with the parameters of thrombin generation or the clotting time depending on phospholipids (monotonous increase). The mean haematocrit increased from 57.8±2.2% on D0 to 65.1±3.9% on D42, which corresponds to a 12.6% increase of the haematocrit during storage.
Increase in the number of red blood cell-derived extracellular vesicles (annexin V+ and annexin V−) during storage
On D0, the number of events positive for CD235a (corresponding to glycophorin A, expressed on the membrane of REV) was similar in each RBC concentrate. The mean number of REV was 1,779±619 (minimum: 807, maximum: 2,766)/μL and the majority of them (97.6%) were annexin V−.
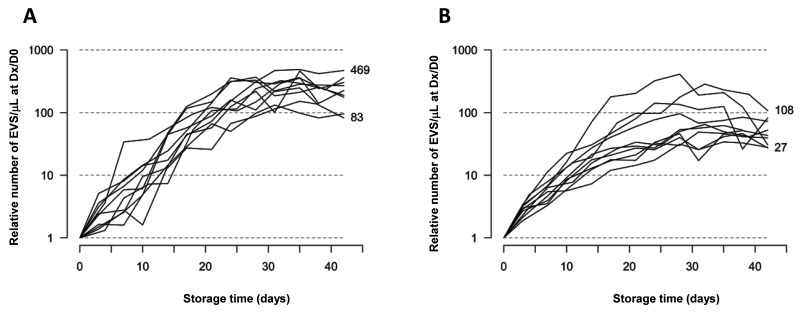
During storage, the number of annexin V+ and annexin V− REV increased exponentially in each RBC concentrate to reach a plateau around D28 and towards the end of the storage period (Figure 2). On D42, 92.2% of REV were still annexin V−. However, the increase in the number of REV over time varied considerably from concentrate to concentrate. For example, the number of annexin V− REV multiplied by 40 in RC10 but by 410 in RC09 (mean of 124). The mean increase of the number of annexin V+ REV during storage was 326-fold (range, 132-fold to 487-fold).
Figure 2.
Changes in the number of red blood cell (RBC)-derived extracellular vesicels (REV)/μL detected by flow cytometry during RBC storage.
There was an exponential increase of the number of REV during storage in each RBC concentrate. Annexin V+ REV are shown in (A) and annexin V− REV in (B). Each line corresponds to one RBC concentrate. The results are expressed as the ratio between the number of REV/μL at Dx and the number of REV/μL at D0 (logarithmic scale), thus showing the relative increase of the number of REV at different time-points of storage compared to the number of REV on the day the RBC were collected. A plateau is reached around D28 and towards the end of storage period. Depending on the RBC concentrate analysed, the number of annexin V+ REV increased by 83- to 469-fold during storage while the number of annexin V− REV increased by 27- to 108-fold.
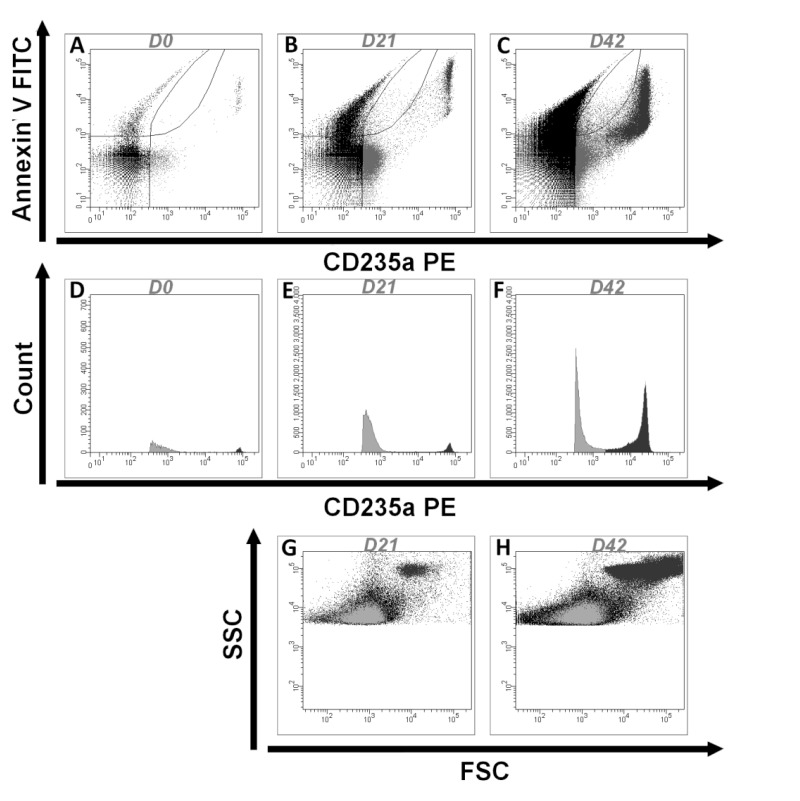
Among annexin V− REV, two different populations of vesicles were identified. The majority population showed moderate glycophorin A expression while the minority population expressed glycophorin A strongly. This second population was present on D0 (with a mean of 236 EV/μL) and increased exponentially during storage (from 270-fold to 21,054-fold, depending on the concentrate). The EV of this subpopulation were slightly higher than EV defined by Megamix-Plus bead calibration. An example of the results obtained for RC07 is illustrated in Figure 3.
Figure 3.
Results obtained from flow cytometry for one donor (RC07). (A–C).
The REVs detected at D0 (A), D21 (B) and D42 (C) are shown according to their labeling by annexin V and CD235a. The number of REVs greatly increases during storage. Among REV (CD235a+ events), the majority are annexin V− at the end of storage. (D–F) The REV detected on D0 (D), D21 (E) and D42 (F) are shown according to their count and their labelling of CD235a. Two subpopulations of annexin V− REV can be identified; one majority population that expresses CD235a (glycophorin A) moderately (in light grey) and the other population that expresses CD235a strongly (in dark grey). (G,H) The distribution of the REV is illustrated by a forward scatter (FSC) and side scatter (SSC) plot on D21 (G) and D42 (H), showing the heterogeneity of the FSC-SSC of the two populations of REV expressing glycophorin A (in grey). REV: extracellular vesicles of red blood cell origin.
The STA®-Procoag-PPL clotting time, which was inversely correlated with the number of annexin V− REV that strongly expressed glycophorin A (see above), was only weakly correlated with the number of all annexin V− REV (partial r=−0.15. p=0.079) or with the number of annexin V+ REV (partial r=−0.29. p<0.001).
Moreover, very few white blood cells (< 20/μL) or platelets (< 2,000/μL) were found in RBC concentrates on D0 (data not shown). A mean of 643 EV/μL of platelet origin (CD41+) was observed on D0 and their number did not increase significantly to D42 (mean of 917/μL).
Red blood cell-derived extracellular vesicles isolated from red blood cell concentrates are able to initiate thrombin generation, and this occurs increasingly during storage
The addition of EV isolated from each RBC concentrate on D0, in the presence of NPP, was able to initiate thrombin generation. We observed some inter-concentrate variability for each parameter of thrombin generation but the general pattern of changes during storage was similar and the curves tended to converge from D14.
There was a linear increase in peak of thrombin generation. The endogenous thrombin potential (ETP) increased logarithmically to around D14, then more slowly to the end of the storage period. The lag time and the time to peak decreased logarithmically. The changes in these parameters are summarised in Table I.
Table I.
Evolution of the four parameters of thrombin generation during storage.
| D0: mean±SD (min.–max.) | D42: mean±SD (min.–max.) | Global evolution (min.–max.) | |
|---|---|---|---|
| Peak (nM) | 27.8±6.8 (15.6–37.3) | 370.6±21.6 (323.7–402.1) | Increase of 12.9 (9.9–20.7) times |
| ETP (nM) | 440.2±130.0 (219.7–654.7) | 1,347.6±85.4 (1,203.7–1,492.0) | Increase of 2.8 (2.1–5.5) times |
| Lag time (min) | 23.2±4.0 (18.3–30.2) | 7.3±0.9 (6.2–9.1) | Decrease of 3.1 (2.6–4.1) times |
| Time to peak (min) | 29.0±4.0 (23.6–36.0) | 9.3±1.0 (8.0–11.2) | Decrease of 3.1 (2.7–3.8) times |
During storage of the 12 RBC concentrates, there was a 12.9-fold increase in peak of thrombin generated, a 2.8-fold increase in the endogenous thrombin potential (ETP), and 3.1-fold decreases of both the lag time and time to peak in the calibrated automated thrombogram assay. The mean values and standard deviations (SD) are indicated. The minimum (min.) and maximum (max.) values are included in parentheses.
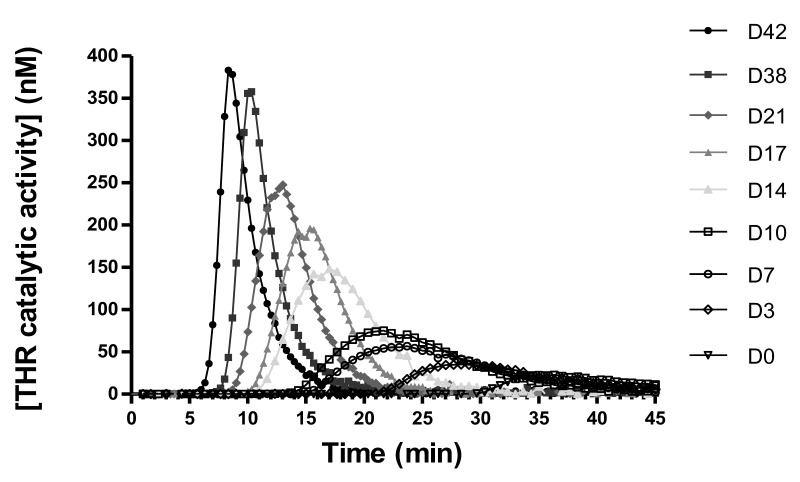
Curves for thrombin generation obtained with RC07 are illustrated in Figure 4 as an example.
Figure 4.
Amount of thrombin generated as a function of time during the thrombin generation assay (RC07).
One RBC concentrate is analysed here (RC07). Each curve represents the amount of thrombin generated during the thrombin generation assay when extracellular vesicles are isolated at various time-points of storage (D0, D3, D7, …) and added to normal pooled plasma. Gradually during storage, the peak of thrombin generated increases, the time before the initiation of thrombin generation (lag time) is shortened, the endogenous thrombin potential (area under the curve) increases and the time to reach the peak of thrombin is shortened. THR: thrombin; RBC: red blood cell.
Filtered PBS and the conservation solution alone were unable to initiate thrombin generation when added to NPP. An inter-plate quality control was performed using “PPP reagent low” added to NPP. The inter-plate coefficient of variation of the measure of ETP was 9.1%. Altogether, all the coefficients of variation measured for thrombin generation assays were acceptable because they were below 10% (data not shown).
The evolution of the peak of thrombin generated was positively correlated with the number of annexin V+ REV detected by flow cytometry (partial r=0.45, p<0.001).
Finally, the clotting time depending on phospholipids decreased steadily throughout storage while the peak of thrombin increased. As expected, the two measures were well correlated (rs=−0.41, p<0.001).
Discussion
In this study, 12 leucoreduced RBC concentrates were assessed at different storage times up to 42 days, which is the limit of the period of conservation of RBC concentrates before transfusion in Europe26. The number of REV detected by flow cytometry clearly and gradually increased during storage, by a mean of 124-fold for annexin V− and 326-fold for annexin V+ REV. Moreover, REV had a procoagulant impact that increased with storage time, as evidenced by the evolution of the thrombin generation parameters and the reduction of the clotting time in the STA®-Procoag-PPL assay. A moderate correlation was observed between the peak of thrombin generated as measured by CAT and the coagulation time measured with the STA®-Procoag-PPL assay.
The different procedures for EV isolation used in these two functional assays may partly explain why only a moderate correlation was observed between their findings. Notably, a further concentration step of EV by ultracentrifugation was applied to find a solution to the lack of sensitivity of the thrombin generation assay16. It should be mentioned that there is no consensus about the method to use to isolate REV10,27–30. This is particularly the case for stored RBC concentrates31,32. Thus, there is a need for standardisation of isolation of REV from stored RBC concentrates as for platelet EV33.
A limitation of our study is that, because of the multiple samples analysed (13 samples per RBC unit), the remaining volume in each unit decreased significantly during storage. We observed a mean increase of 12.6% in the haematocrit during our study. In another, recent study, the haematocrit increased by 6.6% with only four samples taken from each unit34. This multiple sampling may have induced haemolysis and cell stress, which may have changed EV release and storage conditions compared to the normal blood bank conditions. It would be interesting to repeat the study, collecting only two samples on the day of collection and on day 42.
Similarly, the freeze-thaw cycle of REV that was necessary for the characterisation of EV under homogeneous conditions may have induced cryoinjury that was not related to cold storage. However, in plasma samples after double centrifugation, after a single freeze-thaw cycle, only limited increases in platelet EV counts (+15%) and procoagulant activity (+32%) were observed. This was verified up to 12 months of storage at −80 °C and the authors concluded that deepfreeze storage conditions do not strongly influence EV analysis when performed adequately13. However, the analysis of REV from blood units is not exactly comparable to that of platelet EV from plasma samples. It is important to note that all samples in our study were subjected to the same freeze-thaw conditions. In this study, the relative increases of REV number and procoagulant activity were more relevant than the absolute values.
Different measures have been developed to test the quality of stored red blood cells and their procoagulant potential35. The amount of extracellular haemoglobin can be quantified spectrophotometrically and gives an idea of the rate of haemolysis in stored red cell concentrates17. Since it has been shown that 70% of the extracellular haemoglobin found in supernatant is encapsulated in EV36, various techniques have been developed to characterise these vesicles and to understand their potential role in thrombotic complications attributed to transfusion. Flow cytometry is the technique most commonly used to quantify EV but it suffers from a lack of sensitivity. Moreover, strict standardisation of analytical and pre-analytical conditions is necessary because these represent major sources of variability and potential artefacts in EV analysis33,37. Thrombin generation, determined using the CAT, is an alternative to the STA®-Procoag-PPL assay. As recently reviewed, thrombin generation is a promising tool to investigate haemorrhagic or thrombotic disorders38. However, thrombin generation is currently affected by its turnaround time and lack of standardisation. Both techniques also require expertise available only in specialised laboratories.
Our study underlines the potential of the STA®-Procoag-PPL assay as an easy way to determine the procoagulant activity of REV, regardless of the donor or the age of the RBC concentrate. Inter-donor variability is unavoidable. In this study, we observed a variability of the various parameters on the day of collection but also sometimes a different behaviour during storage from donor to donor. The STA®-Procoag-PPL assay is an automated assay, which is both faster and cheaper than the thrombin generation assay, so it could easily be used in research and in many clinical laboratories. Its utility in clinical practice, however, remains to be demonstrated.
The majority of glycophorin A+ events that we detected were annexin V−. In their studies7,9, Rubin et al. observed a majority of annexin V+ events in RBC concentrates while Sweeney et al. described only 50 % of annexin V+ events8. As for platelet EV, it is likely that some REV do not express negative phospholipids at their outer membrane39. It has been suggested that the presence of negative phospholipids at the EV outer membrane provides a surface for procoagulant reactions via tenase and prothrombinase complexes23 but other mechanisms than annexin V are probably also involved in the procoagulant activity of REV. In a recent publication in Blood Transfusion, Grisendi et al.37 noted that only a small fraction of EV was labelled by annexin V. They demonstrated that multiparametric staining, combining carboxyfluorescein succinimidyl ester dye with glycophorin A antibody and annexin V, may be successfully employed to identify REV in packed RBC more specifically.
It may also be relevant that, among annexin V− events, we observed two different populations. Only the population that strongly expressed glycophorin A seemed to have an impact on coagulation time in the STA®-Procoag-PPL assay (but not in thrombin generation). As described in previous reports, RBC release REV of various sizes and forms40. This may be responsible for the different glycophorin A expression of the REV. Indeed, in our flow cytometry study, REV with higher glycophorin A expression also had a higher FSC-SSC distribution (Figure 3). Moreover, it has been previously demonstrated that the size of EV has an impact on their procoagulant activity41.
Several studies have found that transfusions using older blood are associated with adverse clinical outcomes42,43. A meta-analysis involving a total of 409,840 patients suggested that the transfusion of older red cells, compared with newer red cells, was associated with a 16% increase in mortality43. Nevertheless, this fact was not confirmed by the ABLE trial involving 2,430 critically ill adults44. Similarly, the duration of RBC storage was not associated with differences in terms of organ dysfunction in patients undergoing cardiac surgery (RECESS trial)45 and the incidence of major nosocomial infections or organ dysfunction in transfused premature infants was not improved by the use of fresh RBC (ARIPI trial)46. However, in these three large studies, only the benefit of transfusion of very fresh blood (less than 7–10 days of storage) was compared to the standard procedure. The impact of the transfusion of older blood (35–42 days of storage) was not evaluated. Moreover, a lack of power of the studies does not permit the exclusion of a negative impact of transfusion of older blood to some subgroups of vulnerable patients.
Evidence is also conflicting about the risk of deep vein thrombosis related to the transfusion of old blood. In a retrospective cohort study by Spinella et al.47, transfusion of RBC stored for longer than 28 days was associated with an increased incidence of deep vein thrombosis and mortality in critically ill patients. This effect was independent of the red blood cell volume. On the other hand, Katsios et al.48 failed to demonstrate an association between prolonged red cell storage and an increased incidence of deep vein thrombosis in critically ill medical and surgical patients. However, their study suffered a from lack of power, given the relatively low rate of thromboses.
Following this controversy, no changes have been made in transfusion policy with regards to the age of blood. More studies are needed to evaluate the impact of the transfusion of RBC units, focusing on their procoagulant potential, in subgroups of patients at high risk of thrombosis, such as patients with cancer or infection.
Conclusions
To conclude, we show that REV generated during storage of RBC concentrates have a phospholipid-dependent procoagulant activity. The STA®-Procoag-PPL assay is a potentially useful technique to assess the procoagulant activity of RBC concentrates. Thanks to the availability and reproducibility of this technique, it could be used in future clinical studies assessing the impact of transfusion on the incidence of thrombotic events. Even if the method of centrifugation used to isolate EV is available in many laboratories, a shorter period of centrifugation (i.e. double centrifugation at 2,500 g for 15 minutes at room temperature avoiding application of the centrifuge brake as recently proposed33) would facilitate translation into clinical practice.
Acknowledgements
Thanks to Prof. Jean-Claude Osselaer for his logistic help and interesting discussion related to the preliminary studies. The Authors also thank Dr. Elizabeth Wager for language editing.
Footnotes
Funding and resources
This work was supported by grant N. 7.4634.12F from F.R.S.-FNRS Télévie Belgium, a grant from the Fondation Mont-Godinne and the Chair CAF-DCF 2014. Financial support from Alexion Pharmaceuticals (Cheshire, USA) is also gratefully acknowledged.
Authorship contributions
CC and FM contributed equally. BD, AW, NB and LA performed the research. BD designed the research study, analysed the data and wrote the article. DG, JD, VD, BC and JMD contributed essential reagents and tools. BB performed the statistical analysis. BD, BC, CC and FM interpreted the data. All Authors revised the article.
The Authors declare no conflicts of interests.
References
- 1.Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 2.Khorana AA, Francis CW, Blumberg N, et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–81. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Rustum NR, Richard S, Wilton A, et al. Transfusion utilization during adnexal or peritoneal cancer surgery: effects on symptomatic venous thromboembolism and survival. Gynecol Oncol. 2005;99:320–6. doi: 10.1016/j.ygyno.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg. 2007;142:126–32. doi: 10.1001/archsurg.142.2.126. discussion 133. [DOI] [PubMed] [Google Scholar]
- 5.Tan TW, Farber A, Hamburg NM, et al. Blood transfusion for lower extremity bypass is associated with increased wound infection and graft thrombosis. J Am Coll Surg. 2013;216:1005–14. doi: 10.1016/j.jamcollsurg.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar MA, Boland TA, Baiou M, et al. Red blood cell transfusion increases the risk of thrombotic events in patients with subarachnoid hemorrhage. Neurocrit Care. 2013;20:84–90. doi: 10.1007/s12028-013-9819-0. [DOI] [PubMed] [Google Scholar]
- 7.Rubin O, Crettaz D, Canellini J, et al. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang. 2008;95:288–97. doi: 10.1111/j.1423-0410.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney JN, Kouttab N, Kurtis J. Stored red blood cell supernatant facilitates thrombin generation. Transfusion. 2009;49:1569–79. doi: 10.1111/j.1537-2995.2009.02196.x. [DOI] [PubMed] [Google Scholar]
- 9.Rubin O, Delobel J, Prudent M, et al. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2012;53:1744–54. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 10.van der Pol E, Böing AN, Gool EL, Nieuwland R. Recent developments in the nomenclature, presence isolation, detection and clinical impact of extracellular vesicles. J Thromb Haemost. 2016;14:48–56. doi: 10.1111/jth.13190. [DOI] [PubMed] [Google Scholar]
- 11.Patil R, Ghosh K, Shetty S. A simple clot based assay for detection of procoagulant cell-derived microparticles. Clin Chem Lab Med. 2016;54:799–803. doi: 10.1515/cclm-2015-0508. [DOI] [PubMed] [Google Scholar]
- 12.Strasser EF, Happ S, Weiss DR, et al. Microparticle detection in platelet products by three different methods. Transfusion. 2013;53:156–66. doi: 10.1111/j.1537-2995.2012.03720.x. [DOI] [PubMed] [Google Scholar]
- 13.Lacroix R, Judicone C, Poncelet P, et al. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10:437–46. doi: 10.1111/j.1538-7836.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 14.Marchetti M, Tartari CJ, Russo L, et al. Phospholipid-dependent procoagulant activity is highly expressed by circulating microparticles in patients with essential thrombocythemia. Am J Hematol. 2014;89:68–73. doi: 10.1002/ajh.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerotziafas GT, Van Dreden P, Chaari M, et al. The acceleration of the propagation phase of thrombin generation in patients with steady-state sickle cell disease is associated with circulating erythrocyte-derived microparticles. Thromb Haemost. 2012;107:1044–52. doi: 10.1160/TH11-10-0689. [DOI] [PubMed] [Google Scholar]
- 16.Gheldof D, Mullier F, Chatelain B, et al. Inhibition of tissue factor pathway inhibitor increases the sensitivity of thrombin generation assay to procoagulant microvesicles. Blood Coagul Fibrinolysis. 2013;24:567–72. doi: 10.1097/MBC.0b013e328360a56e. [DOI] [PubMed] [Google Scholar]
- 17.Mignon I, Grand F, Boyer F, et al. Thrombin generation and procoagulant phospholipids in patients with essential thrombocythemia and reactive thrombocytosis. Am J Hematol. 2013;88:1007–11. doi: 10.1002/ajh.23553. [DOI] [PubMed] [Google Scholar]
- 18.Han V, Serrano K, Devine DV. A comparative study of common techniques used to measure haemolysis in stored red cell concentrates. Vox Sang. 2010;98:116–23. doi: 10.1111/j.1423-0410.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 19.Lacroix R, Robert S, Poncelet P, et al. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8:2571–4. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 20.Robert S, Lacroix R, Poncelet P, et al. High-sensitivity flow cytometry provides access to standardized measurement of small-size microparticles--brief report. Arterioscler Thromb Vasc Biol. 2012;32:1054–8. doi: 10.1161/ATVBAHA.111.244616. [DOI] [PubMed] [Google Scholar]
- 21.Poncelet P, Robert S, Bouriche T, et al. Standardized counting of circulating platelet microparticles using currently available flow cytometers and scatter-based triggering: forward or side scatter? Cytometry A. 2016;89:148–58. doi: 10.1002/cyto.a.22685. [DOI] [PubMed] [Google Scholar]
- 22.Mullier F, Bailly N, Chatelain C, et al. More on: calibration for the measurement of microparticles: needs, interests, and limitations of calibrated polystyrene beads for flow cytometry-based quantification of biological microparticles. J Thromb Haemost. 2011;9:1679–81. doi: 10.1111/j.1538-7836.2011.04386.x. author reply 1681–2. [DOI] [PubMed] [Google Scholar]
- 23.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101:439–51. [PubMed] [Google Scholar]
- 24.Xiong Z, Oriss TB, Cavaretta JP, et al. Red cell microparticle enumeration: validation of a flow cytometric approach. Vox Sang. 2012;103:42–8. doi: 10.1111/j.1423-0410.2011.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendall MG, Stuart A, Ord JK. Kendall’s Advanced Theory of Statistics. Oxford: Oxford University Press, Inc.; 1992. [Google Scholar]
- 26.Baele PL, Muylle L, Noens L, et al. Guidelines for the transfusion of red cells. Acta Clin Belg. 2008;63:301–12. doi: 10.1179/acb.2008.060. [DOI] [PubMed] [Google Scholar]
- 27.Witwer KW, Buzas EI, Bernis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M. Isolation of extracellular vesicles: determining the correct approach (Review) Int J Mol Med. 2015;36:11–7. doi: 10.3892/ijmm.2015.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu R, Greening DW, Zhu HJ, et al. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152–62. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.György B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muszynski JA, Bale J, Nateri J, et al. Supernatants from stored red blood cell (RBC) units, but not RBC-derived microvsicles, suppress monocyte function in vitro. Transfusion. 2015;55:1937–45. doi: 10.1111/trf.13084. [DOI] [PubMed] [Google Scholar]
- 32.Stewart A, Urbaniak S, Turner M, Bessos H. The application of a new quantitative assay for monitoring of integrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion. 2005;45:1496–503. doi: 10.1111/j.1537-2995.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 33.Lacroix R, Judicone C, Mooberry M, et al. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2013 doi: 10.1111/jth.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mustafa I, Al Marwani A, Mamdouh K, et al. Time dependent assessment of morphological changes: leukodepleted packed red blood cells stored in SAGM. BioMed Res Int. 2016;2016:4529434. doi: 10.1155/2016/4529434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 36.Greenwalt TJ, McGuinness CG, Dumaswala UJ. Studies in red blood cell preservation: 4. Plasma vesicle hemoglobin exceeds free hemoglobin. Vox Sang. 1991;61:14–7. doi: 10.1111/j.1423-0410.1991.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 37.Grisendi G, Finetti E, Manganaro D, et al. Detection of microparticles from human red blood cells by multiparametric flow cytometry. Blood Transfus. 2015;13:274–80. doi: 10.2450/2014.0136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62:699–707. doi: 10.1373/clinchem.2015.248625. [DOI] [PubMed] [Google Scholar]
- 39.Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044–52. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 40.Arraud N, Linares R, Tan S, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–27. doi: 10.1111/jth.12554. [DOI] [PubMed] [Google Scholar]
- 41.Jy W, Horstman LL, Ahn YS. Microparticle size and its relation to composition, functional activity, and clinical significance. Semin Thromb Hemost. 2010;36:876–80. doi: 10.1055/s-0030-1267041. [DOI] [PubMed] [Google Scholar]
- 42.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D, Sun J, Solomon SB, et al. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–95. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–8. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 45.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–29. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fergusson DA, Hebert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–51. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 47.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsios C, Griffith L, Spinella P, et al. Red blood cell transfusion and increased length of storage are not associated with deep vein thrombosis in medical and surgical critically ill patients: a prospective observational cohort study. Crit Care. 2011;15:R263. doi: 10.1186/cc10526. [DOI] [PMC free article] [PubMed] [Google Scholar]