Abstract
A hypercaloric diet combined with a sedentary lifestyle is a major risk factor in the development of insulin resistance, type 2 diabetes mellitus (T2DM) and associated co-morbidities. Standard treatment for T2DM begins with lifestyle modification, and includes oral medications and insulin therapy to compensate for progressive β-cell failure. Current pharmaceutical options for T2DM, however, are limited in that they do not maintain stable, durable glucose control without the need for treatment intensification. Furthermore, each medication is associated with adverse effects ranging from hypoglycaemia to weight gain or bone loss. Unexpectedly, FGF1 and its low mitogenic variants have emerged as potentially safe candidates in restoring euglycaemia, without causing overt adverse effects. In particular, a single peripheral injection of FGF1 can lower glucose to normal levels in hours without the risk of hypoglycaemia. Similarly, a single intracerebroventricular injection of FGF1 can induce long-lasting remission of the diabetic phenotype. This Review discusses potential mechanisms by which centrally administered FGF1 improves central glucose-sensing and peripheral glucose uptake in a sustained fashion. Specifically, we explore the potential crosstalk between FGF1 and glucose-sensing neuronal circuits, hypothalamic neural stem cells and synaptic plasticity. Finally, we highlight therapeutic considerations of FGF1 and compare its metabolic actions to FGF15/FGF19 and FGF21.
Graphical abstract
FGF1 has recently emerged as a potentially safe candidate to restore euglycaemia in type 2 diabetes mellitus. In this Review, Ronald Evans and colleagues discuss possible mechanisms by which central injection of FGF1 can improve central glucose sensing and peripheral glucose uptake, the neuronal circuits involved and therapeutic considerations for translating these findings in rodents to the clinic.
Introduction
Type 2 diabetes mellitus (T2DM) affects over 400 million adults worldwide (~9% of the adult population), a number almost double that seen in 19801,2. The estimated $825 billion spent globally each year in direct expenses related to the management of T2DM will only increase, as the prevalence of the disease is expected to continue to rise in coming decades3. The current pharmacological paradigm of T2DM management involves sequential attempts at normoglycaemia with oral agents, which often culminate in the need for patients to be placed on insulin to approach glycaemic control. An increasing number of new drugs and drug classes have become available to manage the disease; however, despite initial promise, each option remains burdened by a combination of adverse effects and lack of long-term efficacy4,5. In all, the disease has largely remained a chronic and progressive condition. Although stem-cell-derived β-cell replacement could possibly cure diabetes mellitus, successful metrics have not been met. With no widely effective treatment, let alone cure, available and rates of the disease continuing to rise alongside costs, the toll of T2DM seems unyielding.
In this regard, fibroblast growth factor 1 (FGF1) has emerged as a promising solution to the diabetes dilemma. Although FGF1 is considered to be a well-established component of processes such as embryonic development, wound healing, neurogenesis and angiogenesis, the whole-body Fgf1 knockout mouse shows no deficiency in any of these processes6,7. Indeed, only in 2014 was FGF1 shown to be a metabolic hormone crucial for the management of nutrient stress, glycaemic control and insulin sensitivity8. Fgf1 knockout mice develop marked hyperglycaemia and insulin resistance when challenged with a high-fat diet. In ob/ob and db/db mice or diet-induced obesity (DIO) models, peripheral delivery of a single dose of recombinant FGF1 (rFGF1) can normalize blood glucose levels within hours, without inducing hypoglycaemia8. Chronic treatment similarly achieved sustained glucose lowering, with insulin sensitization observed within 3 weeks of initiating therapy8; no desensitization to the effects of FGF1 was observed. This work brought FGF1 to the forefront as a potential new therapeutic approach for insulin sensitization and the treatment of T2DM.
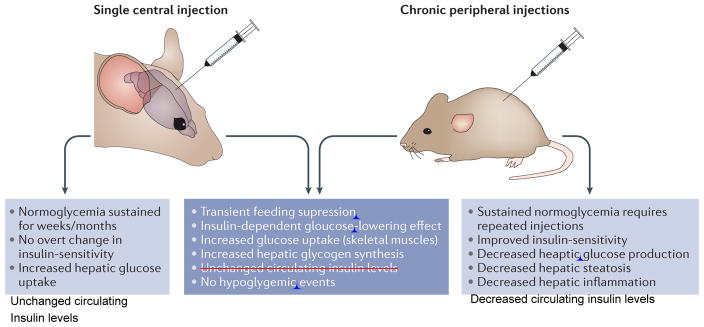
Following on from these original findings, a single central injection of rFGF1 in mice rendered diabetic by DIO and low-dose streptozotocin (STZ) was shown to induce normoglycaemia for up to 18 weeks post-injection9. Long-lasting glucose-lowering effects were also observed after a single central injection of rFGF1 in leptin-deficient ob/ob and leptin-receptor-deficient db/db (on a BKS background) mice, as well as in leptin-receptor-deficient Zucker diabetic fatty rats (ZDF)9. This central effect was associated with increased hepatic glycogen content and was independent of weight loss, reduced food intake, increased insulin sensitivity or increased insulin levels (FIG. 1).
Figure 1. Unique and shared properties of central and peripheral FGF1 injections.
Figure depicting metabolic properties of FGF1 when given either peripherally or centrally.
Although peripheral injection of FGF1 could potentially signal centrally, it is less likely than centrally injected FGF1 to act systemically. This raises the challenging question as to the potential mechanism underlying glycaemic control by the central nervous system (CNS) and whether this can be exploited for therapeutic use. In this Review, we discuss the foundation for FGF1 and the CNS in glycemic control and how these two may interact to jointly improve glucose regulation. We then weigh the metabolic actions of FGF1 against other metabolically active FGFs. We conclude by noting factors that must be evaluated in the further development of FGF based therapeutics for clinical medicine.
Role of FGF1 in glucose control
Feeding suppression
Initial evidence for a central role of FGF1 in feeding suppression stemmed from reports of a postprandial increase in FGF1 and FGF2 levels in the cerebrospinal fluid (CSF) of rats10–12. In this context, glucose was identified as the crucial cue, as both intraperitoneal and intracerebroventricular (ICV) glucose injections were sufficient to induce FGF1 release into the CSF10. Moreover, ventricular microinfusion of FGF1 and FGF2 revealed a dose-dependent suppression of feeding in rats10,12,13.
FGF1 acts in an autocrine and/or paracrine fashion, as binding to heparan sulphate proteoglycans prevents it from entering the circulation, thus necessitating its local production14,15. In the brain, ependymal cells lining the ventricular space constitute the main source of FGF1 production10,16–18. Upon glucose stimulation, FGF1 is secreted by ependymal cells and induces the expression of the early-response markers Fos (which encodes c-Fos) and Hspb1 (which encodes heat shock protein β1) selectively in glucose-sensing tanycytes lining the ventral part of the third ventricle and in periventricular hypothalamic astrocytes10,19–22. The lack of FGF1-induced changes in Fos expression in hypothalamic neurons points to tanycytes and astrocytes as the primary cellular targets of secreted FGF1 in the brain9,19,21. Fos and Hspb1 induction in astrocytes temporally correlates with the feeding inhibition elicited by ICV infusion of FGF1, which is strongest within the initial 2–6 hours but sustained for 24 hours11,19,21. FGF receptor 1 (FGFR1) is widely expressed throughout the hypothalamus23,24; internalization and retrograde transport of radioactively labeled 125I-FGF1 and 125I-FGF2 has been observed in distinct neuronal populations 18 hours, but not 5 hours, after ICV FGF1 administration25. FGF1 has therefore been postulated to suppress food intake in two phases, an early response mediated mainly by hypothalamic astrocytes followed by a neuron-dependent late response19.
Based on the aforementioned findings, the initial negative impact of FGF1 on feeding behaviour is plausibly mediated by its activation of periventricular astrocytes, which in turn are known to modulate the activity of anorexigenic proopiomelanocortin (POMC) and orexigenic agouti-related peptide (AGRP) neurons in the arcuate nucleus26–29. Additionally, the lateral hypothalamic area (LHA) has been prominently implicated in the hypophagia-inducing actions of FGF1. Namely, orexin and melanin-concentrating hormone (MCH) expressing neurons within the LHA are considered important players in the regulation of food intake, arousal and motivated behaviour30. In rats, 125I-FGF1 and 125I-FGF2 are internalized by LHA neurons after ventricular infusion25. Moreover, bilateral LHA administration of antiserum raised against either FGF1, FGF2 or their receptor FGFR1, induced hyperphagia10,31. At the cellular level, FGF1 and FGF2 cause a PKC-dependent inhibition of a significant fraction of glucose-sensitive LHA neurons10. The LHA thus conceivably continues to suppress food intake, for a limited time window, after the initial activation of astrocytes by FGF1 has worn off.
Glucose lowering
In contrast to the feeding effect, the glucose-lowering effect of FGF1 in diabetic settings was discovered only in the past few years, and attempts to identify its cellular and molecular mechanisms are still in the early stages8,9. In addition, the food suppression component of both the central and peripheral FGF1 response is transitory, whereas the glucose-lowering effect is persistent8,9.
In the periphery, the glucose-lowering effect of injected or endogenous FGF1 is in part mediated by the FGF1–FGFR1 signalling cascade. Adipose tissue has been identified as the primary target site of ‘endocrinized’ rFGF1, as AP2-Cre driven Fgfr1 ablation negates its glucose lowering effects in 8-month old DIO mice8. Notably, endogenous FGF1 is induced during the fed state in adipose tissue by the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ), the same nuclear receptor targeted by insulin-sensitizing thiazolidinedione (TZD) drugs32. However, in contrast to TZDs, rFGF1 therapy does not result in adverse effects such as weight gain, bone loss or hepatic steatosis, which creates a very appealing safety profile relative to TZDs.
Endocrinized rFGF1 has been suggested to limit hypothalamic–pituitary–adrenal (HPA) axis activity and produce normoglycaemia in STZ-induced T1DM rats by decreasing hepatic glucose production, hepatic acetyl CoA levels and lipolysis33. However, the idea that suppression of the HPA axis is sufficient to counteract diabetic hyperglycaemia is still controversial34,35. Moreover, central injection of rFGF1 in the lateral or third ventricle of ob/ob mice profoundly lowers blood glucose levels, and does so without affecting plasma corticosterone levels9. A concordant explanation for both models would be that peripherally and centrally injected rFGF1 achieve similar effects through different paths8,9. Thus, peripheral action would be initiated by an FGFR1 signalling cascade in fat, whereas central FGF1 would act through an astrocyte–glial–neuronal circuit36,37.
A second parallel between peripheral and central FGF1 action is that they each seem to rely on intact insulin signalling, as shown by a lack of efficacy in DIO mice treated with the insulin receptor antagonist S961 or in high-dose STZ-treated mice with β-cell ablation9. Additionally, both peripheral and central injections sustain glucose lowering without causing hypoglycaemia8,9. Nonetheless, and despite these parallels, central and peripheral mechanisms reflect significant differences. A single peripheral injection of FGF1 in diabetic rodents triggers acute glucose lowering (within hours) and multiple doses promote insulin sensitization in 3 weeks8. By contrast, a single ICV injection of FGF1 lowers glucose in about a week and sustains this effect beyond 16 weeks, without insulin sensitization9.
Notably, while both FGF1 and FGF2 have been linked to the central regulation of food intake, only FGF1 displays glucose-lowering effects after peripheral injection in ob/ob mice8. This finding is however in contrast to a more recent (2016) metabolomics study, in which intravenous injection of FGF2 into STZ-induced diabetic rats lowered blood glucose levels38. Additionally, to our knowledge, no data describing the effects of central injection of FGF2 in diabetic animals is currently available. Notwithstanding this caveat, deviating functional repertoires of FGF1 and FGF2 despite overlapping receptor specificity could be explained by the possibility that distinct intracellular pathways are engaged following FGF1 and FGF2 receptor binding, evoked by characteristic structural changes in the intracellular receptor domains39.
The sustained normalization of blood glucose levels in diabetic animals after a single ICV injection of FGF1, in the absence of hypoglycaemic episodes, clearly warrants further mechanistic investigations as an attractive alternative to current treatment methods. These results lead us to postulate the existence of an as yet unknown mechanism, by which a single central FGF1 injection might permanently increase peripheral uptake of glucose by the liver and skeletal muscle9.
Central FGF1 effects — possible mechanisms
Glucose-sensing neurons
The significance of the brain in peripheral glucose homeostasis was first demonstrated by Claude Bernard in 1855, who showed that destruction of the hypothalamus in dogs induces hyperglycaemia40. Almost 100 years later, John Mayer proposed the existence of specialized hypothalamic cells that monitor changes in glucose concentrations and commence a corresponding chemical or electrical response41. Definite evidence for the existence of these glucose-sensing neurons (GSNs) was later provided by the identification of hypothalamic neurons that alter their firing activities in response to changes in extracellular glucose concentrations42,43.
Since then, several specific GSN populations have been identified, mainly within the hypothalamus and different brain-stem structures44,45. Depending on whether their firing frequency is increased or decreased in response to rising extracellular glucose levels, they are termed glucose-excited (GE) or glucose-inhibited (GI) neurons, respectively46,47. Neurons can either utilize glucose directly or take it up in the form of lactate, which is produced by neighbouring astrocytes. During euglycaemia, brain glucose levels are believed to be in the range of 0.7–2.5 mM, reaching a maximum of 4.5 mM during severe plasma hyperglycaemia and dropping to 0.2–0.3 mM during plasma hypoglycaemia48,49. Glucose sensing in GE neurons occurs mechanistically similar to that in pancreatic β cells50. High extracellular glucose levels cause an increased intracellular ATP to ADP ratio, closure of ATP-sensitive potassium channels, subsequent depolarization of the plasma membrane and finally opening of voltage-sensitive calcium channels46,51,52. However, additional alternative glucose-sensing mechanisms have been proposed in GE neurons such as the transient response (TRP) channels53 or the dimeric G-protein coupled sweet receptor T1R2–T1R354. Cellular metabolism dependent and independent mechanisms have been reported for GI neurons. In GI neurons of the ventromedial nucleus of the hypothalamus (VMH), firing activity is negatively regulated by high glucose levels that inhibit the AMP-activated kinase (AMPK), which leads to Cl− channel opening and hyperpolarization55,56, whereas the existence of pharmacological glucose detectors has been proposed for orexin neurons57.
Within the hypothalamus, GE and GI neurons have been identified in the arcuate nucleus, the ventromedial hypothalamus, the paraventricular hypothalamus (PVN) and the LHA47. Depending on their anatomical and neurochemical characteristics, the physiological response of GSNs is likely to vary, but altered reproduction, food intake and energy expenditure have so far been shown to be included in their functional repertoire47. In particular, ample evidence exists for the role of GI neurons, most notably in the VMH58–61, in the sympathetic counter-regulatory response to hypoglycaemia, which triggers the secretion of glucagon and epinephrine from pancreatic α cells and the adrenal medulla, respectively, as well as hepatic glucose production62,63.
Of all the GSN populations, only the LHA has so far been directly mechanistically implicated in FGF1 actions. As eluded to earlier, FGF1 application on LHA neurons decreased neuronal activity in 66% of GSNs and only 16% of non-GSNs10. At the same time, none of the tested VMH neurons responded to FGF1. Within the LHA, orexin neurons are inhibited, whereas MCH neurons are excited by physiological changes in glucose64,65, which suggests that orexin neurons are the likely targets of FGF1 actions. Of note, reciprocal synaptic connections exist between orexin neurons and neurons in the ARC, and the orexin receptors OX1R and OX2R are widely expressed in neurons of the ARC, VMH, PVN and dorsomedial hypothalamus (DMH)66,67. In particular, orexin neurons have been shown to control, at least in part via the VMH, the sympathetic output to the liver and skeletal muscles, which modulates glucose production and uptake, respectively68,69. However, the persistent nature of the glucose-lowering effect, long after cellular signalling induced by exogenous FGF1 has abated, clearly suggests that additional mechanisms apart from the mere modulation of the activity of existing neuronal networks are at work.
Tanycytes — neurogenesis
Within the hypothalamus, tanycytes populate the floor and ventro-lateral aspect of the third ventricle, which places them in immediate proximity of the median eminence (ME), ARC, VMH and DMH70,71. They possess a long process that projects into the parenchyma, allowing them to come into close contact with neurons of the hypothalamic nuclei, thus potentially regulating neuroendocrine output and energy homeostasis72. Tanycytes are able to sense altering plasma glucose levels and respond to focally applied glucose by changes in intracellular Ca2+ signalling73,74. Importantly, tanycytes constitute a hypothalamic pool of neurologic progenitor cells in the adult nervous system22,70,75,76, which holds particular relevance when considering the mechanistic ramifications of the long-lasting glucose-lowering effect of FGF1. Lineage-tracing experiments have revealed that the neuronal progeny of tanycytes populate mainly the ARC, but also the VMH, DMH and LHA22,75. Lineage-traced tanycytes have also been shown to give rise to astrocytes and proliferating progenitor cells in the hypothalamic parenchyma22,75,77.
Metabolic stress associated with obesity and diabetes mellitus compromises the functional integrity of hypothalamic circuits that mediate inflammatory and neurodegenerative events, which ultimately contributes to the derailment of energy homeostasis78. In mice, hypothalamic inflammation is evident within the first few days of beginning a high-fat diet (HFD), and prolonged HFD exposure leads to a loss of POMC neurons and apoptosis in mature neurons, which underlines the exceptional vulnerability of the hypothalamus to over-nutrition79–81. The significance of neural regeneration originating from progenitor cells residing in the periventricular zone has been demonstrated most dramatically by the gradual ablation of AGRP neurons, which is compensated for by de novo formation of neurons within the hypothalamic parenchyma82, whereas acute ablation of AGRP neurons in adult mice causes severe anorexia and death83,84. Similarly, weight loss induced by injection of ciliary neurotrophic factor in mice is counteracted by hypothalamic neurogenesis76. At the other end of the spectrum, leptin deficiency or DIO have been shown to disrupt neural stem cell proliferation in adult mice, thus preventing the adaptive remodelling of the arcuate nucleus80,85. Conversely, short-term HFD feeding is reported to promote neurogenesis in tanycytes of the median eminence at pre-adult ages86, potentially indicating an initial compensatory attempt.
Analogous to other neural stem cell populations, tanycyte proliferation is stimulated by insulin-like growth factor 1 (IGF1) and FGF222,87. A similar role for FGF1 in tanycyte self-renewal could promote neurogenesis to repair neural circuits that have deteriorated as a consequence of dietary insults (FIG. 2). Injection of a relatively small number of enhanced green fluorescent protein (eGFP)-labeled leptin receptor (LepR) positive neurons (isolated from embryonic day 13.5 embryos) into the hypothalamus of up to 1-week old LepR-deficient db/db mice was sufficient to cause a marked reduction in blood glucose levels that persisted for 9 and 13 weeks after transplantation88. Mirroring the effect of central FGF1 injection in ob/ob mice, rescue of peripheral glucose homeostasis in adult mice occurred without changes in plasma insulin levels88. Tracing the fate of the injected eGFP-labeled neurons established their synaptic and functional integration into hypothalamic neurocircuits, thereby proving the receptiveness of hypothalamic neuronal circuits to cell-mediated repair following metabolically inflicted damage89.
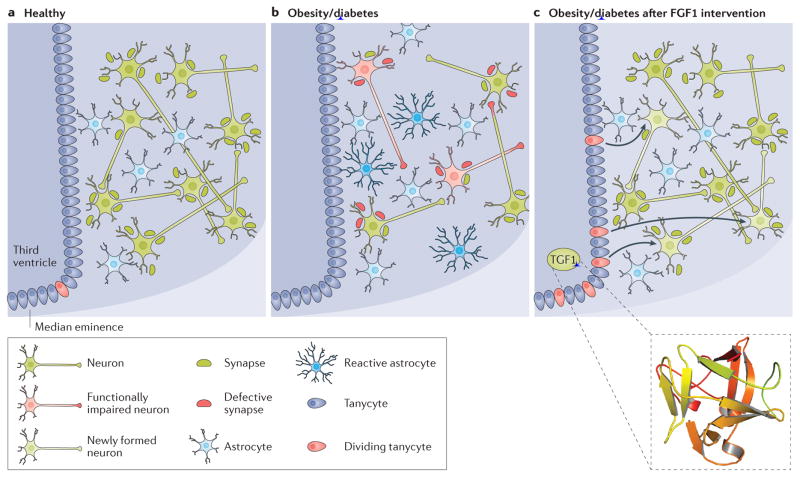
Figure 2. Mechanisms potentially engaged by FGF1 after central injection.
Nutrient excess and lack of exercise are major risk factors for the development of the metabolic syndrome, obesity, diabetes mellitus, hypertension and dyslipidaemia. Pathological changes in peripheral organs are accompanied by hypothalamic inflammation and reduced remodelling of hypothalamic neurocircuits. Astrocytes undergo a process of hypertrophy and hyperplasia, commonly termed reactive astrocytosis or astrogliosis. Increasing neuronal insults, such as inflammatory or excitotoxicity signals contribute to a dysfunctional neuronal firing state and even neurodegeneration. Although rare in adult individuals, neurogenesis, originating from tanycytes in the third ventricular lining or periventricular astrocytes, is believed to amend some of the inflicted damage. However, aggravating metabolic conditions reduce the neurogenic potential of hypothalamic neuroprogenitor cells. Overall, this contributes to decreased central glucose sensing and peripheral glucose clearance. Owing to the limited data currently available, one can only speculate about the actions of fibroblast growth factor 1 (FGF1) within this network. Potentially, central FGF1 remedies the debilitated hypothalamic state in diabetes mellitus by restoring health (or number) of glucose-sensing neurons, (transiently) inducing neurogenesis, suppressing reactive astrocytes and restoring synaptic functionality, which ultimately leads to the observed restoration of normoglycaemia. Structure of human FGF1 (PDB ID 2HZ9).
Synaptic plasticity
Synaptic plasticity has been eluded to as a potential mechanism to explain the long-lasting glucose lowering effect of FGF19. Such plasticity involves changes in synaptic activity and connectivity, thereby providing a mechanism by which neuronal circuits can adapt to and maintain responsiveness across a wide range of stimuli90,91. In contrast to the well-established role of synaptic plasticity in learning and memory formation, its function in the hypothalamic neuronal circuits in control of feeding behaviour has only recently been discovered92,93. The laboratory of Tamás Horváth was the first to show that neurons of the ARC alter their synaptic connections in response to physiological signals of nutrient availability such as ghrelin and leptin, which signal food deprivation and satiety, respectively94. Later, the same group reported that this phenomenon is not an exclusive feature of the ARC. Leptin was found to additionally regulate the synaptic organization of orexin neurons in the LHA95, and ghrelin was shown to modulate synapse formation in the hippocampus96 and ventral tegmental area (VTA)97. Furthermore, hypercaloric challenges in the form of a HFD was also found to induce synaptic remodelling in the ARC98,99. Interestingly, deviations in the synaptic inputs to satiety promoting POMC neurons might contribute to the difference in susceptibility of inbred mouse strains to DIO98.
Depending on the energy state of the organism, synapses are formed or removed and the number of dendrites, as well as the amount of excitatory and inhibitory inputs, can be varied92. Synaptic adaptations are also accompanied by intracellular plasticity, encompassing, for example, mitochondrial fission or fusion in neurons of the ARC and the VMH100–102, or uncoupling of mitochondrial respiration via mitochondrial uncoupling protein 2 in ARC neurons103. In AGRP neurons, synaptic plasticity in the response to ghrelin has been shown to involve a presynaptic AMPK-dependent positive feedback mechanism that allows the glutamatergic activation of AGRP neurons to persist for hours after ghrelin removal and its resetting by leptin administration104. Astrocytes, too, have been connected to the modulation of synaptic plasticity105, by contacting and stripping dysfunctional synapses, releasing glial transmitters and taking up neurotransmitters from the synaptic cleft, thus representing the main defense against excitotoxicity and other neuronal insults106,107. The occurrence of reactive astrogliosis in response to both acute and chronic high-fat feeding79,98 could therefore be potentially damaging to the synaptic plasticity of ARC neurons108. Additional support for the role of astrocytes in synaptic plasticity comes from the findings that hypothalamic astrocytes respond to leptin by changing levels of glutamate and glucose transporters109.
Despite some initial evidence, if and how FGF1 affects synaptic plasticity to induce remission of diabetes mellitus has yet to be determined. Some connections between FGFs and synaptic plasticity, albeit not in the hypothalamus, have already been suggested by earlier studies. FGF2 was reported to promote axonal growth and sprouting after injury110 and to influence hippocampal synaptic plasticity111. FGF1 has been found to modulate the synaptic plasticity of neurons in the cortico–striato–pallidal pathway involving the synergetic activation of FGFR1 and the G protein–coupled α2A adrenergic receptor112. Co-stimulation of both receptors caused a marked synergistic increase in neurite formation and spine density in striato–pallidal neurons, which involved a rapid and long-lasting MEK1/2 mediated ERK1/2 phosphorylation112.
With regards to the enduring nature of the FGF1-driven normalization of blood glucose levels in diabetic animals, the hysteresis effect, which enables sustained activation of AGRP neurons even hours after the initial ghrelin stimulus, is particularly intriguing104,108. Whether central injection of FGF1 elicits a similar signal, causing long-lasting changes in the synaptic plasticity of as yet to be identified neuronal subpopulations and triggering the observed metabolic improvements, is an intriguing possibility. Given their activation by FGF19,19,21 and their effect on neuronal health and functionality, astrocytes represent one avenue by which FGF1 could potentially affect neuronal plasticity in the hypothalamus (FIG. 2).
Central insulin signalling
Considering that FGF1-mediated glucose lowering depends on functional insulin signalling, it is important to note that all of the potentially involved central mechanisms outlined earlier are vulnerable to diminishing insulin signalling. Ablation of insulin receptor signalling in neurons of the ARC113,114, the VTA115 or the dorsal vagal complex in the brainstem116, causes either impaired glucose homeostasis or obesity, whereas deletion of the insulin receptor in steroidogenic factor 1-expressing neurons of the VMH protects against DIO117,118. In 2016, deletion of the insulin receptor in astrocytes was shown to negatively affect their function and morphology, causing changes in glucose transport across the blood–brain barrier and ultimately impeding ARC neurons from monitoring and responding to systemic glucose changes27. Moreover, as discussed earlier, DIO and hyperinsulinaemia put a brake on neurogenesis in the hypothalamus80,85, which could imply that insulin signalling must not come to a complete halt in order for a potential neurogenic effect of FGF1 to occur. Finally, the role of central insulin resistance in neuronal plasticity has become increasingly recognized as a potential cause of the development of cognitive impairment, which involves synapse deterioration and neurodegeneration119,120.
Central FGF1-induced peripheral glucose uptake
Additional studies are required to address how ICV FGF1 induces increases in peripheral glucose clearance in the liver and skeletal muscle, without affecting circulating insulin levels, glucose-induced insulin secretion, insulin sensitivity or hepatic glucose output. Generally, GSNs are best known for their control of both sympathetic (SNS) and parasympathetic (PNS) branches of the autonomic nervous system44. In response to altering glucose levels, the range of actions mediated by the PNS includes the stimulation of pancreatic β-cell proliferation, insulin secretion and the secretion of glucagon during hypoglycaemia. SNS activity stimulates glucagon secretion and inhibits insulin secretion, promotes thermogenesis in brown adipose tissue, stimulates epinephrine secretion by the adrenal glands, enhances lipolysis in white adipose tissue and regulates hepatic glucose production44,121. There are however some indications that the brain has the capacity to lower blood glucose levels via both insulin-dependent and insulin-independent mechanisms20. In rats, electrical stimulation of VMH neurons or leptin injection into the VMH, but not the LHA, has been shown to increase peripheral glucose uptake, including that in skeletal muscle, independently of circulating insulin levels; these effects are abolished by blockade of the SNS122,123. Furthermore, leptin has been shown to rescue and restore normogylcaemia in insulin-deficient mice by reducing hepatic glucose production while increasing tissue glucose124,125.
Metabolic improvements, originating from central FGF1 injections, are also possibly caused by changes in the gut–liver–brain axis20,126. In particular, the hepatic portal vein has a major role in hepatic and peripheral glucose disposal127,128. The portal vein is heavily innervated by vagal afferents expressing nutrient sensors and relaying the information to higher brain centres128. Glucose delivery directly into the portal vein increases net hepatic glucose uptake by a neural mechanism, as denervation of the liver or intraportal infusion of adrenergic blockers and acetylcholine reduces or increases, respectively, net hepatic glucose uptake in response to portal glucose delivery127,129,130.
Nevertheless, FGF1 is likely to engage novel (neural) glucose-regulatory mechanisms or combinations thereof, as similar findings have so far not been reported. Likewise, the involvement of a humoral factor cannot be excluded at this stage.
Barrier to FGF1 success — mitogenicity
Though isolated as an in vitro growth factor, wild-type FGF1 presents the issue of potential in vivo mitogenicity. However, whole-body knockout of FGF1 causes no change in tissue growth and the only known defects are adipose inflammation and a severe form of diabetes mellitus in response to dietary stress32. In addition, several transgenic mouse lines constitutively over-expressing FGF1 have no described tumours or organ growth, which suggests in vivo safety over long periods of exposure131–133. Gene expression array studies have found FGF1 levels increased in breast, prostate and ovarian cancers, but a contribution beyond correlation has not been established129. Perhaps more importantly, targeted structure–function studies clearly suggest that the mitogenic and glucose-lowering potentials of FGF1 are separable. FGF1-induced growth in vitro is predominantly associated with FGFR3 and FGFR4, whereas glucose-lowering is mediated by FGFR18,134,135. Indeed, FGFR3 and FGFR4 binding of FGF1 can be greatly diminished by mutations and/or deletions in FGF1 that leave its glucose lowering potential fully intact8. Thus, the potential for a therapeutically viable fully non-mitogenic human FGF1 variant seems highly plausible. Such a variant could be useful in the context of either a peripheral or central therapeutic injection strategy.
Alternatives to FGF1
As a class, FGF-targeted pharmaceuticals are not completely new prospects. Various members of the FGF family have been explored to treat conditions beyond metabolic disorders. Intravenous recombinant human FGF7 is an FDA-approved treatment for oral mucositis136 whereas other members of the FGF family are being developed for the treatment of ischaemia, cerebrovascular disease and cardiovascular disease135. In addition, various FGFR modulators are in clinical trials for cancer treatment137. Although the high potential benefits of a non-mitogenic FGF1 therapy in the treatment of diabetes mellitus and its complications is tantalizing, the actions of FGF1 must still be validated in clinical trials138,139.
Other FGFs, namely FGF 15/19 (FGF19 being the human form of the rodent FGF15) and FGF21, are known factors in energy homeostasis. To a certain extent, FGF19 and FGF21 have shown metabolic benefits upon central injection (Table 1). ICV injections of FGF19 in both ob/ob and DIO rodents yielded insulin-independent glucose lowering through a CNS mediated mechanism, with acute improvements occurring within a few hours of injection140–143. FGF21 injected ICV to DIO rodents garners metabolic benefits in the form of increased energy output and insulin sensitivity linked to weight loss144,145. In each case, the FGF effect either required multiple injections or did not have duration comparable to a one-time central injection of FGF1. Additionally, concern remains regarding the side effects of therapy with FGF19 and FGF21. FGF19 overexpression has been shown to promote hepatocellular carcinoma146,147, and systemic FGF21 administration has not been fully divested from noticeable bone loss148. However, non-mitogenic FGF19 variants have been developed and acute benefits of FGF21 are currently being explored135. At this point, whether central adverse effects mirror these peripheral ones is unclear.
Table 1.
Comparison of FGF1 to FGF15/19 & FGF21 in diabetic animal models
| General properties1,2 | FGF1 | FGF15/19 | FGF21 |
|---|---|---|---|
| Receptor specificity | All 7 isoforms; Glucose lowering via FGFR1 | Primarily FGFR1 and FGFR4 | Primarily FGFR1 |
| Receptor binding requirements | Heparin dependent | β-klotho co-receptor dependent | β-klotho co-receptor dependent |
| Classification | Autocrine/Paracrine | Autocrine/Endocrine | Autocrine/Endocrine |
| Induction prompt & tissue | Fed state - Adipose | Fed state - Gut | Fasted state - Liver |
| Central actions3,7 | |||
| Feeding suppression | Transient | Transient | None* |
| Glucose lowering (duration) | Months | Hours | Hours |
| Hypoglycemic events | No | NA | NA |
| Insulin sensitizer** | No | No | No |
| Peripheral actions2,8–12 | |||
| Feeding suppression | Transient | None | None*** |
| Glucose lowering (duration) | 3–7 Days | Hours | Hours |
| Hypoglycemic events | None | NA | NA |
| Insulin sensitizer** | Yes | No | No |
Increased food intake
Defined as increased insulin sensitivity not secondary to body weight loss (e.g. TZDs)
Food consumption increased when normalized to dropping body weight
NA, no available data
References
Degirolamo, C., Sabbà, C. & Moschetta, A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 15, 51–69 (2016).
Suh, J. M. et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513, 436–439 (2014).
Scarlett, J. M. et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat. Med. 22, 800–806 (2016).
Ryan, K. K. et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154, 9–15 (2013).
Morton, G. J. et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest 123, 4799–4808 (2013).
Marcelin, G. et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab 3, 19–28 (2014).
Owen, B. M. et al. FGF21 Acts Centrally to Induce Sympathetic Nerve Activity, Energy Expenditure, and Weight Loss. Cell metabolism 20, 670–677 (2014).
Kir, S. et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331, 1621–1624 (2011).
Fu, L. et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145, 2594–2603 (2004).
Xu, J. et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab 297, E1105–E1114 (2009).
Coskun, T. et al. Fibroblast Growth Factor 21 Corrects Obesity in Mice. Endocrinology 149, 6018–6027 (2008).
Xu, J. et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 (2009).
Clinical considerations for FGF1
Therapeutically, an intracranial injection might not be necessary in order to achieve a robust central effect. Achieving normoglycaemia resembling that of central FGF1 injection could possibly occur via an intranasal route. Derivatives of FGF1 given intranasally are able to locally induce angiogenesis and neuronal survival in rodents, with penetrance across the blood–brain barrier greatly enhanced when attached to defined transporter proteins149,150. Migration into the CNS is believed to occur through a combination of movement along the olfactory nerve, nasal mucosa capillaries and through cerebrospinal fluid via the cribriform plate149,151. Intranasal delivery of large biologic proteins is conceptually advantageous; however, this approach has yet to be adopted in an approved prescription drug. Also, whether a single nasal injection would be sufficient to confer the equivalent long-term benefits seen with central injection is unclear. In addition, even if sufficient levels of FGF1 could be transferred into the CNS this might or might not be optimal for key target sites. Developing a therapeutically effective FGF1 targeted to the CNS by means other than direct intracranial application would first require a better understanding of the specific brain regions mediating the peptide’s metabolic actions. Nonetheless, the idea of a non-invasive route remains appealing in that it would greatly improve accessibility, as self-delivery of doses would be possible.
Controlling blood glucose by either peripheral or central delivery will go far to alleviate short-term complications of diabetes mellitus, such as hypoglycaemic episodes, hyperosmolar hyperglycaemic states, diabetic ketoacidosis and diabetic comas. Ultimately though, the success of any intervention to treat or possibly cure diabetes mellitus will rely on more than regulating glucose levels. The value of any solution must also be judged by its ability to limit chronic complications of diabetes mellitus, both microvascular (retinopathy, nephropathy and neuropathy) and macrovascular (namely cardiovascular disease). Traditionally, the standard of treatment has focused heavily on achieving glucose, and in turn HbA1c targets. Current evidence indicates that microvascular complications can be greatly limited by reaching designated HbA1c goals. The same clear, direct benefit of consistent glycaemic control in risk reduction as measured by HbA1c cannot, however, be applied as confidently to macrovascular concerns152–154. It must be noted that in either case, increasing evidence points towards large intra-day fluctuations in glucose, specifically in postprandial glucose, as a driver of complications, independent of chronic hyperglycaemia. These acute changes are believed to create periods of exacerbated inflammation, oxidative stress and off-target glycation. The detriment of intra-day hyperglycaemia applies even in individuals with acceptable HbA1c levels, who might be subject to multiple peaks and troughs throughout the day despite apparently sufficient metabolic control155–157. Current antidiabetic options, insulin in particular, frequently subject patients to these large variations in glucose levels. This phenomenon has not been observed in preclinical FGF1 studies thus far.
Along with large glycaemic swings, the contribution of T2DM to macrovascular complications can also be attributed to disruptions in PPARγ pathways that promote inflammation via vascular endothelial cells158,159. As already discussed, FGF1 works along the PPARγ axis. Hepatically, rFGF1 is able to reduce inflammation, thus by extension it could confer similar benefits on the cardiovascular system160. Furthermore, the ability of peripherally injected FGF1 to relieve insulin resistance8 and normalize insulin levels in patients with T2DM would logically be expected to reduce the risk of stroke161,162, diabetic retinopathy and hypertension163.
Limitations of the current data
The failure of central FGF1 to work in DIO mice raises a major concern as to whether it would be effective in patients with T2DM and obesity. Alas, no data on peripheral or central actions of FGF1 in humans or non-human primates is presently available. It is therefore important to emphasize that most of the findings discussed in this Review were obtained from work performed in rodents; any extrapolation to humans must be done so critically. Despite the vast amount of knowledge obtained from animal models, only a finite number of antidiabetic drugs in preclinical development have successfully advanced to clinical use. To some extent, species-specific variations in glucose regulation can be blamed for the limited interspecies translatability. Notable examples include differences in the major site of peripheral glucose disposal, namely the liver in rodents and skeletal muscle in humans. Differences also exist in the hepatic glucose production rate, islet architecture, islet innervation and glucose sensing by pancreatic β cells164,165.
Species differences on a genomic and proteomic level, as well as deviations in pathway engagement have been described with regards to glucose sensing in pancreatic β cells, which suggests a similar scenario for their central counterparts164. Furthermore, inbred diabetic mouse or rat models are often diabetic of monogenetic origin present from birth. These strains acquire rapid onset of obesity early in life mainly due to hyperphagia and decreased energy expenditure, with only moderate vascular and inflammatory complications. These models thus do not fully reflect the multifactorial disease aetiology in humans, in which environmental influences are superimposed on genetic risk factors and disease onset is more gradual and confounded by microvascular and macrovascular defects166,167. This limitation is particularly relevant in the development and treatment of T2DM. With the jury still out on the actions of FGF1 on the HPA axis, one must also consider that the adverse actions of toxic glucose analogues such as STZ (which are used for the induction of a diabetic pathophysiology in rodents) are not confined to the pancreas and include disruption to the HPA axis in their repertoire168.
Conclusions
Clear mechanistic understandings of the endogenous and pharmacologic actions of FGF1 have yet to be described. However, the remarkable ability of peripherally delivered FGF1 to rapidly restore normal glycaemic levels in diabetic mouse models and function as an insulin sensitizer, combined with the longevity in glucose control achieved with central delivery, alludes to exciting opportunities for entirely new therapeutic approaches in the treatment of T2DM. This enthusiasm will gain credibility with preclinical results in higher-order mammals, and the development of truly non-mitogenic analogues.
Review criteria.
PubMed was searched for relevant topics, using the search terms “FGF1”, “FGF”, “type 2 diabetes”, “glucose-sensing neurons”, “glucose-lowering mechanisms”, “glucose homeostasis”, “glucose uptake”, “glycemic control”, “neuronal control”, “central control”, “food intake”, “appetite”, “neurogenesis”, ”synaptic plasticity”, “neuronal plasticity”, “tanycytes”,”hypothalamus”, “hypothalamic inflammation” and “intranasal”, and combinations thereof. No publication time constraints were applied. References cited in this article include both original research and reviews by experts in the field.
Key points.
Peripherally or centrally injected FGF1 confers potent metabolic benefits in type 2 diabetes mellitus
FGF1 produced by ependymal cells of the central nervous system interacts with tanycytes, astrocytes and glucose-sensing neurons of the hypothalamus to influence feeding and glycaemic control
Functional recovery of hypothalamic glucose-sensing neurons, as well as neural regeneration and synaptic plasticity, might be fundamental in achieving sustained remission of type 2 diabetes mellitus
FGF1 has the potential to improve glycaemic control, in addition to microvascular and macrovascular complications, in patients with type 2 diabetes mellitus
Acknowledgments
E.G. is supported by the Swiss National Science Foundation (grant P2EZP3_172178). C.P.M. is a Howard Hughes Medical Institute Medical Research Fellow. R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology, and is supported by NIH grants (DK057978, HL088093, HL105278, and ES010337), an NCI Cancer Center Support Grant (CA014195), the Glenn Foundation for Medical Research, and grants from Steven and Lisa Altman and the Leona M. and Harry B. Helmsley Charitable Trust (2012-PG-MED002).
Biographies
Emanuel Gasser received his Ph.D. in molecular biology at the Institute for Molecular Health Sciences at ETH Zurich, Switzerland. He is currently working as a postdoctoral research fellow at the Salk Institute for Biological Sciences, La Jolla, California, USA. His current research focuses on the role of nuclear receptors within the neuroendocrine system in the control of energy homeostasis.
Christopher P. Moutos is a medical student at the University of Arkansas for Medical Sciences and current Howard Hughes Medical Institute Medical Research Fellow at the Salk Institute for Biological Sciences, La Jolla, California, USA. His current research interests include understanding the pathophysiology of metabolic diseases and using this knowledge to improve clinical outcomes.
Michael Downes received his Ph.D. in molecular biology from the University of Queensland, Australia and is a senior staff scientist at the Salk Institute for Biological Sciences, La Jolla, California, USA. His research interests are focused on the roles of nuclear hormone receptors in health and disease.
Ronald M. Evans is an Investigator of the Howard Hughes Medical Institute and March of Dimes Chair in Molecular and Developmental Biology at the Salk Institute where his research centers on the roles of nuclear hormone receptors in development, metabolism and the treatment of disease. He received his Ph.D. from UCLA.
Footnotes
Author contributions
E.G. and C.P.M. contributed equally to this article. E.G., C.P.M., M.D. and R.M.E. researched data for the article and wrote the manuscript. All authors contributed to discussion of the content and reviewed and/or edited the manuscript before submission.
Competing interests statement
M.D. and R.M.E are co-inventors of mutated FGF1 proteins and methods of their use (US Patent No. 8,906,854) and might be entitled to royalties.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Competing interests statement
M.D. and R.M.E are co-inventors of mutated FGF1 proteins and methods of their use, and might be entitled to royalties.
Subject ontology terms
Health sciences/Endocrinology/Endocrine system and metabolic diseases/Diabetes/Type 2 diabetes
[URI/692/163/2743/137/773]
Health sciences/Anatomy/Nervous system/Central nervous system
[URI/692/698/1688/1366]
Health sciences/Anatomy/Nervous system/Peripheral nervous system
[URI/692/698/1688/1959]
Health sciences/Medical research/Experimental models of disease
[URI/692/308/1426]
Health sciences/Health care/Therapeutics/Hormonal therapies
[URI/692/700/565/238]
References
- 1.Global Report on Diabetes. World Health Organization; 2016. [Google Scholar]
- 2.Jaacks LM, Siegel KR, Gujral UP, Narayan KMV. Type 2 diabetes: A 21st century epidemic. Best Practice & Research Clinical Endocrinology & Metabolism. 2016;30:331–343. doi: 10.1016/j.beem.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 3.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM. Diabetes: Advances in Diagnosis and Treatment. JAMA. 2015;314:1052–1062. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- 5.Cuddihy RM, Philis-Tsimikas A, Nazeri A. Type 2 Diabetes Care and Insulin Intensification. The Diabetes Educator. 2011;37:111–123. doi: 10.1177/0145721710388426. [DOI] [PubMed] [Google Scholar]
- 6.Itoh N, Nakayama Y, Konishi M. Roles of FGFs As Paracrine or Endocrine Signals in Liver Development, Health, and Disease. Front Cell Dev Biol. 2016;4:31. doi: 10.3389/fcell.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 8.Suh JM, et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014;513:436–439. doi: 10.1038/nature13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarlett JM, et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med. 2016;22:800–806. doi: 10.1038/nm.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oomura Y, et al. A new brain glucosensor and its physiological significance. Am J Clin Nutr. 1992;55:278S–282S. doi: 10.1093/ajcn/55.1.278s. [DOI] [PubMed] [Google Scholar]
- 11.Hanai K, et al. Central action of acidic fibroblast growth factor in feeding regulation. Am J Physiol. 1989;256:R217–23. doi: 10.1152/ajpregu.1989.256.1.R217. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki K, et al. Effects of fibroblast growth factors and platelet-derived growth factor on food intake in rats. Brain Research Bulletin. 1991;27:327–332. doi: 10.1016/0361-9230(91)90120-9. [DOI] [PubMed] [Google Scholar]
- 13.De Saint Hilaire Z, Nicolaïdis S. Enhancement of slow wave sleep parallel to the satiating effect of acidic fibroblast growth factor in rats. Brain Research Bulletin. 1992;29:525–528. doi: 10.1016/0361-9230(92)90094-e. [DOI] [PubMed] [Google Scholar]
- 14.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tooyama I, et al. Production of antisera to acidic fibroblast growth factor and their application to immunohistochemical study in rat brain. Neuroscience. 1991;40:769–779. doi: 10.1016/0306-4522(91)90011-c. [DOI] [PubMed] [Google Scholar]
- 17.Fallon JH, et al. Localization of Acidic Fibroblast Growth Factor within the Mouse Brain Using Biochemical and Immunocytochemical Techniques. Growth Factors. 2009;6:139–157. doi: 10.3109/08977199209011017. [DOI] [PubMed] [Google Scholar]
- 18.Chen MS, et al. Human FGF1 promoter is active in ependymal cells and dopaminergic neurons in the brains of F1B-GFP transgenic mice. Developmental Neurobiology. 2015;75:232–248. doi: 10.1002/dneu.22225. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, et al. Feeding suppression by fibroblast growth factor-1 is accompanied by selective induction of heat shock protein 27 in hypothalamic astrocytes. European Journal of Neuroscience. 2001;13:2299–2308. doi: 10.1046/j.0953-816x.2001.01606.x. [DOI] [PubMed] [Google Scholar]
- 20.Scarlett JM, Schwartz MW. Gut-brain mechanisms controlling glucose homeostasis. F1000Prime Rep. 2015;7:12. doi: 10.12703/P7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki S, Li AJ, Akaike T, Imamura T. Intracerebroventricular infusion of fibroblast growth factor-1 increases Fos immunoreactivity in periventricular astrocytes in rat hypothalamus. Neurosci Lett. 2001;300:29–32. doi: 10.1016/s0304-3940(01)01535-x. [DOI] [PubMed] [Google Scholar]
- 22.Robins SC, et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo A, et al. Immunohistochemical localization in the rat brain of an epitope corresponding to the fibroblast growth factor receptor-1. Neuroscience. 1994;60:49–66. doi: 10.1016/0306-4522(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson IA, Johnson EM. Fibroblast growth factor receptor-bearing neurons in the CNS: Identification by receptor-mediated retrograde transport. Journal of Comparative Neurology. 1991;313:693–706. doi: 10.1002/cne.903130412. [DOI] [PubMed] [Google Scholar]
- 26.Kim JG, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17:908–910. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Cáceres C, et al. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell. 2016;166:867–880. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Qi Y, Yang Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Reports. 2015;11:798–807. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Sweeney P, Qi Y, Xu Z, Yang Y. Activation of hypothalamic astrocytes suppresses feeding without altering emotional states. Glia. 2016;64:2263–2273. doi: 10.1002/glia.23073. [DOI] [PubMed] [Google Scholar]
- 30.Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19:198–205. doi: 10.1038/nn.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki K, et al. Actions of Acidic Fibroblast Growth Factor Fragments on Food Intake in Rats. Obesity. 1995;3:697S–706S. doi: 10.1002/j.1550-8528.1995.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 32.Jonker JW, et al. A PPARγ–FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry RJ, et al. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic–pituitary–adrenal axis. Nat Commun. 2015;6:6980. doi: 10.1038/ncomms7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry RJ, et al. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Med. 2014;20:759–763. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morton GJ, Meek TH, Matsen ME, Schwartz MW. Evidence against hypothalamic-pituitary-adrenal axis suppression in the antidiabetic action of leptin. J Clin Invest. 2015;125:4587–4591. doi: 10.1172/JCI82723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barak Y, et al. PPARγ Is Required for Placental, Cardiac, and Adipose Tissue Development. Molecular Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 37.Forman BM, et al. 15-Deoxy-Δ12,14-Prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 38.Lin X, et al. Metabolic effects of basic fibroblast growth factor in streptozotocin-induced diabetic rats: A (1)H NMR-based metabolomics investigation. Sci Rep. 2016;6:36474. doi: 10.1038/srep36474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarabipour S, Hristova K. Mechanism of FGF receptor dimerization and activation. Nat Commun. 2016;7:10262. doi: 10.1038/ncomms10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernard C. Leçons de physiologie expérimentale appliquée à la médecine. J.B. Baillière et fils; Paris: 1855. [Google Scholar]
- 41.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 42.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- 43.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and Osmosensitive Neurones of the Rat Hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 44.Steinbusch L, Labouèbe G, Thorens B. Brain glucose sensing in homeostatic and hedonic regulation. Trends in Endocrinology & Metabolism. 2015;26:455–466. doi: 10.1016/j.tem.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Marty N, Dallaporta M, Thorens B. Brain Glucose Sensing, Counterregulation, and Energy Homeostasis. Physiology. 2007;22:241–251. doi: 10.1152/physiol.00010.2007. [DOI] [PubMed] [Google Scholar]
- 46.Jordan SD, Könner AC, Brüning JC. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci. 2010;67:3255–3273. doi: 10.1007/s00018-010-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C. Hypothalamic glucose sensing: making ends meet. Front Syst Neurosci. 2014;8:402. doi: 10.3389/fnsys.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silver IA, Erecińska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase Is the Likely Mediator of Glucosensing in Both Glucose-Excited and Glucose-Inhibited Central Neurons. Diabetes. 2002;51:2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- 50.Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose Sensing in Pancreatic β-Cells. Diabetes. 2001;50:1–11. doi: 10.2337/diabetes.50.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Parton LE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 52.Kong D, et al. Glucose Stimulation of Hypothalamic MCH Neurons Involves KATP Channels, Is Modulated by UCP2, and Regulates Peripheral Glucose Homeostasis. Cell metabolism. 2010;12:545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fioramonti X, Lorsignol A, Taupignon A, Pénicaud L. A New ATP-Sensitive K+ Channel–Independent Mechanism Is Involved in Glucose-Excited Neurons of Mouse Arcuate Nucleus. Diabetes. 2004;53:2767–2775. doi: 10.2337/diabetes.53.11.2767. [DOI] [PubMed] [Google Scholar]
- 54.Ren X. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3 doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol, Cell Physiol. 2009;297:C750–8. doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of Pre- and Postsynaptic Influences on Glucosensing Neurons in the Ventromedial Hypothalamic Nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- 57.González JA, Jensen LT, Fugger L, Burdakov D. Metabolism-Independent Sugar Sensing in Central Orexin Neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan O, Sherwin R. Influence of VMH fuel sensing on hypoglycemic responses. Trends in Endocrinology & Metabolism. 2013 doi: 10.1016/j.tem.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCrimmon RJ, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57:444–450. doi: 10.2337/db07-0837. [DOI] [PubMed] [Google Scholar]
- 60.Kang L, et al. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 61.Meek TH, et al. Functional identification of a neurocircuit regulating blood glucose. Proc Natl Acad Sci U S A. 2016;113:E2073–82. doi: 10.1073/pnas.1521160113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verberne T, Sabetghadam A, Korim W. Neural pathways that control the glucose counterregulatory response. Front Neurosci. 2014;8 doi: 10.3389/fnins.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatric endocrinology reviews: PER. 2011;9:463–73. quiz 474–5. [PMC free article] [PubMed] [Google Scholar]
- 64.Burdakov D, et al. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 65.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological Changes in Glucose Differentially Modulate the Excitability of Hypothalamic Melanin-Concentrating Hormone and Orexin Neurons In Situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential Distribution and Regulation of OX1 and OX2 Orexin/Hypocretin Receptor Messenger RNA in the Brain upon Fasting. Hormones and Behavior. 2000;37:335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- 67.Berthoud HR, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiuchi T, et al. Hypothalamic Orexin Stimulates Feeding-Associated Glucose Utilization in Skeletal Muscle via Sympathetic Nervous System. Cell metabolism. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Yi CX, et al. A Major Role for Perifornical Orexin Neurons in the Control of Glucose Metabolism in Rats. Diabetes. 2009;58:1998–2005. doi: 10.2337/db09-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36:91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodman T, Hajihosseini MK. Hypothalamic tanycytes—masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front Neurosci. 2015;9:995. doi: 10.3389/fnins.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rizzoti K, Lovell-Badge R. Pivotal role of median eminence tanycytes for hypothalamic function and neurogenesis. Mol Cell Endocrinol. 2016 doi: 10.1016/j.mce.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 73.Dale N. Purinergic signaling in hypothalamic tanycytes: Potential roles in chemosensing. Semin Cell Dev Biol. 2011;22:237–244. doi: 10.1016/j.semcdb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 74.Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol (Lond) 2011;589:2275–2286. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haan N, et al. Fgf10-Expressing Tanycytes Add New Neurons to the Appetite/Energy-Balance Regulating Centers of the Postnatal and Adult Hypothalamus. J Neurosci. 2013;33:6170–6180. doi: 10.1523/JNEUROSCI.2437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the Hypothalamus of Adult Mice: Potential Role in Energy Balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 77.Pak T, Yoo S, Miranda-Angulo AM, Wang H, Blackshaw S. Rax-CreERT2 Knock-In Mice: A Tool for Selective and Conditional Gene Deletion in Progenitor Cells and Radial Glia of the Retina and Hypothalamus. PLoS ONE. 2014;9:e90381. doi: 10.1371/journal.pone.0090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends in Endocrinology & Metabolism. 2013;24:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, Tang Y, Cai D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moraes JC, et al. High-Fat Diet Induces Apoptosis of Hypothalamic Neurons. PLoS ONE. 2009;4:e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pierce AA, Xu AW. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 2010;30:723–730. doi: 10.1523/JNEUROSCI.2479-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gropp E, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 84.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 85.McNay DEG, Briançon N, Kokoeva MV, Maratos-Flier E, Flier JS. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest. 2012;122:142–152. doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee DA, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pérez Martín M, et al. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. European Journal of Neuroscience. 2010;31:1533–1548. doi: 10.1111/j.1460-9568.2010.07220.x. [DOI] [PubMed] [Google Scholar]
- 88.Czupryn A, et al. Transplanted hypothalamic neurons restore leptin signaling and ameliorate obesity in db/db mice. Science. 2011;334:1133–1137. doi: 10.1126/science.1209870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sternson SM. Neuron transplantation partially reverses an obesity disorder in mice. Cell metabolism. 2012;15:133–134. doi: 10.1016/j.cmet.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 90.Feldman DE. Synaptic Mechanisms for Plasticity in Neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Citri A, Malenka RC. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology. 2007;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 92.Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Zeltser LM, Seeley RJ, Tschöp MH. Synaptic plasticity in neuronal circuits regulating energy balance. Nat Neurosci. 2012;15:1336–1342. doi: 10.1038/nn.3219. [DOI] [PubMed] [Google Scholar]
- 94.Pinto S, et al. Rapid Rewiring of Arcuate Nucleus Feeding Circuits by Leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 95.Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons. Cell metabolism. 2005;1:279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 96.Diano S, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 97.Abizaid A, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Horvath TL, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benani A, et al. Food Intake Adaptation to Dietary Fat Involves PSA-Dependent Rewiring of the Arcuate Melanocortin System in Mice. J Neurosci. 2012;32:11970–11979. doi: 10.1523/JNEUROSCI.0624-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schneeberger M, et al. Mitofusin 2 in POMC Neurons Connects ER Stress with Leptin Resistance and Energy Imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toda C, et al. UCP2 Regulates Mitochondrial Fission and Ventromedial Nucleus Control of Glucose Responsiveness. Cell. 2016;164:872–883. doi: 10.1016/j.cell.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dietrich MO, Liu ZW, Horvath TL. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell. 2013;155:188–199. doi: 10.1016/j.cell.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrews ZB, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger States Switch a Flip-Flop Memory Circuit via a Synaptic AMPK-Dependent Positive Feedback Loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Theodosis DT, Poulain DA, Oliet SHR. Activity-Dependent Structural and Functional Plasticity of Astrocyte-Neuron Interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- 106.García-Cáceres C, Fuente-Martín E, Argente J, Chowen JA. Emerging role of glial cells in the control of body weight. Mol Metab. 2012;1:37–46. doi: 10.1016/j.molmet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: New Roles for the Synaptic Stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 108.Dietrich MO, Horvath TL. Synaptic plasticity of feeding circuits: hormones and hysteresis. Cell. 2011;146:863–865. doi: 10.1016/j.cell.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 109.Fuente-Martín E, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest. 2012;122:3900–3913. doi: 10.1172/JCI64102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fagan AM, et al. Endogenous FGF-2 Is Important for Cholinergic Sprouting in the Denervated Hippocampus. J Neurosci. 1997;17:2499–2511. doi: 10.1523/JNEUROSCI.17-07-02499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Terlau H, Seifert W. Fibroblast Growth Factor Enhances Long-term Potentiation in the Hippocampal Slice. European Journal of Neuroscience. 1990;2:973–977. doi: 10.1111/j.1460-9568.1990.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 112.Flajolet M, et al. FGF acts as a co-transmitter through adenosine A2A receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Könner AC, et al. Insulin Action in AgRP-Expressing Neurons Is Required for Suppression of Hepatic Glucose Production. Cell metabolism. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 114.Hill JW, et al. Phosphatidyl Inositol 3-Kinase Signaling in Hypothalamic Proopiomelanocortin Neurons Contributes to the Regulation of Glucose Homeostasis. Endocrinology. 2009;150:4874–4882. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Könner AC, et al. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell metabolism. 2011;13:720–728. doi: 10.1016/j.cmet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 116.Filippi BM, Yang CS, Tang C, Lam TKT. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell metabolism. 2012;16:500–510. doi: 10.1016/j.cmet.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Klöckener T, et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vogt MC, Brüning JC. CNS insulin signaling in the control of energy homeostasis and glucose metabolism - from embryo to old age. Trends Endocrinol Metab. 2013;24:76–84. doi: 10.1016/j.tem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 119.Blázquez E, Velázquez E, Hurtado-Carneiro V, Ruiz-Albusac JM. Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol (Lausanne) 2014;5:161. doi: 10.3389/fendo.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Felice FG, Ferreira ST. Inflammation, Defective Insulin Signaling, and Mitochondrial Dysfunction as Common Molecular Denominators Connecting Type 2 Diabetes to Alzheimer Disease. Diabetes. 2014;63:2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- 121.Cryer PE. The Barrier of Hypoglycemia in Diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- 123.Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Brain Res. 1994;649:343–347. doi: 10.1016/0006-8993(94)91085-5. [DOI] [PubMed] [Google Scholar]
- 124.Fujikawa T, et al. Leptin Engages a Hypothalamic Neurocircuitry to Permit Survival in the Absence of Insulin. Cell metabolism. 2013;18:431–444. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.German JP, et al. Leptin Activates a Novel CNS Mechanism for Insulin-Independent Normalization of Severe Diabetic Hyperglycemia. Endocrinology. 2011;152:394–404. doi: 10.1210/en.2010-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Breen DM, Rasmussen BA, Côté CD, Jackson VM, Lam TKT. Nutrient-Sensing Mechanisms in the Gut as Therapeutic Targets for Diabetes. Diabetes. 2013;62:3005–3013. doi: 10.2337/db13-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 128.Grayson BE, Seeley RJ, Sandoval DA. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci. 2013;14:24–37. doi: 10.1038/nrn3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cardin S, et al. Portal glucose infusion increases hepatic glycogen deposition in conscious unrestrained rats. Journal of Applied Physiology. 1999;87:1470–1475. doi: 10.1152/jappl.1999.87.4.1470. [DOI] [PubMed] [Google Scholar]
- 130.Moore MC, et al. Neural and pancreatic influences on net hepatic glucose uptake and glycogen synthesis. Am J Physiol. 1996;271:E215–22. doi: 10.1152/ajpendo.1996.271.2.E215. [DOI] [PubMed] [Google Scholar]
- 131.Kirov A, et al. Transgenic Expression of Nonclassically Secreted FGF Suppresses Kidney Repair. PLoS ONE. 2012;7:e36485. doi: 10.1371/journal.pone.0036485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buehler A, et al. Angiogenesis-independent cardioprotection in FGF-1 transgenic mice. Cardiovasc Res. 2002;55:768–777. doi: 10.1016/s0008-6363(02)00494-7. [DOI] [PubMed] [Google Scholar]
- 133.Fernandez B, et al. Transgenic Myocardial Overexpression of Fibroblast Growth Factor-1 Increases Coronary Artery Density and Branching. Circ Res. 2000;87:207–213. doi: 10.1161/01.res.87.3.207. [DOI] [PubMed] [Google Scholar]
- 134.Wu A-L, et al. Amelioration of Type 2 Diabetes by Antibody-Mediated Activation of Fibroblast Growth Factor Receptor 1. Science Translational Medicine. 2011;3 doi: 10.1126/scitranslmed.3002669. [DOI] [PubMed] [Google Scholar]
- 135.Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15:51–69. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- 136.Spielberger R, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 137.Chae YK, et al. Fibroblast Growth Factor Receptor (FGFR) as a Therapeutic Target in Lung and Head and Neck Cancer. American Journal of Hematology/Oncology. 2016;12 [Google Scholar]
- 138.Katoh M. Therapeutics Targeting FGF Signaling Network in Human Diseases. Trends in Pharmacological Sciences. 2016;37:1081–1096. doi: 10.1016/j.tips.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 139.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nature Reviews Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 140.Morton GJ, et al. FGF19 action in the brain induces insulin-independent glucose lowering. J Clin Invest. 2013;123:4799–4808. doi: 10.1172/JCI70710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ryan KK, et al. Fibroblast Growth Factor-19 Action in the Brain Reduces Food Intake and Body Weight and Improves Glucose Tolerance in Male Rats. Endocrinology. 2013;154:9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marcelin G, et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab. 2014;3:19–28. doi: 10.1016/j.molmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fu L, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 144.Owen BM, et al. FGF21 Acts Centrally to Induce Sympathetic Nerve Activity, Energy Expenditure, and Weight Loss. Cell metabolism. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarruf DA, et al. Fibroblast Growth Factor 21 Action in the Brain Increases Energy Expenditure and Insulin Sensitivity in Obese Rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nicholes K, et al. A Mouse Model of Hepatocellular Carcinoma. Am J Pathol. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu X, et al. Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19) Proc Natl Acad Sci U S A. 2010;107:14158–14163. doi: 10.1073/pnas.1009427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wei W, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lou G, et al. Intranasal administration of TAT-haFGF14–154 attenuates disease progression in a mouse model of Alzheimer’s disease. Neuroscience. 2012;223:225–237. doi: 10.1016/j.neuroscience.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, et al. Cell-penetrating peptide TAT-mediated delivery of acidic FGF to retina and protection against ischemia–reperfusion injury in rats. Journal of Cellular and Molecular Medicine. 2010;14:1998–2005. doi: 10.1111/j.1582-4934.2009.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cheng X, et al. Acidic fibroblast growth factor delivered intranasally induces neurogenesis and angiogenesis in rats after ischemic stroke. Neurological Research. 2013;33:675–680. doi: 10.1179/1743132810Y.0000000004. [DOI] [PubMed] [Google Scholar]
- 152.Lipska KJ, Krumholz HM. Is Hemoglobin A1c the Right Outcome for Studies of Diabetes? JAMA. 2017 doi: 10.1001/jama.2017.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Avogaro A, Fadini GP, Sesti G, Bonora E, Prato S. Continued efforts to translate diabetes cardiovascular outcome trials into clinical practice. Cardiovascular Diabetology. 2016;15:111. doi: 10.1186/s12933-016-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boussageon R, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169–d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marcovecchio ML, Lucantoni M, Chiarelli F. Role of Chronic and Acute Hyperglycemia in the Development of Diabetes Complications. Diabetes Technology & Therapeutics. 2011;13:389–394. doi: 10.1089/dia.2010.0146. [DOI] [PubMed] [Google Scholar]
- 156.Monnier L, Colette C. Postprandial and basal hyperglycaemia in type 2 diabetes: Contributions to overall glucose exposure and diabetic complications. Diabetes & Metabolism. 2015;41:6S9–6S15. doi: 10.1016/S1262-3636(16)30003-9. [DOI] [PubMed] [Google Scholar]
- 157.Fysekidis M, et al. Increased glycemic variability and decrease of the postprandial glucose contribution to HbA1c in obese subjects across the glycemic continuum from normal glycemia to first time diagnosed diabetes. Metabolism. 2014;63:1553–1561. doi: 10.1016/j.metabol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 158.Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 159.Hamblin M, Chang L, Fan Y, Zhang J, Chen YE. PPARs and the cardiovascular system. Antioxid Redox Signal. 2009;11:1415–1452. doi: 10.1089/ars.2008.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu W, et al. Effective treatment of steatosis and steatohepatitis by fibroblast growth factor 1 in mouse models of nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2016;113:2288–2293. doi: 10.1073/pnas.1525093113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shinozaki K, et al. Role of Insulin Resistance Associated With Compensatory Hyperinsulinemia in Ischemic Stroke. Stroke. 1996;27:37–43. doi: 10.1161/01.str.27.1.37. [DOI] [PubMed] [Google Scholar]
- 162.Zunker P, et al. Hyperinsulinism and Cerebral Microangiopathy. Stroke. 1996;27:219–223. doi: 10.1161/01.str.27.2.219. [DOI] [PubMed] [Google Scholar]
- 163.Tyrberg M, Melander A, Lvestam-Adrian M, Lindblad U. Retinopathy in subjects with impaired fasting glucose: the NANSY-Eye baseline report. Diabetes Obes Metab. 2008;10:646–651. doi: 10.1111/j.1463-1326.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- 164.Chandrasekera PC, Pippin JJ. Of rodents and men: species-specific glucose regulation and type 2 diabetes research. ALTEX. 2014;31:157–176. doi: 10.14573/altex.1309231. [DOI] [PubMed] [Google Scholar]
- 165.Cherrington AD, Moore MC, Sindelar DK, Edgerton DS. Insulin action on the liver in vivo. Biochemical Society Transactions. 2007;35:1171–1174. doi: 10.1042/BST0351171. [DOI] [PubMed] [Google Scholar]
- 166.Lai M, Chandrasekera PC, Barnard ND. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutrition & Diabetes. 2014;4:e135. doi: 10.1038/nutd.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131–145. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thliveris JA, Paz GF, Rempel E, Faiman C. An extra-pancreatic direct effect of streptozotocin on the hypothalamo-hypophyseal-testicular axis in the rat. Anat Anz. 1984;157:213–219. [PubMed] [Google Scholar]
- 169.Ryan KK, et al. Fibroblast Growth Factor-19 Action in the Brain Reduces Food Intake and Body Weight and Improves Glucose Tolerance in Male Rats. Endocrinology. 2013;154:9–15. doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marcelin G, et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol Metab. 2014;3:19–28. doi: 10.1016/j.molmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kir S, et al. FGF19 as a Postprandial, Insulin-Independent Activator of Hepatic Protein and Glycogen Synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu J, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin resistant mouse models----Association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 173.Coskun T, et al. Fibroblast Growth Factor 21 Corrects Obesity in Mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 174.Xu J, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]