PRACTICAL IMPLICATIONS
A high level of specialization may produce a diagnostic bias that hinders a physician's consideration of other possible differential diagnoses.
A healthy, nonacclimatized 56-year-old woman developed mood changes and general weakness followed by vomiting, sensory disturbances, and ultimately unconsciousness within hours during an ascent from 1,600 to 2,800 meters in the Himalayas, Nepal. She reported no headache, ataxia, or visual disturbances during and following the hike, as confirmed by fellow travelers. As high-altitude cerebral edema (HACE) was suspected, she received 8 mg of dexamethasone and was transferred to a hospital specializing in acute mountain sickness (AMS) located at 1,300 meters. During the transfer, she had a generalized seizure. The next morning, her consciousness was still clouded. She exhibited subtle, brief, involuntary muscle twitching in both arms and neck. Because she responded properly to stimuli, this was interpreted as myoclonus. Laboratory testing revealed serum hyponatremia (117 mmol/L), hyposmolarity, and urine hyperosmolality. These disturbances were associated with decreased urine volume, high positive fluid balance, anadipsia, and weight gain. Her history revealed multiple prior vaccinations but no infections.
MRI performed 36 hours after symptom onset showed no brain edema or venous thrombosis. However, there were innumerous variably sized confluent and discrete nonenhancing T2 high signal intensity foci in subcortical and deep white matter (figure, A). The subcortical U-fibers were also affected. These hyperintense fluid-attenuated inversion recovery (FLAIR) foci were less than 20 mm in size and slightly hypointense at the T1 sequence. Two lesions were periventricular and none of the lesions affected the mesial temporal lobe or corpus callosum. The optic nerves were unremarkable. No spine MRI was performed.
Figure. Fluid-attenuated inversion recovery (FLAIR) MRI.
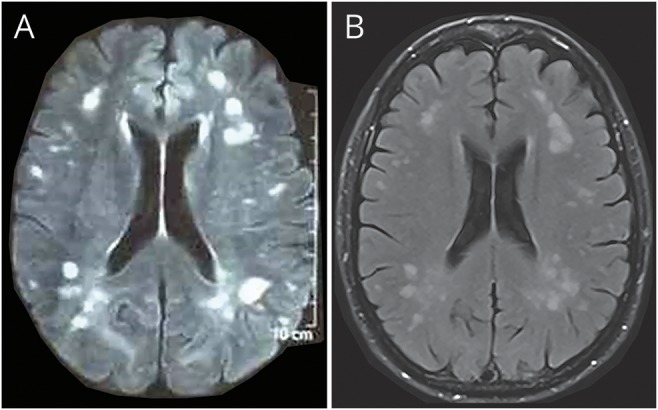
(A) FLAIR MRI taken 36 hours after the onset of symptoms and before methylprednisolone therapy shows innumerous, asymmetric, patchy, and poorly marginated areas of increased signal intensity. (B) FLAIR MRI taken 2.5 months after the onset of symptoms shows residual lesions, but no new lesions.
Neurologists from Nepal and Germany (none experts in AMS) were involved in this case by happenstance. Because the circumstances and symptoms were atypical and rare for HACE (relatively low altitude, lack of headache and ataxia, myoclonus) and because of the MRI findings, the diagnoses of AMS and HACE were discarded and acute demyelinating encephalomyelitis (ADEM) was suspected.1–4 Ultimately the patient was transferred to a neurologic hospital. A CSF analysis showed normal cell count, protein, and glucose levels. CSF testing was negative for herpes simplex, Japanese encephalitis, acid-fast bacilli, Cryptococcus neoformans, Gram stain, and culture. Further detailed CSF testing (e.g., aquaporin 4 immunoglobulin G) was not possible in Nepal. There was no evidence of autoimmune encephalitis.
The patient made a full recovery after a restriction of fluids and 1 g of IV methylprednisolone. Because a second MRI showed new lesions 6 days later, the methylprednisolone therapy was extended for a total of 10 days. She had no neurologic deficits at the 5-month follow-up. A third MRI 2.5 months later showed residual lesions, but no new lesions (figure, B).
Discussion
The probability of a nonacclimatized young woman developing a severe case of AMS during a rapid ascent (<2 hours) to an altitude of 3,000 meters is less than 2%.5 Furthermore, no symptoms of headache, ataxia, cerebral edema, or corpus callosum lesions on MRI are atypical for HACE1,3,4,6 or exercise-induced hyponatremia.7 Even though a headache has been proposed as the leading symptom required for a diagnosis of AMS or HACE according to the Lake Louise criteria,3 there may be a few cases of HACE without headache.4 Another hallmark of HACE is truncal ataxia.4,6 This, however, was not observed in the patient. Perhaps due to specialized knowledge of AMS, these low-probability and atypical circumstances and symptoms were interpreted as compatible with a diagnosis of HACE. Since the circumstances and symptoms both have a low probability of supporting a diagnosis of HACE, their combined presence further reduces the epistemologic probability to an extremely low level. Furthermore, the presence of the lesions in the FLAIR MRI was regarded as a possible indication of other diagnostic considerations. This evokes the theory of the paradigm shift in science by T.S. Kuhn,8 who postulated in 1962 that “Indeed those [phenomena] that will not fit in the box are often not seen at all.” The final diagnosis of ADEM was made by recognizing the atypical symptoms as hints of an alternative diagnosis.
The electrolyte and fluid disturbances indicated a syndrome of inappropriate antidiuretic hormone secretion (SIADH). The innumerous cerebral lesions most probably provoked the SIADH; the linked hyponatremia might have caused the myoclonus,9 clouded consciousness, and generalized seizure. ADEM and hyponatremia are often associated with one another.10
Author contributions
O. Hensel: idea and drafting of the manuscript. P. Niroul: acquisition of data and critical revision of manuscript for intellectual content. R. Paudel: acquisition of data and critical revision of manuscript for intellectual content. T. Sherpa: acquisition of data and critical revision of manuscript for intellectual content. T. Kraya: critical revision of manuscript for intellectual content. P. Presek: critical discussion of the case and revision of the manuscript. S. Zierz: critical revision of manuscript for intellectual content.
Acknowledgment
The NGO Nepalmed e.V. organized a scientific exchange program and brought German and Nepalese neurologists. The authors thank Prof. Dr. M. Pham (Department of Neuroradiology, University of Würzburg) for reevaluating the MRI.
Study funding
No targeted funding reported.
Disclosure
O. Hensel is a member of NGO Nepalmed. P. Niroula, R. Paudel, T. Sherpa, and T. Kraya report no disclosures. P. Presek has received speaker honoraria from Pfizer and Bristol-Myers Squibb. S. Zierz has received speaker honoraria from Genzyme. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Hackett PH, Yarnell PR, Hill R, Reynard K, Heit J, McCormick J. High-altitude cerebral edema evaluated with magnetic resonance imaging: clinical correlation and pathophysiology. JAMA 1998;280:1920–1925. [DOI] [PubMed] [Google Scholar]
- 2.Tenembaum S, Chitnis T, Ness J, Hahn JS; for the International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology 2007;68(suppl 2):S23–S36. [DOI] [PubMed] [Google Scholar]
- 3.Roach RC, Bärtsch P, Oelz O, Hackett PH; the Lake Louise AMS Scoring Consensus Committee. The Lake Louise acute mountain sickness scoring system. In: Sutton S, Houston CS, Coates G, eds. Hypoxia and Molecular Medicine. Burlington: Queen City Press; 1993:272–274. [Google Scholar]
- 4.Wu T, Ding S, Liu J, et al. . Ataxia: an early indicator in high altitude cerebral edema. High Alt Med Biol 2006;7:275–280. [DOI] [PubMed] [Google Scholar]
- 5.Beidleman BA, Tighiouart H, Schmid CH, Fulco CS, Muza SR. Predictive models of acute mountain sickness after rapid ascent to various altitudes. Med Sci Sports Exerc 2013;45:792–800. [DOI] [PubMed] [Google Scholar]
- 6.Luks AM, Swenson ER, Bärtsch P. Acute high-altitude sickness. Eur Respir Rev 2017;26:160096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayus JC, Moritz ML. Exercise-associated hyponatremia masquerading as acute mountain sickness: are we missing the diagnosis? Clin J Sport Med 2008;18:383–386. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn TS. The Structure of Scientific Revolutions, 5th ed Chicago: The University of Chicago Press; 2012:24. [Google Scholar]
- 9.Marsden CD, Hallett M, Fahn S. The nosology and pathophysiology of myoclonus. In: Marsden CD, Fahn S, editors. Movement Disorders. London: Butterworths; 1982:196–248. [Google Scholar]
- 10.Fujiki F, Tsuboi Y, Hori T, Yamada T. Aseptic meningitis as initial presentation of acute disseminated encephalomyelitis. J Neurol Sci 2008;272:129–131. [DOI] [PubMed] [Google Scholar]


