Abstract
Background
We summarize the existing evidence on the potential benefit of oral anticoagulation (OAC) in intracerebral hemorrhage (ICH) survivors with nonvalvular atrial fibrillation (NVAF).
Methods
Systematic review of the literature to address the following issues: (1) prevalence of NVAF in ICH survivors, (2) current prescription of OAC, (3) factors associated with resumption of OAC, (4) risk of ischemic stroke (IS) and recurrent ICH, and (5) ideal timing for restarting OAC in ICH survivors with NVAF.
Results
After screening 547 articles, 26 were included in the review. Only 3 focused specifically on patients with ICH as primary event, NVAF as indication for OAC, and recurrent ICH and IS as primary endpoints. In addition, 19 letters to the editor/reviews/editorials/experts' surveys/experts' opinion were used for discussion purposes.
Conclusions
NVAF is highly prevalent among ICH survivors. The risks of IS, recurrent ICH, and mortality are heightened in this group. Most published data show a net benefit in terms of IS prevention and mortality when anticoagulation is restarted. However, those studies are observational and mostly retrospective, therefore selection bias may play a major role in the results observed in these cohorts. Only randomized controlled trials, either pragmatic or explanatory, can provide more conclusive answers for this important clinical question.
Nonvalvular atrial fibrillation (NVAF) increases 5-fold the risk of ischemic stroke (IS).1,2 Oral anticoagulation reduces that risk by 64%,3 while the risk of intracerebral hemorrhage (ICH) increases 8-fold.4 Most anticoagulation-related intracranial bleedings are ICH (ARICH, 70%), followed by subdural hematomas (SDH).5 ARICH accounts for almost 20% of all ICH,6 and causes high mortality and disability.4,7 The incidence of ARICH has grown in the last years, driven by the increasing prescription of oral anticoagulants (OAC),6 even among ICH survivors.8
ICH survivors have a heightened risk of IS,9–14 recurrent ICH,15 and mortality,14 making the resumption of OAC in this population a major clinical dilemma. Growing evidence suggests that restarting OAC in ICH survivors benefits patients with NVAF. Yet the correct selection of patients and the timing for restarting OAC are both unclear.16 Moreover, direct OAC (DOAC) are promising for secondary prevention after ICH because of a reduction of IS compared to warfarin, which is mainly due to a decrement in ICH. However, clinical data on DOAC in this population are scarce.
Our aim is to provide an updated and critical interpretation of the available evidence to guide management decisions on this important clinical quandary.
Methods
We conducted a systematic review of the literature according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses for systematic review protocols (PRISMA-P) guidelines.17
Search strategy
One reviewer (M.A.H.) identified potentially eligible articles by searching the PubMed database. No limits were applied. Search filters were constructed for (1) ICH, (2) anticoagulation, and (3) NVAF (search strategy: [“hemorrhagic stroke” or “intracerebral hemorrhage” or “ICH” or “intracranial hemorrhage”] and [“anticoagulation” or “oral anticoagulants” or “vitamin K antagonists” or “warfarin” or “novel oral anticoagulants”] and “atrial fibrillation.”). The last access was on April 1, 2017. Additional articles were found by scanning the reference lists of the retrieved publications and experts' consultation.
Study selection
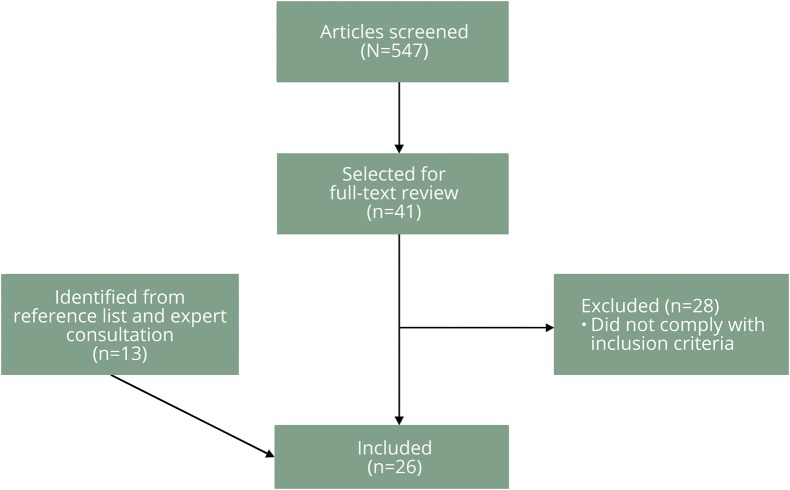
Potentially relevant articles were selected by one reviewer (M.A.H.) after titles and abstracts were screened. If no abstract was available, the publication was still selected for review of the full text (figure 1). We included articles with analytic data addressing the following issues:
Prevalence of NVAF among ICH survivors
Current prescription of OAC in ICH survivors with NVAF
Factors associated with resumption of OAC after ICH
Risk of IS and recurrent ICH in ICH survivors with NVAF with and without OAC
Ideal timing for restarting OAC in ICH survivors with NVAF
Figure 1. Flowchart of studies' selection.
A second reviewer (A.A.R.) confirmed the appropriateness of the included articles.
Results
After screening 547 articles, 26 were included in the review, including 1 letter to the editor with a subgroup analysis of a retrospective study (figure 1, table e-1, links.lww.com/CPJ/A12). In addition, 19 letters to the editor/reviews/editorials/experts' surveys/experts' opinion were used for discussion purposes (e-References, links.lww.com/CPJ/A13).
ICH in perspective: Focus on ARICH and NVAF
ICH represents 10%–15% of all strokes.18 Between 39% and 44% of ICH are related to antithrombotic drugs, and 10%–24% to OAC.6–8,19 NVAF is the principal indication for OAC in 72%–79% of ARICH cases.8,20,21 The annual incidence of ARICH increased from 0.8 in 1988 to 4.4 per 100,000 persons in 1999, probably due to the increased prescription of OAC.6,8 This uptrend is expected to continue because of the increasing incidence of atrial fibrillation driven by the aging of the general population.10 Hematoma volumes are larger in ARICH cases as compared to ICH cases in patients not taking anticoagulants, and this may, at least in part, explain the higher mortality observed in patients with ARICH (70% vs 30%–55% in patients without OAC).4,7,22–24
Current prescription of OAC in ICH survivors with NVAF
The proportion of ICH survivors with NVAF restarted on OAC ranges between 30% and 39% in small retrospective and prospective studies, respectively.25,26 In a recent meta-analysis including 1,027 patients, 23% and 28% of lobar and nonlobar ICH survivors resumed OAC, respectively.27 This percentage was lower (14%–19.4%) in 2 large multicentric studies,19,21 and lowest in 2 nationwide registries from Denmark (n = 2,978) and Sweden (n = 2,777), in which around 11% of patients were prescribed anticoagulation at 3–6 months and 1 year after the index event, respectively.8,20
The rate of prescription of OAC after ICH increased from 8.3% in 2006 to 17.2% in 2011, reflecting a change in practice that may have been related to the publication of studies suggesting a benefit from this therapeutic approach.8 Accordingly, a nationwide survey among 260 neurologic specialists from Japan showed that 91% of the respondents would restart OAC in ICH survivors with NVAF.28 Still, a significant proportion of ICH survivors with NVAF are prescribed (31%–44%)8,20 or switched to antiplatelet drugs (11%) after the hemorrhage.19 This is remarkable because antiplatelet agents do not significantly decrease the risk of IS, but they do increase the risks of recurrent ICH and mortality.15,29,30
Factors associated with resumption of OAC
Common factors associated with prescription of OAC in ICH survivors with NVAF in different studies were less severe ICH,8,31–34 younger age,8,21,25 prior anticoagulation,8 prior IS,8 and nonlobar ICH.27 Lower CHA2DS2-VASc and HAS-BLED scores were associated with anticoagulation resumption in one study,31 but they were not in 2 others.8,27 NVAF patients were less likely to be restarted on OAC compared those with valvular disease.8,25,35 In addition, in a national survey of experts in the field, the risk of recurrent ICH and poor functional condition were considered the main contraindications for OAC resumption.28
Risk balance between ICH and IS: Is restarting OAC beneficial?
Natural history of ICH patients without OAC
In a systematic review including 1,880 ICH survivors (irrespective of NVAF) followed for a mean of 3.4 patient-years, Bailey et al.36 reported an aggregate recurrence of all strokes of 4.3% per patient-year, higher in population-based than in hospital-based studies (6.2% vs 4.0%). The aggregate recurrence of ICH was 2.3% per patient-year, varying from 4.4% to 2.1% in patients with lobar and deep ICH, respectively. On the other hand, the aggregate rates of subsequent IS and mortality were 1.1% and 8.8% per patient-year.36 Similar risks of recurrent ICH and IS were later reported by Vermeer et al.37 Meanwhile, a prospective cohort study by Viswanathan et al.38 reported substantially higher recurrence rates after lobar ICH as compared to nonlobar ICH (cumulative 2-year rate 22% vs 4%; p = 0.007); the evaluation of this risk was later refined by O'Donnell et al.32 in a subgroup of 71 patients from the same cohort with lobar ICH and age >55 years, likely due to cerebral amyloid angiopathy (CAA) (13.6% at 1 year, 20.7% at 2 years, and 36.3% at 3 years). The rationale behind the dichotomization of ICH by localization is that CAA is the main etiology underlying lobar ICH, particularly in people aged >55 years, and, as noted above, those patients have the highest risks of ICH recurrence.
Effect of OAC on ICH survivors with NVAF
There have been no published randomized controlled trials comparing the risks of recurrent ICH and IS among survivors of ICH with NVAF. Available data are observational, mostly retrospective studies.
For some time the literature was limited to a theoretical study based on a Markov model, which concluded that OAC could be harmful for lobar ICH survivors with NVAF. However, this analysis also suggested that OAC should be considered after deep hemispheric ICH when the risk for thromboembolism is particularly high.33 Although helpful to conceptualize this difficult clinical scenario, the model was built using assumptions that were subsequently not confirmed by actual clinical data.
Very few studies have focused specifically on patients with ICH as primary event, NVAF as indication for OAC, and recurrent ICH and IS as primary endpoints. In a Swedish population-based study by Pennlert et al.,39 the 3-year cumulative incidence of recurrent ICH in patients on OAC, antiplatelets, or no treatment were 6.9%, 3.9%, and 4.4%, respectively. The corresponding incidences for thromboembolic events were 6.3%, 18.8%, and 13.8%, respectively.39 The differences across groups were not significant for recurrent ICH, but significantly lower for IS and vascular death in anticoagulated patients. Meanwhile, Kuramatsu et al.21 found a similar risk of recurrent ICH in patients with and without OAC (3.9% per patient-year) whereas the risk of IS was considerably lower with OAC resumption (12.7% vs 3.9% per patient-year). In a retrospective study of 201 ICH survivors with atrial fibrillation (160 lobar) with a mean follow-up of 39.5 ± 31.9 months, Park et al.40,41 reported rates of recurrent ICH of 2.1% per patient-year after lobar ICH and 1.4% per patient-year after deep ICH after OAC resumption; there were no cases of ICH recurrence among nonanticoagulated patients.
A recent meta-analysis pooling individual data from 3 large prospective observational cohorts (total n = 1,027) showed better outcomes at 1 year among patients with ICH who had OAC resumption as compared to those who were not restarted on anticoagulation. Patients restarted on OAC had lower mortality (hazard ratio [HR] 0.22; 95% confidence interval [CI] 0.16–0.30; p < 0.0001) and better functional outcomes (HR for mRS 0–3, 5.12, 95% CI 3.86–6.80; p < 0.0001), and these beneficial effects were confirmed when the analysis was restricted to patients with lobar ICH.27
Effect of OAC on intracranial hemorrhage survivors
Most studies addressing the risk and benefit balance of restarting OAC after ICH included all types of intracranial bleeding (often with a predominance of SDH) among primary events and recurrences, and also patients with different indications for OAC (i.e., not only NVAF). Most of these studies supported a benefit from resuming OAC.14,15,31,35,40
Only 4 studies reported a detrimental effect of OAC, but they had important limitations. OAC resumption among 243 ICH survivors was associated with a 3-fold increase in the risk of recurrent ICH. Remarkably, 22 patients were restarted on OAC, and only 4 of them had NVAF.37 Gathier et al.34 reported a nonsignificant increase in the long-term risk of any stroke among ARICH survivors restarted on OAC or antiplatelets, but the small sample size and the underrepresentation of patients with NVAF limit the usefulness of the analysis. In a study originally designed to assess the ideal timing for OAC reinitiation after intracranial hemorrhage, Majeed et al.42 found that restarting OAC increased the risk of recurrent intracranial hemorrhage by more than 5 times, although the risk of thromboembolic events was reduced by almost 90%. The higher risk of recurrence of SDH compared with ICH may help explain this finding (16% vs 8.4%, p = 0.07). Finally, the CHIRIONE study prospectively evaluated OAC resumption in 267 survivors of intracranial hemorrhage (traumatic or not), and found a “substantial risk of recurrence” (2.56% per patient-year over a median follow-up of 2 years). However, this cohort only included 33% of patients with ICH and only 45% of patients in whom NVAF was the indication for OAC.43 Traumatic SDH exceeded spontaneous ICH as index event (60%), and data about IS were scant, making it difficult to estimate the true balance between ischemic and hemorrhagic risks.43
Two additional studies deserve special mention, because they provide information on the optimal candidates for OAC and the value of greater time in therapeutic international normalized ratio. In a large Taiwanese study (n = 12,917), Chao et al.15 only found benefit associated with OAC in patients with a CHA2DS2-VASc score ≥6. This study did not differentiate across types of intracranial bleeding and did not contemplate the higher morbidity and mortality of intracranial hemorrhage in comparison with IS. Also, the risk reduction of IS in the warfarin group was low. Identifying patients with CHA2DS2-VASc scores between 2 and 5 who can benefit from OAC is an important challenge that was not addressed by this study. Meanwhile, the largest single-center study to date (n = 428) found no differences in the composite endpoint of IS, thromboembolic events and major hemorrhage between intracranial hemorrhage survivors with and without OAC (11.5 vs 7.9 events per 100 patient-years; p = 0.154), but showed that those patients on OAC with ≥60% of time in therapeutic INR had a better cumulative survival free of the composite endpoint (p < 0.001).40 Both studies should be carefully interpreted, since ICH recurrence in Asian populations, in which deep ICH is certainly dominant, without further breakdown by ICH localization, is hardly generalizability to Western populations.
All population-based studies assessing the effect of OAC after ICH showed a net benefit in prevention of ischemic complications, mortality, hospitalization costs, and recurrent hemorrhage.20,29,31 Yet these studies do not provide sufficient information to understand how patients were selected to resume OAC.
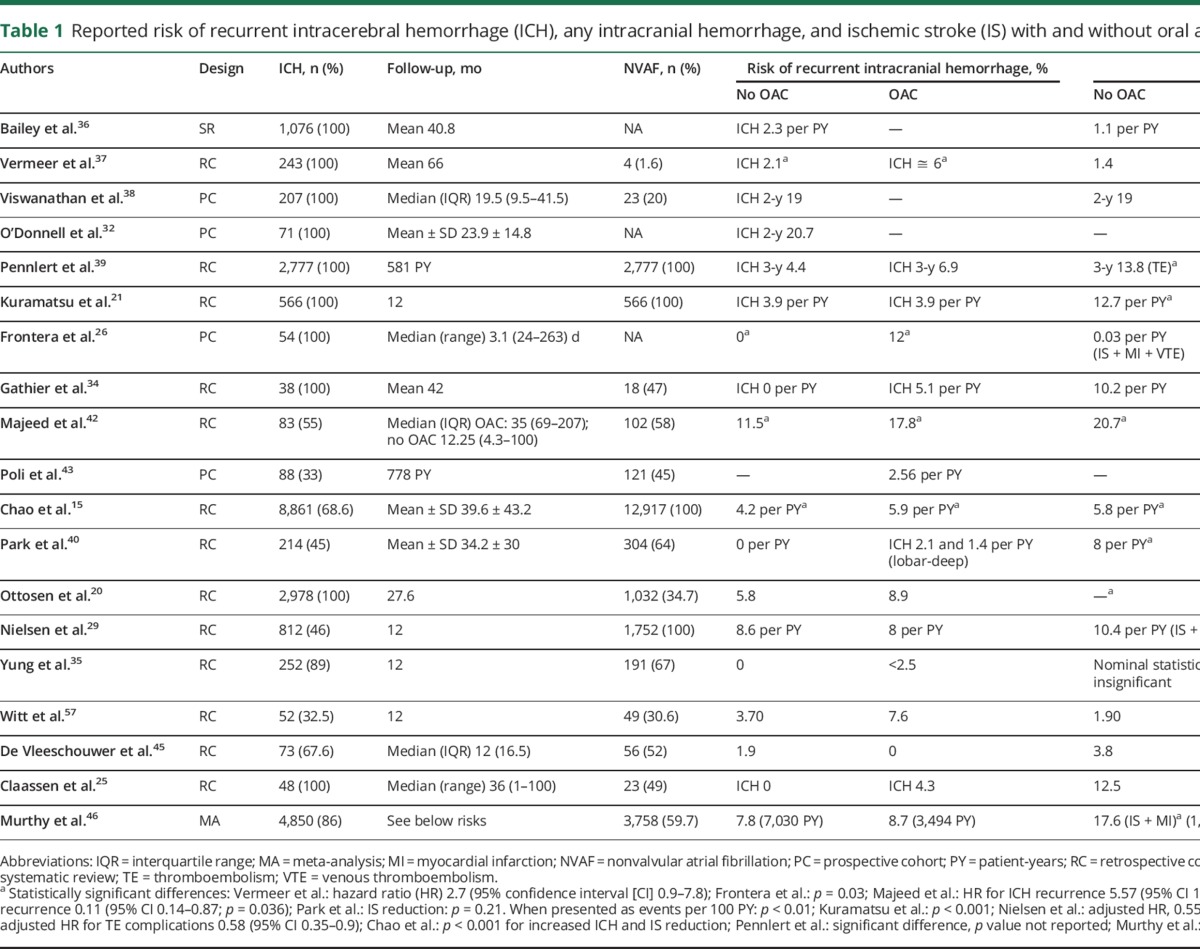
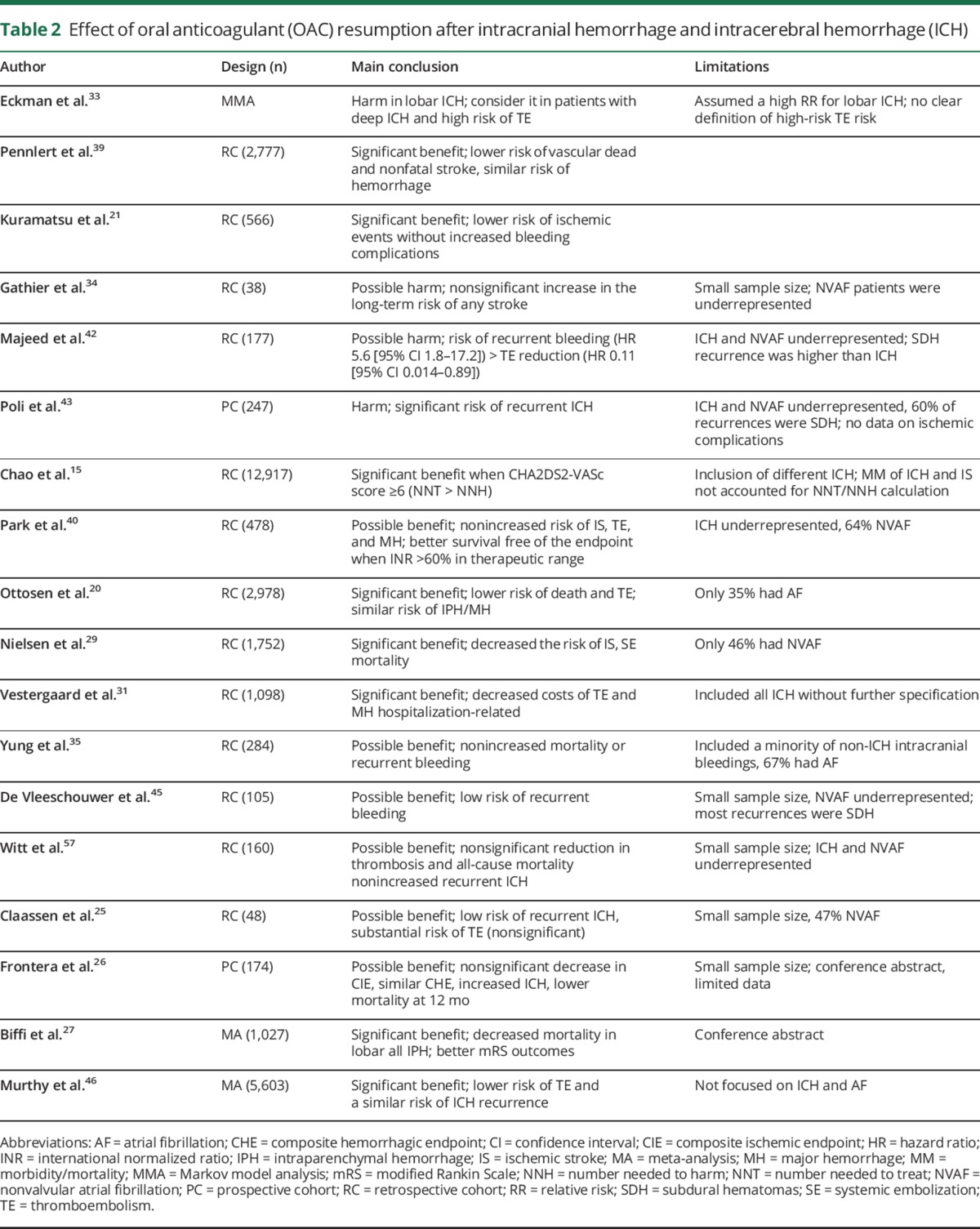
Table 1 summarizes the studies reporting the risks of recurrent ICH or intracranial hemorrhage and of IS among patients with intracranial hemorrhage who are subsequently treated with OAC or not. Table 2 summarizes the main findings on the effects of resumption of OAC in different studies and highlights key study limitations. Taken together, these data suggest that OAC benefits ICH survivors by decreasing IS, thromboembolic complications, and vascular mortality, and improving functional outcomes without a major detrimental effect on recurrent ICH. Of note, all but 5 studies included a large proportion of SDH, a condition with a risk of recurrence 2 or 3 times higher than ICH.42–44 Even so, the risk of recurrent intracranial bleeding remained mostly neutral. A recent meta-analysis pooling data from 5,606 patients (86% with ICH) from most of the cited studies20,21,25,29,35,41,42,45 confirmed a substantially lower risk of thromboembolic complications among survivors of intracranial hemorrhage who were restarted on OAC (relative risk [RR] 0.34; 95% CI 0.25–0.45), while the risk of recurrent intracranial bleeding remained similar (RR 1.01; 95% CI 0.58–1.77).46
Table 1.
Reported risk of recurrent intracerebral hemorrhage (ICH), any intracranial hemorrhage, and ischemic stroke (IS) with and without oral anticoagulants (OAC)
Table 2.
Effect of oral anticoagulant (OAC) resumption after intracranial hemorrhage and intracerebral hemorrhage (ICH)
Ideal timing for restarting anticoagulation
Few studies addressed the optimal timing for restarting anticoagulation after ICH (table 3). Only one, a nationwide study including 2,777, focused on ICH survivors with NVAF. It found that restarting OAC between 7 and 8 weeks after ICH was associated with a reduced the rate of thrombotic events without increasing the risk of recurrent ICH.39
Table 3.
Ideal timing for restating oral anticoagulants (OAC)
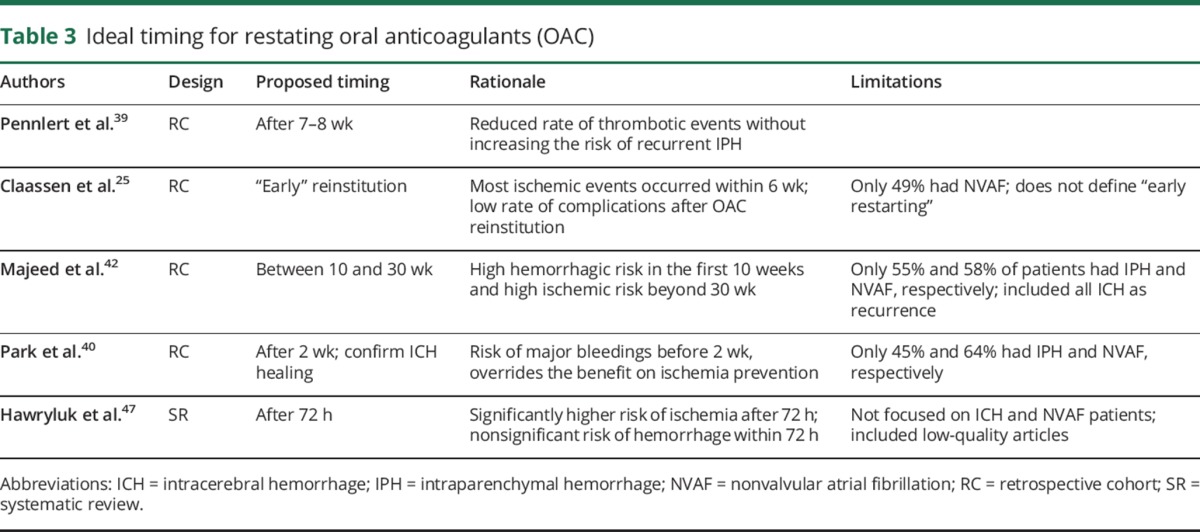
Claassen et al.25 found that most ischemic events in ARICH survivors occurred within 6 weeks of OAC suspension. Also, there was a low rate of hemorrhagic recurrences after resumption, suggesting that early reinitiation may be advantageous. This study included several indications for OAC. Instead, Majeed et al.42 concluded that the optimal timing for restarting anticoagulation should be between 10 and 30 weeks after the intracranial bleeding (55% had ICH), based on the high risk of recurrent hemorrhage in the first 10 weeks that decreased over time, and a rise in ischemic complications beyond 30 weeks. Park et al.40 proposed that OAC be reinitiated after 2 weeks to avoid the overriding effect of intracranial hemorrhage recurrence on the prevention of systemic thromboembolism and IS. In this study, the prescription of OAC without imaging confirmation of intracranial hemorrhage resolution was associated with an increased risk of recurrent bleeding.
In a systematic review including retrospective data of 492 patients (50% with ICH), Hawryluk et al.47 suggested that anticoagulation may be restarted 72 hours after the bleeding event. However, this conclusion was limited by the inclusion of studies of suboptimal quality, different etiologies of intracranial (even spinal) hemorrhage, and different indications for OAC (50.2% NVAF, prosthetic heart valves, and rheumatic heart disease). Noticeably, none of the available studies addressed whether the site of intracranial hemorrhage (ICH vs extra-axial, lobar vs deep) should influence the timing for resumption of OAC.
Opinions differ among specialists. A national survey in Japan revealed that 71% of 260 experts would restart OAC after 8 days and 46% after 15 days; 47% would want proof of ICH resolution on CT scan before resuming OAC.28
Gaps in current knowledge and future directions
NVAF is highly prevalent among ICH survivors. In contrast with prior data, newer population-based studies and meta-analyses show a net benefit in terms of IS prevention and mortality when anticoagulation is restarted. This benefit might apply to patients with lobar and nonlobar ICH.25,42 Yet supporting evidence is limited by the variable quality of the studies, the heterogeneity of the cohorts and endpoints, and most notably its observational nature. Only a minority of patients was prescribed OAC in all reported cohorts, and it is likely that these patients were deemed to be at higher risk of thromboembolism or lower risk of recurrent hemorrhage compared to patients who were not recommended anticoagulation.
The evaluation of the competing risks of recurrent intracranial hemorrhage vs thromboembolism is challenging. The HAS-BLED, CHADS2 and CHA2DS2-VASc scores are commonly used in clinical practice to predict bleeding and ischemic risks, respectively. However, several factors such as advanced age, hypertension, prior ischemic stroke, and diabetes mellitus are commonly present in high-risk patients for both thromboembolism and recurrent ICH. This positive correlation between a high CHA2DS2-VASc and a high HAS-BLED score in ICH survivors with concurrent NVAF has been solidly demonstrated.8
Experts' opinions are divided regarding the benefit of OAC in ICH survivors, which is understandable given the limitations of current evidence. Thus, the decision whether to anticoagulate a patient with NVAF after an ICH should be individualized. To select the better candidates for OAC resumption, factors to consider include localization and severity of the ICH, concomitant comorbidities (especially risk factors for thromboembolism and systemic bleeding), previous IS, findings consistent with cerebral amyloid angiopathy (lobar ICH with subcortical microbleeds, cortical superficial siderosis, convexal subarachnoid hemorrhage, or progressive cognitive impairment), or extensive leukoaraiosis on MRI. There is also an increasing interest in the use of DOAC and left atrial appendage occlusion (LAAO) as therapeutic alternatives for this population (e-References, links.lww.com/CPJ/A13).
DOAC reduce the risk of IS or systemic embolism compared to warfarin, mainly driven by a reduction in ICH (RR 0.49, 95% CI 0.38–0.64; p < 0.0001).48 In the AVERROES trial, apixaban was associated with lower the risk of stroke or systemic embolism compared to aspirin without increasing the risk of ICH.49 Yet the safety of DOAC in ICH survivors is unknown. The APACHE-AF is an ongoing phase II, randomized, multicenter, open-label, parallel-group, clinical trial designed to compare apixaban vs no anticoagulation in patients with AF and a recent ARICH.50
LAAO has been shown noninferior to warfarin for IS prevention in patients with NVAF with low procedural risks.51–53 Two small prospective studies including a total of 66 patients reported that LAAO is safe and may be effective for IS prevention in ICH survivors with NVAF.54,55 The Prevention of Stroke by Left Atrial Appendage Closure in Atrial Fibrillation Patients After Intracerebral Hemorrhage trial is evaluating if LAAO can be a suitable therapeutic option in this population.56
Discussion
Due to its limitations, available evidence does not allow us to draw definite conclusions regarding the safety and efficacy of reinitiating OAC after an intracranial hemorrhage in patients with NVAF. The inclusion of patients with SDH is a particularly important bias given the high risk of recurrence of this type of bleeding. Yet, despite this caveat, OAC resumption was reported to be safe in most studies. A more important limitation is that most published information consists of observational and mostly retrospective studies in which only a minority of patients was restarted on OAC. The selection criteria for restarting OAC were generally not presented in these studies and therefore selection bias may play a major role in the results observed in these cohorts (i.e., the benefits from OAC may only apply to patients with greater chances of benefiting from anticoagulation). Only randomized controlled trials, either pragmatic or explanatory, can provide more conclusive answers for this important clinical question.
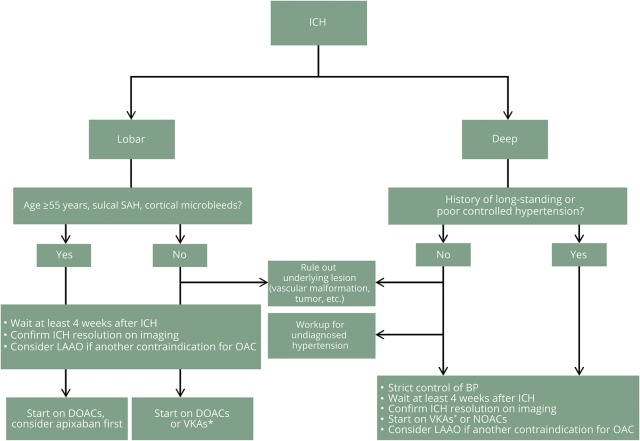
In the absence of conclusive data, the decision of restarting OAC should be tailored to the individual patient. The risk of falls due to aging or prior neurologic deficits should be considered. In figure 2, we present our recommendations for practice, in which we favor resumption of OAC for IS prevention in most ICH survivors with NVAF after a careful assessment of various risk factors for recurrent intracranial hemorrhage and systemic bleeding.
Figure 2. Recommendations for management of intracerebral hemorrhage (ICH) survivors with nonvalvular atrial fibrillation.
*Close monitoring of the international normalized ratio is recommended for vitamin K antagonist (VKA) treatment. BP = blood pressure; DOAC = direct oral anticoagulants; LAAO = left atrial appendage occlusion; NOAC = new oral anticoagulants; OAC = oral anticoagulants; SAH = subarachnoid hemorrhage.
Author contributions
M.A. Hawkes: acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision. A.A. Rabinstein: study conception, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision.
Study funding
No targeted funding reported.
Disclosure
M.A. Hawkes reports no disclosures. A.A. Rabinstein serves on the external committee for adverse event adjudication for PREVAIL trial/CAP2 registry; serves as an Associate Editor of Neurocritical Care and on the editorial boards of Neurology®, Stroke, and CONTINUUM; receives publishing royalties for Practical Neuroimaging in Stroke (Elsevier, 2009) and What To Do? Neurocritical Care (Oxford, 2016); and receives an unrestricted research grant from DJO Global for an investigator-initiated project. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Wolf PA, Dawber TR, Thomas HE, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology 1978;28:973–977. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA. Atrial fibrillation: a major contributor to stroke in the elderly. Arch Intern Med 1987;147:1561. [PubMed] [Google Scholar]
- 3.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 4.Franke CL, de Jonge J, van Swieten JC, Op de Coul AA, van Gijn J. Intracerebral hematomas during anticoagulant treatment. Stroke 1990;21:726–730. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage: facts and hypotheses. Stroke 1995;26:1471–1477. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology 2007;68:116–121. [DOI] [PubMed] [Google Scholar]
- 7.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 2004;164:880–884. [DOI] [PubMed] [Google Scholar]
- 8.Pennlert J, Asplund K, Carlberg B, et al. Antithrombotic treatment following intracerebral hemorrhage in patients with and without atrial fibrillation. Stroke 2015;46:2094–2099. [DOI] [PubMed] [Google Scholar]
- 9.Lerario MP, Gialdini G, Lapidus DM, et al. Risk of ischemic stroke after intracranial hemorrhage in patients with atrial fibrillation. PLoS One 2015;10:e0145579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner T. Resumption of oral anticoagulation after warfarin-associated intracerebral hemorrhage. Stroke 2011;42:3661–3662. [DOI] [PubMed] [Google Scholar]
- 11.Flynn RWV, MacDonald TM, Murray GD, MacWalter RS, Doney ASF. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke 2010;41:2606–2611. [DOI] [PubMed] [Google Scholar]
- 12.Pennlert J, Eriksson M, Carlberg B, Wiklund PG. Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke 2014;45:1839–1841. [DOI] [PubMed] [Google Scholar]
- 13.Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 14.Brønnum Nielsen P, Larsen TB, Gorst-Rasmussen A, Skjøth F, Rasmussen LH, Lip GYH. Intracranial hemorrhage and subsequent ischemic stroke in patients with atrial fibrillation. Chest 2015;147:1651–1658. [DOI] [PubMed] [Google Scholar]
- 15.Chao TF, Liu CJ, Liao JN, et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation 2016;133:1540–1547. [DOI] [PubMed] [Google Scholar]
- 16.Bejot Y, Cordonnier C, Durier J, Aboa-Eboule C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain 2013;136:658–664. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration: International Stroke Incidence Collaboration. Stroke 1997;28:491–499. [DOI] [PubMed] [Google Scholar]
- 19.Pasquini M, Charidimou A, van Asch CJJ, et al. Variation in restarting antithrombotic drugs at hospital discharge after intracerebral hemorrhage. Stroke 2014;45:2643–2648. [DOI] [PubMed] [Google Scholar]
- 20.Ottosen TP, Grijota M, Hansen ML, et al. Use of antithrombotic therapy and long-term clinical outcome among patients surviving intracerebral hemorrhage. Stroke 2016;47:1837–1843. [DOI] [PubMed] [Google Scholar]
- 21.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824. [DOI] [PubMed] [Google Scholar]
- 22.Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997;28:1–5. [DOI] [PubMed] [Google Scholar]
- 23.Juvela S. Risk factors for impaired outcome after spontaneous intracerebral hemorrhage. Arch Neurol 1995;52:1193–1200. [DOI] [PubMed] [Google Scholar]
- 24.Flaherty ML, Tao H, Haverbusch M, et al. Warfarin use leads to larger intracerebral hematomas. Neurology 2008;71:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claassen DO, Kazemi N, Zubkov AY, Wijdicks EFM, Rabinstein AA. Restarting anticoagulation therapy after warfarin-associated intracerebral hemorrhage. Arch Neurol 2008;65:1313–1318. [DOI] [PubMed] [Google Scholar]
- 26.Frontera J, Nocero J, Katyshev V. Resumption of anticoagulation after intracerebral hemorrhage: risks, benefits and impact on outcome. Stroke 2017;48. Presented at the International Stroke Conference 2017; February 2017; Houston, TX. [Google Scholar]
- 27.Biffi A, Kuramatsu J, Leasure A, et al. Resumption of oral anticoagulation after intracerebral hemorrhage is associated with decreased mortality and favorable functional outcome. Presented at the International Stroke Conference 2017; February 2017; Houston, TX. Available at: abstractsonline.com/pp8/#!/4172/presentation/13142. Accessed May 1, 2017.
- 28.Maeda K, Koga M, Okada Y, et al. Nationwide survey of neuro-specialists' opinions on anticoagulant therapy after intracerebral hemorrhage in patients with atrial fibrillation. J Neurol Sci 2012;312:82–85. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen PB, Larsen TB, Skjøth F, Gorst-Rasmussen A, Rasmussen LH, Lip GYH. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: clinical perspective. Circulation 2015;132:517–525. [DOI] [PubMed] [Google Scholar]
- 30.Aguilar M, Hart R. Antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev 2005:CD001925. [DOI] [PubMed] [Google Scholar]
- 31.Vestergaard AS, Skjøth F, Lip GYH, Larsen TB. Effect of anticoagulation on hospitalization costs after intracranial hemorrhage in atrial fibrillation. Stroke 2016;47:979–985. [DOI] [PubMed] [Google Scholar]
- 32.O'Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med 2000;342:240–245. [DOI] [PubMed] [Google Scholar]
- 33.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke 2003;34:1710–1716. [DOI] [PubMed] [Google Scholar]
- 34.Gathier CS, Algra A, Rinkel GJE, van der Worp HB. Long-term outcome after anticoagulation-associated intracerebral haemorrhage with or without restarting antithrombotic therapy. Cerebrovasc Dis 2013;36:33–37. [DOI] [PubMed] [Google Scholar]
- 35.Yung D, Kapral MK, Asllani E, Fang J, Lee DS. Reinitiation of anticoagulation after warfarin-associated intracranial hemorrhage and mortality risk: the Best Practice for Reinitiating Anticoagulation Therapy After Intracranial Bleeding (BRAIN) study. Can J Cardiol 2012;28:33–39. [DOI] [PubMed] [Google Scholar]
- 36.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology 2001;56:773–777. [DOI] [PubMed] [Google Scholar]
- 37.Vermeer SE, Algra A, Franke CL, Koudstaal PJ, Rinkel GJE. Long-term prognosis after recovery from primary intracerebral hemorrhage. Neurology 2002;59:205–209. [DOI] [PubMed] [Google Scholar]
- 38.Viswanathan A, Rakich SM, Engel C, et al. Antiplatelet use after intracerebral hemorrhage. Neurology 2006;66:206–209. [DOI] [PubMed] [Google Scholar]
- 39.Pennlert J, Overholser R, Asplund K, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke 2017;48:314–320. [DOI] [PubMed] [Google Scholar]
- 40.Park YA, Uhm JS, Pak HN, Lee MH, Joung B. Anticoagulation therapy in atrial fibrillation after intracranial hemorrhage. Hear Rhythm 2016;13:1794–1802. [DOI] [PubMed] [Google Scholar]
- 41.Park YA, Joung B. Reply to the Editor: Anticoagulation in atrial fibrillation after intracranial hemorrhage: could the hemorrhage location influence the outcome? Hear Rhythm 2017;14:e46. [DOI] [PubMed] [Google Scholar]
- 42.Majeed A, Kim YK, Roberts RS, Holmstrom M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010;41:2860–2866. [DOI] [PubMed] [Google Scholar]
- 43.Poli D, Antonucci E, Dentali F, et al. Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE Study. Neurology 2014;82:1020–1026. [DOI] [PubMed] [Google Scholar]
- 44.Chan PH, Siu CW. Letter by Chan and Siu regarding article, “Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage.” Circulation 2016;134:e226–e227. [DOI] [PubMed] [Google Scholar]
- 45.De Vleeschouwer S, Van Calenbergh F, van Loon J, Nuttin B, Goffin J, Plets C. Risk analysis of thrombo-embolic and recurrent bleeding events in the management of intracranial haemorrhage due to oral anticoagulation. Acta Chir Belg 2005;105:268–274. [DOI] [PubMed] [Google Scholar]
- 46.Murthy SB, Gupta A, Merkler AE, et al. Restarting anticoagulant therapy after intracranial hemorrhage: a systematic review and meta-analysis. Stroke 2017;48:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawryluk GWJ, Austin JW, Furlan JC, Lee JB, O'Kelly C, Fehlings MG. Management of anticoagulation following central nervous system hemorrhage in patients with high thromboembolic risk. J Thromb Haemost 2010;8:1500–1508. [DOI] [PubMed] [Google Scholar]
- 48.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 49.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 50.van Nieuwenhuizen KM, van der Worp HB, Algra A, et al. Apixaban versus Antiplatelet Drugs or no Antithrombotic Drugs After Anticoagulation-Associated Intracerebral Haemorrhage in Patients with Atrial Fibrillation (APACHE-AF): study protocol for a randomised controlled trial. Trials 2015;16:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmes DR, Kar S, Price MJ, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 52.Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with Watchman left atrial appendage closure technology). J Am Coll Cardiol 2013;61:2551–2556. [DOI] [PubMed] [Google Scholar]
- 53.Fountain RB, Holmes DR, Chandrasekaran K, et al. The PROTECT AF (Watchman left atrial appendage system for embolic Protection in patients with atrial fibrillation) trial. Am Heart J 2006;151:956–961. [DOI] [PubMed] [Google Scholar]
- 54.Horstmann S, Zugck C, Krumsdorf U, et al. Left atrial appendage occlusion in atrial fibrillation after intracranial hemorrhage. Neurology 2014;82:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renou P, Thambo JB, Iriart X, et al. Left atrial appendage closure in patients with atrial fibrillation and previous intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2017;26:545–551. [DOI] [PubMed] [Google Scholar]
- 56.Prevention of stroke by left atrial appendage closure in atrial fibrillation patients after intracerebral hemorrhage. Full text view: ClinicalTrials.gov [online]. Available at: clinicaltrials.gov/ct2/show/NCT02830152?term=amplatzer+AND+ICH&rank=1. Accessed April 11, 2017.
- 57.Witt DM, Clark NP, Martinez K, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for intracranial hemorrhage. Thromb Res 2015;136:1040–1044. [DOI] [PubMed] [Google Scholar]