Abstract
SIGNIFICANCE
The α2-adrenergic receptor agonist brimonidine has been reported to induce conjunctival blanching in cataract, strabismus, laser refractive, and filtration procedures. Clinicians are often faced with red eyes with no apparent underlying pathology. Low-dose brimonidine reduced ocular redness in such subjects with efficacy maintained over 1 month and negligible rebound redness.
PURPOSE
The aim of this study was to evaluate the safety and efficacy of brimonidine tartrate ophthalmic solution 0.025% for the treatment of ocular redness.
METHODS
In this single-center, double-masked, phase 3 clinical trial, adult subjects with baseline redness of more than 1 unit in both eyes (0- to 4-unit scale) were randomized 2:1 to brimonidine 0.025% or vehicle. A single dose was administered in-office (day 1); thereafter subjects instilled treatment four times a day for 4 weeks, with clinic visits on days 15, 29, and 36 (7 days post-treatment). Efficacy end points included investigator-evaluated redness 5 to 240 minutes post-instillation on day 1 (primary); investigator-evaluated change from baseline 1, 360, and 480 minutes post-instillation on day 1, and 1 and 5 minutes post-instillation on days 15 and 29; total clearance of redness, and subject-assessed redness. Safety/tolerability measures included adverse events, rebound redness, and drop comfort.
RESULTS
Sixty subjects were randomized (n = 40 brimonidine, n = 20 vehicle). Investigator-assessed redness was lower with brimonidine versus vehicle over the 5- to 240-minute post-instillation period (mean [SE], 0.62 [0.076] vs. 1.49 [0.108]; P < .0001) and at each time point within that period (P < .0001). At 1, 360, and 480 minutes post-instillation, respectively, the mean differences (95% confidence interval) between treatments were −0.73 (−1.05 to −0.41), −0.57 (−0.84 to −0.29), and −0.39 (−0.67 to −0.10), respectively. No tachyphylaxis was evident with brimonidine on days 15 and 29, and minimal rebound redness was observed following discontinuation. Adverse events were infrequent, and brimonidine was rated as very comfortable.
CONCLUSIONS
Brimonidine 0.025% appeared safe and effective for reduction of ocular redness, with an 8-hour duration of action, no evidence of tachyphylaxis, and negligible rebound redness.
Ocular redness is commonly due to inflammation of the conjunctiva resulting in vasodilation of the conjunctival blood vessels, with multiple possible causes including allergy, infection, dryness or fatigue, prolonged visual tasking, trauma, foreign body, and contact lens wear.1,2 Once all pathological causes of redness have been ruled out, conjunctival vasoconstriction is of therapeutic value, providing temporary relief of congestion. Current ophthalmic decongestants are adrenergic receptor agonists and include the sympathomimetic amine phenylephrine and the imidazolines naphazoline, tetrahydrozoline, and oxymetazoline. These agents are α1- or mixed α1/α2-adrenergic receptor agonists and reduce ocular redness through vasoconstriction.3,4 However, their use is associated with tachyphylaxis (tolerance or loss of effectiveness) with continued use and/or rebound redness upon treatment discontinuation, which restricts their long-term use.4–8 These adverse effects are likely related to the actions of these agents at the α1-adrenergic receptor. Tachyphylaxis is thought to be due to a tolerance-related dampening of the α1-adrenergic receptor response, possibly through internalization of α1-adrenergic receptors and subsequent down-regulation of surface α1-adrenergic receptors.9,10 Because of the predominant vasoconstrictive effects of α1-adrenergic receptors on arterial beds,11 rebound redness is thought to result from a generalized ischemia brought about by vasoconstriction with secondary activation of an inflammatory cascade and/or loss of basal vascular tone due to receptor down-regulation.5,8
Brimonidine tartrate is a selective imidazoline α2-adrenergic receptor agonist currently marketed as a 0.1 to 0.2% ophthalmic solution for the treatment of ocular hypertension.12–14 Brimonidine acts on the iris–ciliary body α2-adrenergic receptors to mediate intraocular pressure lowering through reduction of aqueous humor production and also increases uveoscleral outflow over the longer term.15,16 Brimonidine tartrate 0.5% gel is approved as a vasoconstrictor for the treatment of nontransient facial erythema secondary to rosacea.17 As brimonidine affects vasoconstriction primarily via the α2-adrenergic receptor, it may have decreased potential for rebound redness or tachphylaxis.8,18,19 Brimonidine's effect on conjunctival blood vessels is dose dependent; at the high doses used for intraocular pressure lowering, it has been associated with hyperemia.20,21 In contrast, at low doses, it has been shown to be associated with conjunctival whitening or “blanching”22,23 and to control bleeding during ophthalmic surgery or associated with intravitreal injections.22,24–29 These and other studies have driven the development of a low-dose brimonidine formulation for the reduction of ocular redness, which was recently approved by the Food and Drug Administration.
The objective of this phase 3 clinical trial was to evaluate the efficacy and safety of brimonidine tartrate ophthalmic solution 0.025% for treating ocular redness in a population of subjects with redness of an undetermined nature representative of a real-world population. Redness reduction was evaluated in-office through 8 hours following a single dose. Thereafter, treatment was instilled four times daily for 1 month in order to assess the potential for tachyphylaxis with continued use. Post-treatment assessments were also carried out to identify if rebound redness occurred following treatment discontinuation.
METHODS
Study Design
This was a single-center (Total Eye Care, PA, Memphis, TN), double-masked, randomized, vehicle-controlled, parallel-group, efficacy and safety study (Clinicaltrials.gov NCT01959230). The study was performed in compliance with the Declaration of Helsinki, International Conference of Harmonization, Good Clinical Practice Guidance, and all applicable local, state, and federal requirements. Each subject or his/her legal representative provided written informed consent prior to any study-related procedures. The study protocol, informed consent, and related documents were approved by the investigational review board, Alpha IRB (San Clemente, CA).
Subjects
Subjects were 18 years or older with stable ocular health (defined as no ocular conditions requiring therapy or surgical intervention) and either a history of ocular vasoconstrictor use or a desire to use over-the-counter vasoconstrictors for redness relief within the previous 6 months. Participants were required to be able to self-administer or have a care provider available to administer study treatments. Female subjects of childbearing potential underwent urine pregnancy testing at day 1 (visit 1), and at exit, and all subjects agreed to use at least one medically acceptable form of birth control. All subjects had ocular health otherwise within normal limits, including a best corrected visual acuity of 0.3 logarithm of the minimum angle of resolution or better in each eye, as measured using an Early Treatment Diabetic Retinopathy Study chart, at visit 1. Subjects had a baseline redness score of greater than 1 in both eyes as assessed by investigators using the Ora Calibra Ocular Hyperemia Scale (0- to 4-unit scale of none, mild, moderate, severe, and extremely severe, allowing half-unit increments). This scale, which has been used in a previous study,30 is based on photographic standards and is a global assessment of ocular redness taking into account the redness manifested in the bulbar and palpebral conjunctiva.
Subjects were excluded if they had known contraindications or sensitivity to brimonidine or any ingredients in the formulation, or if they had an ocular surgical intervention during the study or within 3 months of visit 1, and/or a history of refractive surgery within the past 6 months. Subjects with a history or presence of any ophthalmic or systemic disorder or disease that, in the opinion of the investigator, could have confounded study data, interfered with the subject's study participation, or affected the subject's safety or trial parameters were excluded. Subjects were also excluded if they had prior (within 5 days of beginning study treatment) or anticipated concurrent use of artificial tear products, other ocular vasoconstrictors, ocular decongestants, ocular antihistamines, ocular corticosteroids, phenylephrine dilating drops, any other topical ophthalmic agents, or contact lenses; had prior (within 7 days of beginning study treatment) or anticipated concurrent use of systemic antihistamines or decongestants; had prior (within 14 days of beginning study treatment) or anticipated concurrent use of systemic corticosteroids, cancer chemotherapy, or any other systemic medications that the investigator felt may have confounded study data or interfered with subject's study participation; or had prior (within 30 days of beginning study treatment) or anticipated concurrent use of an investigational drug or device. Subjects who had an abnormal blood pressure (defined as ≤90 or ≥160 mmHg systolic or ≤60 or ≥100 mmHg diastolic), had intraocular pressure that was less than 5 mmHg or greater than 22 mmHg, or had a diagnosis of glaucoma were also excluded.
Study Treatments and Assessments
Brimonidine tartrate ophthalmic solution 0.025% and its vehicle (formulation without brimonidine tartrate) were manufactured by Bausch & Lomb, Inc. (Tampa, FL), and supplied in identical 10-mL sterile bottles. For masking purposes, bottles of active treatment and vehicle were identical in appearance.
Eligible subjects completed a screening visit and four study visits (including three on-treatment visits and one follow-up safety visit) over approximately 36 days. Informed consent was obtained, and demographics and medical and ophthalmic history were collected at the screening visit.
On day 1 (visit 1), subjects who met the inclusion criteria were randomized 2:1 according to a computer-generated randomization code to bilateral treatment with one drop of brimonidine tartrate ophthalmic solution 0.025% or vehicle. A single dose was instilled on day 1 in-office by the subjects under the supervision of a trained technician. The investigator graded ocular redness prior to treatment instillation and at 1, 5, 15, 30, 60, 90, 120, 180, 240, 360, and 480 minutes post-treatment instillation using the Ora Calibra Ocular Hyperemia Scale, and subjects assessed drop comfort on a 0- to 10-unit scale (from very comfortable to very uncomfortable) upon instillation and at 30 seconds and 1 minute after treatment instillation. In addition, subjects completed the Ora Calibra Drop Descriptor Query at 3 minutes post-instillation. Subjects were asked to choose three words that best described how the drop felt using prompted (e.g., burning, filmy, comfortable, cool, refreshing, smooth) or spontaneous word descriptors.
Subjects instilled study treatment four times daily (approximately 4 hours apart) at home beginning the day following visit 1 and continued dosing four times daily for up to 4 weeks except on days 15 ± 2 (visit 2) and 29 ± 2 (visit 3) when one of the doses was instilled in-office by a trained technician. At these visits, as well as the day 36 ± 1 visit (visit 4; approximately 7 days after treatment discontinuation), ocular redness was assessed in-office by the investigator. During the on-treatment visits (i.e., days 15 and 29), redness assessments were conducted prior to and at 1 and 5 minutes after in-office drug instillation (approximately 4 hours from the previous at-home dose).
Subjects were provided with a diary at visits 1 to 3 to document dosing between study visits and to record their ocular redness during these study periods. Subject dosing compliance was determined by an in-office review of the dosing diary at the next visit and calculated as the number of doses taken divided by the number of doses expected multiplied by 100. Any subject who had missed more than 20% or had dosed at greater than 120% in either of their two on-treatment diaries was considered noncompliant. Subjects scored their ocular redness using a photographic (0 to 4 units, no half-unit increments) scale (Ora Calibra Ocular Hyperemia Scale) four times daily for the duration of the study.
At each study visit, medication, medical histories, and treatment-emergent adverse events were collected; best corrected visual acuity was assessed; and slit-lamp examinations of the lid and lid margin, bulbar and palpebral conjunctiva, cornea, and anterior chamber were conducted (before and after drop instillation for visits 1 to 3). In addition, vital signs (resting blood pressure and pulse) were monitored, and the subjects' alertness was evaluated on a 6-point scale (fully alert, alert, lethargy, obtunded, stupor, coma) at each on-treatment study visit (visits 1 to 3). A full physical examination, dilated ophthalmoscopy and intraocular pressure measurement were also performed at visits 1 and 3.
Outcome Measures
The primary efficacy end point was the investigator-assessed ocular redness evaluated at 5, 15, 30, 60, 90, 120, 180, and 240 minutes post-instillation at visit 1. Secondary end points included the change from pre-instillation ocular redness at 1, 360, and 480 minutes at visit 1 and at 1 and 5 minutes at visits 2 and 3; ocular redness scores recorded in the subject diaries throughout the treatment period; and investigator-assessed total clearance of ocular redness at visits 1, 2, and 3. Visits 2 and 3 investigator and subject redness scores were used to evaluate the potential for tachyphylaxis of effect after dosing for approximately 15 and 29 days, respectively.
Tolerability measurements included drop comfort. Safety assessments included physical examination, vital signs, adverse events, best corrected visual acuity, slit-lamp examination, intraocular pressure, dilated ophthalmoscopy, alertness evaluation, and rebound redness based on investigator- and subject-assessed ocular redness scores following treatment discontinuation. Rebound redness was defined as an increase of 1 unit or greater in investigator-assessed ocular redness scores at visit 4 compared with the pre-instillation score at visit 1 or an increase of 1 unit or greater in subject-assessed mean ocular redness score over the follow-up period after dosing had ceased (determined by first calculating the average daily score from both eyes and then averaging all daily scores between visits 3 and 4) compared with the pre-instillation score at visit 1 as recorded in patient diaries.
Statistical Methods
The primary efficacy analysis was performed in the intent-to-treat population, which included all randomized subjects who received at least one dose of study medication and completed at least one post-instillation ocular redness evaluation at visit 1. The safety population included all randomized subjects who received at least 1 dose of study treatment. Investigator-assessed ocular redness scores were the average of both eyes. Actual scores and change from baseline scores for brimonidine-treated eyes versus vehicle-treated eyes were compared using a mixed-effect repeated-measure model over the first 4 hours post-treatment instillation at visit 1 (from 5 to 240 minutes), accounting for repeated measures and adjusting for baseline score and with last observation carried forward for missing data. The model contained treatment, time point, treatment by time point interaction, and baseline score as covariates using the unstructured variance-covariance matrix. The least squares mean, standard errors (SEs), least squares mean differences with 95% confidence interval (CI), and P values were calculated. Analysis was repeated with observed data only. The mixed-effect repeated-measure model was also used to analyze the entire time course (1 to 480 minutes; observed data only). The differences between brimonidine and vehicle investigator-assessed ocular redness scores were also analyzed using two-sample t tests for each time point at each visit (last observation carried forward). A sample size of 40 subjects in the active group and 20 subjects in the vehicle group was determined to have approximately 95% power to detect a difference of 1.0 unit in mean ocular redness using a two-sample t test, assuming an SD of 1.0 unit.
Two-sample t tests were used to analyze the change from baseline in ocular redness at the corresponding visit for the 1-, 360-, and 480-minute time points at visit 1 and the 1- and 5-minute time points at days 15 and 28 (observed data only) and drop comfort at visit 1. Subject-assessed ocular redness for brimonidine and vehicle-treated eyes was compared by first calculating the average of the mean of the four daily scores in both eyes over the periods between visits 1 to 2 (days 1 to 15), visits 2 to 3 (days 15 to 29), and visits 3 to 4 (days 29 to 36) and then comparing these values using a mixed-effect repeated-measure model, accounting for repeated measures with last observation carried forward for missing data. The percentage of subjects with total clearing for each post-instillation time point at each visit was analyzed using Fisher exact tests (observed data only).
Adverse events were summarized using discrete summaries at the subject and event level by using the Medical Dictionary for Regulatory Activities (version 16.1) coding for the system organ class and preferred term for each treatment group, as well as by severity (mild, moderate, severe) and relationship to drug. The investigator deemed an adverse event treatment related if a reasonable possibility existed that the study treatment caused the adverse event and not related if, in their opinion, a reasonable possibility did not exist.
RESULTS
Subject Disposition and Demographics
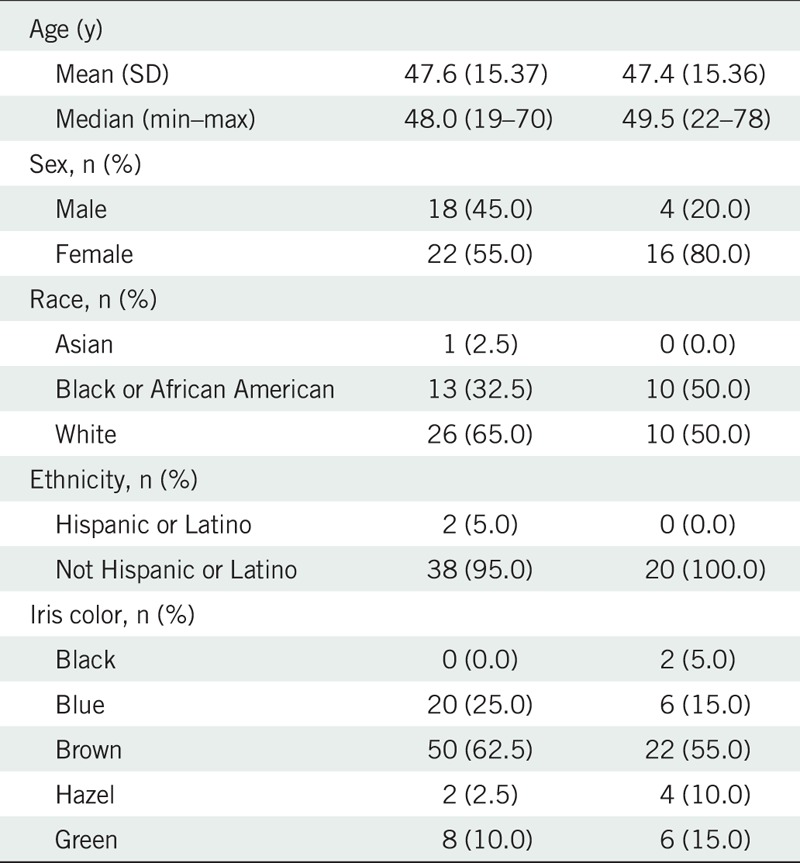
Sixty subjects were randomized (n = 40 brimonidine; n = 20 vehicle), received at least one dose, and had at least one post-instillation redness assessment. All subjects received their assigned treatment; hence, the Safety population and intent-to-treat population were the same. Table 1 provides a summary of demographic information. Of the 60 subjects randomized, 55 subjects (91.7%) completed the study. Reasons for discontinuation included withdrawal of consent (n = 2, brimonidine), administrative reasons (no show; n = 2, brimonidine), and failure to follow required study procedures (n = 1, vehicle). There were no major protocol violations.
TABLE 1.
Subject demographics of the intent-to-treat population
Compliance with dosing instructions was assessed at 95.2% across all subjects. The majority (90%) of subjects in the brimonidine group and all of the subjects in the vehicle group were compliant within 80 to 120% of the required dosing regimen. Four subjects (10%) in the brimonidine group were undercompliant (<80%).
Efficacy Results
Mean (SD) ocular redness scores at baseline were comparable between treatment groups (brimonidine: 1.82 [0.41]; vehicle: 1.71 [0.37]). The mixed-effect repeated-measure model least squares mean (SE) ocular redness was significantly lower in brimonidine-treated eyes versus vehicle-treated eyes over the 5- to 240-minute post-instillation period (0.62 [0.08] vs. 1.49 [0.11]; P < .0001). There was also a significantly greater change from baseline in redness of −1.16 (0.08) for brimonidine versus −0.29 (0.11) for vehicle (P < .0001) over this time frame. The least squares mean differences (95% CI) between brimonidine and vehicle were −0.87 (−1.13 to −0.60) both for the actual redness score and for the change from baseline in redness. In addition, mean ocular redness scores were significantly lower in brimonidine-treated eyes compared with vehicle-treated eyes at each of the individual primary efficacy time points from 5 to 240 minutes post-instillation (P < .0001 for each, t test; Fig. 1). The mean difference in change from baseline in ocular redness was also significantly greater for brimonidine as compared with vehicle at each individual time point (P < .0001 for each, t test). All changes from baseline to individual primary time points over the 5- to 240-minute period were at least 1 unit in brimonidine-treated eyes. Results for primary end point analysis using data as observed were consistent with findings using last observation carried forward.
FIGURE 1.
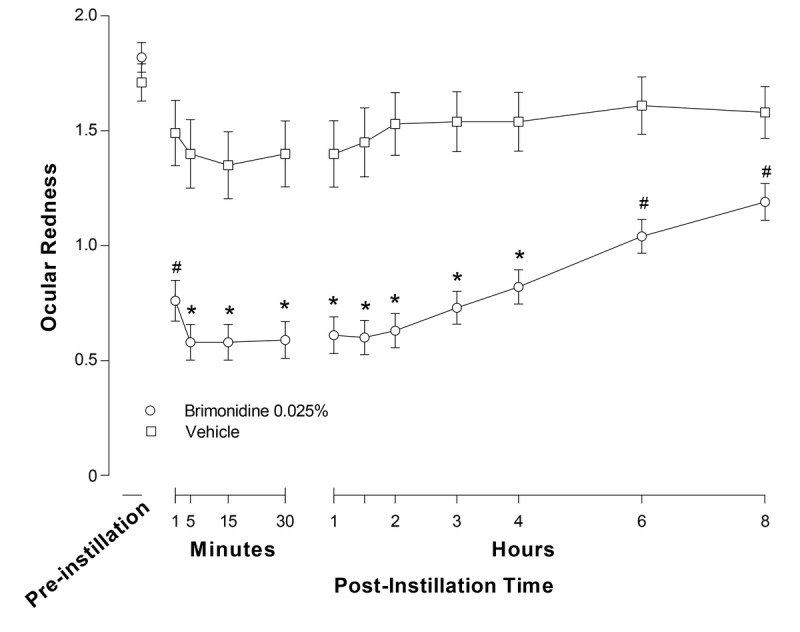
Investigator-evaluated ocular redness (0- to 4-unit scale) at day 1 by treatment in the intent-to-treat (ITT) population. Data are means (SE); *P < .0001 vs. vehicle at the indicated time point (last observation carried forward); #P ≤ 0.01 vs. vehicle at the indicated time point (observed data only).
Similar results were observed for the secondary measurements of ocular redness at 1 minute, 6 hours (360 minutes), and 8 hours (480 minutes) at visit 1 (observed data only), also shown in Fig. 1. At 1 minute post-instillation, there was a significantly greater reduction in mean ocular redness with brimonidine compared with vehicle (mean difference [95% CI], −0.73 [−1.05 to −0.41]; P = .0001), as well as a significantly greater change from baseline in mean redness with brimonidine compared with vehicle (mean difference [95% CI], −0.84 [1.16 to −0.52], P < .0001). Ocular redness was also significantly lower in brimonidine-treated eyes versus vehicle-treated eyes at 6 hours (360 minutes) and 8 hours (480 minutes) post-instillation (mean difference [95% CI], −0.57 [−0.84 to −0.29] and −0.39 [−0.67 to −0.10]; P ≤ 0.0098 for both). In addition, the change from baseline in mean redness was significantly greater with brimonidine than vehicle at these two time points (mean difference [95% CI], −0.68 [−0.95 to −0.41] and −0.50 [−0.80 to −0.20] at 6 and 8 hours post-instillation, respectively; P ≤ 0.0008 for both). Analysis of the entire post-instillation time course at day 1 (from 1 minute to 8 hours) demonstrated a statistically significant difference between treatment groups (0.72 [0.073] vs. 1.52 [0.104] for brimonidine and vehicle, respectively; P < .0001).
In order to determine whether continued dosing of brimonidine was associated with tachyphylaxis of effect, ocular redness was evaluated by investigators after 15 days (visit 2) and 29 days (visit 3) of dosing. Mean (SD) ocular redness scores on these respective days in brimonidine-treated eyes were 1.57 (0.65) and 1.64 (0.46) pre-instillation decreasing to 0.80 (0.52) and 0.76 (0.51) at 1 minute post-instillation and 0.54 (0.43) and 0.43 (0.41) at 5 minutes post-instillation. These scores were comparable to day 1 scores of 1.82 (0.41), 0.76 (0.56), and 0.58 (0.50) at the pre-instillation and 1- and 5-minute time points, respectively. In addition, at days 15 and 29, brimonidine-treated eyes had significantly lower redness scores than vehicle-treated eyes at both the 1- and 5-minute post-instillation time points. Day 15 mean differences (95% CI) in redness scores and change from baseline in redness scores, respectively, were −0.29 (−0.56 to −0.03) and −0.62 (−0.87 to −0.37) at 1 minute post-instillation and −0.46 (−0.70 to −0.22) and −0.78 (−1.07 to −0.50) at 5 minutes post-instillation (P ≤ 0.0174). Day 29 mean differences (95% CI) in redness scores and change from baseline in redness scores, respectively, were −0.35 (−0.62 to −0.09) and −0.64 (−0.88 to −0.40) at 1 minute post-instillation and −0.56 (−0.78 to −0.33) and −0.84 (−1.06 to −0.62) at 5 minutes post-instillation (P ≤ 0.0038).
Fig. 2 presents mean (SE) subject-assessed ocular redness scores by day throughout the dosing period and the period following treatment discontinuation. The brimonidine group reported a significantly lower mean of the mean daily ocular redness score versus the vehicle group post-instillation over the periods between visit 1 and visit 2 (mean difference [95% CI], −1.00 [−1.46 to −0.54]; P < .0001) and between visits 2 and 3 (mean difference [95% CI], −0.83 [−1.28 to −0.38]; P = .0005), demonstrating that redness reduction with brimonidine was maintained over the entire treatment period. Following discontinuation of treatment, between visits 3 and 4 (days 29 to 36), there was no difference in mean (SE) daily ocular redness scores between the brimonidine and vehicle groups (1.69 [0.89] vs. 1.69 [0.90]).
FIGURE 2.
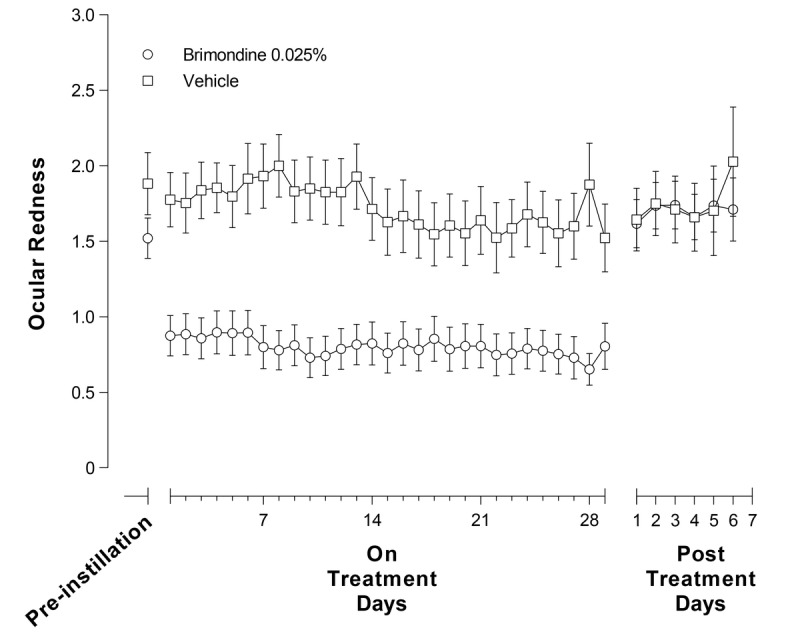
Subject-graded ocular redness (0- to 4-unit scale) over the whole treatment period by treatment in the intent-to-treat population with observed data only. Data are means (SE).
A significantly greater number of subjects had total clearance of redness based on investigator evaluation (observed data only) in the brimonidine group as compared with the vehicle group at visit 1, at 5 (30% vs. 5%, P < .043), 15 (30% vs. 5%, P < .043), 30 (27.5% vs. 5%, P < .047), and 120 minutes (23.1% vs. 0%, P < .022), and at visits 2 and 3, at 5 minutes post-instillation (24.3% vs. 0%, P < .021; 30.6% vs. 5.3%, P < .041).
Safety Results
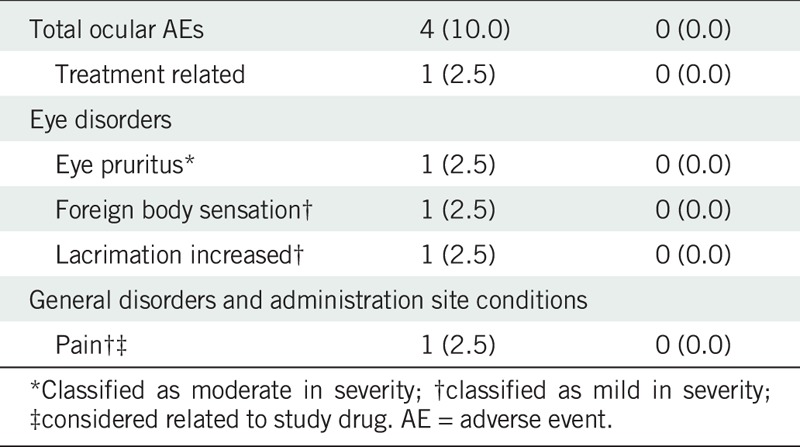
There were no serious adverse events during the course of the study and no withdrawals due to an adverse event. A total of 12 (20%) of the 60 randomized subjects experienced 13 adverse events, of which 11 were treatment-emergent adverse events (n = 4 ocular, n = 7 nonocular). Four subjects (10%) in the brimonidine group had one ocular treatment-emergent adverse event each (pruritus, foreign body sensation, increased lacrimation, and pain), all mild to moderate in severity (Table 2). The adverse event of pain was considered to be related to the study medication and further characterized as mild stinging. With the exception of pruritus, all ocular adverse events resolved spontaneously. Six subjects (15.0%) each in the brimonidine group and one subject (5.0%) in the vehicle group had a nonocular treatment-emergent adverse event. These included nasopharyngitis, ligament sprain, muscle strain, abdominal discomfort, temporomandibular joint pain, and bronchitis in the brimonidine group and nasal congestion in the vehicle group. None were considered related to study treatment, and all were mild to moderate in severity.
TABLE 2.
Ocular treatment-emergent adverse events
There were no instances of losses of greater than 0.05 in best corrected visual acuity logarithm of the minimum angle of resolution. No safety concerns were raised as a result of any of the clinical examinations (slit-lamp biomicroscopy, intraocular pressure measures, and dilated ophthalmoscopy). While no reductions in mean intraocular pressure were observed, an increase was noted in both treatment groups at day 29 compared with baseline (mean [SD] change from baseline [in mmHg] brimonidine: OD = 1.6 [2.27], OS = 1.6 [2.29]; vehicle: OD = 1.8 [2.76], OS = 1.5 [2.74]) and was presumed related to normal intraocular pressure fluctuation. Further, all subjects were determined to be “fully alert” by investigators at every visit. There were no meaningful changes in vital signs (pulse rate, blood pressure) or physical examinations observed at any time point.
Rebound redness after treatment discontinuation was minimal based on both investigator and subject assessments. At visit 4, approximately 7 days after treatment discontinuation, investigator-assessed mean (SD) ocular redness was 1.55 (0.51) in brimonidine-treated eyes and 1.17 (0.48) in vehicle-treated eyes. Visit 4 mean (SD) ocular redness in eyes previously treated with brimonidine was comparable to day 1 (visit 1) baseline redness prior to brimonidine treatment, or 1.82 (0.41). One subject in the brimonidine group (2.88%) and no subject in the vehicle group experienced redness rebound at day 36 based on investigator assessment. Evaluation of subject-reported ocular redness during the 7-day post-treatment period yielded similar results, with four subjects in the brimonidine group (11.1%) and three in the vehicle group (15.8%) experiencing rebound redness. The mean of the mean daily ocular redness score over the post-treatment period for brimonidine was similar to the mean daily pre-instillation score between days 1 and 15 (1.69 [0.885] and 1.52 [0.857]).
Drop Tolerability
Mean (SD) drop comfort scores in the brimonidine group and vehicle group, respectively, were 1.1 (2.11) and 1.3 (1.71) upon instillation, 1.1 (1.89) and 1.0 (1.26) at 30 seconds post-instillation, and 0.9 (1.67) and 1.0 (1.13) at 1 minute post-instillation. There were no differences between treatments in drop comfort at any of these assessment time points (P ≥ 0.75).
The three most common positive word descriptors used in the brimonidine group were comfortable, cool, and gentle (used by 67.5%, 55.0%, and 32.5% of subjects, respectively). The three most common negative word descriptors were burning, irritating, and stinging (used by 5.0%, 12.5%, and 12.5% of subjects, respectively).
DISCUSSION
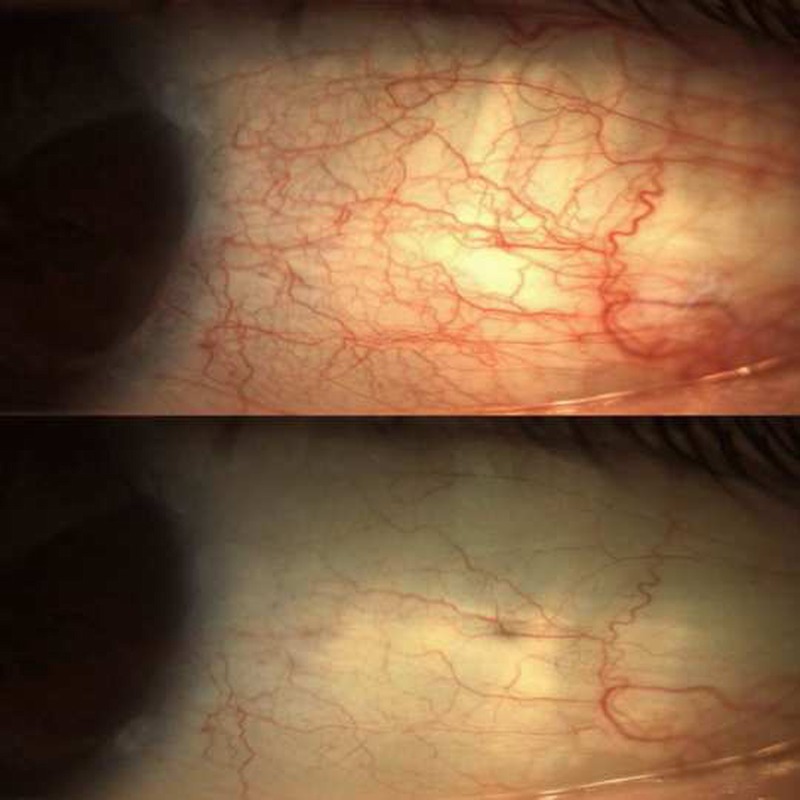
This randomized, parallel-group, vehicle-controlled, double-masked study evaluated the safety and efficacy of brimonidine tartrate ophthalmic solution 0.025% for reduction of ocular redness. Participating subjects had ocular redness of an undetermined nature for which there was no apparent underlying pathology but with otherwise stable ocular health. Based on investigator evaluations, treatment with brimonidine 0.025% resulted in statistically significantly lower ocular redness and a greater change from baseline in ocular redness compared with vehicle over the 5- to 240-minute post-instillation time period, as well as at each time point within that period. Furthermore, brimonidine resulted in a clinically significant (>1-unit change) change from baseline in ocular redness at each time point over this time period. Ocular redness scores were also significantly lower compared with vehicle at 1 minute post-instillation and at 6 and 8 hours post-instillation, supporting an onset of action of brimonidine as early as 1 minute post-instillation and lasting as long as 8 hours. In addition, complete clearing of ocular redness was experienced by approximately one fourth of brimonidine-treated subjects as early as 5 minutes following drop instillation (see Fig. 3 for photographs from a representative eye before and 5 minutes after instillation of brimonidine 0.025%). While there are no published data to inform on the expected time to tachyphylaxis with ophthalmic decongestants, redness reduction was maintained with continued use over 4 weeks, suggesting that no tachyphylaxis occurred with this selective α2-adrenergic receptor agonist. Importantly, minimum redness rebound was observed after cessation of therapy, with only one brimonidine subject experiencing an increase in ocular redness of more than 1 unit. Subject self-assessed ocular redness scores were consistent with investigator-evaluated findings; brimonidine 0.025% maintained redness reduction over the entire 4-week treatment period, with minimal reports of redness rebound after treatment discontinuation, similar in incidence as that in the vehicle group.
FIGURE 3.
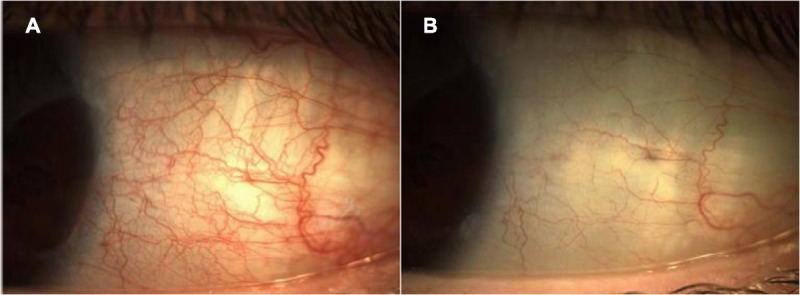
Photographs from a representative eye with baseline ocular redness graded as moderate (score of 2) before (A) and 5 minutes after (B) instillation of one drop of brimonidine tartrate ophthalmic solution 0.025% (score of 0 or none).
In terms of tolerability, brimonidine was rated as very comfortable and comparable to vehicle as evaluated by two independent assessments, comfort score and drop description. With regard to adverse events, brimonidine 0.025% appeared safe when administered four times daily for a month in this study. Of the four treatment-emergent ocular adverse events with brimonidine, only one was considered to be related to treatment (mild pain) and resolved spontaneously despite continued treatment. There have been reports of ocular surface allergic disease in association with the use of high doses of brimonidine,31,32 yet only two subjects treated with brimonidine 0.025% in the current study reported an ocular adverse event potentially associated with allergic disease (increased lacrimation and pruritus), neither of which was judged by the investigator to be related to study drug. There were no reports of allergic conjunctivitis or of papillary or follicular conjunctival response. Considering that brimonidine 0.15% has a lower rate of associated allergic disease than brimonidine 0.2%,33,34 the potential for allergic disease with brimonidine 0.025% (6- to 8-fold lower than in current marketed formulations) may be minimal. However, further studies, including longer duration studies, may be warranted to fully evaluate the risk of allergic reactions with low-dose brimonidine. While mydriasis is a known adverse effect of α1-adrenergic receptor agonists,35 this was not reported with brimonidine, in keeping with its known selectivity for the α2-adrenergic receptor.36 Miosis, which is reported for higher doses of brimonidine, particularly under scotopic conditions,37–39 was also not reported in this study. Finally, although fatigue and drowsiness have been reported with topical use of high-dose brimonidine,23,40,41 especially in pediatric and geriatric patients,42,43 all subjects in the current study were deemed “fully alert” at all visits, and there were no reports of somnolence. Overall, no clinically meaningful safety signals were observed, suggesting that brimonidine could be safely used at this concentration and dosing regimen for reduction of ocular redness.
Clinical trials defining the duration of action and adverse effects for currently marketed ophthalmic α1-adrenergic receptor agonists or nonselective α1-/α2-adrenergic receptor agonists on ocular redness are scarce. Oxymetazoline was evaluated in randomized controlled clinical trials in the early 1980s and was found to reduce ocular redness for up to 6 hours. However, with only 1 week of treatment, a complete evaluation of tachyphylaxis and rebound redness was not performed.44–47 In the present study, brimonidine, a selective α2-adrenergic receptor agonist, was shown to have an 8-hour duration of action and continued to demonstrate effective redness reduction through 1 month of therapy. The data that led to the hypothesis that α2-adrenergic receptor agonists might be associated with less tachyphylaxis and redness rebound8,18,19 appear to be substantiated clinically in the present study. While the precise mechanism for the lack of tachyphylaxis and minimal redness rebound with brimonidine is currently unclear, studies in nonocular tissues suggest that α2-adrenergic receptor agonists such as brimonidine mediate vasoconstriction primarily on the venular side,10,11,18,19 and therefore generalized ischemia is not expected to occur. The sustained efficacy of higher concentrations of brimonidine than used in this study on intraocular pressure lowering and in the treatment of rosacea further supports that this α2-adrenergic receptor agonist does not impact receptor down-regulation to the same extent as observed for α1-receptor agonists.12–14,21,48,49
To our knowledge, this is the longest-duration, controlled clinical trial conducted with a topical vasoconstrictor to date. There is an unmet need for a topical vasoconstrictor that can be safely used for long periods for reduction of conjunctival hyperemia. In this study, brimonidine 0.025% reduced ocular redness both clinically and statistically with a rapid onset of action (1 minute) and long duration of action (up to 8 hours). In conclusion, brimonidine tartrate ophthalmic solution 0.025% dosed four times daily appears safe and effective with continued month-long use for the relief of ocular redness, with no evidence of tachyphylaxis of effect with continued use and minimal rebound redness after treatment discontinuation.
Footnotes
Submitted: May 2, 2017
Accepted: October 16, 2017
Funding/Support: Funding was provided by Bausch & Lomb, Inc., a division of Valeant Pharmaceuticals International, Inc. Bausch & Lomb, Inc., in collaboration with Ora, Inc., participated in study design/conduct, provided manuscript preparation support, and reviewed the final manuscript prior to submission.
Conflict of Interest Disclosure: EM has received research grants from the following companies: Aciex, Acucela, Alcon Research Ltd., Allergan, AstraZeneca, Bausch + Lomb, Inotek Pharma, InSite Vision, Lexicon Pharma, Mimetogen, and Ocular Therapeutix. MEC is an employee of Bausch + Lomb. PJG is an employee of Ora. JBC is a paid consultant for Ora.
Author Contributions: Investigation: EM; Writing – Review & Editing: EM, MEC, PJG, JBC; Writing – Original Draft: MEC; Conceptualization: PJG; Methodology: PJG; Supervision: JBC.
Clinicaltrials.gov Identifier: NCT01959230, registered October 8, 2013.
REFERENCES
- 1.Cronau H, Kankanala RR, Mauger T. Diagnosis and Management of Red Eye in Primary Care. Am Fam Physician 2010;81:137–44. [PubMed] [Google Scholar]
- 2.Galor A, Jeng BH. Red Eye for the Internist: when to Treat, when to Refer. Cleve Clin J Med 2008;75:137–44. [DOI] [PubMed] [Google Scholar]
- 3.Bartels SP. Adrenergic agents. In: Albert DM, Jakobiec FA, eds. Principals and Practices of Ophthalmology, Basic Sciences, Philadelphia, PA: W. B. Saunders Company; 1994;993–1012. [Google Scholar]
- 4.Stafford-Smith M, Bartz R, Wilson K, et al. Alpha-adrenergic mRNA Subtype Expression in the Human Nasal Turbinate. Can J Anaesth 2007;54:549–55. [DOI] [PubMed] [Google Scholar]
- 5.Soparkar CN, Wilhelmus KR, Koch DD, et al. Acute and Chronic Conjunctivitis Due to Over-the-counter Ophthalmic Decongestants. Arch Ophthalmol 1997;115:34–8. [DOI] [PubMed] [Google Scholar]
- 6.Spector SL, Raizman MB. Conjunctivitis Medicamentosa. J Allergy Clin Immunol 1994;94:134–6. [DOI] [PubMed] [Google Scholar]
- 7.Tappeiner C, Sarra GM, Abegg M. Abuse of Vasoconstrictive Eyedrops Mimicking an Ocular Pemphigoid. Eur J Ophthalmol 2009;19:129–32. [DOI] [PubMed] [Google Scholar]
- 8.Vaidyanathan S, Williamson P, Clearie K, et al. Fluticasone Reverses Oxymetazoline-induced Tachyphylaxis of Response and Rebound Congestion. Am J Respir Crit Care Med 2010;182:19–24. [DOI] [PubMed] [Google Scholar]
- 9.Fratelli M, De Blasi A. Agonist-induced Alpha 1-Adrenergic Receptor Changes. Evidence for Receptor Sequestration. FEBS Lett 1987;212:149–53. [DOI] [PubMed] [Google Scholar]
- 10.Insel PA. Seminars in Medicine of the Beth Israel Hospital, Boston. Adrenergic Receptors—Evolving Concepts and Clinical Implications. N Engl J Med 1996;334:580–5. [DOI] [PubMed] [Google Scholar]
- 11.Guimaraes S, Moura D. Vascular Adrenoceptors: An Update. Pharmacol Rev 2001;53:319–56. [PubMed] [Google Scholar]
- 12.Adkins JC, Balfour JA. Brimonidine. A Review of Its Pharmacological Properties and Clinical Potential in the Management of Open-angle Glaucoma and Ocular Hypertension. Drugs Aging 1998;12:225–41. [DOI] [PubMed] [Google Scholar]
- 13.Fudemberg SJ, Batiste C, Katz LJ. Efficacy, Safety, and Current Applications of Brimonidine. Expert Opin Drug Saf 2008;7:795–9. [DOI] [PubMed] [Google Scholar]
- 14.Rahman MQ, Ramaesh K, Montgomery DM. Brimonidine for Glaucoma. Expert Opin Drug Saf 2010;9:483–91. [DOI] [PubMed] [Google Scholar]
- 15.Crosson CE, Heath AR, DeVries GW, et al. Pharmacological Evidence for Heterogeneity of Ocular Alpha 2 Adrenoceptors. Curr Eye Res 1992;11:963–70. [DOI] [PubMed] [Google Scholar]
- 16.Potter DE, Crosson CE, Heath AR, et al. Review: Alpha 2 and Da2 Agonists as Antiglaucoma Agents: Comparative Pharmacology and Clinical Potential. J Ocul Pharmacol 1990;6:251–7. [DOI] [PubMed] [Google Scholar]
- 17.Tong LX, Moore AY. Brimonidine Tartrate for the Treatment of Facial Flushing and Erythema in Rosacea. Expert Rev Clin Pharmacol 2014;7:567–77. [DOI] [PubMed] [Google Scholar]
- 18.Corboz MR, Mutter JC, Rivelli MA, et al. Alpha2-adrenoceptor Agonists as Nasal Decongestants. Pulm Pharmacol Ther 2007;20:149–56. [DOI] [PubMed] [Google Scholar]
- 19.Corboz MR, Rivelli MA, Mingo GG, et al. Mechanism of Decongestant Activity of Alpha 2-adrenoceptor Agonists. Pulm Pharmacol Ther 2008;21:449–54. [DOI] [PubMed] [Google Scholar]
- 20.Melamed S, David R. Ongoing Clinical Assessment of the Safety Profile and Efficacy of Brimonidine Compared with Timolol: Year-three Results. Brimonidine Study Group II. Clin Ther 2000;22:103–11. [DOI] [PubMed] [Google Scholar]
- 21.Schuman JS, Horwitz B, Choplin NT, et al. A 1-Year Study of Brimonidine Twice Daily in Glaucoma and Ocular Hypertension. A Controlled, Randomized, Multicenter Clinical Trial. Chronic Brimonidine Study Group. Arch Ophthalmol 1997;115:847–52. [DOI] [PubMed] [Google Scholar]
- 22.Dahlmann-Noor AH, Cosgrave E, Lowe S, et al. Brimonidine and Apraclonidine as Vasoconstrictors in Adjustable Strabismus Surgery. J AAPOS 2009;13:123–6. [DOI] [PubMed] [Google Scholar]
- 23.Derick RJ, Robin AL, Walters TR, et al. Brimonidine Tartrate: A One-month Dose Response Study. Ophthalmology 1997;104:131–6. [DOI] [PubMed] [Google Scholar]
- 24.Desco MC, Navea A, Ferrer E, et al. Effect of Prophylactic Brimonidine on Bleeding Complications After Cataract Surgery. Eur J Ophthalmol 2005;15:228–32. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Kekunnaya R, Sachdeva V, et al. Strabismus Surgery Hemostasis. Ophthalmology 2012;119:649–50. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Kim CY, Seong GJ, et al. Effect of Prophylactic Brimonidine Instillation on Bleeding during Strabismus Surgery in Adults. Am J Ophthalmol 2007;144:469–70. [DOI] [PubMed] [Google Scholar]
- 27.Kim CS, Nam KY, Kim JY. Effect of Prophylactic Topical Brimonidine (0.15%) Administration on the Development of Subconjunctival Hemorrhage After Intravitreal Injection. Retina 2011;31:389–92. [DOI] [PubMed] [Google Scholar]
- 28.Norden RA. Effect of Prophylactic Brimonidine on Bleeding Complications and Flap Adherence After Laser in Situ Keratomileusis. J Refract Surg 2002;18:468–71. [DOI] [PubMed] [Google Scholar]
- 29.Pasquali TA, Aufderheide A, Brinton JP, et al. Dilute Brimonidine to Improve Patient Comfort and Subconjunctival Hemorrhage After LASIK. J Refract Surg 2013;29:469–75. [DOI] [PubMed] [Google Scholar]
- 30.Abelson MB, Gomes PJ, Vogelson CT, et al. Clinical Efficacy of Olopatadine Hydrochloride Ophthalmic Solution 0.2% Compared with Placebo in Patients with Allergic Conjunctivitis or Rhinoconjunctivitis: A Randomized, Double-masked Environmental Study. Clin Ther 2004;26:1237–48. [DOI] [PubMed] [Google Scholar]
- 31.Katz LJ. Brimonidine Tartrate 0.2% Twice Daily vs Timolol 0.5% Twice Daily: 1-Year Results in Glaucoma Patients. Brimonidine Study Group. Am J Ophthalmol 1999;127:20–6. [DOI] [PubMed] [Google Scholar]
- 32.LeBlanc RP. Twelve-month Results of an Ongoing Randomized Trial Comparing Brimonidine Tartrate 0.2% and Timolol 0.5% Given Twice Daily in Patients with Glaucoma or Ocular Hypertension. Brimonidine Study Group 2. Ophthalmology 1998;105:1960–7. [DOI] [PubMed] [Google Scholar]
- 33.Kim CY, Hong S, Seong GJ. Brimonidine 0.2% Versus Brimonidine Purite 0.15% in Asian Ocular Hypertension. J Ocul Pharmacol Ther 2007;23:481–6. [DOI] [PubMed] [Google Scholar]
- 34.Katz LJ. Twelve-month Evaluation of Brimonidine-Purite Versus Brimonidine in Patients with Glaucoma or Ocular Hypertension. J Glaucoma 2002;11:119–26. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa H, Miller DD, Patil PN. Comparison of Post-junctional Alpha-adrenoceptors in Iris Dilator Muscle of Humans, and Albino and Pigmented Rabbits. Naunyn Schmiedebergs Arch Pharmacol 1996;354:765–72. [DOI] [PubMed] [Google Scholar]
- 36.Burke J, Schwartz M. Preclinical Evaluation of Brimonidine. Surv Ophthalmol 1996;41(Suppl. 1):S9–18. [DOI] [PubMed] [Google Scholar]
- 37.Kesler A, Shemesh G, Rothkoff L, et al. Effect of Brimonidine Tartrate 0.2% Ophthalmic Solution on Pupil Size. J Cataract Refract Surg 2004;30:1707–10. [DOI] [PubMed] [Google Scholar]
- 38.McDonald JE, 2nd, El-Moatassem Kotb AM, Decker BB. Effect of Brimonidine Tartrate Ophthalmic Solution 0.2% on Pupil Size in Normal Eyes under Different Luminance Conditions. J Cataract Refract Surg 2001;27:560–4. [DOI] [PubMed] [Google Scholar]
- 39.Thordsen JE, Bower KS, Warren BB, et al. Miotic Effect of Brimonidine Tartrate 0.15% Ophthalmic Solution in Normal Eyes. J Cataract Refract Surg 2004;30:1702–6. [DOI] [PubMed] [Google Scholar]
- 40.Cantor LB. The Evolving Pharmacotherapeutic Profile of Brimonidine, an Alpha 2-Adrenergic Agonist, After Four Years of Continuous Use. Expert Opin Pharmacother 2000;1:815–34. [DOI] [PubMed] [Google Scholar]
- 41.Lee DA, Gornbein J, Abrams C. The Effectiveness and Safety of Brimonidine as Mono-, Combination, or Replacement Therapy for Patients with Primary Open-angle Glaucoma or Ocular Hypertension: A Post Hoc Analysis of an Open-label Community Trial. Glaucoma Trial Study Group. J Ocul Pharmacol Ther 2000;16:3–18. [DOI] [PubMed] [Google Scholar]
- 42.Enyedi LB, Freedman SF. Safety and Efficacy of Brimonidine in Children with Glaucoma. J AAPOS 2001;5:281–4. [DOI] [PubMed] [Google Scholar]
- 43.Bowman RJ, Cope J, Nischal KK. Ocular and Systemic Side Effects of Brimonidine 0.2% Eye Drops (Alphagan) in Children. Eye (Lond) 2004;18:24–6. [DOI] [PubMed] [Google Scholar]
- 44.Breakey AS, Cinotti AA, Hirshman M. A Double-blind, Multi-centre Controlled Trial of 0.25% Oxymetazoline Ophthalmic Solution in Patients with Allergic and Non-infectious Conjunctivitis. Pharmatherapeutica 1980;2:353–6. [PubMed] [Google Scholar]
- 45.Duzman E, Anderson J, Vita JB, et al. Topically Applied Oxymetazoline. Ocular Vasoconstrictive Activity, Pharmacokinetics, and Metabolism. Arch Ophthalmol 1983;101:1122–6. [DOI] [PubMed] [Google Scholar]
- 46.Duzman E, Warman A, Warman R. Efficacy and Safety of Topical Oxymetazoline in Treating Allergic and Environmental Conjunctivitis. Ann Ophthalmol 1986;18:28–31. [PubMed] [Google Scholar]
- 47.Rybiczka R, Mauracher E. Oxymetazoline Ophthalmic Solution Versus Naphazoline Solution in Non-infectious Conjunctivitis. Pharmatherapeutica 1983;3:376–81. [PubMed] [Google Scholar]
- 48.Fowler J, Jr., Jackson M, Moore A, et al. Efficacy and Safety of Once-daily Topical Brimonidine Tartrate Gel 0.5% for the Treatment of Moderate to Severe Facial Erythema of Rosacea: Results of Two Randomized, Double-blind, and Vehicle-controlled Pivotal Studies. J Drugs Dermatol 2013;12:650–6. [PubMed] [Google Scholar]
- 49.Moore A, Kempers S, Murakawa G, et al. Long-term Safety and Efficacy of Once-daily Topical Brimonidine Tartrate Gel 0.5% for the Treatment of Moderate to Severe Facial Erythema of Rosacea: Results of a 1-Year Open-label Study. J Drugs Dermatol 2014;13:56–61. [PubMed] [Google Scholar]


