Abstract
Problem
Developing a national pragmatic clinical trial infrastructure is central to understanding the effectiveness of interventions applied under usual conditions and where people receive health care. To address this challenge, three Florida universities—the University of Florida Clinical and Translational Science Institute, Florida State University (with its community-based distributive medical education model), and the University of Miami—created (2010–2013) a statewide consortium, the OneFlorida Clinical Research Consortium, to support the conduct of pragmatic clinical trials and provide mentored research experiences for medical and graduate students in real-world practice settings.
Approach
OneFlorida has four programs, which report to a steering committee with membership from each partner, community members, and the state Medicaid agency and Department of Health to ensure shared governance. The Clinical Research Program provides support to conduct research in the network and uses champions to engage community clinicians. The Citizen Scientist Program has community members who provide input on health topics of importance to them, study design, recruitment and retention strategies, and the interpretation of findings. The Data Trust Program contains electronic health record and health care claims data for 10.6 million Floridians. The Minority Education Program, in collaboration with three historically black colleges and universities, offers minority junior faculty mentoring in pragmatic clinical trials and implementation science.
Outcomes
OneFlorida has implemented 27 studies with diverse patient populations and in diverse community practice settings.
Next Steps
To identify evidence-based best practices from the clinical trials conducted in the network, foster their implementation, and expand research training opportunities.
Problem
Developing a national pragmatic clinical trial infrastructure is central to understanding the effectiveness of interventions applied under the usual conditions in the places where people receive health care.1 Conducting clinical trials in such real-world settings may also accelerate the translation of findings into practice because the interventions are tested in the settings in which care routinely occurs. Florida is the third most populous state in the United States; it is also racially, ethnically, and geographically diverse, making it an ideal setting to conduct pragmatic clinical trials.2 However, to leverage the state’s diversity, multiple academic institutions, health systems, and clinicians need to be aligned.
To address this challenge, three universities created a statewide clinical research consortium to support the conduct of pragmatic clinical trials and provide mentored research experiences for medical and graduate students in real-world practice settings. The initial partnership was formed in 2010 between the University of Florida (UF) National Institutes of Health (NIH)-funded Clinical and Translational Science Institute (CTSI) and Florida State University (FSU), which uses a community-based distributive medical education (CBDME) model.3 In the CBDME model, students receive basic scientific training at the medical school and clinical training with community physician faculty members in clinics and practices throughout Florida. In 2013, the University of Miami (UM) joined the partnership. Together, these three universities created the OneFlorida Clinical Research Consortium (hereafter, OneFlorida). The purpose of this paper is to describe OneFlorida’s innovative development, its programs, and the sociodemographic and health status of the consortium’s patients.
Approach
OneFlorida background and development
The UF CTSI is an intellectual home for clinical and translational research and training that integrates the scientific and educational activities of 16 colleges, 2 academic institutions, and 2 regional health care systems across Florida. FSU, through its CBDME model, is affiliated with over 2,000 physicians and their associated practices and institutions throughout the state. Approximately 2 million Floridians are seen in these settings annually.
In October 2010, with an NIH supplement to the UF CTSI and a grant from the state of Florida designed to promote collaboration among state universities, the UF CTSI and FSU joined together to leverage their academic, clinical, and educational strengths to create a network for pragmatic clinical trials. The network had some early success with studies conducted both at UF and in FSU’s CBDME practice sites using health information technology to facilitate evidence-based pediatric concussion and adolescent health risk assessments in primary care.4,5 A genomic medicine intervention focused on appropriate prescribing of clopidogrel was also conducted.6 These three studies each involved randomization of the practice sites to usual care or to the intervention (i.e., health information technology support for the assessments and genotyping support for clopidogrel). These study topics were identified through focus groups with clinicians in the network to identify their practice concerns. Despite these early successes, the network had geographic limitations and did not include the racially and ethnically diverse south Florida and Miami areas. Then, in June 2013, the UM joined the partnership, expanding the network’s patient population and clinical settings (Figure 1).
Figure 1.
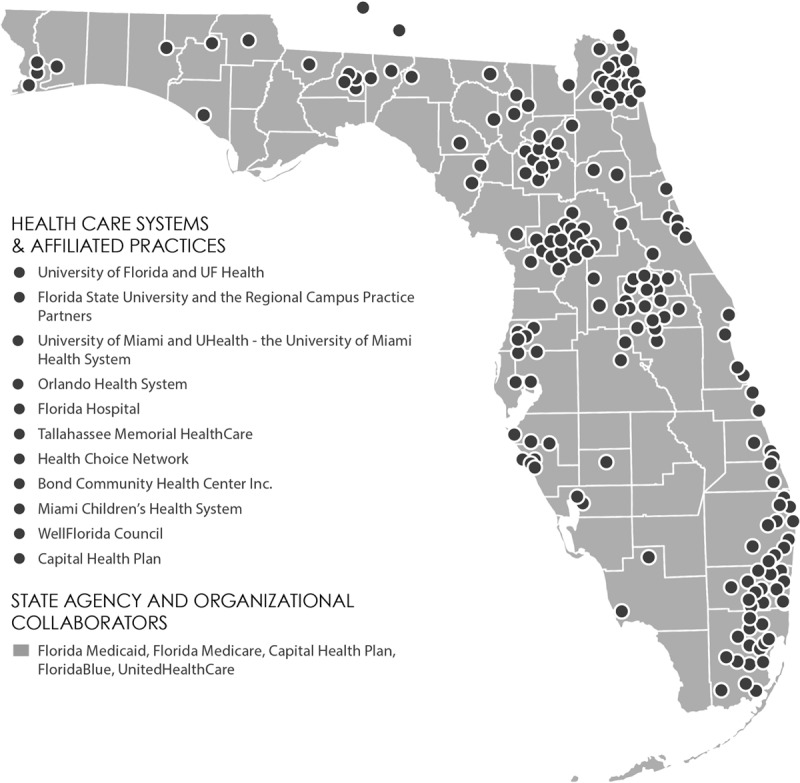
Map of the OneFlorida Clinical Research Consortium network—a statewide consortium (made up of the University of Florida Clinical and Translational Science Institute, Florida State University, and University of Miami) to support the conduct of pragmatic clinical trials and provide mentored research experiences for medical and graduate students in real-world practice settings—2013. A color version of this map can be found at http://onefloridaconsortium.org/about-the-consortium/. Reprinted with permission from UFHealth/University of Florida.
OneFlorida structure
In December 2013, the partners (the UF CTSI, FSU, UM, and 1,240 practice sites and 8 health systems) established the OneFlorida organizational structure, which includes a steering committee with representation from each partner, community members, and the state Medicaid agency and Department of Health to ensure shared governance. The steering committee sets the strategic direction of OneFlorida, establishes its policies and procedures, and approves study protocols. The UF CTSI conducts annual statewide consumer telephone surveys and clinician focus groups on the top health care concerns, which previously included hypertension, diabetes, obesity, cancer, and heart failure. In the focus groups, clinicians are also asked to provide information about the care delivery challenges they face, including caring for rural populations, care coordination, and following evidence-based screening guidelines. The steering committee uses the information on health concerns to give priority to study protocols that address one or more of these concerns. Further, the list of health and care delivery concerns are shared with scientists at UF, FSU, and UM, and UF CTSI pilot funding is given to support studies focused on these priorities. The scientists then work with OneFlorida administrative staff to identify the best settings to approach for study participation based on each study’s design and specific aims.
OneFlorida also has four programs—the OneFlorida Clinical Research Program, OneFlorida Citizen Scientist Program, OneFlorida Data Trust Program, and OneFlorida Minority Education Program—each of which reports to the steering committee and has coleaders and membership from each partner to ensure broad participation.
The OneFlorida Clinical Research Program uses several proven strategies to support pragmatic clinical trials in the network, including using clinician champions to engage community clinicians in research.7 The clinician champions are local physician leaders throughout the state who recruit physicians and practice sites for study participation. This group consists of the six regional campus deans from FSU’s CBDME model, who live and work in communities throughout Florida and arrange for medical student placement. Their in-depth local knowledge makes them critical to engaging physicians and practice sites in OneFlorida studies. The clinical champions are supported in their work by community practice facilitators who reside in the communities and assist with practice and patient recruitment, facilitate study implementation, and provide study monitoring. The UF CTSI Clinical Trials Management Office provides support to OneFlorida and the Clinical Research Program related to participant eligibility and recruitment tracking, regulatory and billing compliance, and electronic data capture.
The Clinical Research Program also offers maintenance of certification (MOC) support, which has been particularly important for engaging physicians. MOC Part IV projects have been integrated into all of OneFlorida’s research studies. For recertification, most physicians must complete at least one MOC project. The community practice facilitators help physicians implement an MOC project based on the study in which they participated. Physicians also receive general training in quality improvement and support with data collection and analysis from OneFlorida administrative staff. Those participating in recertification must also meet regularly in a peer-to-peer learning environment to discuss their projects. All training and peer-to-peer interactions are done virtually, online, and via e-mail.
The OneFlorida Citizen Scientist Program ensures equitable partnerships among scientists, clinicians, and community members. The Citizen Scientist Program was named by the community members to reflect their role in scientific discovery and make a statement that they are people first and not always patients. There are 12 citizen scientists who participate on the steering committee and in the other three programs. They provide input on health topics of importance to them, study design, recruitment and retention strategies, and the interpretation of findings. Scientists using the network report that citizen scientist participation has helped them refine their study topics and address potential barriers to study participation, particularly related to recruitment processes.
The OneFlorida Data Trust Program has a centralized repository, which contains the electronic health record (EHR) and health care claims data from the health system partners. All of the OneFlorida health system partners signed data use agreements, which allowed for the transfer of EHR Health Insurance Portability and Accountability Act–limited data sets, which are formatted according to the National Patient-Centered Clinical Research Network (PCORnet) Common Data Model, to the Data Trust Program repository. Data elements from the claims and EHRs include diagnoses, procedures, place of service, vital signs, laboratory values, and condition lists. Currently, health care claims and/or EHR data from 2012 to 2016 for 10.6 million Floridians reside in the Data Trust Program repository. EHR data are available for 5.1 million of these individuals. The Data Trust Program team has considerable experience with data standards—for example, with the i2b2 common data model, the RxNorm medication terminology, and the Logical Observation Identifiers Names and Codes standards to ensure that the data are well organized for researchers.
We are currently implementing a privacy-preserving algorithm to address the duplication of patients in the OneFlorida network, which is estimated to be approximately 10% based on the market areas of the partners. The health systems submitting their EHR data are beginning to use a UF-supplied software program to combine patient information such as date of birth, last name, and address to create a unique numeric string that can allow for patient tracking across practice settings without violating patient privacy. In tests, we have correctly linked 92% of patients using this method.8 The Data Trust Program is approved by UF’s institutional review board (IRB no. 201500466).
The OneFlorida Minority Education Program, in collaboration with three Florida historically black colleges and universities, offers minority junior faculty mentoring in pragmatic clinical trials and implementation science. Junior faculty from these institutions apply for the program by providing a statement on their research interests and career goals. On the basis of their applications, they are matched with a senior faculty member from UF, FSU, and/or UM to conduct research using OneFlorida’s Data Trust Program and clinical settings.
OneFlorida also has a scientific advisory committee, which reviews the network’s accomplishments. The key metrics of success are patient and clinician engagement in study topic selection and implementation, participant recruitment, peer-review publications, presentations, medical and graduate students receiving mentoring, and the extent to which study findings are implemented in clinical practice.
OneFlorida patient population
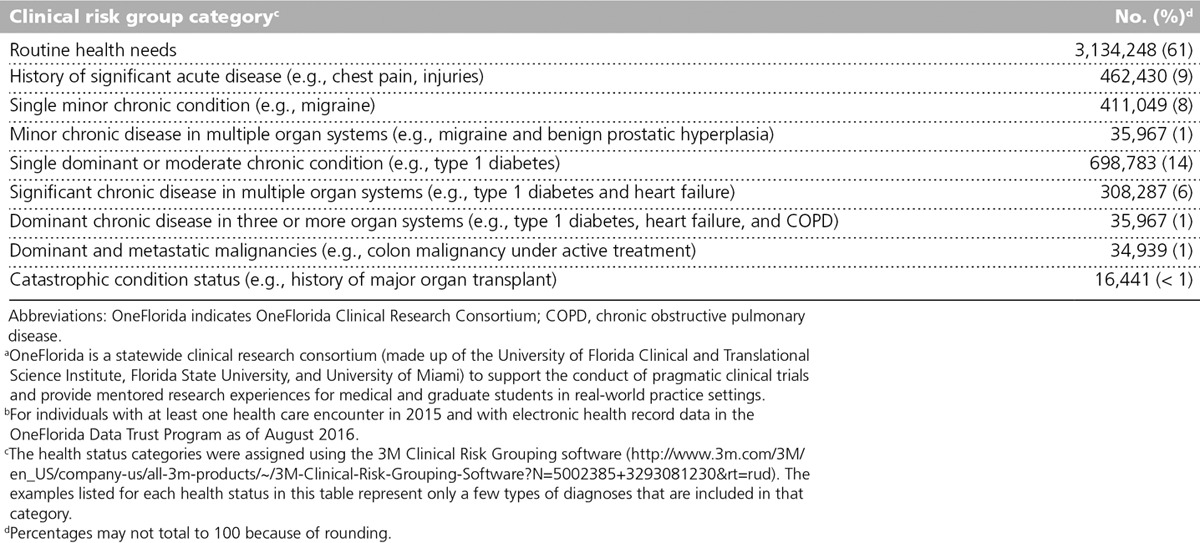
Table 1 shows the sociodemographic characteristics of the individuals with EHR data in the Data Trust Program, as compared with the total Florida and U.S. populations. A higher percentage of these individuals are 18 years or older, female, and black or African American relative to Florida and the United States overall. The percentage of Hispanics seen in OneFlorida is lower than that of Florida and the United States overall (see Table 1). The health status of OneFlorida individuals was classified using the 3M Clinical Risk Grouping diagnostic classification system, which groups International Classification of Disease 9 and 10 codes from inpatient and outpatient facility, pharmaceutical, and professional claims and from procedure codes into categories (Table 2). The categories are informative in terms of grouping individuals into routine, acute, and chronic health care needs. Overall, 30% of adults and children in OneFlorida currently have or have a history of at least one chronic condition, compared with about half of all adults and 15% of children nationally.9,10
Table 1.
OneFloridaa Patient Sociodemographic Characteristics,b Compared With Those of the Total Florida and U.S. Populationsc
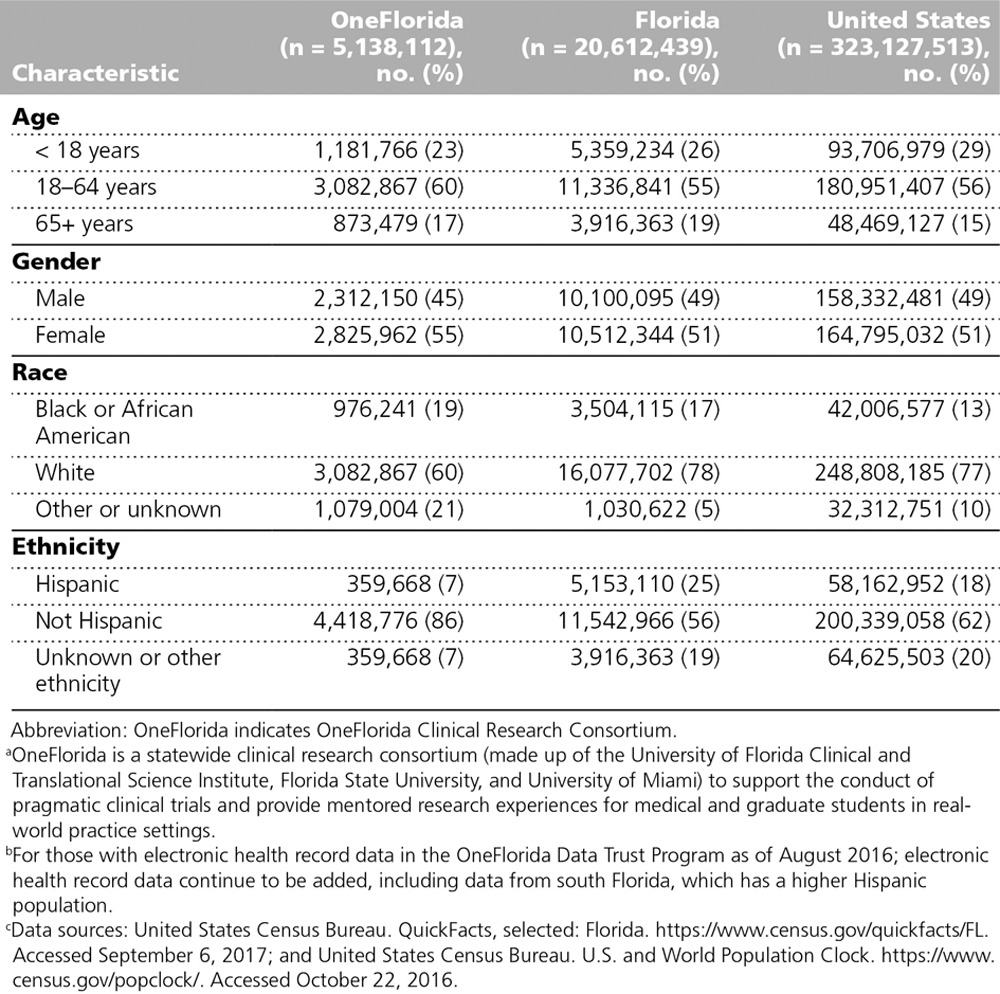
Table 2.
OneFloridaa Patient (n = 5,138,112) Health Status by Clinical Risk Group Categoriesb
Outcomes
The novel partnership that began with the UF CTSI and FSU CBDME model and later expanded to include UM resulted in a nationally recognized innovative research infrastructure for pragmatic clinical trials. In October 2015, OneFlorida received funding from the Patient-Centered Outcomes Research Institute to become 1 of 13 clinical data research network sites and part of PCORnet, a national network which is health system based and includes patient-powered research networks that patients and their clinical partners lead to address specific health conditions.
Further, we have implemented 27 studies throughout the OneFlorida network, including a randomized trial that provides support to clinicians to improve the uptake of the human papillomavirus vaccine and a pragmatic clinical trial comparing the effectiveness of two different daily doses of aspirin, which is widely used to prevent heart attacks and strokes in individuals with heart disease, known as ADAPTABLE (Aspirin Dosing: A Patient-Centric Trial Assessing Benefits and Long-Term Effectiveness). These and other studies are taking place with diverse patient populations and in diverse community practice settings, thus addressing our primary goal of examining the effectiveness of interventions applied under the usual conditions in the places where people receive health care.
Next Steps
Our next steps are to identify evidence-based best practices from the clinical trials conducted in our network and foster their implementation throughout the OneFlorida network. Further, we are monitoring the success of our trainees in terms of developing their own studies and expanding on our four programs to enhance our research training opportunities for medical students in pragmatic clinical trials.
Footnotes
Funding/Support: Information reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the National Institutes of Health National Center for Advancing Translational Sciences under award number UL1TR001427. Information reported in this publication was also supported in part by the OneFlorida clinical data research network, funded by the Patient-Centered Outcomes Research Institute under award number CDRN-1501-26692.
Other disclosures: None reported.
Ethical approval: The OneFlorida Data Trust Program is approved by the University of Florida’s institutional review board (IRB no. 201500466).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Patient-Centered Outcomes Research Institute.
References
- 1.Concannon TW, Guise JM, Dolor RJ, et al. A national strategy to develop pragmatic clinical trials infrastructure. Clin Transl Sci. 2014;7:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Census Bureau. Population and Housing Unit Estimates. https://www.census.gov/programs-surveys/popest.html. Accessed August 21, 2017.
- 3.Hurt MM, Harris JO. Founding a new college of medicine at Florida State University. Acad Med. 2005;80:973–979. [DOI] [PubMed] [Google Scholar]
- 4.Snyder AR, Bauer RM; Health IMPACTS for Florida Network. A normative study of the sport concussion assessment tool (SCAT2) in children and adolescents. Clin Neuropsychol. 2014;28:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadivar H, Thompson L, Wegman M, et al. Adolescent views on comprehensive health risk assessment and counseling: Assessing gender differences. J Adolesc Health. 2014;55:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahin MH, Johnson JA. Clopidogrel and warfarin pharmacogenetic tests: What is the evidence for use in clinical practice? Curr Opin Cardiol. 2013;28:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan WV, Pearson TA; NHLBI Implementation Science Workgroup. Literature Review: Evidence-Based Implementation Strategies. 2013Bethesda, MD: National Heart, Lung, and Blood Institute. [Google Scholar]
- 8.Yuan J, Malin B, Modave F, et al. Towards a privacy preserving cohort discovery framework for clinical research networks. J Biomed Inform. 2017;66:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Chronic Disease Prevention and Health Promotion. https://www.cdc.gov/chronicdisease/overview/index.htm. Accessed September 1, 2017.
- 10.Data Resource Center for Child & Adolescent Health. 2009/10 National Survey of Children With Special Health Care Needs. http://www.childhealthdata.org/browse/data-snapshots/cshcn-profiles?rpt=9&geo=. Accessed September 1, 2017.


