A retrospective on the scientific importance and impact of Hecht, Shlaer, and Pirenne’s classic 1942 paper, “Energy, Quanta, and Vision.”
Abstract
Vertebrate rod photoreceptors evolved the astonishing ability to respond reliably to single photons. In parallel, the proximate neurons of the visual system evolved the ability to reliably encode information from a few single-photon responses (SPRs) as arising from the presence of an object of interest in the visual environment. These amazing capabilities were first inferred from measurements of human visual threshold by Hecht et al. (1942), whose paper has since been cited over 1,000 times. Subsequent research, in part inspired by Hecht et al.’s discovery, has directly measured rod SPRs, characterized the molecular mechanism responsible for their generation, and uncovered much about the specializations in the retina that enable the reliable transmission of SPRs in the teeth of intrinsic neuronal noise.
Introduction
The Journal of General Physiology has a rich tradition of publication in sensory physiology, particularly in vision science. This includes much research on rod and cone visual pigments and their photochemistry, on visual retinoid physiology, and on phototransduction. The Journal continues to attract such research, not only because of this history, but also because of its transparent reviewing principles and its outstanding production quality. Cited 1,195 times according to Google Scholar, the paper on the absolute sensitivity of human vision that discovered the ability of rod photoreceptors to respond to single photons (Hecht et al., 1942) stands out for its importance and enduring impact.
A cardinal principle of quantum mechanics is that all exchange of energy between the electromagnetic field and matter takes place in discrete packets or quanta. This principle had been firmly established by 40 years of physics and spectroscopic chemistry when Hecht et al. made their meticulous measurements of the threshold energy for detection of a small, brief 500 nm visual target by dark-adapted human observers1. Reported as the energy at the 60th percentile of ogival frequency of seeing curves (Fig. 1 A), the thresholds measured at the cornea for their four primary observers ranged from 2.1 to 5.7 × 10−17 J, equivalent to 48–148 photons according to Planck’s formula (Table 1).
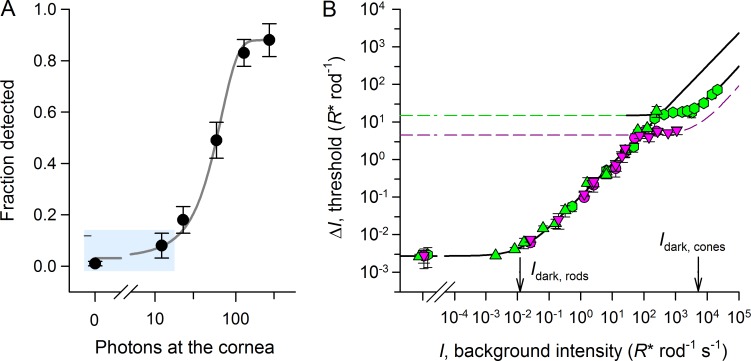
Figure 1.
Selected results from a mouse behavioral study of visual threshold (modified from Naarendorp et al., 2010). (A) Frequency of seeing curve. Data represent 1,369 trials collected in which a mouse running on a wheel had to detect a <1 ms, 500 nm target of varied energy subtending 5° of visual angle on the ventral retinal. Flashes were presented at random times while the mouse was running on a wheel, and the mouse was rewarded with access to water if it exited the wheel in less than one revolution after the presentation. Error bars are ±2 SEM and thus approximate 95% confidence intervals. The data point plotted at zero represents stimulus-null trials and thus estimates the false positive rate, 1.0%. The light blue rectangle highlights the highly reliable difference (P < 0.0001) between the false positive rate (1%) and the detection rate (8%) for the lowest stimulus energy, 11 photons, discussed in the section "A single photon can occasionally be detected . . ." (B) Dark and increment threshold curves of mice for 365-nm (purple) and 510-nm (green) flashes in the presence of backgrounds of varied intensity, specified in R* units. The smooth curves plot the generalized Weber-Fechner relation, where I is the background intensity, ΔI is the threshold, ΔI0 is its value in darkness, Idark is the dark light or Eigengrau level, and n = 0.9 is the slope of the linear portion of the log–log plot of the curves. For the lower curve, the 365-nm and 510-nm data are coincident with the abscissa expressed in rod R* units, indicating that rods provide the signals for detection with backgrounds producing up to ∼100 R* rod−1 s−1. The breaks in the curve reveal the points at which cone vision for the two wavelength test flashes becomes more sensitive than rod vision. The value of the rod dark light Idark corresponds exactly to the spontaneous rate of rhodopsin isomerization, 0.012 s−1, measured by Burns et al. (2002) in suction electrode recordings from mouse rods. The lower branch of the increment threshold curve reveals that signaling via SPRs dominates most of the background intensity range over which rod-generated vision is more sensitive than that of cones; because the normal SPR has a duration of ∼200 ms, backgrounds of up to 5 R* rod−1 s−1 represent conditions when all visual information is carried by SPRs.
Table 1. Summary of experiments measuring absolute visual threshold.
| Species | Reference | Retinal eccentricity | Stimulus duration | Stimulus wavelength | Retinal image areaa | Rods subtended by targetb | Average number of photons at the corneac |
|---|---|---|---|---|---|---|---|
| degrees | ms | nm | μm2 | ||||
| Human | Hecht et al., 1942 | 20 (T) | 1 | 510 | 1,800 | 240 | 90 ± 15 (4) |
| Hallett, 1962 | 20 (T) | 2.6 | 520 | 250 | 35 | 90 (1) | |
| Hallett, 1962 | 20 (T) | 2.6 | 520 | 65,000 | 8,775 | 100 (1) | |
| Sakitt, 1972 | 7 (N) | 16 | 495 | 540 | 50 | 55, 66 (3) | |
| Teich et al., 1982 | 17.5 (T) | 1 | 514 | 92 | 8 | 110 ± 10 (4) | |
| Sharpe et al., 1993 | 12 (N) | 10 | 507 | 1,800 | 235 | 43 ± 5 (3) | |
| Koenig and Hofer, 2011 | 11 (T) | 34 | 490 | 510 | 66 | 50 ± 16 (6) | |
| Tinsley et al., 2016d | 23 (T) | <1e | 504 | Not specified | – | 73 ± 9 (3) | |
| Mousef | Naarendorp et al., 2010 | Inferior | <1e | 500 | 2,200 | 960 | 31 ± 7 (5) |
| Naarendorp et al., 2010 | Inferior | <1e | 500 | 19,000 | 8,350 | 67 ± 6 (6) |
The second column identifies the study. In the third column, N and T represent nasal and temporal, respectively. This table does not report the number of experiments per subject, nor measurements per experiment; it consequently does not fully capture the precision of the values. For example, in the experiments of Hecht et al., for two of the subjects, thresholds were measured in seven different experiments, whereas in only three and four experiments for two other subjects. In the mouse experiments, for one animal, 1,369 trials from ∼30 experimental sessions comprised the frequency of seeing data (this included 35% blank trials, for which the false positive rate was 1%). In addition, the psychophysical methodology (yes/no; 2AFC; rating scale) varied among the studies. Results and analyses in papers from Sakitt (1972) onward concur that statistically reliable information about the target was occasionally available to the subject at stimulus energies threefold or more lower than classical threshold values (midpoint of the frequency of seeing curves) generated by subjects using a high criterion.
For the human experiments, target areas in deg2 were converted to mm2 using the standard adult schematic eye, with a scaling factor of 0.291 mm/deg (Wyszecki and Stiles, 1982).
To calculate the number of rods subtending the target, the retinal area was multiplied by the rod density at the appropriate retinal eccentricity as given in Curcio et al. (1990). For the smaller targets, the areas and number of rods subtended are nominal, and likely considerably larger because of optical aberrations.
The last column of the table gives the average threshold at the cornea of the study. Error terms are the SEMs over subjects, and the values in parentheses are the number of subjects. For the mouse experiments, flashes were generated by time-gated LED pulses, which ranged in duration from 10 µs to 1 ms to control the total flash energy. The photon energy density at the cornea was multiplied by an effective dark-adapted pupil area of 2 mm2; the mouse retinal rod density of ∼340,000 mm−2 was taken from Jeon et al. (1998); this is more than twofold larger than the maximal human rod density, ∼140,000 mm−2, which occurs at ∼18° eccentricity on the temporal retina (Curcio et al., 1990).
Tinsley et al. (2016) used a quantum optical technique, spontaneous parametric down conversion, in which a nonlinear crystal is used to down convert a higher energy (shorter wavelength) photon into two lower energy (longer wavelength) photons, one of which was delivered to the eye and the second (the “idler”) used to determine when a down conversion took place. Although this technology cannot create single-photon trials at will, at the low source strength used, it produced mainly blank trials (92%), one-photon trials (8%), and extremely rare multiphoton trials. In a total of 2,420 postselected one-photon trials (out of a total of 30,767 trials), the average probability of a correct response was 0.516 ± 0.010 (mean ± SEM), a value just greater than chance success (0.5) at the P = 0.05 statistical significance level. Similar results were obtained with a Poisson light source delivering a mean of one photon at the cornea, increasing the overall significance level of one-photon detection to 0.01. The authors also obtained intriguing results suggesting that the capture of a single photon can elevate the probability of detecting another photon over an interval of several seconds. The conventional threshold of the subjects was measured with a temporal 2AFC procedure and stimuli ranging from 20 to 140 photons at the cornea. The thresholds (defined at the 75th percentile of the detection functions; see Fig. S3 [A–C] in Tinsley et al., 2016) were close to those obtained in the other experiments cited in this table.
Stimuli of <1 ms are effectively instantaneous for mammalian rods, whose SPRs peak at ∼100 ms in vivo (Peinado Allina et al., 2017)
Mice maintained a false positive rate of 1–2%, likely because each trial involved running a random number in the hundreds of cycles on a wheel to achieve a water reward, and so both false positive (type I) and false negative (type II) errors were energetically costly.
Hecht et al. drew several enduring mechanistic conclusions. First, as there were inevitable losses in light transmission through the ocular media, the number of photons captured by rhodopsin2 in rods was less than the number incident on the cornea by a factor of 2–10 or more. Second, because visual threshold required the capture of only a small number of photons, Poisson statistics implied substantial fluctuations in the number captured trial-by-trial from each nominal energy flash. Third, because the region of the retina upon which the target was imaged subtended several hundred rod photoreceptors, it was highly improbable that any individual rod captured more than one photon at threshold. Thus, Hecht et al. incontrovertibly drew a profound conclusion that has stood the test of time, namely that rod photoreceptors had attained the limit of light sensitivity dictated by quantum physics. In other words, rods were capable of generating single-photon responses (SPRs) that propagated through the retina and created reliable signals in the central nervous system (CNS).
The impact of Hecht et al.’s investigation on sensory physiology has been extensive and includes inspiring numerous investigations into aspects of the molecular mechanisms of phototransduction, guiding research into the retinal circuitry underlying night vision, and providing novel insight into mechanisms that cope with (and even exploit) noise in neuronal circuits. These topics will be reviewed in this paper after a brief summary of subsequent experiments that confirmed and extended the findings of Hecht et al. (1942).
Threshold as signal/noise discrimination
The findings of Hecht et al. (1942) have been replicated in several human psychophysical studies and in a recent behavioral study of mice (Fig. 1 and Table 1). Several factors contribute to variation in threshold between experiments, including the target size, duration, and retinal location; optima for these factors reflect variation in rod density and in properties of the underlying retinal circuitry such as receptive field size, which vary systematically with eccentricity in humans. Another critical factor affecting threshold is the observer’s criterion for reporting a target as seen (i.e., giving a “yes” response) on any given trial. Hecht et al. (1942) hypothesized that the Poisson fluctuations in photon capture were the primary source of variation underlying the trial-by-trial variability in detection of targets of the same nominal energy and that differences among their observers arose from the adoption of different criteria for the strength of the sensation required for a yes response. However, as developed initially in vision research by Barlow (1956) and further by him and many others, threshold detection is best understood as a signal/noise discrimination (Green and Swets, 1966), with noise arising from intrinsic retinal processes (Barlow et al., 1957). In the signal detection framework, systematic manipulation of the criterion (e.g., with confidence-rating scales) revealed that observers could indeed reliably detect stimuli whose average energy at the cornea was threefold or more lower than the average 90 photons of Hecht et al.’s experiments (Sakitt, 1972; Teich et al., 1982; Tinsley et al., 2016). Strong support for the validity of this framework arose from the “receiver operating characteristic” relationship between detection probability and false positive rate (Green and Swets, 1966); observers could detect lower energy stimuli with high probability on stimulus-null trials only at the cost of increased false positive responses, which were reasonably presumed to be triggered by retinal noise. In this context, Hecht et al.’s observers were understood to have adopted high criteria to avoid making false positive responses to very dim flashes that they hadn’t “really” seen. Models of detection that incorporate intrinsic neural noise naturally explained the relationship between detection and false positive rates at low stimulus energies (Sakitt, 1972; Teich and Prucnal, 1977; Field et al., 2005; Naarendorp et al., 2010; Koenig and Hofer, 2011). In such models, an event counter serves as a detector that reaches the detect state when the count on a trial exceeds a criterion number as postulated by Hecht et al. However, spontaneous noise events not generated by the stimulus contribute to each count. Indeed, as originally hypothesized by Barlow et al. (1957) and subsequently confirmed (below), rhodopsin, the marvelous molecule that can signal the capture of one photon, can also spontaneously trigger photon-like events in the absence of light. In summary, although confirming the fundamental findings of Hecht et al. (1942) and strongly affirming their conclusion that rods are able to generate SPRs, subsequent experiments and analyses revealed that intrinsic retinal noise, as well as Poisson fluctuations in the number of captured photons, was an important determinant of absolute visual sensitivity.
A single photon can occasionally be detected by a human observer
A recent human threshold study used an optical technology that enabled identification of trials in which exactly one photon was delivered to the eye (Tinsley et al., 2016). In their two-alternative forced choice (2AFC) detection task, subjects in one-photon trials made the correct choice 51.6% of the time, which was a success rate 1.6% higher than the 50% chance performance level, yet statistically reliable (P = 0.05, 2,420 one-photon trials). These and other results in the study make the case that a well-trained observer in a task that pushes their criterion to its lowest level can, albeit infrequently, correctly discriminate trials producing a single SPR from trials with noise alone (Tinsley et al., 2016). Although reaching the ultimate physically allowable limit of visual system performance, these results are not incommensurate with prior studies (Table 1). For example, the mouse whose data are shown in Fig. 1 A was successful 8% of the time on trials when 12 photons on average were delivered to its eye, a success rate reliably greater than its false alarm rate, 1.0% (130 stimulus trials, 500 blank trials; P < 10−4), and the average number of SPRs in nominal 12-photon trials was likely one third or less than the number of photons arriving at the cornea. A broad issue raised by one-photon detection trials is whether the CNS can extract information useful to the organism from a stimulus that can only be detected a small fraction of the time it is available. The conventional definition of threshold as the energy corresponding to the midpoint of the frequency of seeing curve corresponds to an ∼50% false negative rate and, to some extent, embodies the hypothesis that stimuli that give rise to much higher false negative rates may not have much value to an organism. It is notable that the subjects of the Tinsley et al. (2016) study, even in the performance-enhancing 2AFC task, had thresholds comparable to those of subjects in the other studies (Table 1). For an organism in a more natural context, both false negatives (type II errors) and false positives (type I errors) will usually have a cost. In this context, it is notable that mice, like the subjects of Hecht et al.’s study, naturally maintained a low false positive rate of 1–2% (Naarendorp et al., 2010) and thus implicitly maintained a high criterion.
The direct measurement of SPRs
Electrical recordings of SPRs from vertebrate photoreceptors were made for the first time by Baylor, Lamb, and Yau from single toad rods with suction electrodes (Baylor et al., 1979b; Yau et al., 1977). In a companion study, the authors characterized the rod circulating (dark) current and the intensity dependence and kinetics of its suppression by light (Baylor et al., 1979a), confirming the discovery by Hagins et al. (1970) in rat retinal slices of the light-suppressible, outer segment–inward, inner segment–outward dark current. The critical evidence for SPRs was that for appropriately weak flash strengths, the probability of a response followed Poisson statistics with the frequencies of trials with no events and of trials with events appropriately dependent on flash strength Baylor et al., 1979b. Subsequent work characterized the SPRs of primate rods (Baylor et al., 1984), followed by numerous studies of SPRs in mouse rods (e.g., Rieke and Baylor, 1998; Field et al., 2005; Gross et al., 2015). One key finding of all these studies was that a single rhodopsin isomerization generated an SPR that suppressed 2–5% of the dark current at the response peak. Because the inward current was found to be uniformly distributed along the outer segment (Baylor et al., 1979a), such suppression implied the axial spread of a signal along the outer segment from the disc membrane where the isomerized rhodopsin (R*) resided and thus further implied the existence of an internal messenger. A second key finding was that SPRs were highly reproducible in shape and amplitude, with a coefficient of variation (SD/mean) of amplitude of 0.2 (amphibian rods) to 0.3 (mammalian rods). A third fundamental finding of the suction electrode studies was that in darkness, rods generated spontaneous events whose amplitude and kinetics were indistinguishable from the light-stimulated SPRs. These spontaneous photon-like events, which were naturally attributed to thermally triggered activation of rhodopsin, were relatively infrequent: 0.02 s−1 in toad rods at room temperature (Baylor et al., 1980), 0.006 s−1 in primate rods (Baylor et al., 1980), and 0.012 s−1 in mouse rods (Burns et al., 2002) at body temperature. Because toad rods contain 2 × 109 rhodopsin molecules (mammalian rods, ∼108), these dark events revealed that the rhodopsin molecule, today understood to be a GPCR covalently bound to its natural “inverse ligand,” 11-cis-retinal, is extraordinarily stable; the thermal activation rate at room temperature corresponds to a rate constant of once per 50,000 years. Nonetheless, for their rarity in individual rods, because of the very high convergence of signaling in successive layers of the retina, these spontaneous events create “dark light” that sets a limit on the sensitivity of the dark-adapted eye and the visual system as a whole, as further discussed below.
Inspiration for research on phototransduction
Phototransduction is the molecular and cellular process by which the isomerization of the covalently liganded 11-cis-retinal chromophore of an opsin GPCR leads to the production of an electrical response in a rod or cone photoreceptor cell. Grounded on the principle that isomerization of the chromophore is the initial event in rod-mediated vision (Wald and Brown, 1958; Wald et al., 1963), at the time when SPRs were first reported, research into the molecular mechanisms of phototransduction was advancing rapidly, culminating in the discoveries of amplified G protein and cGMP phosphodiesterase activation and of the CNG-gated channels of the plasma membrane (Yee and Liebman, 1978; Fung et al., 1981; Fesenko et al., 1985; Stryer, 1986). The electrical recording of SPRs, however, provided much inspiration and a clear focus for mechanistic investigations. For example, the hypothesis that a diffusible internal messenger carried the signal between rod disc membranes and the plasma membrane received strong support from the fact that an SPR involved the closure of channels at micrometer distances along the outer segment from the disc membrane locus of the R* (Baylor et al., 1979b; Lamb et al., 1981; Pugh and Cobbs, 1986). Similarly, the realization that a single R* could lead to suppression of a few percent of the outer segment membrane current implied that the biochemical mechanism was strongly amplifying, so that the theory of phototransduction had to account for this amplification (Lamb and Pugh, 1992; Pugh and Lamb, 1993; Leskov et al., 2000). Another key aspect of the SPR that inspired research into molecular mechanisms was its reproducibility; how could the activity time course of a single GPCR give rise to so stereotypic an electrical response when one-step stochastic deactivation of R* predicts a coefficient of variation of unity? Although some debate continues on these issues, it now seems clear that SPR reproducibility is achieved primarily by three mechanisms: (1) high gain of the fully active R*, such that it initially activates G proteins at the rate of hundreds per second; (2) timed deactivation of R* by multiple phosphorylation, leading to gradually increasing affinity of the positively charged binding surface of the capping protein arrestin for the increasingly phosphorylated state; and (3) feedback activation of guanylate cyclase, whereby the decline in Ca2+i that accompanies the closure of CNG channels dampens the variability that would otherwise result from stochastically longer R* lifetimes (Gross et al., 2012).
Implications for retinal circuitry and transmission of SPR information to the CNS
A profound implication of the experiments of Hecht et al. (1942) and its replications and extensions is that a small number of rod SPRs can generate a signal that propagates reliably through the retina and into the CNS, where the ultimate correlates of detection and behavioral response generation must occur. There are two related aspects to this implication: (1) the cellular and subcellular anatomy of the neural circuitry of the post-rod SPR signals and (2) the signal processing in that circuitry. These topics have been the focus of a great body of research (Tsukamoto et al., 2001; Field et al., 2005; Field and Sampath, 2017). In regard to the anatomy underlying SPR signaling, research has revealed that evolution fashioned a unique retinal circuit to carry the SPR signal in the most dark-adapted state, namely via rod bipolar cells (RBCs)→AII amacrine cells→ON-α/β retinal ganglion cells (RGCs; Tsukamoto et al., 2001; Takeshita et al., 2017). This “starlight” pathway is highly convergent, such that thousands of rods signal to a single ON-β or ON-α RGC, and clearly operates over much of the intensity regime of night vision (Fig. 1 B). This circuit also appears grafted into more primitive versions of the retina, affording an interesting case in which the evolution of a multicellular neuronal circuit can be related to a specific function: SPR signaling (Lamb et al., 2007). In regard to the processing, both the overall architecture of the SPR-signaling circuits and their subcellular elements have been found to have properties that aid in noise reduction and signal transmission efficiency. For example, the rod→RBC synapse has a nonlinearity that serves to minimize transmission of non-SPR rod membrane noise (Field and Rieke, 2002; Berntson et al., 2004). Other functional aspects of the circuitry, such as coincidence detection mechanisms at the RGC level, remain under intense investigation (Ala-Laurila and Rieke, 2014) and are contributing novel mechanistic insight into how intrinsic noise is processed by neuronal circuits to improve signal detection.
Summary and conclusions
This admittedly inadequate paper has attempted to briefly summarize a large body of scientific investigations, ranging from characterization of the molecular events initiated by a single captured photon to signal transmission in the visual systems of humans and mice, that can trace their lineage back to the seminal work of Hecht et al. (1942). (For other recent reviews of this topic, see Nelson [2016] and Field and Sampath [2017].) The deduced and subsequently directly measured ability of a single photoisomerized rhodopsin molecule to generate a reliable signal that propagates through the visual system is an extraordinary feat of evolution. This discovery will no doubt continue to inspire novel investigations into the rich array of mechanisms that make it possible, from the extremely stable nature of the inverse-liganded rhodopsin GPCR and its photoactivation into a form that triggers a high-gain and highly reproducible SPR, to signal transmission mechanisms in the retina and visual pathways that filter noise and amplify rod SPR signals. Perhaps two examples of extant questions arising from this heritage can serve to amplify this point.
In their work characterizing the spontaneous activation of rhodopsin, Baylor et al. (1980) reported that the activation energy (22 kcal/mol) and activation entropy (−35 e.u.) of the spontaneous photon-like event-generating process were essentially the same as those for 11-cis-retinal in detergent (Hubbard, 1966), observations that greatly strengthen the conclusion that thermal isomerization of the rhodopsin chromophore underlies these SPR-like events. However, the rate constant of isomerization of the chromophore in digitonin was ∼1,000-fold higher than that derived from the spontaneous event rate of rods. Given the strong evidence that the spontaneous events indeed arose from chromophore isomerization, collectively, the results imply that the rhodopsin-binding pocket greatly stabilizes the 11-cis-retinal chromophore. Indeed, in the absence of this stabilization, the rate of spontaneous events in a typical mammalian rod would be at least 6 s−1, a rate that would swamp the ultrasensitive rod bipolar cells whose signaling current is half-maximal when, on average, each of the 20–50 rods in its receptive field captures one photon. Clearly, the physicochemical mechanisms of stabilization have been of profound importance for vision, for without this stabilization, night vision would literally be blinded by a blizzard of rod-generated dark light. It remains a challenge for the future to understand the mechanisms of chromophore stabilization by the protein rhodopsin, for example, by reducing some of the vibrational modes of the chromophore (Kim et al., 2003; Ala-Laurila et al., 2004). This effort should be abetted by the rich universe of opsin GPCRs, which vary not only in their spectral tuning but also in their chromophore stability (Ala-Laurila et al., 2004).
The insight that evolution fashioned a highly convergent neuronal circuit (RBC→AII amacrine→ON-RGC) specifically tailored for signaling SPRs raises many challenging questions for developmental and evolutionary biology. Because the circuit has such a clear and distinct function, it may serve as a textbook case of the coevolution of molecular, cellular, and transcellular features that serve a well-defined physiological function: high-fidelity transmission of SPR events in a noisy background. Biophysically accurate modeling of the circuits may continue to identify novel molecular mechanisms at various scales that contribute to the remarkable signal/noise ratio that has been achieved under selection pressure for vision to operate in photon-starved environments, and thus for SPR signal transmission from rods to the CNS.
Acknowledgments
Olaf S. Andersen served as editor.
Footnotes
Hecht et al. cited 12 prior studies of absolute threshold and culled them to the three they deemed to have made the most carefully controlled and calibrated measurements; the thresholds at the cornea of these studies ranged from 17 to 90 quanta (the latter for 530-nm light).
Although its nature as a membrane protein had not been established by 1942, rhodopsin was nonetheless well known from spectroscopy as the light-absorbing, bleachable visual pigment present in rods. Furthermore, experiments comparing its absorption spectrum with the scotopic (nighttime) visibility function had certainly established that light captured by rhodopsin initiated vision under dark-adapted conditions (see Fig. 5 in Hecht et al., 1942).
References
- Ala-Laurila P., and Rieke F.. 2014. Coincidence Detection of Single-Photon Responses in the Inner Retina at the Sensitivity Limit of Vision. Curr. Biol. 24:2888–2898. 10.1016/j.cub.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Laurila P., Donner K., and Koskelainen A.. 2004. Thermal activation and photoactivation of visual pigments. Biophys. J. 86:3653–3662. 10.1529/biophysj.103.035626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H.B. 1956. Retinal noise and absolute threshold. J. Opt. Soc. Am. 46:634–639. 10.1364/JOSA.46.000634 [DOI] [PubMed] [Google Scholar]
- Barlow H.B., Fitzhugh R., and Kuffler S.W.. 1957. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat’s retina. J. Physiol. 137:327–337. 10.1113/jphysiol.1957.sp005816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D.A., Lamb T.D., and Yau K.W.. 1979b. Responses of retinal rods to single photons. J. Physiol. (Lond.). 288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D.A., Lamb T.D., and Yau K.W.. 1979a The membrane current of single rod outer segments. J. Physiol. 288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D.A., Matthews G., and Yau K.W.. 1980. Two components of electrical dark noise in toad retinal rod outer segments. J. Physiol. 309:591–621. 10.1113/jphysiol.1980.sp013529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D.A., Nunn B.J., and Schnapf J.L.. 1984. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J. Physiol. 357:575–607. 10.1113/jphysiol.1984.sp015518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A., Smith R.G., and Taylor W.R.. 2004. Transmission of single photon signals through a binary synapse in the mammalian retina. Vis. Neurosci. 21:693–702. 10.1017/S0952523804215048 [DOI] [PubMed] [Google Scholar]
- Burns M.E., Mendez A., Chen J., and Baylor D.A.. 2002. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 36:81–91. 10.1016/S0896-6273(02)00911-X [DOI] [PubMed] [Google Scholar]
- Curcio C.A., Sloan K.R., Kalina R.E., and Hendrickson A.E.. 1990. Human photoreceptor topography. J. Comp. Neurol. 292:497–523. 10.1002/cne.902920402 [DOI] [PubMed] [Google Scholar]
- Fesenko E.E., Kolesnikov S.S., and Lyubarsky A.L.. 1985. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 313:310–313. 10.1038/313310a0 [DOI] [PubMed] [Google Scholar]
- Field G.D., and Rieke F.. 2002. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 34:773–785. 10.1016/S0896-6273(02)00700-6 [DOI] [PubMed] [Google Scholar]
- Field G.D., and Sampath A.P.. 2017. Behavioural and physiological limits to vision in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160072 10.1098/rstb.2016.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field G.D., Sampath A.P., and Rieke F.. 2005. Retinal processing near absolute threshold: From behavior to mechanism. Annu. Rev. Physiol. 67:491–514. 10.1146/annurev.physiol.67.031103.151256 [DOI] [PubMed] [Google Scholar]
- Fung B.K., Hurley J.B., and Stryer L.. 1981. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc. Natl. Acad. Sci. USA. 78:152–156. 10.1073/pnas.78.1.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.M., and Swets J.A.. 1966. Signal Detection Theory and Psychophysics. John Wiley, New York. 521 pp. [Google Scholar]
- Gross O.P., Pugh E.N. Jr., and Burns M.E.. 2012. Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron. 76:370–382. 10.1016/j.neuron.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O.P., Pugh E.N. Jr., and Burns M.E.. 2015. cGMP in mouse rods: the spatiotemporal dynamics underlying single photon responses. Front. Mol. Neurosci. 8:6 10.3389/fnmol.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W.A., Penn R.D., and Yoshikami S.. 1970. Dark current and photocurrent in retinal rods. Biophys. J. 10:380–412. 10.1016/S0006-3495(70)86308-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P.E. 1962. Scotopic acuity and absolute threshold in brief flashes. J. Physiol. 163:175–189. 10.1113/jphysiol.1962.sp006966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S., Shlaer S., and Pirenne M.H.. 1942. Energy, Quanta, and Vision. J. Gen. Physiol. 25:819–840. 10.1085/jgp.25.6.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. 1966. The stereoisomerization of 11-cis-retinal. J. Biol. Chem. 241:1814–1818. [PubMed] [Google Scholar]
- Jeon C.J., Strettoi E., and Masland R.H.. 1998. The major cell populations of the mouse retina. J. Neurosci. 18:8936–8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Tauber M.J., and Mathies R.A.. 2003. Analysis of the mode-specific excited-state energy distribution and wavelength-dependent photoreaction quantum yield in rhodopsin. Biophys. J. 84:2492–2501. 10.1016/S0006-3495(03)75054-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig D., and Hofer H.. 2011. The absolute threshold of cone vision. J. Vis. 11:21 10.1167/11.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T.D., and Pugh E.N. Jr. 1992. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 449:719–758. 10.1113/jphysiol.1992.sp019111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T.D., McNaughton P.A., and Yau K.W.. 1981. Spatial spread of activation and background desensitization in toad rod outer segments. J. Physiol. 319:463–496. 10.1113/jphysiol.1981.sp013921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T.D., Collin S.P., and Pugh E.N. Jr. 2007. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat. Rev. Neurosci. 8:960–976. 10.1038/nrn2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskov I.B., Klenchin V.A., Handy J.W., Whitlock G.G., Govardovskii V.I., Bownds M.D., Lamb T.D., Pugh E.N. Jr., and Arshavsky V.Y.. 2000. The gain of rod phototransduction: reconciliation of biochemical and electrophysiological measurements. Neuron. 27:525–537. 10.1016/S0896-6273(00)00063-5 [DOI] [PubMed] [Google Scholar]
- Naarendorp F., Esdaille T.M., Banden S.M., Andrews-Labenski J., Gross O.P., and Pugh E.N.. 2010. Dark Light, Rod Saturation, and the Absolute and Incremental Sensitivity of Mouse Cone Vision. J. Neurosci. 30:12495–12507. 10.1523/JNEUROSCI.2186-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P.C. 2016. Old and new results about single-photon sensitivity in human vision. Phys. Biol. 13:025001 10.1088/1478-3975/13/2/025001 [DOI] [PubMed] [Google Scholar]
- Peinado Allina G., Fortenbach C., Naarendorp F., Gross O.P., Pugh E.N. Jr., and Burns M.E.. 2017. Bright flash response recovery of mammalian rods in vivo is rate limited by RGS9. J. Gen. Physiol. 149:443–454. 10.1085/jgp.201611692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E.N. Jr., and Cobbs W.H.. 1986. Visual transduction in vertebrate rods and cones: a tale of two transmitters, calcium and cyclic GMP. Vision Res. 26:1613–1643. 10.1016/0042-6989(86)90051-9 [DOI] [PubMed] [Google Scholar]
- Pugh E.N. Jr., and Lamb T.D.. 1993. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta. 1141:111–149. 10.1016/0005-2728(93)90038-H [DOI] [PubMed] [Google Scholar]
- Rieke F., and Baylor D.A.. 1998. Single-photon detection by rod cells of the retina. Rev. Mod. Phys. 70:1027–1036. 10.1103/RevModPhys.70.1027 [DOI] [Google Scholar]
- Sakitt B. 1972. Counting every quantum. J. Physiol. 223:131–150. 10.1113/jphysiol.1972.sp009838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe L.T., Stockman A., Fach C.C., and Markstahler U.. 1993. Temporal and spatial summation in the human rod visual system. J. Physiol. 463:325–348. 10.1113/jphysiol.1993.sp019597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. 1986. Cyclic GMP cascade of vision. Annu. Rev. Neurosci. 9:87–119. 10.1146/annurev.ne.09.030186.000511 [DOI] [PubMed] [Google Scholar]
- Takeshita D., Smeds L., and Ala-Laurila P.. 2017. Processing of single-photon responses in the mammalian On and Off retinal pathways at the sensitivity limit of vision. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160073 10.1098/rstb.2016.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich M.C., and Prucnal P.R.. 1977. PHOTON-COUNTING AND ENERGY DETECTION IN VISION. J. Opt. Soc. Am. 67:1426. [Google Scholar]
- Teich M.C., Prucnal P.R., Vannucci G., Breton M.E., and McGill W.J.. 1982. Multiplication noise in the human visual system at threshold: 1. Quantum fluctuations and minimum detectable energy. J. Opt. Soc. Am. 72:419–431. 10.1364/JOSA.72.000419 [DOI] [PubMed] [Google Scholar]
- Tinsley J.N., Molodtsov M.I., Prevedel R., Wartmann D., Espigule-Pons J., Lauwers M., and Vaziri A.. 2016. Direct detection of a single photon by humans. Nat. Commun. 7:12172 10.1038/ncomms12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y., Morigiwa K., Ueda M., and Sterling P.. 2001. Microcircuits for night vision in mouse retina. J. Neurosci. 21:8616–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G., and Brown P.K.. 1958. Human rhodopsin. Science. 127:222–249. 10.1126/science.127.3292.222 [DOI] [PubMed] [Google Scholar]
- Wald G., Brown P.K., and Gibbons I.R.. 1963. The problem of visual excitation. J. Opt. Soc. Am. 53:20–35. 10.1364/JOSA.53.000020 [DOI] [PubMed] [Google Scholar]
- Wyszecki G., and Stiles W.S.. 1982. Color Science: Concepts and Methods, Quantitative Data and Formulae. Second edition John Wiley, New York. 968 pp. [Google Scholar]
- Yau K.W., Lamb T.D., and Baylor D.A.. 1977. Light-induced fluctuations in membrane current of single toad rod outer segments. Nature. 269:78–80. 10.1038/269078a0 [DOI] [PubMed] [Google Scholar]
- Yee R., and Liebman P.A.. 1978. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J. Biol. Chem. 253:8902–8909. [PubMed] [Google Scholar]