Abstract
Chronic stress stimulates corticotrophin-releasing hormone (CRH)–expressing neurons in the paraventricular nucleus (PVN) of the hypothalamus and leads to hypothalamic-pituitary-adrenal (HPA) axis hyperactivity, but the mechanisms underlying this action are unknown. Because chronic stress enhances N-methyl-d-aspartate receptor (NMDAR) activity in various brain regions, we hypothesized that augmented NMDAR activity contributes to the hyperactivity of PVN-CRH neurons and the HPA axis in chronic stress. We performed whole-cell patch-clamp recordings on PVN-CRH neurons expressing CRH promoter-driven enhanced green fluorescent protein in brain slices from rats exposed to chronic unpredictable mild stress (CUMS) and unstressed rats. CUMS rats had significantly higher expression levels of the NMDAR subunits GluN1 in the PVN than unstressed rats. Furthermore, puff NMDA-elicited currents, evoked NMDAR currents, and the baseline frequency of the miniature excitatory postsynaptic currents (mEPSCs) in PVN-CRH neurons were significantly larger in CUMS rats than in unstressed rats. The NMDAR-specific antagonist 2-amino-5-phosphonopentanoic acid (AP5) significantly decreased the frequency of mEPSCs of PVN-CRH neurons in CUMS rats but did not change the frequency or amplitude of mEPSCs in unstressed rats. Bath application of AP5 normalized the elevated firing activity of PVN-CRH neurons in CUMS rats but not in unstressed rats. In addition, microinjection of the NMDAR antagonist memantine into the PVN normalized the elevated corticosterone (CORT) levels in CUMS rats to the levels in unstressed rats, but did not alter CORT levels in unstressed rats. Our findings suggest that synaptic NMDAR activity is enhanced in CUMS rats and contributes to the hyperactivity of PVN-CRN neurons and the HPA axis.
We used in vivo and in vitro approaches to assess NMDAR activity in hypothalamic CRH neurons and find that enhanced NMDAR activity contributes to HPA axis hyperactivity during chronic stress.
Chronic stress may result in hypothalamic-pituitary-adrenal (HPA) axis hyperactivity (1, 2), which is associated with many neurologic disorders, such as depression (3, 4) and Alzheimer’s disease (5–8). The HPA axis plays a critical role in the neuroendocrine response to stress stimuli (2). The paraventricular nucleus (PVN) of the hypothalamus is a key brain region that determines HPA axis activity (9). The corticotrophin-releasing hormone (CRH)–expressing neurons located in the PVN are crucial in mediating the HPA axis response to stress (2). The PVN-CRH neurons are innervated by inhibitory γ-aminobutyric acid (GABA)–ergic and excitatory noradrenergic and glutamatergic inputs (10, 11). However, the synaptic mechanisms underlying HPA axis hyperactivity in response to chronic stress remain unknown.
Glutamate, a major excitatory neurotransmitter in the central nervous system, is involved in regulating HPA axis activity (12). Previous studies have shown that glutamate-immunoreactive synapses (11, 13) and glutamate receptors, including N-methyl-d-aspartate receptor (NMDAR) messenger RNA (mRNA) and protein, are distributed in the PVN (2, 14, 15). Chronic variable stress increases the number of glutamatergic terminals opposing PVN-CRH neurons (16, 17). Furthermore, chronic stress increases the activity of ionotropic glutamate receptors including NMDAR in the hippocampus and amygdala and leads to learning and memory impairment and depression-like behavior (18, 19). NMDARs are heterotetrameric, commonly composed of two GluN1 subunits and two GluN2 (GluN2A or GluN2B) subunits, and distributed in both presynaptic terminals and postsynaptic soma (20–22). It has been shown that chronic variable stress decreases GluN2B mRNA levels, but did not alter GluN1 and GluN2A mRNA levels in the PVN (11). However, it is not clear if chronic stress alters NMDAR protein expression levels and synaptic NMDAR function in the PVN. Because chronic stress enhances NMDAR activity in various brain regions such as hippocampus (18, 23), frontal cortices (24), as well as the hypothalamus (11), we hypothesized that augmented NMDAR activity in the PVN contributes to the hyperactivity of PVN-CRH neurons and the HPA axis during chronic stress. Thus, in the current study, we determined whether chronic stress affects the NMDAR subunit protein expression levels in the PVN. Using a recently developed approach to identify PVN-CRH neurons, we determined the extent to which chronic stress increases NMDAR activity and the role of NMDARs in the control of HPA axis hyperactivity during chronic stress.
Materials and Methods
Animals
Seven- to 10-week-old male Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN) and housed in standard conditions (22°C to 24°C, 12-hour light/dark cycle) at the Research Animal Support Facility of The University of Texas MD Anderson Cancer Center. The experimental protocol and surgical procedure were approved by MD Anderson’s Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center (#00001111-RN01) and conformed to the National Institutes of Health guidelines on the ethical use of animals.
Chronic unpredictable mild stress procedures
In the chronic unpredictable mild stress (CUMS) procedure, rats were exposed to two of eight stressors varied from day to day for 11 consecutive days according to an established paradigm (25). These stressors were forced swim (5 minutes), restraint stress (60 minutes), isolation housing (overnight), food and water deprivation (12 hours), cage rotation (50 minutes), cold isolation (15 minutes), light off (2 hours), and light on (overnight). To avoid any effect of the last stressor in the CUMS procedure, the measurements, including those of circulating corticosterone (CORT), sucrose preference test, and electrophysiological recordings, were performed 3 to 5 days after the cessation of CUMS procedure. The plasma CORT levels and sucrose preference test determined to confirm that the CUMS procedure induced behavioral and hormonal alteration. The rats were trained by drinking 0.7% sucrose solution for 48 hours before the sucrose preference test. Then, each rat was simultaneously exposed to two bottles: one contained 0.7% sucrose solution, and another one contained tap water for 24 hours. These two bottles were randomly positioned (right or left) after 12 hours to avoid side preferences. Consumption of tap water or sucrose solution was determined by subtracting the volume of residual solution at the end of testing from the initial volume. The sucrose preference was determined as a percentage of consumed sucrose solution intake over the total amount of liquid intake (water intake plus sucrose intake).
Identification of PVN-CRH neurons
We identified PVN-CRH neurons by assessing the specific expression of enhanced green fluorescent protein (eGFP) driven by the rat CRH promoter, as described previously (26–29). In brief, a plasmid containing a sequence of eGFP driven by the rat CRH-promoter was constructed and packaged into an adeno-associated viral (AAV) vector and concentrated to a high titer (1013) of genome copies per milliliter. The serotype of the AAV used was chimeric AAV1/2 containing both AAV1 and AAV2 capsid proteins with a ratio of 1:1. The AAV-CRH-eGFP vector was packaged by GeneDetect Limited (Auckland, New Zealand) (26). The vector was bilaterally microinjected into the PVN of rats anesthetized with 2% isoflurane through a hole drilled through the skull following the coordinates: 1.6 to 2.0 mm caudal to the bregma and 0.5 mm lateral to the midline. The tip of a 0.5-μL microsyringe controlled by a nanoinjector was advanced into the PVN according to the stereotaxic coordinates 1.8 to 2.0 mm caudal from the bregma, 0.5 mm lateral to the midline, and 7.3 to 7.5 mm deep from the surface of the cortex. We delivered 100 nL of the AAV vector into each side of the PVN over the course of 2 minutes. The rats were returned to their home cages after they woke up from the anesthesia. We allowed 20 to 30 days for eGFP to be expressed in PVN-CRH neurons.
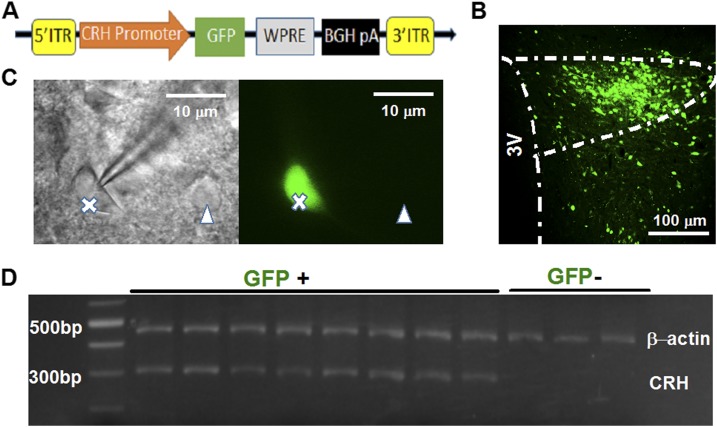
Next, we performed single-cell reverse transcription polymerase chain reaction (PCR) to detect CRH mRNA expression in PVN neurons using the Single Cell-to CTTM kit (Invitrogen, Carlsbad, CA). The eGFP-labeled neuron membrane was ruptured by a glass pipette containing 2 μL of diethylpyrocarbonate-treated water, and the cytoplasm was extracted into this pipette (30). The cytoplasm was processed for reverse transcription, and then PCR amplifying of mRNA of β-actin was conducted with the primers 5′-ATCTATGAGGGTTACGCGCTC-3′ (forward) and 5′-TGCTAGGAGCCAGGGCAGTA-3′ (reverse) and CRH with primers 5′-CGCGATGTGGCAAAAAGCTA-3′ (forward) and 5′-ACCTAAAAGGGACACCCCCT-3′ (reverse). These primers were synthesized using Integrated DNA Technologies (Coralville, IA). Final PCR products were electrophoresed on 2% agarose gel in tris(hydroxymethyl)aminomethane-acetate-EDTA buffer with 40.0 mM tris(hydroxymethyl)aminomethane-acetate and 1 mM EDTA (pH 8.0) containing 0.5 μg/mL ethidium bromide and displayed under UV light. The gel images were photographed.
Electrophysiological recording in brain slices
Brain slices containing the hypothalamic PVN were cut from the brain tissue harvested from the AAV CRH promoter GFP-injected rats. In brief, the brain tissue of the rat was immediately removed after the rat was decapitated under 2% isoflurane anesthesia. Then, the brain was emerged in ice-cold artificial cerebral spinal fluid (aCSF) containing 124.0 mM NaCl, 3.0 mM KCl, 1.3 mM MgSO4, 2.4 mM CaCl2, 1.4 mM NaH2PO4, 10.0 mM glucose, and 26.0 mM NaHCO3 (300 mOsm, gassed by 95% O2 and 5% CO2). The brain tissue was trimmed to a tissue block containing the hypothalamus and glued onto the stage of a vibrating microtome (VT1000; Leica Biosystems Inc., Buffalo Grove, IL). Coronal hypothalamic slices were sectioned at a thickness of 300 μm and transferred to a chamber containing aCSF continuously gassed with a mixture of 95% O2 and 5% CO2 at 34°C for at least 1 hour before electrophysiological recording.
Whole-cell recordings were performed in eGFP-labeled neurons in hypothalamic slices, which were placed in the recording chamber and fixed to the bottom of the recording chamber by a mesh mounted on a stainless steel weight. The recording chamber was perfused with aCSF at a speed of 3.0 mL/min at 34°C maintained by an inline solution heater and a temperature controller (model TC-324; Warner Instruments, Hamden, CT). The eGFP-labeled PVN neurons were firstly identified by an upright microscope (BX51WI; Olympus, Tokyo, Japan) with a combination of epifluorescence illumination and differential interference contrast optics. The recording electrode was pulled from borosilicate capillaries (1.2 mm outer diameter, 0.68 mm inner diameter; World Precision Instruments, Sarasota, FL) by using a micropipette puller (P-97; Sutter Instruments, Novato, CA). The resistance was 3 to 5 MΩ when filled with intracellular solution containing: 140.0 mM potassium gluconate, 2.0 mM MgCl2, 0.1 mM CaCl2, 10.0 mM HEPES, 1.1 mM EGTA, 0.3 mM Na2-GTP, and 2.0 mM Na2-ATP adjusted to pH 7.25 with 1 M KOH, 270 to 290 mOsm. The miniature excitatory postsynaptic currents (mEPSCs) were recorded at a holding potential of −60 mV in the presence of 1.0 μM tetrodotoxin (TTX). The NMDA currents were elicited by puff application of 100 μM NMDA through pressure system IIe (Toohey Company, Fairfield, NJ). The puff pipette (15-μm tip diameter) was placed ∼100 to 150 μm away from the recorded neuron. Positive pressure (3 to 5 psi) was applied for 100 to 200 ms to eject NMDA onto the recorded neuron. In addition, because the NMDA channel is blocked by Mg2+ at negative holding potentials and coactivated by glycine, puff NMDA-elicited currents were recorded in Mg2+-free solution and in the presence of glycine 10 μM as described previously (31, 32).
To study glutamatergic synaptic inputs to the PVN-CRH neurons, we recorded evoked excitatory postsynaptic currents (EPSCs) induced by electric stimulation (0.5 to 1.0 mA at a duration of 0.2 ms at 0.2 Hz) through a bipolar tungsten electrode connected to a stimulator (Grass Instruments, Quincy, MA). The stimulation electrode tip was placed on the ventral side to the recorded PVN neurons. The distance between the tip and recorded neuron was ∼150 μm. The evoked α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)–EPSCs were recorded at a holding potential of −60 mV in the presence of 10 μM gabazine to block GABAA receptor–mediated inhibitory postsynaptic currents. The evoked NMDAR-EPSCs were recorded at a holding potential of 40 mV in the presence of 10 μM gabazine and 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). A serial of stimulation with intensity from low to high was delivered to recorded neurons to determine the threshold and peak response for evoked EPSCs. The stimulation intensity that induced half of the peak response was used to evoke EPSCs. The stimulation intensities did not differ among different treatment groups. The recording pipette was filled with an internal solution containing 110.0 mM Cs2SO4, 2.0 mM MgCl2, 0.1 mM CaCl2, 1.1 mM EGTA, 10.0 mM HEPES, 2.0 mM MgATP, and 0.3 mM Na2GTP adjusted to 7.25 with 1.0 mM CsOH and 280 to 300 mOsm. To block Na+ channels in the postsynaptic neurons, which could be potentially opened by electrical stimulation (32, 33), we included 10.0 mM lidocaine N-ethyl bromide (QX-314; Abcam, Cambridge, MA) in the internal solution.
We recorded the spontaneous firing discharge of eGFP-labeled PVN-CRH neurons under current-clamp conditions without applying holding currents. To avoid affecting the firing activity, we did not use TTX in the perfusion solution and QX-314 in the internal recording solution. The firing activity of the PVN-CRH neurons was recorded ∼5 to 10 minutes after the whole-cell access was established and reached a steady state. The electrical signals were amplified by a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), filtered at 2 kHz, digitalized at 10 kHz using Digidata 1320A (Axon Instruments, Union City, CA), and stored at Axon data format in a computer for further analysis. We performed electrophysiological recording on one neuron in each brain slice. At least five rats were used for each recording protocol. We freshly prepared all drugs in aCSF before each experiment and delivered them to the recording chamber at the final concentrations. 2-Amino-5-phosphonopentanoic acid (AP5), TTX, QX-314, gabazine, and CNQX were purchased from Abcam.
Western immunoblotting
Each rat was anesthetized with 2% isoflurane and quickly decapitated. The brain was sectioned at the level of 1.08 to 2.12 mm caudally to the bregma. PVN tissues were micropunched bilaterally with a slice punch (0.5-mm diameter) spanning from 1.08 to 2.12 mm caudal to the bregma under a dissection microscope. Bicinchoninic acid assay was used to extract total protein (Thermo Fisher Scientific, Waltham, MA). The extracted protein was separate by 4% to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Immobilon P; Millipore, Burlington, MA). The immunoblots were probed with rabbit anti-GluN1 (Sigma-Aldrich, St. Louis, MO) and rabbit anti– glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies (Abcam) overnight. The next day, goat anti-rabbit horseradish peroxidase antibody (Abcam) was applied to the immunoblots for 2 hours at room temperature. The protein bands were detected using a chemiluminescence kit (Life Technologies, Waltham, MA), quantified using ImageJ software (National Institutes of Health, Bethesda, MD), and normalized by the GAPDH optical density within the same sample.
Cannulation and PVN microinjection
Rats were anesthetized with 2% isoflurane and implanted with a 26-gauge double-barrel guide cannula (1.0 mm between barrels, 7.0 mm long; RWD Life Science Ltd, San Diego, CA). The tips of the guide cannula were 1.0 mm dorsal to the bilateral PVN. After the guide cannula was affixed to the skull with dental acrylic, a dummy cannula was inserted into the two barrels of the guide cannula, and a dust cap was used to cover the external end of the dummy cannula. After recovering from anesthesia, the rats were returned to their cages and allowed to recover for 1 to 2 weeks before the microinfusions were performed. During the microinfusion procedure, an injection needle with a bilateral cannula targeting 1.0 mm beyond the tip of the guide cannula was placed into the guide cannula. Memantine (100 pmol in 50 nL of aCSF) or vehicle (50 nL aCSF) was injected bilaterally into the PVN. The injection sites in the PVN were verified at the end of each experiment by infusion of 50 nL fluorescent microspheres (0.04 μm, wavelength 580 nm, red).
Measurement of CORT levels
Blood samples were collected before CUMS, 5 to 10 days after cessation of CUMS, and at multiple time points (30, 60, and 120 minutes) after PVN injection of vehicle and memantine in CUMS rats and unstressed rats. Blood samples were collected from the saphenous vein of each rat at 10:00 am. Blood samples of only 100 μL were collected to avoid influencing blood volume loss on CORT levels. The serum was obtained by subjecting the blood in serum collection tubes to centrifugation at 19,064g for 5 minutes. Circulating CORT concentrations were measured by an enzyme immunoassay (Enzo Life Sciences, Farmingdale, NY) and compared with a standard curve of known CORT concentrations per the manufacturer’s instructions. The sensitivity of this immunoassay was 26.99 pg/mL, and the intra-assay coefficients of variation were 4.6% in the unstressed plus vehicle group, 3.6% in the unstressed plus memantine group, 5.3% in the CUMS plus vehicle group, and 5.1% in the CUMS plus memantine group.
Data analysis
Data are presented as the means ± standard errors of the mean. Spontaneous firing activity and mEPSCs were analyzed offline using a peak detection program (MiniAnalysis; Synaptosoft Inc., Decatur, GA). The firing rate and frequency of the mEPSCs of PVN-CRH neurons were averaged over 3 minutes before, during, and after drug application. The liquid junction potential was corrected depending on the ionic composition of the internal and external solution. We used the software pClamp (version 10) to determine the peak amplitude of evoked EPSCs and puff NMDA currents. A paired t test was used to compare the CORT level and sucrose preference values before and after CUMS treatment, and an unpaired t test was used to compare NMDAR and AMPAR expression levels and the function between unstressed rats and CUMS rats. For comparisons of more than two groups, we performed repeated-measures analysis of variance with the Dunnett post hoc test or one-way analysis of variance with the Tukey post hoc test to compare responses within or between experimental groups using Prism software version 6 (GraphPad Software, San Diego, CA). P values <0.05 were considered statistically significant.
Results
PVN-CRH neuron identification
PVN-CRH neurons were reliably identified by specifically expressing eGFP under the control of the rat CRH promoter (Fig. 1A). To validate that the eGFP-tagged PVN neurons were CRH-expressing neurons, single-cell PCR was used to detect CRH mRNA in eGFP-tagged PVN neurons. The intracellular content of a single eGFP-tagged PVN neuron was extracted into the glass pipette for mRNA extraction (Fig. 1C). CRH mRNAs were detected in all eight eGFP-labeled neurons but not in three eGFP-negative neurons. The β-actin mRNA, used as a control, was detectable in both the eGFP-positive and eGFP-negative neurons (Fig. 1D).
Figure 1.
Reliable identification of PVN-CRH neurons. (A) Major components of the structure of the AAV vector used to express CRH promoter-driven eGFP in PVN neurons. (B) Representative image shows the eGFP expression in the PVN 3 weeks after microinjection of the AAV-CRH-eGFP vector into the PVN. (C) eGFP-labeled PVN-CRH neurons (marked by X) and non-eGFP–labeled neurons (∆) used for single-cell PCR examination. (D) Single-cell reverse transcription PCR revealed that eGFP-labeled neurons (GFP+), but not non-GFP–labeled neurons (GFP−), were CRH mRNA-positive. 3V, third ventricle; BGH pA, bovine growth hormone polyadenylation; ITR, inverted terminal repeat; WPRE, woodchuck postregulatory element.
CUMS increases NMDAR expression levels in the PVN
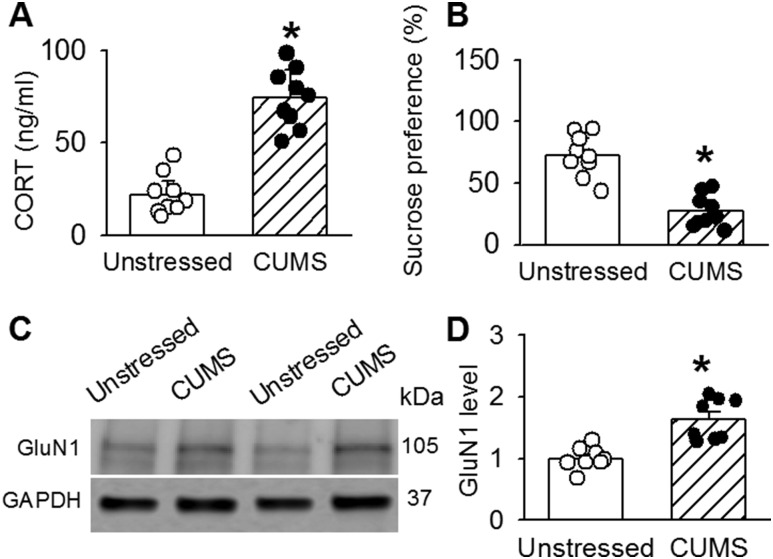
Chronic stress can induce hyperactivity of the HPA axis (34) and alter NMDAR activity in a variety of brain regions (18, 35, 36). Because NMDARs are composed of two GluN1 and two GluN2 (GluN2A or GluN2B) subunits (21), we determined GluN1 expression levels to assess whether CUMS alters the protein levels of the NMDARs in the PVN. CUMS treatment significantly increased circulating CORT levels (n = 9; t(16) = 14.90; P < 0.0001), whereas it decreased sucrose preference (n = 9; t(16) = 8.27; P < 0.0001; Fig. 2A and 2B). Each immunoblot detecting GluN1 displayed a single band. The density of these bands for GluN1 in PVN tissues were significantly higher in CUMS rats than in unstressed rats (n = 8 samples; GluN1: t(14) = 4.73; P = 0.0003; Fig. 2C and 2D).
Figure 2.
CUMS increases the protein levels of NMDAR subunits in the PVN. Summary data of (A) CORT levels and (B) sucrose preference before and after CUMS (n = 8 in each group). (C) Representative Western immunoblot gel images and (D) quantification of band density show the protein levels (normalized to GAPDH) of GluN1 in PVN tissue in unstressed rats and CUMS rats (n = 8 samples in each group). The molecular weights are indicated to the right of the gel images. *P < 0.05 compared with the value in unstressed rats (unpaired t test).
CUMS enhance postsynaptic NMDAR activity in PVN-CRH neurons
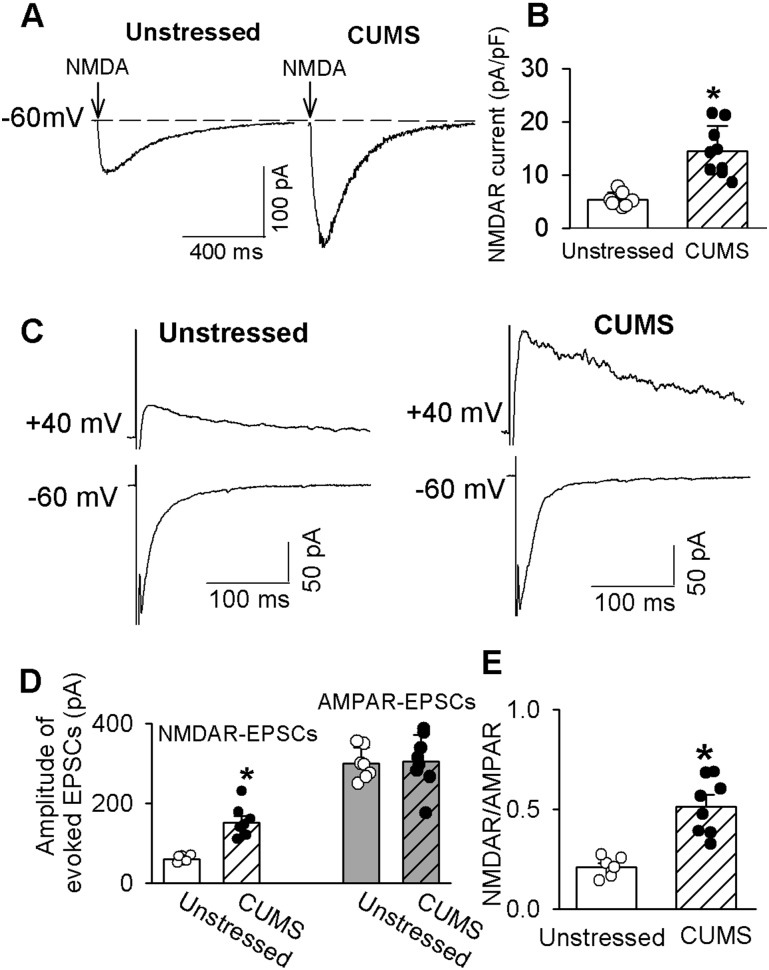
Because NMDARs are expressed in both presynaptic terminals and postsynaptic soma (21, 37), we then determined whether CUMS altered postsynaptic NMDAR activity. The NMDAR currents were elicited by puff application of 100 μM NMDA in Mg2+-free external solution at a holding potential of −60 mV. Puff NMDA-elicited currents in the PVN-CRH neurons were significantly larger in CUMS rats than in unstressed rats (n = 7 neurons from 6 rats in unstressed and n = 9 neurons from 6 rats in CUMS group; t(14) = 4.94; P = 0.0002; Fig. 3A and 3B).
Figure 3.
CUMS enhances synaptic NMDAR activity in PVN-CRH neurons. (A) Original current traces and (B) summary data show currents elicited by puff 100 µM NMDA in eGFP-tagged PVN-CRH neurons from CUMS (n = 9 neurons) and unstressed rats (n = 7 neurons). (C) Representative traces and (D) summary data of evoked AMPAR-EPSCs (holding potential of −60 mV) and NMDAR-EPSCs (holding potential of 40 mV) in eGFP-labeled neurons from CUMS rats (n = 8 neurons) and unstressed rats (n = 7 neurons). (E) Group data show the ratios of NMDAR-EPSCs to AMPAR-EPSCs in neurons from unstressed and CUMS rats in D. *P < 0.05 compared with unstressed rats.
We next compared the electrical evoked AMPAR- and NMDAR-mediated EPSCs in PVN-CRH neurons in CUMS rats and unstressed rats. The AMPAR-EPSCs were recorded at a holding potential of −60 mV in the presence of 10 μM gabazine. Bath application of 20 μM CNQX abolished the evoked AMPAR-EPSCs. The NMDAR-EPSCs were recorded at a holding potential of 40 mV in the presence of 10 μM gabazine and 20 μM CNQX. Bath application of 50 μM AP5 eliminated NMDAR-EPSCs. The amplitude of evoked AMPAR-EPSCs of labeled PVN neurons was similar in unstressed rats (n = 7 neurons) and CUMS rats (n = 8 neurons). In contrast, the amplitude of evoked NMDAR-EPSCs was significantly greater in CUMS rats than in unstressed rats. The ratio of NMDAR-EPSCs to AMPAR-EPSCs in CUMS rats was significantly larger than that in unstressed rats (n = 7 neurons in unstressed rats and n = 8 neurons in CUMS rats; t(13) = 5.45; P = 0.001; Fig. 3).
CUMS induces tonic activation of presynaptic NMDAR in PVN-CRH neurons
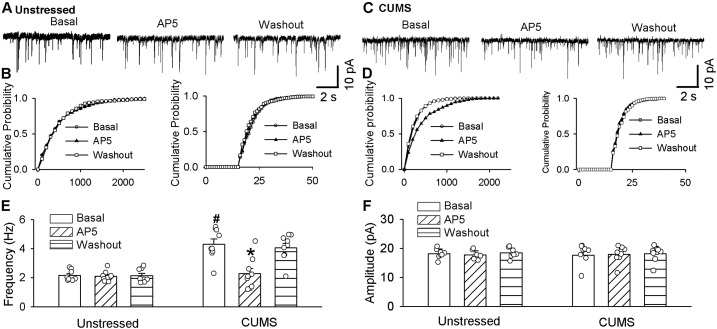
Presynaptic NMDARs in the PVN are silent under physiological conditions but become tonically activated in certain conditions (38). To determine the role of presynaptic NMDARs in regulating presynaptic glutamate release to PVN-CRH neurons, we recorded mEPSCs to reflect spontaneous quanta release of glutamate from presynaptic terminals (39). Postsynaptic NMDAR activity was blocked by including 1 mM MK-801 to the internal solution in the recording pipette (40). The baseline frequency of mEPSCs was significantly higher in CUMS rats (n = 9 neurons) than in unstressed rats (n = 8 neurons; t(15) = 5.54; P < 0.0001; Fig. 4), whereas the baseline amplitude of mEPSCs did not differ significantly between these two groups. To determine the role of NMDARs in the increased frequency of mEPSCs in CUMS rats, we blocked NMDARs by bath application of 50 μM AP5, a selective NMDAR antagonist. AP5 application significantly decreased the frequency of mEPSCs in eGFP-tagged PVN-CRH neurons in CUMS rats from 4.31 ± 0.35 Hz to 2.27 ± 0.36 Hz (n = 9 neurons from 6 CUMS-treated rats; F(2, 16) = 27.71; P < 0.0001; Fig. 4B, 4D, and 4F). However, AP5 application had no significant effect on the frequency or amplitude of mEPSCs in unstressed rats (Fig. 4A, 4C, and 4E). We also performed cumulative probability analysis for frequency and amplitude of mEPSCs for each neuron recorded. The cumulative probability analysis of mEPSCs revealed that the distribution pattern of the interevent interval of mEPSCs shifted toward the right during AP5 application in CUMS rats, whereas the distribution pattern of the amplitude of mEPSCs was not significantly changed during AP5 application (Fig. 4D). Neither the distribution pattern of the interevent interval nor the amplitude of mEPSCs was altered during the application of AP5 in unstressed rats (Fig. 4C). These data suggest that presynaptic NMDAR activity is tonically activated and involved in CUMS-induced increases in presynaptic glutamate synaptic inputs to PVN-CRH neurons.
Figure 4.
NMDAR contributes to the increased frequency of mEPSCs in PVN-CRH neurons in CUMS rats. Original traces and cumulative probability plots show the effect of bath application of 50 µM AP5 on the mEPSCs of PVN-CRH neurons from (A and B) unstressed rats and (C and D) CUMS rats. Summary data show the effects of AP5 on the (E) frequency and (F) amplitude of mEPSCs in the PVN-CRH neurons of unstressed rats (n = 8 neurons) and CUMS rats (n = 9 neurons). *P < 0.05 compared with basal value in the same group; #P < 0.05 compared with baseline in unstressed rats.
Blockade of NMDARs normalizes the increased firing activity of PVN-CRH neurons and elevated CORT levels in CUMS rats
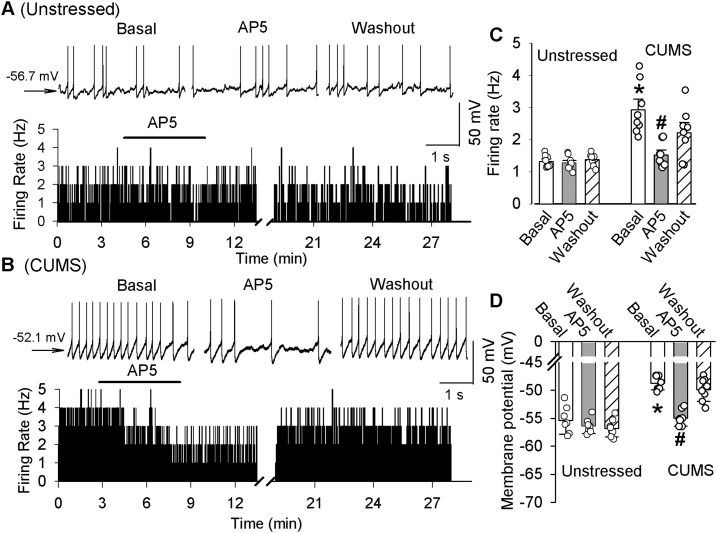
To determine the role of NMDARs in the hyperactivity of PVN-CRH neurons, we recorded the firing activity of PVN-CRH neurons before and after NMDAR blockade. The majority of the PVN-CRH neurons displayed spontaneous firing activity in both unstressed rats (8 of 11 cells; 74.2%) and CUMS-treated rats (8 of 10 cells; 80%). The baseline firing rate of the PVN-CRH neurons was significantly higher in CUMS rats (n = 8 neurons from 6 rats) than in unstressed rats (n = 8 neurons from 6 rats; t(14) = 5.51; P < 0.0001). Blockade of NMDARs with 50 µM AP5 did not significantly change the firing activity and membrane potential in PVN-CRH neurons in unstressed rats (Fig. 5). However, bath application of 50 µM AP5 normalized the firing rate in CUMS rats to the levels of unstressed rats, from 2.92 ± 0.34 to 1.52 ± 0.15 Hz (n = 8 neurons; F(2, 14) = 34.01; P < 0.0001; Fig. 5). Furthermore, AP5 significantly hyperpolarized the membrane potential from −48.5 ± 1.1 to 54.8 ± 1.2 mV (F(2, 14) = 21.86; P < 0.0001) in these neurons in CUMS rats.
Figure 5.
Blockade of NMDARs reduces the hyperactivity of PVN-CRH neurons in CUMS rats. Representative raw traces and a histogram of firing rates show the effect of 50 µM AP5 on the spontaneous firing rate of labeled PVN-CRH neurons of (A) unstressed and (B) CUMS rats. Summary data show the effects of 50 µM AP5 on the (C) firing rates and (D) membrane potentials of PVN-CRH neurons of unstressed (n = 8 neurons) and CUMS rats (n = 8 neurons). *P < 0.05 compared with the corresponding value in the unstressed group; #P < 0.05 compared with the basal values in CUMS rats.
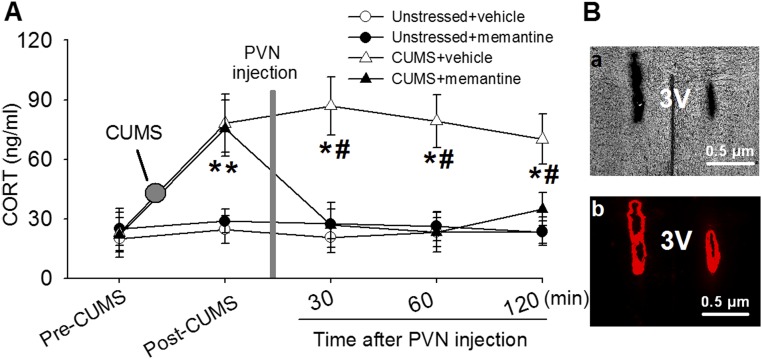
We then determined whether increased NMDAR activity in the PVN contributes to the CUMS-induced hyperactivity of the HPA axis. Memantine is a competitive NMDAR antagonist and is used clinically to improve stress-induced cognitive and psychological symptoms (41, 42). To determine the roles of NMDARs in the CUMS-induced hyperactivity of the HPA axis, memantine was microinjected into bilateral PVN through an implanted cannula in unstressed and CUMS rats. Circulating CORT levels were measured in blood samples collected from the saphenous vein before and after memantine microinfusion. The CORT levels were remarkably elevated in CUMS rats (n = 6; 76.09 ± 14.69 ng/mL) compared with unstressed rats (n = 6; 24.64 ± 7.05 ng/mL; t(10) = 10.54; P < 0.0001; Fig. 6A). Figure 6B shows examples of microphotographs of PVN microinfusion sites. Microinjection of 100 pmol memantine in 50 nL aCSF (43) decreased the CORT level from 95.57 ± 14.13 to 43.20 ± 9.90 ng/mL in CUMS rats (F(5,25) = 80.51; P < 0.0001; n = 6). However, memantine did not significantly alter the CORT level in unstressed rats (Fig. 6).
Figure 6.
An NMDAR antagonist normalizes the CUMS-induced elevation of circulating CORT levels. (A) Summary data show plasma CORT levels during the basal condition and before and after bilateral microinjection of the NMDAR antagonist memantine into the PVN in unstressed rats (n = 6) and CUMS rats (n = 6). (B) Images of coronal brain slice showing the microinfusion sites marked by fluorescent microspheres (0.04 μm, wavelength 580 nm, red) in the PVN in (a) light optics and (b) fluorescent illumination. *P < 0.05 compared with the basal value within the same group; #P < 0.05 compared with the respective values at the same time in unstressed rats. 3V, third ventricle.
Discussion
This study shows that CUMS augments synaptic NMDAR activity in PVN-CRH neurons and that the increase in synaptic NMDAR activity contributes significantly to HPA axis hyperactivity. We found that CUMS treatment not only increased NMDAR subunit protein levels in the PVN but also enhanced pre- and postsynaptic NMDAR-mediated glutamatergic synaptic inputs to PVN-CRH neurons. In addition, we found that NMDAR blockade normalized the hyperactivity of PVN-CRH neurons and hypercortisolemia in CUMS rats. These data suggest that enhanced synaptic NMDAR activity is an important mechanism underlying the hyperactivity of the HPA axis during chronic stress.
We found that the protein expression levels of the NMDAR subunits GluN1 in the PVN were significantly higher CUMS rats than in unstressed rats. Previous study using in situ hybridization revealed that chronic variable stress decreases the mRNA expression of the GluN2B subunit in the PVN but has no effect on the mRNA expression of GluN1 and GluN2A (11). It should be noted that changes in mRNA levels may not represent the same tendency changes of protein expression levels and different stressors and/or the durations of stressors may result in discrepant NMDAR expression in the PVN. In addition, chronic stress has been shown to decrease protein expression levels of the GluN1, GluN2A, and GluN2B subunits in the prefrontal cortex (24), which suggests that the stress responses of NMDAR in various brain regions differ. The hypothalamic PVN contains heterogeneous neurons including PVN-CRH neurons, and thus, the upregulation of NMDARs in PVN tissue may not represent an increase in the NMDAR expression level in PVN-CRH neurons in CUMS rats.
PVN-CRH neurons are innervated by glutamatergic nerve terminals (11) and express a high density of glutamate receptors, including NMDARs and AMPARs (14). We found that CUMS augmented evoked NMDAR-EPSCs in PVN-CRH neurons but had no notable effect on evoked AMPAR-EPSCs, suggesting that CUMS enhanced the NMDAR-mediated glutamatergic synaptic inputs to PVN-CRH neurons. Although AMPARs may contribute to the HPA axis hyperactivity induced by acute immobilization stress (44), we found that CUMS had little effect on evoked AMPAR-EPSCs in PVN-CRH neurons. Previous study has shown that repeated restraint stress increases the frequency of glutamatergic spontaneous EPSCs in parvocellular PVN neurons, although the nature of the peptides synthesized and released by these cells has not been determined (45). We found that CUMS significantly increased the frequency of glutamatergic mEPSCs to PVN-CRH neurons without altering their amplitude. According to the quanta hypothesis, changes in the frequency of mEPSCs represent an increase in the probability of glutamate release (39, 46). This increased presynaptic glutamate release may contribute to increased evoked NMDAR-EPSCs in PVN-CRH neurons in CUMS rats. CUMS had little effect on the amplitude of mEPSCs, suggesting that CUMS did not alter postsynaptic AMPAR activity. Because NMDARs are distributed in both presynaptic terminals and postsynaptic soma (21, 37), we also determined the role of NMDARs in CUMS-induced increased presynaptic glutamate release and found that blocking NMDARs with its specific antagonist AP5 significantly decreased the frequency of mEPSCs in PVN-CRH neurons in CUMS rats but not in unstressed rats. These data suggest that presynaptic NMDAR activity is elevated in CUMS rats and contributes to enhanced glutamate release to PVN-CRH neurons. The elevated NMDAR activity may be due to an increase in the number of glutamatergic buttons, as opposed to the soma and dendrites, of PVN-CRH neurons in chronically stressed rats (16, 19). In addition, it is possible that the phosphorylation level of NMDARs might contribute to the increase in NMDAR-mediated glutamatergic synaptic inputs.
One salient finding of our study was that CUMS significantly increased postsynaptic NMDAR activity in PVN-CRH neurons. We found that the amplitudes of evoked NMDAR-EPSCs and NMDA currents induced by puff application of NMDA in PVN-CRH neurons were significantly higher in CUMS rats than in unstressed rats. This increase in NMDAR currents may have been largely due to the increased expression levels of NMDAR subunits during CUMS. Another possibility is that CUMS increases the activity of kinases that are involved in the regulation of NMDAR activity in the PVN. Chronic restraint stress increases the activity of protein kinase C (47) and cyclin-dependent kinase 5 (48, 49), which closely regulate the trafficking of NMDARs (50, 51), as many phosphorylation sites in NMDAR subunits are substrates of kinases such as protein kinase C (51) and cyclin-dependent kinase 5 (52, 53). Therefore, future studies are needed to determine whether CUMS-induced NMDAR activity is due to an increase in the phosphorylation level of the NMDARs induced by the elevated activity of protein kinase C and/or cyclin-dependent kinase 5 in the PVN. We also found that blocking NMDAR with AP5 induced hyperpolarization in the PVN-CRH neurons in CUMS rats but had no significant effect in unstressed rats, suggesting that NMDARs are tonically activated and contribute to depolarized membrane potentials in PVN-CRH neurons in CUMS rats. The enhanced NMDAR activity in PVN-CRH neurons may decrease inhibitory GABAB receptor activity because blocking NMDAR increases GABAB receptor–mediated currents (27). It is also possible that enhanced NMDAR activity contributes to the depolarizing shift of GABA reversal potential in the PVN-CRH neurons in CUMS rats (26, 54, 55). Both reduced GABAB receptor activity and depolarizing shift of GABA reversal potential increase excitability of PVN-CRH neurons and induce HPA axis hyperactivity during chronic stress (26, 27).
We found that NMDAR blockade significantly decreased the basal spontaneous firing activity of PVN-CRH neurons in brain slices from CUMS rats but not unstressed rats. These data suggest that enhanced NMDAR activity contributes to the hyperactivity of PVN-CRH neurons in CUMS rats, whereas in unstressed rats, NMDAR is not involved in the control of the basal firing activity of PVN-CRH neurons. Because PVN-CRH neurons are key components of the HPA axis and the activity of PVN-CRH neurons are critical in controlling HPA axis activity and circulating CORT levels (56), blocking NMDAR would decrease circulating CORT levels in CUMS rats. Memantine is a competitive NMDA antagonist used to treat patients with Alzheimer’s disease (57, 58), psychological disorders, and cognitive impairment (41, 42), we used this agent to assess the effect of blocking NMDARs in the PVN on circulating CORT levels. We found that microinjection of memantine into the PVN significantly reduced circulating CORT levels in CUMS rats but not unstressed rats. These data suggest that increased NMDAR activity in the PVN is critically involved in the hypercortisolemia in CUMS rats.
In summary, findings from our study suggest that CUMS enhances the excitatory glutamatergic synaptic inputs to PVN-CRH neurons. Increased glutamatergic synaptic inputs, including increased NMDAR-mediated presynaptic glutamate release and upregulation of postsynaptic NMDAR activity, can lead to hyperactivity of the PVN-CRH neurons, which likely contributes to the elevated activity of the HPA axis. Thus, the pre- and postsynaptic plasticity of the NMDARs may play an important role in the maintaining the hyperactivity of the HPA axis during chronic stress. These findings provide insight into the synaptic plasticity in the hypothalamus in chronic stress and suggest strategies to reduce hyperactivity of the HPA axis.
Acknowledgments
The authors thank Joe Munch in the Department of Scientific Publications for editing the manuscript.
Financial Support: This work was supported by National Institutes of Health Grants R01-MH-096086 and R01-HL-139523 (to D.-P.L.) and sponsored by a program for introduction of overseas scholars to Hebei Province, China (CY201720 to Y.G.). In addition, this work was supported in part by the National Institutes of Health/National Cancer Institute through MD Anderson Cancer Center Support Grant P30-CA-016672, which helps fund the institution’s Research Animal Support Facility.
Author Contributions: J.-J.Z., Y.G., T.A.K., and D.-P.L. were involved in study design. J.-J.Z. and Y.G. performed electrophysiological recordings and biochemical assays. J.-J.Z., X.Z., and D.-P.L. performed data analysis. J.-J.Z., Y.G., X.Z., T.A.K., and D.-P.L. wrote the manuscript.
Disclosure Summary:
The authors have nothing to disclose.
Glossary
Abbreviations:
- AAV
adeno-associated viral
- aCSF
artificial cerebral spinal fluid
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- AP5
2-amino-5-phosphonopentanoic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CORT
corticosterone
- CRH
corticotrophin-releasing hormone
- CUMS
chronic unpredictable mild stress
- eGFP
enhanced green fluorescent protein
- EPSC
excitatory postsynaptic current
- GABA
γ-aminobutyric acid
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HPA
hypothalamic-pituitary-adrenal
- mEPSC
miniature excitatory postsynaptic current
- mRNA
messenger RNA
- NMDAR
N-methyl-d-aspartate receptor
- PCR
polymerase chain reaction
- PVN
paraventricular nucleus
- TTX
tetrodotoxin
References
- 1.Dallman MF. Stress update Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab. 1993;4(2):62–69. [DOI] [PubMed] [Google Scholar]
- 2.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. [DOI] [PubMed] [Google Scholar]
- 4.Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–626. [DOI] [PubMed] [Google Scholar]
- 5.Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJ, Tilders FJ, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. Am J Psychiatry. 1995;152(9):1372–1376. [DOI] [PubMed] [Google Scholar]
- 6.Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiatry. 2006;163(12):2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elgh E, Lindqvist Astot A, Fagerlund M, Eriksson S, Olsson T, Näsman B. Cognitive dysfunction, hippocampal atrophy and glucocorticoid feedback in Alzheimer’s disease. Biol Psychiatry. 2006;59(2):155–161. [DOI] [PubMed] [Google Scholar]
- 8.Lanté F, Chafai M, Raymond EF, Pereira AR, Mouska X, Kootar S, Barik J, Bethus I, Marie H. Subchronic glucocorticoid receptor inhibition rescues early episodic memory and synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2015;40(7):1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6(1):269–324. [DOI] [PubMed] [Google Scholar]
- 10.Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol. 2000;419(3):344–351. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J Comp Neurol. 2005;484(1):43–56. [DOI] [PubMed] [Google Scholar]
- 12.Herman JP, Larson BR, Speert DB, Seasholtz AF. Hypothalamo-pituitary-adrenocortical dysregulation in aging F344/Brown-Norway F1 hybrid rats. Neurobiol Aging. 2001;22(2):323–332. [DOI] [PubMed] [Google Scholar]
- 13.Decavel C, van den Pol AN. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J Comp Neurol. 1992;316(1):104–116. [DOI] [PubMed] [Google Scholar]
- 14.Herman JP, Eyigor O, Ziegler DR, Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 2000;422(3):352–362. [PubMed] [Google Scholar]
- 15.Tasker JG, Boudaba C, Schrader LA. Local glutamatergic and GABAergic synaptic circuits and metabotropic glutamate receptors in the hypothalamic paraventricular and supraoptic nuclei. Adv Exp Med Biol. 1998;449:117–121. [DOI] [PubMed] [Google Scholar]
- 16.Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517(2):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miklós IH, Kovács KJ. Reorganization of synaptic inputs to the hypothalamic paraventricular nucleus during chronic psychogenic stress in rats. Biol Psychiatry. 2012;71(4):301–308. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese F, Guidotti G, Molteni R, Racagni G, Mancini M, Riva MA. Stress-induced changes of hippocampal NMDA receptors: modulation by duloxetine treatment. PLoS One. 2012;7(5):e37916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S. Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos Trans R Soc Lond B Biol Sci. 2013;369(1633):20130151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist. 2008;14(6):609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasgow NG, Siegler Retchless B, Johnson JW. Molecular bases of NMDA receptor subtype-dependent properties. J Physiol. 2015;593(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurophysiol. 2008;100(6):3175–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CH, Hsiao YH, Chen YW, Yu YJ, Gean PW. Social isolation-induced increase in NMDA receptors in the hippocampus exacerbates emotional dysregulation in mice. Hippocampus. 2015;25(4):474–485. [DOI] [PubMed] [Google Scholar]
- 24.Lee YA, Goto Y. Chronic stress modulation of prefrontal cortical NMDA receptor expression disrupts limbic structure-prefrontal cortex interaction. Eur J Neurosci. 2011;34(3):426–436. [DOI] [PubMed] [Google Scholar]
- 25.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16(4):525–534. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Zhou JJ, Zhu Y, Kosten T, Li DP. Chronic unpredictable mild stress induces loss of GABA inhibition in corticotrophin-releasing hormone-expressing neurons through NKCC1 upregulation. Neuroendocrinology. 2017;104(2):194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, Zhou JJ, Zhu Y, Wang L, Kosten TA, Zhang X, Li DP. Neuroadaptations of presynaptic and postsynaptic GABAB receptor function in the paraventricular nucleus in response to chronic unpredictable stress. Br J Pharmacol. 2017;174(17):2929–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou JJ, Gao Y, Kosten TA, Zhao Z, Li DP. Acute stress diminishes M-current contributing to elevated activity of hypothalamic-pituitary-adrenal axis. Neuropharmacology. 2017;114:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coldren KM, Li DP, Kline DD, Hasser EM, Heesch CM. Acute hypoxia activates neuroendocrine, but not presympathetic, neurons in the paraventricular nucleus of the hypothalamus: differential role of nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2017;312(6):R982–R995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Káradóttir R, Attwell D. Combining patch-clamping of cells in brain slices with immunocytochemical labeling to define cell type and developmental stage. Nat Protoc. 2006;1(4):1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergeron R, Coyle JT, Tsai G, Greene RW. NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology. 2005;30(1):7–16. [DOI] [PubMed] [Google Scholar]
- 32.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586(6):1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li DP, Zhou JJ, Pan HL. Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Physiol. 2015;593(19):4439–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwendt M, Jezová D. Gene expression of two glutamate receptor subunits in response to repeated stress exposure in rat hippocampus. Cell Mol Neurobiol. 2000;20(3):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe Y, Weiland NG, McEwen BS. Effects of adrenal steroid manipulations and repeated restraint stress on dynorphin mRNA levels and excitatory amino acid receptor binding in hippocampus. Brain Res. 1995;680(1-2):217–225. [DOI] [PubMed] [Google Scholar]
- 37.Banerjee A, Larsen RS, Philpot BD, Paulsen O. Roles of presynaptic NMDA receptors in neurotransmission and plasticity. Trends Neurosci. 2016;39(1):26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao X, Zhou JJ, Li DP, Pan HL. Src kinases regulate glutamatergic input to hypothalamic presympathetic neurons and sympathetic outflow in hypertension. Hypertension. 2017;69(1):154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sulzer D, Pothos EN. Regulation of quantal size by presynaptic mechanisms. Rev Neurosci. 2000;11(2-3):159–212. [DOI] [PubMed] [Google Scholar]
- 40.Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 2010;30(12):4460–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramaswamy S, Madabushi J, Hunziker J, Bhatia SC, Petty F. An open-label trial of memantine for cognitive impairment in patients with posttraumatic stress disorder. J Aging Res. 2015;2015:934162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Battista MA, Hierholzer R, Khouzam HR, Barlow A, O’Toole S. Pilot trial of memantine in the treatment of posttraumatic stress disorder. Psychiatry. 2007;70(2):167–174. [DOI] [PubMed] [Google Scholar]
- 43.Chen HS, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 1997;499(Pt 1):27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zelena D, Makara GB, Jezova D. Simultaneous blockade of two glutamate receptor subtypes (NMDA and AMPA) results in stressor-specific inhibition of prolactin and corticotropin release. Neuroendocrinology. 1999;69(5):316–323. [DOI] [PubMed] [Google Scholar]
- 45.Kusek M, Tokarski K, Hess G. Repeated restraint stress enhances glutamatergic transmission in the paraventricular nucleus of the rat hypothalamus. J Physiol Pharmacol. 2013;64(5):565–570. [PubMed] [Google Scholar]
- 46.Lustig C, Parnas H, Segel LA. Neurotransmitter release: development of a theory for total release based on kinetics. J Theor Biol. 1989;136(2):151–170. [DOI] [PubMed] [Google Scholar]
- 47.Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA. 2009;106(42):17957–17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adzic M, Djordjevic J, Djordjevic A, Niciforovic A, Demonacos C, Radojcic M, Krstic-Demonacos M. Acute or chronic stress induce cell compartment-specific phosphorylation of glucocorticoid receptor and alter its transcriptional activity in Wistar rat brain. J Endocrinol. 2009;202(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papadopoulou A, Siamatras T, Delgado-Morales R, Amin ND, Shukla V, Zheng YL, Pant HC, Almeida OF, Kino T. Acute and chronic stress differentially regulate cyclin-dependent kinase 5 in mouse brain: implications to glucocorticoid actions and major depression. Transl Psychiatry. 2015;5(6):e578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112(3):810–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4(4):382–390. [DOI] [PubMed] [Google Scholar]
- 52.Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci USA. 2001;98(22):12742–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J Neurosci. 2008;28(2):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitamura A, Ishibashi H, Watanabe M, Takatsuru Y, Brodwick M, Nabekura J. Sustained depolarizing shift of the GABA reversal potential by glutamate receptor activation in hippocampal neurons. Neurosci Res. 2008;62(4):270–277. [DOI] [PubMed] [Google Scholar]
- 55.Sun D, Murali SG. Stimulation of Na+-K+-2Cl- cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am J Physiol. 1998;275(3 Pt 1):C772–C779. [DOI] [PubMed] [Google Scholar]
- 56.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. [DOI] [PubMed] [Google Scholar]
- 57.Lipton SA, Chen HS. Paradigm shift in neuroprotective drug development: clinically tolerated NMDA receptor inhibition by memantine. Cell Death Differ. 2004;11(1):18–20. [DOI] [PubMed] [Google Scholar]
- 58.Witt A, Macdonald N, Kirkpatrick P. Memantine hydrochloride. Nat Rev Drug Discov. 2004;3(2):109–110. [DOI] [PubMed] [Google Scholar]