Abstract
Estradiol acts as a neuromodulator in brain regions important for cognition and sensory processing. Estradiol also shapes brain sex differences but rarely have these concepts been considered simultaneously. In male and female songbirds, estradiol rapidly increases within the auditory forebrain during song exposure and enhances local auditory processing. We tested whether G-protein-coupled estrogen receptor 1 (GPER1), a membrane-bound estrogen receptor, is necessary and sufficient for neuroestrogen regulation of forebrain auditory processing in male and female zebra finches (Taeniopygia guttata). At baseline, we observed that females had elevated single-neuron responses to songs vs males. In males, narrow-spiking (NS) neurons were more responsive to conspecific songs than broad-spiking (BS) neurons, yet cell types were similarly auditory responsive in females. Following acute inactivation of GPER1, auditory responsiveness and coding were suppressed in male NS yet unchanged in female NS and in BS of both sexes. By contrast, GPER1 activation did not mimic previously established estradiol actions in either sex. Lastly, the expression of GPER1 and its coexpression with an inhibitory neuron marker were similarly abundant in males and females, confirming anatomical similarity in the auditory forebrain. In this study, we found: (1) a role for GPER1 in regulating sensory processing and (2) a sex difference in auditory processing of complex vocalizations in a cell type–specific manner. These results reveal sex specificity of a rapid estrogen signaling mechanism in which neuromodulation accounts and/or compensates for brain sex differences, dependent on cell type, in brain regions that are anatomically similar in both sexes.
Neuronal auditory responses and coding are modulated by a membrane estrogen receptor in male birds. Sensory regions can be physiologically differentiated for sex-specific encoding of complex stimuli.
Sex differences in anatomical features have been observed throughout the central nervous system in vertebrates. For regions that do not differ structurally between males and females, anatomical similarity could belie underlying sex differences in physiology and modulation (1). In many songbird species, song production is male specific and is associated with robust sex differences in the song motor pathway (2, 3). By contrast, the structure and size of the songbird auditory network are considered largely similar in males and females (4, 5). Specifically, the songbird caudomedial nidopallium (NCM; analogous to secondary auditory cortex) is not sexually dimorphic in structure, yet some NCM firing states appear divergent between males and females (6, 7). It is currently unclear whether and how these observed differences manifest in the auditory response properties of single NCM neurons. In both sexes, NCM is selective for complex song stimuli (8–11), and it is hypothesized to be a locus for auditory memory and discrimination (12–15). Auditory processing is essential for both males and females, yet song is a sexual stimulus that can have differing contextual meaning and salience depending on the sex of the listener and its hormonal state (7, 11, 15–21). A focused examination of sex differences in songbird auditory physiology can likely provide insights into sex differences in hearing, language development, and neuroendocrine mechanisms in other vocal-learning species, including humans (22–24).
Hormones and genes organize brains into “male-like” and “female-like” states (25–27). Sexually dimorphic neural circuits can support differentiated motor behaviors and traits (28), but they can also compensate to maintain similarity between sexes when hormonal, genetic, and/or morphologic factors differ (29). A key hormonal mechanism associated with sex differences in vertebrates is the neural production of estradiol, which can shape long-term gene expression in the brain (4, 25, 30–32). In humans and songbirds, estrogens are locally produced within the auditory cortex, including at synaptic terminals (33–35). Evidence for sex differences in estradiol production and action in the songbird NCM is mixed. The abundance of aromatase-positive neurons in NCM is similar in male and female zebra finches; however, notably, males have elevated aromatase expression and enzymatic activity within presynaptic terminals (34, 36), as well as higher-density clustering of aromatase cell bodies (37). In vivo, local NCM estradiol is acutely elevated when birds hear conspecific song (38, 39), and exogenous estradiol increases auditory neuronal responsiveness (8, 39) in a similar manner in adult males and females. However, brain-derived estradiol fluctuations differ between the sexes in juveniles (40), as well as in response to visual inputs (38). Against this backdrop, one current route to clarify the relationship between sex differences in brain aromatase and rapid neuroestrogen signaling is to examine the sex-specific regulation of estrogen receptors (ERs) and signaling pathways (4).
Classical nuclear receptor agonists for ERα and ERβ do not mimic rapid estradiol actions in NCM of males (41), suggesting that an alternative receptor may mediate these fast changes in auditory responsiveness. The membrane-bound G-protein-coupled ER1 (GPER1) can mediate rapid estrogen signaling in other species (42) and is expressed in mammalian, avian, and teleost brain (43–46). In mammals, rapid estrogen signaling is mediated by GPER1 in both the hippocampus (47–50) and striatum (44), and the acute actions of estrogens on hippocampal synaptic transmission are sex specific, mediated, in part, by GPER1 (51). Therefore, GPER1 may have an essential function in zebra finch auditory processing. Previous studies show that GPER1 is widely expressed throughout the auditory forebrain, and sex differences in expression appear during key development milestones in zebra finch song learning (46). Despite these insights, GPER1 activation has never been tested in a sensory context in any system. The exploration of this question in songbirds offers the opportunity to study how GPER1 regulates the auditory firing properties of individual neurons in response to ethologically relevant stimuli.
For these reasons, we tested two primary hypotheses: that (1) the response properties of single NCM neurons differ between males and females, and (2) auditory processing and coding are regulated by GPER1 at the level of single neurons in NCM. We report sex differences in auditory processing and information coding that are cell-type specific. We further show that GPER1 is necessary to maintain this sex difference but that activation of GPER1 alone does not mimic the actions of estradiol.
Materials and Methods
Animals, study designs, and drugs
Adult (>120 days post-hatch) male and female zebra finches were housed in single-sex cages in flight aviaries with food and water available ad libidum (14-hour day/10-hour night). All animals were gonadally intact. Protocols for animal care and use were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts. Males (n = 27) and females (n = 27) were collected across four electrophysiological studies that had the same within-subject design [artificial cerebrospinal fluid (aCSF), drug, aCSF]. To examine potential sex differences in firing at predrug conditions, results from the first aCSF trial were pooled across all four studies (n = 27 males and 27 females each). The three drug-treatment studies with antagonist G36 (100 µM; males n = 5, females n = 5) and agonist G1 at two doses (low dose 100 nM: males n = 10, females n = 8; high dose 100 µM: males n = 5, females n = 6) include all data from pre-drug, drug, and post-drug trials. To determine drug selection and doses, we relied on a mixture of in vitro cell and in vivo animal work to inform our choices. GPER1 is a highly conserved protein, and zebra finch GPER1 is 82.5% homologous with the human form of the receptor (Basic Local Alignment Search Tool), and there is also 83.6% homology of the binding motifs of human and zebra finch GPER1 for both G1 and G36 [UniProt sequence reported in Mendez-Luna et al. (52)]. G36 is a more specific antagonist than another known antagonist, G15, at higher doses, as it has a bulky isopropyl moiety similar to G1 and thus, is less likely to bind to ERα and ERβ (53), so we selected G36 as the antagonist. For G1, there is a known in vitro limit to specificity of G1 (as opposed to G36), so we selected two doses that represent a high dose (100 µM), which can elicit nonspecific estrogen binding to other ERs in vitro but is comparable with doses in other bird studies that measure behavioral outcomes [∼60 µM in quail third ventricle (54, 55)], as well as mammals (∼19 µM) (47), and a low dose, which is within the specificity range (100 nM) (53). One study has demonstrated agonism of G1 and antagonism of G15—another GPER1 antagonist—in the zebra finch when administered through a silastic capsule dorsal to the hippocampus (55). Additional animals (males n = 7, females n = 6) were added from Trial 1 aCSF-only recordings for a larger comparison across studies. A separate set of males (n = 7) and females (n = 6) were collected from the same aviaries for the immunofluorescence study.
Surgery
We used protocols adapted from previously published methods (8, 9, 39, 56, 57). Animals underwent stereotaxic surgery to affix headposts and draw markings on the skull for NCM coordinates. Animals were removed from the larger aviary just before surgery and were isolated from food for ∼20 minutes to prevent aspiration during anesthesia. Based on weight, 35 to 45 µL equithesin was injected into the pectoralis muscle. Approximately 20 minutes following injection, birds were affixed to a stereotax at a 50° head angle.
A local lidocaine injection (10 to 20 µL, 2% in ethanol; Sigma-Aldrich) was subcutaneously administered under the scalp, and the skull was exposed. The bifurcation of the midsagittal sinus (“zero point”) was identified by cutting a small window into the upper leaflet of the skull. From here, hatched marks were made in the upper leaflet of the skull over the NCM coordinates (rostral 0.8 mm; lateral 0.8 mm) from the zero point on both left and right hemispheres. A small hole was also made in the upper skull leaflet above the cerebellum, and a silver wire was inserted for grounding during physiology recordings and secured using cyanoacrylate. The scalp was resealed using cyanoacrylate, and dental cement secured the headpost. Birds recovered from anesthesia within 4 hours and were placed in an isolation chamber, housed with at least one other bird for 1 to 2 days until recording.
Electrophysiology and retrodialysis
We performed anesthetized in vivo, single-electrode electrophysiology on our subjects with a retrodialysis probe implanted in NCM. Previous studies testing neuromodulation of auditory neurons in songbirds have used this method to achieve long-term (2 to 3 hours), stable recording sites alongside acute vehicle, drug, and washout administration with retrodialysis (8, 9, 39, 57). There is a strong effect of habituation on repeated presentations of the same song in awake songbirds (58), which is not present in anesthetized birds (8, 39). As we wanted to test the same songs presented across drugs trials, we selected anesthetized preparation to avoid this confound. We have also previously demonstrated estradiol enhancement of auditory responsiveness to song in urethane-anesthetized animals. We used urethane, which has nonspecific effects on multiple neurotransmitter systems (59). Before recordings, each subject was administered 20% urethane over the course of 2 to 2.5 hours in at least three doses at 30 µL each. Total volume of anesthesia ranged from 90 to 120 µL, depending on the size of the bird. Birds were then secured by the headpost to a custom stereotax (Herb Adams Engineering) at a fixed 50° angle. Each subject was kept on a heating pad to maintain body temperature (DC Neurocraft). All experiments took place inside of a sound-attenuation booth (Industrial Acoustics) on an air table (TMC).
The lower skull leaflet and dura were dissected away from the marked exposure. The left NCM was always exposed first, and most subjects had recordings from the left hemisphere (50/54 recordings). If recordings were unsuccessful in the left hemisphere, then the right hemisphere was exposed (4/54 recordings). Right-hemisphere recordings were not systematically different from left-hemisphere recordings for auditory responsiveness and were therefore pooled in our analyses. A prefilled microdialysis probe (CMA 7 with 1 mm membrane; microdialysis probe; CMA Microdialysis) was inserted caudal to the hatched markings and descended ∼1.5 mm ventral into NCM. The flow rate of aCSF was 2 μL/min. After 30 minutes of delay from implantation, a single carbon fiber electrode (Carbostar-1; Kation Scientific) was descended immediately caudal (∼200 to 500 µm) to the probe to search for auditory sites. Sites were selected between ∼0.8 and 1.5 mm ventral from the surface. An auditory site was selected by online confirmation that peristimulus time histograms displayed a positive auditory response to auditory-playback stimuli. All recordings were amplified, bandpass filtered (300 to 5000 Hz; A-M Systems), and digitized at 20 kHz (Micro 1401, Spike2 software; Cambridge Electronic Design). Before recordings, song files for auditory playback were normalized to ∼70 decibels. All multitrial experiments use the same recording site and isolated single units for the entirety of the experiment.
Once the site was selected, Trial 1 of the experiments began. Trial 1 consisted of 30 minutes of retrodiaylsis of aCSF, followed by 15 minutes of continuous retrodialysis of aCSF with concurrent playback of song stimuli. Stimuli were three distinct male conspecific songs selected from recordings of birds from the colony at least 3 years before (i.e., outside of age ranges for our subjects) and a white noise sound file totaling four sound files. Each sound file was played back for 20 repetitions pseudorandomly at an interstimulus interval of 10 ± 2 seconds, totaling ∼15 minutes of stimuli exposure. After the end of Trial 1, the aCSF was switched for the drug trial [100 µM G36 (Azano Scientific); 100 µM or 100 nM G1 (Azano Scientific); stock made in dimethyl sulfoxide and then diluted to concentrations in aCSF for a final dimethyl sulfoxide concentration of 0.5%]. Trial 2 followed the same time course and playback. Trial 3 consisted of a washout with aCSF for the same time course. Following Trial 3 washout, recording sites were lesioned (10 µA for 10 seconds) for confirmation of electrode placement.
Histology
Birds were decapitated immediately following the lesion. Brains were extracted and stored in 20% sucrose/10% formalin solution in 4°C. After fixation, brains were frozen in optimal cutting temperature and stored at −80°C until sectioning. We sectioned at 45 µm using a cryostat (Leica CM 3050S) and mounted onto Fisher superfrost slides. We performed a Nissl stain using 0.25% thionin, and slides were then dehydrated through a series of ethanol washes, followed by Hemo D, and coverslipped using Permount (Fisher Scientific). Sections were visualized under a brightfield microscope (Zeiss Axio Laboratory.A1) to confirm probe-site implantation into NCM. Lesion sites were difficult to confirm as a result of the lack of gliosis. However, all recording sites were confirmed online to exhibit highly phasic, bursting response properties that are characteristic of NCM neurons, and all probes were histologically confirmed to be within NCM.
Auditory responsiveness
Recordings were processed in Spike2 (version 7.04) software. First-pass analysis consisted of measurement of auditory responsiveness in multiunit recordings. Thresholds were set at least twofold above the noise band for rastering multiunit activity. Single units were isolated by sorting spikes, based on waveform shape in Spike2, with thresholds twofold above the noise band (n = 116). A principal component analysis (PCA) confirmed isolated units, and we obtained two to three units from each subject (Fig. 1). All single units included in our analysis had an interspike interval > 1 ms. We performed an unbiased categorization solely on the PCA to isolate the most distinct waveforms (Fig. 1A, right). We ran paired t tests for each unit between spontaneous and stimulus-evoked firing frequencies across all stimulus presentations to determine whether units were statistically responsive (P < 0.05) to auditory stimuli for any of the treatment trials. One unit in the G1 low-dose study did not meet this criterion and was removed from our analyses.
Figure 1.
Characteristics of broad and narrow cells. (A, left) Waveform averages for two example units from the same recording site. The purple unit is a narrow cell, and the red unit is a broad cell. The center line indicates the mean waveform, whereas the shaded area represents the standard deviation (SD) for all incidences of that shape in the recording. (A, right) An example of a PCA for the red and purple cells of that recording. (B) Histogram of quarter-spike width durations (in milliseconds). A dip in the histogram at 0.5 ms was used as an indicator of cutoff between narrow- and broad-unit classification. (C) Latency (in milliseconds) to fire after (left) the first syllable, and stimulus-evoked firing frequency are depicted as means and standard error of the mean. Gray bars are for broad units and white are narrow units. Samples sizes for each group are depicted for each bar; *P < 0.05.
For rasterized multiunit and single-unit spike trains, several parameters were measured. Peristimulus histograms were aligned with each sound stimulus, measuring 2 seconds preceding the stimulus for spontaneous activity and 2 seconds after stimulus onset for stimulus-evoked activity. Firing frequency (in hertz) was measured for both multiunit and single-unit firing during spontaneous and stimulus-evoked periods. Auditory responsiveness is represented by the z score as follows:
where Mean(S) is the mean number of spikes during the stimulus presentation, Mean(B) is the mean number of spikes during the spontaneous firing period, Var(S) is the variance of the stimulus-evoked activity period, Var(B) is the variance of the spontaneous activity, and Cov(S,B) is their covariance. We were principally interested in neuromodulation of song-evoked responses rather than differential representation of individual song subtypes. Our descriptive means did not demonstrate systematic differences among the three conspecific song types, so we averaged firing frequency and z scores per unit across the three conspecific songs presented for each unit. White-noise response had a lower firing frequency and z score, so it was not included in the average.
Response latency for each unit was computed, as described by Ono et al. (60). In brief, for each stimulus (20 trials each) 5 ms-binned peristimulus time histograms were generated and smoothed with a five-point boxcar filter. The means and standard deviation (SD) of the spontaneous firing rate of 100 ms, preceding the stimulus onset, were computed. The latency to fire was defined as the midpoint of the first bin from 0 to 400 ms after stimulus onset when the firing rate exceeded the means + three times the SD of the spontaneous period. If the response did not exceed the threshold within the first 400 ms of stimulus presentation, then latency was not calculated for that stimulus; this contingency only occurred in three units. For conspecific songs, latency was measured relative to the onset of the first nonintroductory note. For each cell, the latencies to fire for each stimulus were averaged (Fig. 1C, left).
Auditory coding
We used a custom pattern classifier coded in Python, based on a similar classifier, originally described by Caras et al. (61) to determine how auditory-evoked events predict stimuli discrimination in our population of neurons [for similar methods in NCM, see Lee et al. (62) as well]. For each single unit, the classifier pseudorandomly selected one response to each stimulus to serve as templates. The remaining recordings (19 trials per stimulus = 76) were compared with the four templates and categorized based on two types of similarity measures: count and timing. This procedure was repeated 1000 times, and a mean accuracy score was generated for each stimulus. Spike-count accuracy was calculated by using the number of spikes within the 2 seconds of song presentation. The stimulus type of the template that yielded the most similar number of spikes to the trial in each comparison was considered the predicted stimulus. Ties were resolved pseudorandomly. We also performed a binned-count accuracy, as described in Lee et al. (62), but did not find that this analysis significantly altered effects observed in any of the neuromodulatory experiments (data not shown). Spike-timing accuracy was determined in a similar manner, but the binary signals were convolved with Gaussian filters before comparison. We systematically varied the SD (σ) of the filter for each cell to determine the optimal integration window (values used: 1, 2, 4, 8, 16, 32, 64, 128, and 256 ms). The filter that yielded the highest accuracy was selected for each cell (Fig. 1A; all cells had a σ of 16 ms, with the exception of four cells that had a σ of either 1 or 2 ms). Templates and trials were compared using the Rcorr method [Caras et al. (61)] as follows:
Where represents the vectors of the trial and the template responses after filtering, which are dot multiplied and then divided by the product of their lengths. This calculation returns a value between zero and one, which represents a range from total dissimilarity to total similarity, respectively. For figures representing accuracy coding, these values are depicted as 0% to 100%. The stimulus type of the template that provided the highest Rcorr (trial, template) value was considered the predicted stimulus for the trial in analysis. A confusion matrix was generated for each unit that illustrates the percentage of accuracy for each predicted and observed song type. All timing and count accuracy data presented here are an average of the correctly assigned percentages across all sound types [three conspecific songs (CONs; CON1, CON2, CON3) and white noise (WN)] to represent an overall accuracy score. Excluding responses to WN, the overall accuracy score did not result in changes to the effects reported below, so all classifier data presented below include responses to WN.
After the generation of the confusion matrices, the accuracies of each unit were statistically tested by use of a trial-shuffling approach [modified from Caras et al. (61)]. In brief, responses were shuffled and randomly assigned to stimuli. The classifier ran 1000 times using the random responses as input (timing classifiers were run 1000 times for each σ value mentioned previously). Finally, the distribution of original accuracies was compared with the distribution of the random accuracies. As sample sizes were large (1000 × 1000), we opted to use the Cohen d as an indicator of effect size as follows:, where denotes the means of the distributions, and is the pooled SD. Accuracies were considered significantly greater than random when Cohen d was >0.2, which is indicative of a small, significant positive effect size (63). Similar to Caras et al. (61), single units were categorized as timing, count, bicoding, or neither, based on the effect size threshold (Cohen d > 0.2; i.e., if a unit was significant for both timing and count, then it was categorized as a “bicoding” cell; if a unit was significant for neither timing nor count, then it was categorized as a “neither” cell).
Spike width
We measured spike quarter-width duration for each single unit (averaged across all firing incidents of the first trial), which is the duration of the waveform at 25% of the highest absolute peak value. Quarter-width duration of the first largest peak [spike quarter width (SQW); both positive and negative deflection] was the most reliable measurement for the diversity of waveform shapes (Fig. 1A) and has been validated in zebra finch high vocal center to assign “broad” units as projection neurons and “narrow” units as interneurons (64–66). The resultant distribution of SQW was bimodal with a dip at ∼0.50 ms (Fig. 1B). However, recent work, especially in NCM, has also used spike peak-to-peak duration to classify cell types, so therefore, we performed peak-to-peak measurements as well. Although a consensus about NCM categorizations is still emerging, two characteristics have been generally consistent with broad vs narrow categories in recent studies: broad cells consistently have a lower firing frequency and a higher latency to fire after stimulus onset (60, 67, 68). When we examined our cells using the peak-to-peak classification with a cutoff between broad and narrow at 0.4-ms duration, we observed that broad peak-to-peak units could be classified either with a bimodal shape (two clear high and low peaks) or with a strong unipolar peak and a weak afterhyperpolarization event. Unipolar broad cells had longer durations than previous cutoffs (∼0.75 ms) (60). The peak-to-peak classification scheme did not concomitantly segregate cell types based on latency or firing rates, as has been previously reported. By contrast, the division of our population of recorded cells using SQW with a 0.5-ms cutoff clearly distinguished waveform latency [F(1,109) = 6.59, P = 0.012; Fig. 1C, left] and spontaneous firing frequency [F(1,112) = 5.95, P = 0.016; data not shown], where the unipolar broad neurons had a higher latency to fire and higher spontaneous firing frequency. Stimulus-evoked firing frequency was not different between cell types [F(1,112) = 0.079, P = 0.78; Fig. 1C, right]. Because of the consistency of categorizing based on shape and the physiological parameters of latency and firing frequency between both types of classification (peak to peak vs quarter width), we proceeded with SQW for broad- vs narrow-unit classification. Prior work has presented these classifications as putative projection neurons (broad) and putative interneurons (narrow). Our latency data support this hypothesis, but our firing frequency results do not. Moreover, the direction of the spontaneous firing difference is not consistent with previous observations (broad > narrow), and stimulus-evoked firing was similar between broad and narrow neurons. Therefore, we restrict our interpretation of broad vs narrow units as distinct neuronal subtypes but do not make inferences about their putative identities as excitatory/projection neurons vs inhibitory/interneurons.
Immunofluorescence
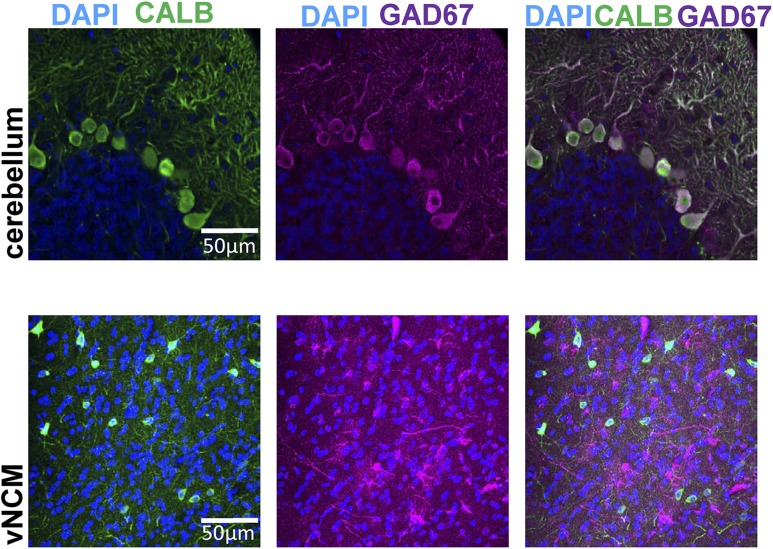
We isolated males (n = 7) and females (n = 7) from single-sexed aviaries for ∼12 to 24 hours before collection. We exposed birds to song before euthanasia for another study not relevant to these analyses. We perfused animals with ice-cold saline and then 4% paraformaldehyde, followed by an overnight fix in paraformaldehyde, and sinking in 30% sucrose in 0.1 M phosphate buffer (PB). We sectioned the brains at 35 µm into cryoprotectant solution. We were interested in double labeling for GPER1 and markers for inhibitory neurons. All antibodies were first confirmed to be specific to zebra finch tissue by running a series of sections that removed one of the primary or secondary antibodies in all of the double-label combinations that we tested. In no case, when we omitted the primary antibody, did we observe cellular staining. Pilot studies indicated that there was no coexpression of GPER1 in any brain regions with specific interneuron markers [parvalbumin, 1:10,000, Research Resource Identifier (RRID): AB_2174013; Millipore] and calbindin (CALB; 1:2000, RRID: AB_476894; Sigma-Aldrich; data not shown). Therefore, we used a polyclonal antibody against glutamate decarboxylase with molecular weight of 67 (GAD67), the enzyme that synthesizes GABA, is expressed in cell bodies of rat and humans (69, 70), and has two analogs, GAD65 and GAD67, that are found across vertebrates (71). We determined from the manufacturer and verified with our own control experiment that this antibody only marks a subpopulation of GABAergic cells [i.e., coexpressed with CALB Purkinje neurons in the cerebellum but not CALB cells in the telencephalon (see Fig. 7)]. CALB has been previously verified as GABAergic in zebra finch (72), so this marker is not representative of all GABAergic cells but rather, a subtype. There was no blocking peptide available for preabsorption control. For our GPER1 antibody, one study has used this antibody, which is targeted to the human GPER1 protein in the zebra finch and confirmed with Western blot and preabsorption control (46). The antibody targets the extracellular domain of the GPER1 protein located between amino acids 175 and 225 (Uniprot: NP_001496.1), which is a domain of the receptor that is 82% homologous between human (Uniprot Q99527) and zebra finch (Uniprot H0ZDD2). Sections were transferred from cryoprotectant and went through a series of 0.1 M PB washes. Sections were blocked with 10% normal goat serum for 2 hours and then incubated in anti-GPER1, raised in rabbit (catalog no. MC-4268; RRID: AB_591551; MBL International), at a 1:2000 dilution, which previously has been validated in zebra finches (46) and anti-GAD67 raised in chicken (catalog no. ab75712; RRID: AB_1310248; Abcam) at 1:100 for 48 hours at 4°C. Sections were then washed in a 0.1% Triton X-100 PB, followed by an incubation in 1:200 goat anti-rabbit Alexa 488 secondary antibody (RRID: AB_2576217; Life Technologies) and 1:200 goat anti-chicken Alexa 594 (RRID: AB_142803; Life Technologies). After a final wash in 0.1% Triton X-100 PB, sections were mounted on slides with Prolong Diamond with 4′,6-diamidino-2-phenylindole (DAPI) mounting media (Invitrogen). Images were taken of NCM using a Nikon A1SP confocal microscope at ×10 and ×60. z-Stacks (15 µm) as a projection image at ×60 to illustrate antibody staining of GPER1 and relative expression level within the regions that were recorded from using electrophysiology is depicted (see Fig. 3). All GPER1+ and GAD67+ cell counts in ×60 z-stack images of the dorsal NCM (dNCM) and ventral NCM (vNCM) were scored by an experimenter blinded to treatment conditions, and all quantified counts are normalized to DAPI.
Figure 7.
Anti-GAD67 targets some GABAergic neurons but not all. Images are (upper) a ×60 original magnification section of the cerebellum and (lower) a ×60 original magnification z-stack maximal projection from vNCM. DAPI (blue), CALB (green), and GAD67 (magenta) are shown. Notably, there is complete coexpression between CALB and GAD67 in the Purkinje cell bodies of the cerebellum, known GABAergic neurons, but no coexpression in vNCM. This depicts that anti-GAD67 is only targeting a subset of GABAergic neurons that in the NCM are distinct from other GABAergic subtypes, such as those labeled with CALB antibodies.
Figure 3.
Representative images of GPER1 expression in dNCM and vNCM. GPER1 is expressed in regions that are targeted for recording. (Left) Original magnification (×10), 3 × 3 stitch of medial section of forebrain (sagittal plane). Boxes indicate regions from which subsequent images were taken. (Middle and right) Each image was taken at ×60 original magnification within respective NCM regions. Images are z-stack maximum projections with 15-µm thickness.
Data analysis
All statistical analyses were performed using SPSS Statistics 22 and Origin 2017 (IBM). For sex comparisons across all studies during Trial 1 aCSF, between-subject analyses of variance (ANOVAs) were performed as well as sex-by–cell type comparisons and Student t test for sex comparisons of multiunit-dependent measures. For neuromodulation studies (G36 and G1), a three-way mixed-factors ANOVA was performed initially for each study. We had power to detect this three-way interaction for auditory responsiveness in the G36 study, so we performed two-way ANOVAs for trial by cell-type fixing the factor sex for all analyses to determine cell type–specific effects within each sex. We were underpowered in other incidences to detect the three-way interaction; however, for other dependent measures in the G36 study (timing accuracy, count accuracy, firing rate), we performed the same two-way ANOVAs and detected significant two-way interactions. In the two-way ANOVAs, when a significant interaction for cell type and interaction was detected, we performed one-way ANOVAs, fixing the factor cell type to test whether each cell type was significantly altered by drug administration. If a significant treatment effect was detected in the one-way analysis, we then performed Tukey honest significant difference post hoc analyses on the within-subject groups (pre-drug vs drug). When comparing magnitude of effects, Cohen d was used for significant post hoc comparisons. For categorization of cells as bicoding, timing, count, or neither, a χ2 analysis was performed on all cells, based on sex or spike-width categorization. Anatomical comparisons were performed by mixed-effects two-way ANOVAs. Significance is reported as P < 0.05. Trends are noted as P < 0.10.
Results
NCM has both sex and cell-type differences in firing frequency and auditory responses
We first examined firing parameters in NCM for potential sex differences under aCSF (i.e., nondrug) conditions. In our analysis of multiunit data, we found that males and females had similar spontaneous firing rates [male 11.49 ± 1.44 and female 9.37 ± 1.21; t(52) = 1.13, P = 0.27], stimulus-evoked firing rates [male 26.05 ± 3.03 and female 21.33 ± 2.4; t(52) = 1.22, P = 0.23], auditory responsiveness [z score; male 0.40 ± 0.043 and female 0.46 ± 0.053; t(52) = 0.68, P = 0.50], timing accuracy [male 0.75 ± 0.033 and female 0.66 ± 0.03; t(51) = 1.83, P = 0.073], and count accuracy [male 0.44 ± 0.022 and female 0.41 ± 0.023; t(51) = 0.74, P = 0.46; data not shown]. In contrast, when we examined data from our population of isolated single NCM units, sex differences emerged (Figs. 1C and 2).
Figure 2.
The firing rates of NCM neurons differ between males and females, and the coding properties of NCM neurons differ by cell-type classification. All panels depict bar graphs representing means and standard error of the mean for the average of all three conspecific song types. (A) Stimulus-evoked firing frequency (song) is higher than baseline firing frequency (no song) for both sexes. Female cells (n = 59) have a higher firing frequency than male cells (n = 57) for both spontaneous and stimulus-evoked conditions. (B) Male narrow single units (n = 25) have a higher normalized auditory responsiveness (z score) than broad units (n = 32). Female broad (n = 37) and narrow (n = 22) units have similar auditory responsiveness. (C) Percentage of cells that are categorized as four coding types: bicoding (purple), count (red), timing (blue), and neither (white), separated by (upper) sex or (lower) cell type. Males and females have a similar distribution of coding types, but broad vs narrow units have significantly different distribution of coding types (χ2 = 12.2, P = 0.007). Specifically, narrow-spiking cells have more bicoding cells (85.1%) than broad-spiking cells (56.5%). (D and E) Means and standard errors for the average (D) timing accuracy and (E) count accuracy of the correctly assigned sound types. Broad cells (gray) have lower count and timing accuracy than narrow cells (white) for both males and females. Confusion matrices above each bar depict a representative cell of the mean for that group. Colors on confusion matrix are a heat map of accuracy from 0% to 100%. Gray, dotted lines represent chance-level decoding accuracy (25%); #P = 0.07; **P < 0.01; ***P < 0.001. 1, conspecific song 1; 2, conspecific song 2; 3, conspecific song 3; O, observed; P, predicted; W, white noise.
For single units in NCM, neurons in females had an overall higher firing frequency than did males [F(1,112) = 5.88, P = 0.017; Fig. 2A]. For stimulus-evoked firing NCM, neurons in females also had a higher firing frequency [F(1,112) = 6.6, P = 0.01; Fig. 2A] and a trend for spontaneous firing [F(1,112) = 3.34, P = 0.07; Fig. 2A]. Despite differences in firing frequency, both sexes were similar in overall normalized auditory responsiveness [z score; F(1,112) = 1.86, P = 0.18; data not shown]. We next evaluated the cell type–dependent auditory response properties in males and females. Narrow-spiking (NS) cells had a higher auditory responsiveness than broad-spiking (BS) cells [F(1,112) = 7.16, P = 0.009; data not shown], and there was a trending interaction between sex and cell type for z score [F(1,112) = 2.84, P = 0.09], although with low statistical power (0.39). Therefore, we evaluated the cell type–dependent auditory response properties in individual tests fixing the factor sex to determine if the cell-type main effect was driven by one sex. This analysis revealed that NS cells in males were more responsive than BS cells [t(55) = −3.58, P = 0.001] and also that NS and BS cells in females were similarly responsive [t(57) = −0.63, P = 0.53; Fig. 2B]. Therefore, NCM neurons differed between males and females in terms of firing rates, as well as the auditory response properties of NS vs BS neurons.
Males and females did not differ in how accurately NCM neurons represent individual stimuli via rate-based coding [count; F(1,112) = 0.18, P = 0.67] or for timing coding [timing; F(1,112) = 1.85, P = 0.18]. For both count and timing accuracy, narrow cells were more accurate than broad cells in both sexes [count: F(1,112) = 22.66, P < 0.001; timing: F(1,112) = 40.0, P < 0.001; Fig. 2D and 2E). Timing accuracy was higher than count accuracy [F(1,112) = 49.72, P < 0.001] for both sexes and cell types.
Cells were categorized into four groups, based on the results of the trial-shuffling analysis (see Materials and Methods): bicoding (both timing and count), timing, count, and neither, similar to Caras et al., (61). Cells that code for stimulus types using timing accuracy (timing and bicoding cells) made up 93.1% of all cells collected. Timing-only cells were 25% of the population, and count-only cells were 1.7%. Bicoding cells were the majority of cells, with 68.1%. Males and females did not significantly differ in the proportion of stimulus coding types (χ2 = 1.56, P = 0.67; Fig. 2C). The combination of both sexes (NS vs BS cells) significantly differed in the proportion of cell classification types (χ2 = 12.2, P = 0.007; Fig. 2C). This effect was likely driven by the proportion of bicoding cells that constituted 85.1% of all NS cells but only 56.5% of all BS cells. In summary, NCM cells for both sexes preferentially used timing as opposed to count-information coding, meaning that the timing of individual action potentials during the auditory stimulus provided more consistent and reliable coding for that stimulus type compared with a purely rate-based code (count). NS cells, in particular, used both kinds of information (bicoding) in greater proportion than did BS cells.
GPER1 is necessary for auditory responsiveness and coding in a sex- and cell type–specific manner
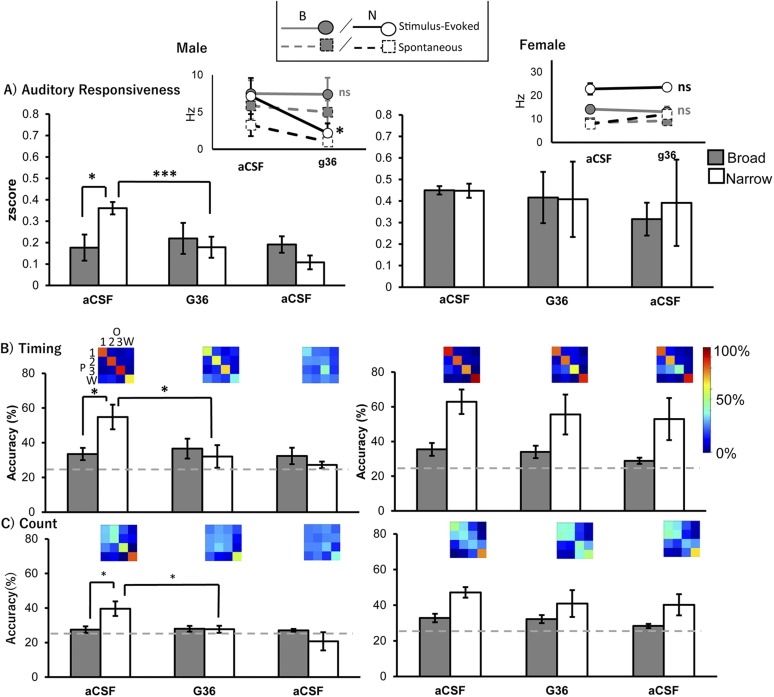
GPER1 expression occurs in NCM (46), and conventional nuclear ER agonists do not mimic the rapid actions of estrogens on NCM auditory processing (41). We confirmed the expression of GPER1 in dNCM and vNCM using immunofluorescence (Fig. 3). We then tested the extent to which GPER1 activation is necessary for sex differences in auditory responsiveness and coding by administration of G36, a GPER1 antagonist, via retrodialysis, coupled to extracellular recordings in adult males and females. For auditory responsiveness in males, we detected a significant effect of treatment [F(2,20) = 7.32, P = 0.004] and a treatment by cell type interaction [F(2,20) = 10.59, P = 0.001; Fig. 4A, left]. NS cells in males showed a G36-dependent decrease in auditory responsiveness [F(2,8) = 23.28, P < 0.001; pre vs G36, t(8) = 6.74, P = 0.0036; Fig. 4A, left], but BS cells were unaffected [F(2,12) = 0.471, P = 0.64; Fig. 4A, left]. In females, we did not detect a significant effect of treatment [F(2,20) = 0.003, P = 0.997; Fig. 4A, right] or treatment by cell-type interaction [F(2,20) = 1.25, P = 0.31; Fig. 4A, right]. During the pre-aCSF condition, NS cells had a higher z score than BS cells in males [t(8.353) = −2.743, P = 0.024] but not in females [t(10) = −0.755, P = 0.468], reconfirming the sex difference reported in our overall analyses above.
Figure 4.
Auditory responsiveness and coding are suppressed during GPER1 inactivation in narrow cells in the NCM of males only. (A, Left) Male auditory responsiveness (z score) with broad cells (gray, n = 7) and narrow cells (white, n = 5). (A, Inset) Firing frequency (in hertz) of single units for males for aCSF and G36 trials. Dashed lines are spontaneous activity (no song), and solid lines are stimulus-evoked activity (songs). Gray lines are broad cells, and black lines are narrow cells. There is not a significant difference in firing frequency between the pre-aCSF and G36 for broad cells, but there is a significant difference for narrow cells in stimulus-evoked firing. (Right) Female auditory responsiveness (z score) with broad cells (gray, n = 8) and narrow cells (white, n = 4). (Inset) Firing frequency (in hertz) of single units for females for aCSF and G36 trials. Dashed lines are spontaneous activity (no song), and solid lines are stimulus-evoked activity (songs). Gray lines are broad cells, and black lines are narrow cells. (B and C) Bar graphs for (B) timing accuracy and (C) count accuracy for broad (gray) and narrow (white) units in (left) males and (right) females. Confusion matrices are representative examples of the means of the narrow single units only. Colors on the confusion matrix are a heat map of accuracy from 0% to 100%. Male broad (n = 7), male narrow (n = 5), female broad (n = 8), and female narrow (n = 4). Bar graphs depict means and standard errors. Gray, dotted lines represents chance-level decoding accuracy (25%); *P < 0.05; **P < 0.01; ***P < 0.001. 1, conspecific song 1; 2, conspecific song 2; 3, conspecific song 3; B, broad cell; N, narrow cell; ns, not significant; O, observed; P, predicted; W, white noise.
We next sought to determine whether the GPER1-dependent decrease in auditory responsiveness could be explained by changes in firing frequency in both the spontaneous period and stimulus-evoked period. We found a significant interaction between firing frequency and sex [F(1,40) = 9.48, P = 0.006] and a trend for firing frequency and cell-type interaction [F(1,40) = 3.31, P = 0.08]. We fixed our factor for sex and examined how cell types within sexes exhibited changes in firing rate with drug administration for spontaneous and stimulus-evoked firing frequencies. GPER1 inactivation significantly decreased stimulus-evoked firing in male NS cells [stimulus-evoked main effect: F(2,8) = 9.78, P = 0.0071, pre vs G36, t(8) = 4.93, P = 0.020 (Fig. 4A, left inset); spontaneous main effect: F(2,8) = 5.76, P = 0.028, pairwise pre vs G36, t(8) = 4.01, P = 0.052 (Fig. 4A, left inset)]. All other comparisons were not significant [male BS spontaneous: F(2,12) = 1.36, P = 0.29, male BS stimulus evoked: F(2,12) = 1.27, P = 0.32 (Fig. 4A, left inset); female BS spontaneous: F(2,14) = 0.87, P = 0.44, female BS stimulus evoked: F(2,14) = 0.44, P = 0.65, female NS spontaneous: F(2,6) = 0.44, P = 0.66, female NS stimulus evoked: F(2,6) = 0.19, P = 0.83 (Fig. 4A, right inset)].
Therefore, we reasoned that GPER1 inactivation should also change the coding properties of NCM single units, sex specifically. Accordingly, we found that in males, both count and timing accuracy showed a significant effect of G36 treatment [count: F(2,18) = 18.68, P < 0.001; timing: F(2,18) = 14.27, P < 0.001] and a significant cell type by treatment interaction [count: F(2,18) = 18.31, P < 0.001 (Fig. 4C, left); timing: F(2,18) = 14.59, P < 0.001 (Fig. 4B, left)]. NS neurons in males exhibited a G36-dependent decrease in both count and timing accuracy [count: F(2,6) = 15.20, P = 0.0045, pre vs G36, t(6) = 6.18, P = 0.011 (Fig. 4C); timing: F(2,6) = 11.09, P = 0.010, pre vs G36, t(6) =4.84, P = 0.033 (Fig. 4B, left)] during GPER1 inactivation; however, there was not a detectable effect in BS cells [count: F(2,12) = 0.26, P = 0.78 (Fig. 4C, right); timing: F(2,12) = 1.90, P = 0.19 (Fig. 4B, right)]. In females, there was an overall treatment effect for count accuracy [count: F(2,20) = 3.89, P = 0.037] and a trending effect for timing [F(2,20) = 3.40, P = 0.054] but not a cell type–by-treatment interaction [count: F(2,20) = 0.95, P = 0.40; timing: F(2,20) = 0.42, P = 0.66]. Moreover, when examining pairwise comparisons for each trial for all female cells, we did not detect an effect of G36 application [count: pre vs g36, t(20) = 1.09, P = 0.53 (Fig. 4C, right); timing: pre vs g36, t(20) = 1.15, P = 0.70 (Fig. 4B, right)].
In summary, inactivation of GPER1 produced a rapid and robust decrease in firing frequency, z score, and coding accuracy, specifically in NS NCM cells in males alone. Together, these results are consistent with the hypothesis that acute membrane ER signaling is key to auditory responsiveness and coding in a specific population of NS neurons in the NCM of males.
GPER1 activation is not sufficient to enhance auditory responsiveness or coding accuracy of NCM neurons
To test whether GPER1 activation is sufficient to enhance auditory responsiveness in NCM, akin to native estradiol [e.g., Remage-Healey et al. (8, 73)], we tested two doses of the selective GPER1 agonist G1 (100 nM and 100 µM, referred to as low and high dose, respectively, later). The high dose did not alter single-unit auditory responsiveness [F(2,36) = 1.31, P = 0.28 (Fig. 5A); count accuracy F(2,38) = 0.006, P = 0.99 (data not shown)] or timing accuracy [F(2,38) = 1.61, P = 0.21 (data not shown)]. Additionally, there were no significant interactions for sex by treatment [z score: F(2,38) = 1.98, P = 0.15; count: F(2,38) = 2.00, P = 0.15; timing: F(2,38) = 0.004, P = 0.60] or cell type by treatment [z score: F(2,38) = 0.47, P = 0.63; count: F(2,38) = 0.091, P = 0.91; timing: F(2,38) = 0.64, P = 0.53] for the high dose. Notably, at high doses (>10 µM), G1 can have nonselective binding and weak antagonism (53), which may mask GPER1-specific effects. Therefore, we selected a lower dose of G1 (100 nM) in a new set of experiments to test GPER1 sufficiency. However, similar to the high dose, again, we observed that the low dose did not alter auditory responsiveness [F(2,66) = 3.14, P = 0.05; pre vs G1 low, t(66) = 0.44, P = 0.95; Fig. 5B], count accuracy [F(2,66) = 0.839, P = 0.44; Fig. 6B], or timing accuracy [F(2,66) = 1.98, P = 0.15; Fig. 6A]. There was also no significant sex-by-treatment interaction for the low dose [z score: F(2,66) = 2.42, P = 0.09; count: F(2,66) = 2.08, P = 0.13; timing: F(2,66) = 1.53, P = 0.23]. Cell type–by-treatment interactions in z score [F(2,66) = 2.70, P = 0.075] and count accuracy [F(2,66) = 0.18, P = 0.83] were also not significant; however, there was a significant interaction in timing accuracy [F(2,66) = 5.22, P = 0.007]. BS cells were unaffected by G1 [F(2,32) = 0.70, P = 0.50], but there was a trending treatment effect in NS cells [F(2,36) = 3.27, P = 0.051]. When we fixed the analysis by the factor sex, we observed a significant effect of G1 in males [F(2,22) = 3.66, P = 0.04; Fig. 6A, left] but not females [F(2,12) = 0.63, P = 0.55; Fig. 6A, right]. We note that the effect size for this G1 treatment of male narrow cells was smaller (Cohen d = 0.90) compared with the substantial effect size of G36 observed for male NS cells (Cohen d = 1.49). In summary, although we observed that GPER1 is key for both auditory responsiveness and coding stimulus information in NS neurons in male NCM, our experiments with G1 indicated that GPER1 activation alone is not sufficient to mimic the rapid effects of estradiol on NCM neurons in either sex.
Figure 5.
No changes in auditory responsiveness with GPER1 activation. (A) Individual single units depicted across the three treatment trials for the high dose of G1 (100 µM) for both (left) males and (right) females. Individual data are depicted because of the low number of male narrow cells (n = 2). (Insets) Descriptive bar graphs of means and standard errors for visual comparison with other figures. (B) Means and standard errors for the low dose of G1 (100 nM) for (left) males and (right) females. Bar graphs depict means and standard error of the mean of conspecific song auditory responsiveness. Broad cells are depicted by gray bars and narrow cells by white bars. High dose: male broad (n = 8), male narrow (n = 2), female broad (n = 8), and female narrow (n = 5). Low dose: male broad (n = 9), male narrow (n = 12), female broad (n = 11), and female narrow (n = 9).
Figure 6.
Only male narrow cells decrease in auditory coding accuracy with GPER1 activation. All bar graphs depict means and standard error of the mean for the average accuracy across all correctly assigned sound types for both broad (gray) and narrow (white) units. Confusion matrices are representative examples of the means of the narrow single units only. Colors on confusion matrices are a heat map of accuracy from 0% to 100%. Graphs depict the lower dose of the G1 (100 nM) experiment. (A) G1 application decreased timing accuracy in males. (B) Count accuracy was not affected for either sex or cell type by G1 application. Low dose: male broad (n = 9), male narrow (n = 12), female broad (n = 11), and female narrow (n = 9). Gray, dotted lines represent chance-level decoding accuracy (25%); *P < 0.05. 1, conspecific song 1; 2, conspecific song 2; 3, conspecific song 3; O, observed; P, predicted; W, white noise.
GPER1 is expressed in inhibitory neurons in NCM, but the expression is not sexually dimorphic
As we observed a sex-specific effect of GPER1 inactivation on auditory responsiveness and coding accuracy, we hypothesized that this could be a result of differences in GPER1 expression between male and female NCM. As G36 specifically modulated NS cells in males, and prior work has putatively identified NS cells as inhibitory interneurons, we performed colabeling immunofluorescence for GPER1 and the GABAergic neuronal marker, GAD67, which we show only marks a subset of inhibitory neurons (Fig. 7). First, we observed that vNCM had higher expression of GPER1 and GAD67 than dNCM [GPER1: F(1,10) = 9.32, P = 0.012; GAD67: F(1,10) = 5.18, P = 0.046]. Second, we observed that ∼20% of GAD67-positive cells in NCM were also positive for GPER1, consistent with the hypothesis that estrogens act, in part, to shape inhibition in NCM (Fig. 8) and that coexpression of GPER1 and GAD67 was similar between regions of vNCM and dNCM [F(1,10) = 0.32, P = 0.59]. Third, we also found that in both dNCM and vNCM, GPER1 expression and GAD67 expression were not different between the sexes [dNCM: GPER1: F(1,12) = 0.11, P = 0.75, GAD67: F(1,12) = 2.91, P = 0.12; vNCM: GPER1 F(1,11) = 0.21, P = 0.66, GAD67: F(1,11) = 0.19, P = 0.68]. Last, we also did not find sex differences in GPER1–GAD67-colabeled cells [percent of GAD67, dNCM: F(1,12) = 0.14, P = 0.71, vNCM: F(1,11) = 1.19, P = 0.30 (Fig. 8F); percent of GPER1, dNCM, male: 8.24 ± 1.97 and female: 7.97 ± 5.96, F(1,12) = 0.002, P = 0.96, vNCM, male: 17.71 ± 6.85 and female: 8.41 ± 3.04, F(1,11) = 1.54, P = 0.24 (data not shown)]. Together, these results indicate that GPER1 protein expression alone cannot explain sex differences observed in auditory physiology in NCM.
Figure 8.
GPER1 expression and colocalization in GABAergic neurons are each not sexually dimorphic. (A) Representative images of dNCM for labeling of GPER1 (green), GAD67 (magenta), and DAPI (blue). Each image was taken at ×60 original magnification within respective regions. Images are z-stack maximal projections with 15 µm thickness. Triangles point to examples of a cell that expresses both GPER1 and GAD67. Arrows point to examples of a single label. Dashed boxes indicate sections of the image that are presented in (B) and (C) that have been zoomed in and resized for colocalization clarity. (B) Zoomed-in images from the representative female dNCM in (A). Triangles point to a cell expressing both GAD67 and GPER1. (C) Zoomed-in images from the representative male dNCM in (A). Arrows point to a single label of a GPER1+ cell. (D and E) Means and standard error of the mean error bars for (left) GPER1-positive neurons and (right) GAD67-positive neurons as a percentage of DAPI. (F) Means and standard error of the mean error bars for GPER1/GAD67-positive neurons as a percentage of GAD67 cells. Red bars are females (dNCM n = 6; vNCM n = 6), and blue bars are males (dNCM n = 7; vNCM n = 6); *P < 0.05.
Discussion
We observed several sex differences in neurophysiological parameters in the songbird auditory forebrain. First, female single units identified from extracellular recordings had a higher firing frequency. Second, male single-unit auditory responsiveness was elevated in NS compared with BS cells. We also found that GPER1 inactivation caused decreases in auditory responsiveness and coding accuracy in male NS cells; however, it was not effective in either cell type in females. Furthermore, when responses to stimuli were computationally decoded, spike timing and count classification accuracies were higher in NS than BS in NCM for both sexes.
Our findings indicate that brain regions that are similar in morphology and cytoarchitecture between males and females can exhibit marked sex differences in the physiology of single neurons. Prior work on the songbird NCM and caudomedial mesopallium reported sex differences in auditory-evoked, immediate early-gene expression (10, 74, 75) and multiunit response magnitude (7). Other regions of the songbird auditory network also exhibit sex differences in single-neuron call responses and tone-amplitude coding (61, 76). Our work builds on this foundation to show that separate classes of NCM neurons (NS and BS) can respond to conspecific songs in a sex-specific way.
Male zebra finches sing learned vocalizations during courtship, and females do not ordinarily sing (77). Therefore, our report of sex differences in song responsiveness and coding in NCM could reflect divergent role(s) for song in auditory salience, valence, and life history in males vs females. Outside of the auditory system, sex differences in the single-unit representation of sensory stimuli have been most clearly delineated for sex-specific pheromonal responses in reptiles (78) and rodents (79). Interestingly, these differences are also associated with estrogen-dependent signaling mechanisms, such as those shown here (79, 80). Estradiol has long been implicated in its role for organizing sexually dimorphic brain regions, circuits, and behavior; however, more recently, its role as a rapid neuromodulator has become apparent. Although auditory processing is regulated by rapid estrogen signaling in both male and female zebra finches, our study indicates that such sensory brain regions that are under sex-specific experiential and evolutionary pressures may have sex differences in neuromodulation.
In addition to the sex differences described above, we examined the coding properties of NCM neurons. We used a pattern classifier (61, 62) to determine how NCM units discriminate among conspecific songs and whether this differed between the sexes and cell types. Despite sex differences in neuronal firing profiles, pattern-classifier coding was similarly accurate for recordings from male and female NCM. This result indicates that even though there are sex differences in neuron responsiveness to songs, these neuronal “strategies” do not impact the neuronal coding for discrimination among conspecific songs. Future work examining different categories of songs that may be more salient for one sex (i.e., tutor song, mate song, preferential male song, etc.) may uncover sex differences in coding properties in NCM. Our analysis also indicated that NCM cells, in general, were more temporally consistent when responding to the same conspecific song. When characterizing cells as count, timing, or bicoding, there was a strong bias toward cells that exhibited timing accuracy significantly above chance and not count accuracy alone. This was consistent with previous NCM recordings in males (62) and with Caras et al. (61), who found that both primary and secondary auditory regions showed a greater proportion of neurons that were categorized as timing or bicoding for tone stimuli. We now report cell type–specific differences in auditory coding of individual songs in NCM and that this feature is not different between males and females.
Prior studies of BS and NS in songbird auditory cortex have focused exclusively on males. BS cells contribute to background-invariant coding of vocalizations (67), selectivity to songs after tutoring (14), and sensitivity to song sequence (60). Our study in both sexes showed that BS and NS neurons differentially responded to auditory stimuli in two specific ways. First, NS cells responded to conspecific song more strongly in males only. Second, NS cells exhibited higher timing and rate-coding accuracy, indicating that NS are better at distinguishing among stimuli. Previous findings showed that BS neurons in NCM had greater selectivity for specific song types after tutoring (14). Taken together, BS neurons may be selective for specific songs, whereas NS neurons have greater coding accuracy and favor a bicoding computational mode. Although NS cells are putatively inhibitory interneurons, this assignment has not been validated in NCM, and there are exceptions to spike-width duration in the classification of these cell types [e.g., narrow, fast-spiking cells can be excitatory neurons in the primate cortex (81)]. NCM is also thought to be a locus for auditory memory (12–14), so the cell type–specific coding of conspecific songs in NCM will be important to explore to understand the neural basis of perception and individual recognition.
Our study demonstrates a role for GPER1 in sensory processing. We found that the blocking of GPER1 signaling led to decreases in firing rate, auditory responsiveness, timing accuracy, and count accuracy in male NS neurons, revealing that for this sex and cell type, GPER1 is critical for auditory activation and coding of stimuli. Sex-specific mechanisms for rapid estradiol signaling have been described in other brain regions, such as the hippocampus (82, 83). These rapid actions can facilitate changes in behavior, and similar to the modulation of NCM by neuroestrogens (8, 39, 56), neuroestrogens also alter hippocampal memory formation and consolidation (47, 55, 84–87). We found that even though there is a sex- and cell type–specific mechanism of GPER1 in auditory-specific neuron firing, we did not detect sex differences in GPER1 expression in the NCM. Sex differences in physiology also exist in other species and brain regions in which anatomical descriptions and protein expression are not sexually dimorphic. For example, despite similarities between the sexes in expression of GPER1 and aromatase (49, 88), there are noted sex differences in physiological responses to estradiol in the hippocampus (51, 89–91). Our study is consistent with these findings and emphasizes the importance of physiological investigations to understand sex differences in the brain.
Although our findings implicate GPER1 in auditory processing, the signaling mechanism may be independent of the previously established role of estradiol in the songbird forebrain. The agonist for GPER1, G1, did not mimic the overall enhancement of auditory processing by estradiol in both sexes (8, 39). One resultant hypothesis is that estradiol acts through multiple membrane ERs concurrently, as classical nuclear ER agonists do not enhance auditory responsiveness in males (41). Future work should consider concurrent activation of multiple ER subtypes for auditory processing in NCM. There is evidence for a multi-ER mechanism in synaptic hippocampal neurotransmission (50), and whereas GPER1 and estradiol can enhance hippocampal-dependent object recognition, estradiol depends on mitogen-activated protein kinase kinase-extracellular signal-regulated kinase signaling, whereas GPER1 depends on the c-Jun N-terminal kinase pathway (47), indicating that there are nonestradiol actions of GPER1 activation. One surprising observation in this study was a decrease in timing accuracy during application of the low-dose GPER1 agonist. This occurred in male NS cells, which were also sensitive to the antagonist. Although counterintuitive that the agonist and antagonist would have similar directional effects on timing accuracy, given the smaller effect size (and lack of agonist-dependent changes in z score, firing frequency, and count accuracy), this is consistent with the hypothesis that GPER1 activation initiates alternative mechanisms that are independent of estradiol signaling in the zebra finch. Another interpretation of a lack of G1 enhancement of song responsiveness may be a result of the high production of locally synthesized estradiol in NCM. The administration of an aromatase inhibitor to block local estradiol synthesis could help disentangle these findings.
We did not observe a washout of antagonist on auditory responses and coding. Rather, some effects not only persisted into the post-aCSF but also sometimes became stronger. There is some evidence that GPER1 has effects on synaptic plasticity via changes in spine dynamics. Rapid estrogenic actions have been implicated before in long-term potentiation (82, 92), as well as a mechanism for learning and memory (93, 94), so the lack of a washout here could be explained by a fundamental, long-lasting change in the NCM modulatory “state” after application of the antagonist. Future experiments using this approach could extend the time course of observations to examine potential washout effects beyond the 30- to 40-minute observations in this study.
Prior work in songbirds has identified other sex-specific differences in auditory coding that may be mediated by peripheral estrogens. Previous findings in Field L and caudomedial mesopallium demonstrated that bicoding cells in females are sensitive to breeding photoperiod (when peripheral estrogen levels are high), where both timing accuracy and temporal resolution increased compared with the nonbreeding season. This effect of season does not occur in males (61). We find that GPER1 inhibition decreased auditory responsiveness and coding for both timing and count-classification accuracy but only in males. Whereas the previous study infers that these effects are mediated via hormonal actions, our study suggests an ER mechanism that directly affects coding in the auditory forebrain in a sex-specific way. Gonadal production of testosterone and estradiol can mediate brain-derived estradiol production, as well as membrane steroid receptor activation. Peripheral hormones have long been understood to affect auditory activity (16). Gonadal hormones can also affect aromatase expression in the brain (34), which can impact the local supply of estrogen available for neurons. In our study, both males and females were gonadally intact, and future work could determine how gonadal hormones contribute to sex differences in NCM firing properties and GPER1-mediated changes in auditory responsiveness.
Our electrophysiology results led to the hypothesis that a sex difference in GPER1 receptor expression occurs in subregions of NCM. We found that a GPER1-positive cell number was similar between males and females, and we established that ∼20% of GAD67-positive cells in NCM are also positive for GPER1, consistent with the hypothesis that estrogens act, in part, to shape inhibition in NCM (Fig. 8). However, we did not observe sex differences in the number of cells coexpressing GPER1 and GAD67, as well as a lack of expression with interneuron markers parvalbumin or CALB (data not shown). Other inhibitory interneuron subtypes, such as somatostatin and vasoactive intestinal peptide, which are important auditory cell types in mammalian cortex (95–97), could be explored further to account for the sex differences observed here. Excitatory cell types and their relationship to GPER1 have not been characterized in finches, despite their obvious importance. Local network connections between GPER1-positive cells and other NCM cell subtypes now become an active area of interest in this work. Overall, these results indicate that despite a similarity in GPER1 expression, sex differences in physiological responses to antagonism persist.
The temporal cortex of humans can produce estradiol locally, including at synaptic terminals (33), much like the zebra finch NCM (34, 36). Although there has not been an anatomical description of GPER1 in the human brain, GPER1 has been shown to be expressed in the primate cortex associated with synaptic densities (45). Our findings suggest that sex is a fundamental factor when examining mechanisms of audition and that it is worthwhile to explore neuroestrogen signaling and GPER1 in the primate auditory cortex, considering sex as a biological factor. In the context of life history and reproductive strategies for the sex and species of interest, brain areas that process sensory information should be explored further for sexually dimorphic mechanisms and in particular, considering neuromodulatory mechanisms of estrogens.
Acknowledgments
We thank Racquel Bitar for technical assistance with immunofluorescence and Dr. Jim Chambers for assistance on confocal microscopy.
Financial Support: This work was supported by National Institutes of Health Grant R01NS082179 (to L.R.-H.) and Coordination for Development of Higher Education Personnel (13640/13-5, CAPES-Brazil; to M.M.-L.).
Disclosure Summary:
The authors have nothing to disclose.
Glossary
Abbreviations:
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- BS
broad spiking
- CALB
calbindin
- CON
conspecific song
- DAPI
4′,6-diamidino-2-phenylindole
- dNCM
dorsal caudomedial nidopallium
- ER
estrogen receptor
- GAD65/67
glutamate decarboxylase with molecular weight of 65/67
- GPER1
G-protein-coupled estrogen receptor 1
- NCM
caudomedial nidopallium
- NS
narrow spiking
- PB
phosphate buffer
- PCA
principal component analysis
- RRID
Research Resource Identifier
- SD
standard deviation
- SQW
spike quarter width
- vNCM
ventral caudomedial nidopallium
- WN
white noise
References
- 1.Dorris DM, Cao J, Willett JA, Hauser CA, Meitzen J. Intrinsic excitability varies by sex in prepubertal striatal medium spiny neurons. J Neurophysiol. 2015;113(3):720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194(4261):211–213. [DOI] [PubMed] [Google Scholar]
- 3.Hamaide J, De Groof G, Van Steenkiste G, Jeurissen B, Van Audekerke J, Naeyaert M, Van Ruijssevelt L, Cornil C, Sijbers J, Verhoye M, Van der Linden A. Exploring sex differences in the adult zebra finch brain: in vivo diffusion tensor imaging and ex vivo super-resolution track density imaging. Neuroimage. 2017;146:789–803. [DOI] [PubMed] [Google Scholar]
- 4.Krentzel AA, Remage-Healey L. Sex differences and rapid estrogen signaling: a look at songbird audition. Front Neuroendocrinol. 2015;38:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenowitz EA, Remage-Healey L. It takes a seasoned bird to be a good listener: communication between the sexes. Curr Opin Neurobiol. 2016;38:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagostin AA, Mello CV, Leão RM. Increased bursting glutamatergic neurotransmission in an auditory forebrain area of the zebra finch (Taenopygia guttata) induced by auditory stimulation. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2012;198(9):705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoder KM, Phan ML, Lu K, Vicario DS. He hears, she hears: are there sex differences in auditory processing? Dev Neurobiol. 2015;75(3):302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA. 2010;107(8):3852–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda MZ, Jeon SD, Cowell RA, Remage-Healey L. Norepinephrine modulates coding of complex vocalizations in the songbird auditory cortex independent of local neuroestrogen synthesis. J Neurosci. 2015;35(25):9356–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomaszycki ML, Sluzas EM, Sundberg KA, Newman SW, DeVoogd TJ. Immediate early gene (ZENK) responses to song in juvenile female and male zebra finches: effects of rearing environment. J Neurobiol. 2006;66(11):1175–1182. [DOI] [PubMed] [Google Scholar]
- 11.Terpstra NJ, Bolhuis JJ, Riebel K, van der Burg JM, den Boer-Visser AM. Localized brain activation specific to auditory memory in a female songbird. J Comp Neurol. 2006;494(5):784–791. [DOI] [PubMed] [Google Scholar]
- 12.Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7(5):347–357. [DOI] [PubMed] [Google Scholar]
- 13.Bolhuis JJ, Moorman S. Birdsong memory and the brain: in search of the template. Neurosci Biobehav Rev. 2015;50:41–55. [DOI] [PubMed] [Google Scholar]
- 14.Yanagihara S, Yazaki-Sugiyama Y. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat Commun. 2016;7:11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell BA, Phan ML, Vicario DS. Neural responses in songbird forebrain reflect learning rates, acquired salience, and stimulus novelty after auditory discrimination training. J Neurophysiol. 2015;113(5):1480–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23(6):1523–1529. [DOI] [PubMed] [Google Scholar]
- 17.Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511(2):173–186. [DOI] [PubMed] [Google Scholar]
- 18.Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds [published correction appears in Front Neuroendocrinol 2011;32(4):466]. Front Neuroendocrinol. 2011;32(3):287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolhuis JJ, Zijlstra GG, den Boer-Visser AM, Van Der Zee EA. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc Natl Acad Sci USA. 2000;97(5):2282–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riebel K, Smallegange IM, Terpstra NJ, Bolhuis JJ. Sexual equality in zebra finch song preference: evidence for a dissociation between song recognition and production learning. Proc Biol Sci. 2002;269(1492):729–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoder KM, Lu K, Vicario DS. Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport. 2012;23(16):922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wermke K, Hain J, Oehler K, Wermke P, Hesse V. Sex hormone influence on human infants’ sound characteristics: melody in spontaneous crying. Biol Lett. 2014;10(5):20140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quast A, Hesse V, Hain J, Wermke P, Wermke K. Baby babbling at five months linked to sex hormone levels in early infancy. Infant Behav Dev. 2016;44:1–10. [DOI] [PubMed] [Google Scholar]
- 24.Charitidi K, Meltser I, Tahera Y, Canlon B. Functional responses of estrogen receptors in the male and female auditory system. Hear Res. 2009;252(1-2):71–78. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy MM. How it’s made: organisational effects of hormones on the developing brain. J Neuroendocrinol. 2010;22(7):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann N Y Acad Sci. 2004;1016(1):540–559. [DOI] [PubMed] [Google Scholar]
- 27.Maekawa F, Tsukahara S, Kawashima T, Nohara K, Ohki-Hamazaki H. The mechanisms underlying sexual differentiation of behavior and physiology in mammals and birds: relative contributions of sex steroids and sex chromosomes. Front Neurosci. 2014;8:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. I. Complete demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci. 1983;3(2):417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145(3):1063–1068. [DOI] [PubMed] [Google Scholar]
- 30.Micevych PE, Wong AM, Mittelman-Smith MA. Estradiol membrane-initiated signaling and female reproduction. Compr Physiol. 2015;5(3):1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18(5):690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hornung MW, Jensen KM, Korte JJ, Kahl MD, Durhan EJ, Denny JS, Henry TR, Ankley GT. Mechanistic basis for estrogenic effects in fathead minnow (Pimephales promelas) following exposure to the androgen 17alpha-methyltestosterone: conversion of 17alpha-methyltestosterone to 17alpha-methylestradiol. Aquat Toxicol. 2004;66(1):15–23. [DOI] [PubMed] [Google Scholar]
- 33.Yague JG, Muñoz A, de Monasterio-Schrader P, Defelipe J, Garcia-Segura LM, Azcoitia I. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138(2):389–401. [DOI] [PubMed] [Google Scholar]
- 34.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423(4):619–630. [DOI] [PubMed] [Google Scholar]
- 35.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63(2):149–155. [DOI] [PubMed] [Google Scholar]
- 36.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272(1576):2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda MZ, Krentzel AA, Oliver TJ, Scarpa GB, Remage-Healey L. Clustered organization and region-specific identities of estrogen-producing neurons in the forebrain of zebra finches (Taeniopygia guttata). J Comp Neurol. 2017;525(17):3636–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11(11):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107(6):1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao A, Paon A, Remage-Healey L. Dynamic variation in forebrain estradiol levels during song learning. Dev Neurobiol. 2015;75(3):271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remage-Healey L, Jeon SD, Joshi NR. Recent evidence for rapid synthesis and action of oestrogens during auditory processing in a songbird. J Neuroendocrinol. 2013;25(11):1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava DP, Evans PD. G-protein oestrogen receptor 1: trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol. 2013;25(11):1219–1230. [DOI] [PubMed] [Google Scholar]
- 43.Mangiamele LA, Gomez JR, Curtis NJ, Thompson RR. GPER/GPR30, a membrane estrogen receptor, is expressed in the brain and retina of a social fish (Carassius auratus) and colocalizes with isotocin. J Comp Neurol. 2017;525(2):252–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almey A, Milner TA, Brake WG. Estrogen receptor α and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum. Neurosci Lett. 2016;622:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crimins JL, Wang AC, Yuk F, Puri R, Janssen WG, Hara Y, Rapp PR, Morrison JH. Diverse synaptic distributions of G protein-coupled estrogen receptor 1 in monkey prefrontal cortex with aging and menopause. Cereb Cortex. 2017;27(3):2022–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acharya KD, Veney SL. Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. 2012;72(11):1433–1446. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Szinte JS, Boulware MI, Frick KM. 17β-Estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms. J Neurosci. 2016;36(11):3309–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briz V, Liu Y, Zhu G, Bi X, Baudry M. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release. J Cell Biol. 2015;210(7):1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA. G-Protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci. 2015;35(6):2384–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar A, Bean LA, Rani A, Jackson T, Foster TC. Contribution of estrogen receptor subtypes, ERα, ERβ, and GPER1 in rapid estradiol-mediated enhancement of hippocampal synaptic transmission in mice. Hippocampus. 2015;25(12):1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oberlander JG, Woolley CS. 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci. 2016;36(9):2677–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Méndez-Luna D, Martínez-Archundia M, Maroun RC, Ceballos-Reyes G, Fragoso-Vázquez MJ, González-Juárez DE, Correa-Basurto J. Deciphering the GPER/GPR30-agonist and antagonists interactions using molecular modeling studies, molecular dynamics, and docking simulations. J Biomol Struct Dyn. 2015;33(10):2161–2172. [DOI] [PubMed] [Google Scholar]
- 53.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127(3-5):358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seredynski AL, Balthazart J, Ball GF, Cornil CA. Estrogen receptor β activation rapidly modulates male sexual motivation through the transactivation of metabotropic glutamate receptor 1a. J Neurosci. 2015;35(38):13110–13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bailey DJ, Makeyeva YV, Paitel ER, Pedersen AL, Hon AT, Gunderson JA, Saldanha CJ. Hippocampal aromatization modulates spatial memory and characteristics of the synaptic membrane in the male zebra finch. Endocrinology. 2017;158(4):852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32(24):8231–8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pawlisch BA, Remage-Healey L. Neuroestrogen signaling in the songbird auditory cortex propagates into a sensorimotor network via an ‘interface’ nucleus. Neuroscience. 2015;284:522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci USA. 1995;92(8):3406–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94(2):313–318 (table of contents). [DOI] [PubMed] [Google Scholar]
- 60.Ono S, Okanoya K, Seki Y. Hierarchical emergence of sequence sensitivity in the songbird auditory forebrain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2016;202(3):163–183. [DOI] [PubMed] [Google Scholar]
- 61.Caras ML, Sen K, Rubel EW, Brenowitz EA. Seasonal plasticity of precise spike timing in the avian auditory system. J Neurosci. 2015;35(8):3431–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee V, Pawlisch BA, Macedo-Lima M, Remage-Healey L. Norepinephrine enhances song responsiveness and encoding in the auditory forebrain of male zebra finches. J Neurophysiol. 2018;119(1):209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 64.Rauske PL, Shea SD, Margoliash D. State and neuronal class-dependent reconfiguration in the avian song system. J Neurophysiol. 2003;89(3):1688–1701. [DOI] [PubMed] [Google Scholar]
- 65.Mooney R, Prather JF. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci. 2005;25(8):1952–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Day NF, Terleski KL, Nykamp DQ, Nick TA. Directed functional connectivity matures with motor learning in a cortical pattern generator. J Neurophysiol. 2013;109(4):913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider DM, Woolley SM. Sparse and background-invariant coding of vocalizations in auditory scenes. Neuron. 2013;79(1):141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mouterde SC, Elie JE, Mathevon N, Theunissen FE. Single neurons in the avian auditory cortex encode individual identity and propagation distance in naturally degraded communication calls. J Neurosci. 2017;37(13):3491–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7(1):91–100. [DOI] [PubMed] [Google Scholar]
- 70.Schwab C, Yu S, Wong W, McGeer EG, McGeer PL. GAD65, GAD67, and GABAT immunostaining in human brain and apparent GAD65 loss in Alzheimer’s disease. J Alzheimers Dis. 2013;33(4):1073–1088. [DOI] [PubMed] [Google Scholar]
- 71.Bosma PT, Blázquez M, Collins MA, Bishop JD, Drouin G, Priede IG, Docherty K, Trudeau VL. Multiplicity of glutamic acid decarboxylases (GAD) in vertebrates: molecular phylogeny and evidence for a new GAD paralog. Mol Biol Evol. 1999;16(3):397–404. [DOI] [PubMed] [Google Scholar]
- 72.Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J Neurobiol. 2006;66(2):182–195. [DOI] [PubMed] [Google Scholar]
- 73.Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31(27):10034–10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avey MT, Phillmore LS, MacDougall-Shackleton SA. Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav Brain Res. 2005;165(2):247–253. [DOI] [PubMed] [Google Scholar]
- 75.Gobes SM, Ter Haar SM, Vignal C, Vergne AL, Mathevon N, Bolhuis JJ. Differential responsiveness in brain and behavior to sexually dimorphic long calls in male and female zebra finches. J Comp Neurol. 2009;516(4):312–320. [DOI] [PubMed] [Google Scholar]
- 76.Giret N, Menardy F, Del Negro C. Sex differences in the representation of call stimuli in a songbird secondary auditory area. Front Behav Neurosci. 2015;9:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford, UK: Oxford University Press. [Google Scholar]
- 78.Huang GZ, Zhang JJ, Wang D, Mason RT, Halpern M. Female snake sex pheromone induces membrane responses in vomeronasal sensory neurons of male snakes. Chem Senses. 2006;31(6):521–529. [DOI] [PubMed] [Google Scholar]
- 79.Bergan JF, Ben-Shaul Y, Dulac C. Sex-specific processing of social cues in the medial amygdala. eLife. 2014;3:e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergan JF. Neural computation and neuromodulation underlying social behavior. Integr Comp Biol. 2015;55(2):268–280. [DOI] [PubMed] [Google Scholar]
- 81.Vigneswaran G, Kraskov A, Lemon RN. Large identified pyramidal cells in macaque motor and premotor cortex exhibit “thin spikes”: implications for cell type classification. J Neurosci. 2011;31(40):14235–14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vierk R, Glassmeier G, Zhou L, Brandt N, Fester L, Dudzinski D, Wilkars W, Bender RA, Lewerenz M, Gloger S, Graser L, Schwarz J, Rune GM. Aromatase inhibition abolishes LTP generation in female but not in male mice. J Neurosci. 2012;32(24):8116–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meitzen J, Grove DD, Mermelstein PG. The organizational and aromatization hypotheses apply to rapid, nonclassical hormone action: neonatal masculinization eliminates rapid estradiol action in female hippocampal neurons. Endocrinology. 2012;153(10):4616–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav. 2016;83:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frick KM, Kim J, Tuscher JJ, Fortress AM. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22(9):472–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33(38):15184–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kramár EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience. 2013;239:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tabatadze N, Sato SM, Woolley CS. Quantitative analysis of long-form aromatase mRNA in the male and female rat brain. PLoS One. 2014;9(7):e100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex differences in molecular signaling at inhibitory synapses in the hippocampus. J Neurosci. 2015;35(32):11252–11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25(20):5066–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tozzi A, de Iure A, Tantucci M, Durante V, Quiroga-Varela A, Giampà C, Di Mauro M, Mazzocchetti P, Costa C, Di Filippo M, Grassi S, Pettorossi VE, Calabresi P. Endogenous 17β-estradiol is required for activity-dependent long-term potentiation in the striatum: interaction with the dopaminergic system. Front Cell Neurosci. 2015;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28(35):8660–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frick KM. Epigenetics, oestradiol and hippocampal memory consolidation. J Neuroendocrinol. 2013;25(11):1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503(7477):521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen IW, Helmchen F, Lütcke H. Specific early and late oddball-evoked responses in excitatory and inhibitory neurons of mouse auditory cortex. J Neurosci. 2015;35(36):12560–12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Phillips EA, Hasenstaub AR. Asymmetric effects of activating and inactivating cortical interneurons. eLife. 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]