Abstract
Research has established that the body is fundamentally involved in perception: bodily experience influences activation of the shared neural system underlying action perception and production during action observation, and bodily characteristics influence perception of the spatial environment. However, whether bodily characteristics influence action perception and its underlying neural system is unknown, particularly in early ontogeny. We measured grip strength in 12-month-old infants and investigated relations with mu rhythm attenuation, an electroencephalographic correlate of the neural system underlying action perception, during observation of lifting actions performed with differently weighted blocks. We found that infants with higher grip strength exhibited significant mu attenuation during observation of lifting actions, whereas infants with lower grip strength did not. Moreover, a progressively strong relation between grip strength and mu attenuation during observation of lifts was found with increased block weight. We propose that this relation is attributable to differences in infants’ ability to recognize the effort associated with lifting objects of different weights, as a consequence of their developing strength. Together, our results extend the body’s role in perception by demonstrating that bodily characteristics influence action perception by shaping the activation of its underlying neural system.
Introduction
More than bodily movements through space, human actions convey important information about individuals and their surroundings. Interpretations of observed actions are not uniform across individuals, however, but vary according to the observer’s bodily characteristics and experiences. For example, imagine two spectators at a weightlifting competition: a seasoned weightlifter and a novice weightlifter. Both observe a competitor approach, grasp, and heft a 300 lb barbell into the air, before progressing to successfully lifting a 400 lb barbell. For both spectators, it is clear that the barbells are heavy, and that the competitor’s goal is to lift the barbells. However, whereas the novice is unable to recognize the increased effort exhibited by the competitor when lifting the 400 lb, relative to the 300 lb, barbell, the seasoned spectator marvels upon witnessing the second lift, in recognition of the increased effort associated with lifting the 400 lb barbell. As this example illustrates, perceptions of others’ actions vary according to the observer’s prior experience and abilities.
Bodily experience shapes action perception
Given the centrality of action understanding to everyday functioning, a critical question concerns how this understanding is achieved. There is now a wealth of evidence demonstrating that bodily experience producing an action is linked to, and causally influences, action perception (e.g. Casile & Giese, 2006; Hommel, Müsseler, Aschersleben & Prinz, 2001). This is particularly true in infancy: training infants to produce goal-directed actions enables them to detect the goal structure of similar actions produced by others (Sommerville, Woodward & Needham, 2005; Sommerville, Hildebrand & Crane, 2008). Evidence suggests that this close alliance is established via a shared underlying neural system (Buccino, Binkofski, Fink, Fadiga, Fogassi et al., 2001; Iacoboni, Woods, Brass, Bekkering, Mazziotta et al., 1999; Vanderwert, Fox & Ferrari, 2013), the activation of which is shaped by one’s experience and expertise with the observed action, both in adults (Calvo-Merino, Glaser, Grèzes, Passingham & Haggard, 2005; Cannon, Yoo, Vanderwert, Ferrari, Woodward et al., 2014; Cross, Hamilton & Grafton, 2006) and in infants (Paulus, Hunnius, van Elk & Bekkering, 2012; van Elk, van Schie, Hunnius, Vesper & Bekkering, 2008). Thus, bodily experience profoundly influences action perception by tuning the underlying neural system.
Bodily characteristics shape spatial perception
Work from a different line of inquiry suggests that the body is fundamentally involved in another perceptual arena: bodily states and characteristics influence perception of the spatial environment (Kretch, Franchak & Adolph, 2014; Proffitt & Linkenauger, 2013). For example, Adolph and colleagues have demonstrated that infants’ perception of their environment is heavily influenced by bodily characteristics, such as their posture: infants who primarily walk spend more time looking at distal and elevated surfaces than same-aged, primarily crawling infants who spend more time looking at the ground (Kretch et al., 2014). Similarly, infants who sit independently spend more time looking at the objects in their grasp than infants who cannot yet sit alone (Soska, Adolph & Johnson, 2010). These perceptual differences that come as a consequence of infants’ posture are in turn associated with unique cognitive achievements (Adolph, Cole, Komati, Garciaguirre, Badaly et al., 2012; Campos, Anderson, Barbu-Roth, Hubbard, Hertenstein et al., 2000; Soska et al., 2010). Relatedly, there is evidence that perception of the environment is influenced by bodily characteristics in adults: inclines are perceived to be steeper by adults wearing a heavy backpack relative to those who are unencumbered, by adults who are fatigued relative to those who are rested, and by the elderly relative to the young (Bhalla & Proffitt, 1999). Taken together, these findings raise the possibility that bodily characteristics may shape not only infants’ perception of the spatial environment but also their perception of others’ actions.
Investigating the neural system underlying action perception in infancy
Due to limitations with using functional neuroimaging techniques in infancy, researchers have relied on electroencephalography (EEG) to study the development of the neural system underlying action perception. Specifically, the mu rhythm, recorded between 8 and 13 Hz in adults and between 6 and 9 Hz in infants (Marshall & Meltzoff, 2011; Southgate, Johnson, Karoui & Csibra, 2010), has been tied to the shared neural system underlying action perception and production, due to its selective attenuation during action perception and production (Marshall, Young & Meltzoff, 2011; Muthukumaraswamy, Johnson & McNair, 2004), its prominence over the sensorimotor cortex (Pineda, 2005), and relations with other methods investigating this system (Keuken, Hardie, Dorn, Dev, Paulus et al., 2011; Braadbaart, Williams & Waiter, 2013). Thus, the mu rhythm is ideal for investigating the development and neural basis of action perception.
Overview of the current study
The question addressed in the current study concerns the impact of bodily characteristics on mu attenuation during action observation in infancy. Past work has demonstrated that bodily experience influences mu attenuation during action observation: for example, mu attenuation during observation of crawling is related to infants’ crawling experience (van Elk et al., 2008). The novel question addressed in our experiment was whether bodily characteristics, particularly those that influence both the frequency and nature of motor experience, are associated with the degree of activation of the neural system underlying action perception and production during action observation. Infancy is an ideal time to investigate the effect of bodily characteristics on action perception and its underlying neural system, as there is wide variability in bodily characteristics (World Health Organization, 2006) and significant changes in action perception (Loucks & Sommerville, 2012) during this developmental period. Moreover, infants have fewer means than children and adults to mitigate bodily constraints in order to achieve their goals (e.g. grab a stool to increase height).
In the current experiment, EEG activity was recorded while infants took part in an action task during which they alternately lifted, and watched an experimenter lift, blocks of different weights; infants’ grip strength was measured midway through the action task via a novel grip strength assessment. Prior work with adults has demonstrated that observing lifting actions performed with heavy objects, relative to lifting actions performed with light objects, results in increased activation of the shared neural system underlying action perception and production (Alaerts, Senot, Swinnen, Craighero, Wenderoth et al., 2010a; Senot, D’Ausilio, Franca, Caselli, Craighero et al., 2011). One possible interpretation of this finding is that the action perception systems of adults are sensitive to differences in object weight per se (Cole, 2008; Flanagan & Beltzner, 2000). Another interpretation is that activation of the neural system underlying action perception and production is modulated by differences in the phenomenological experiences associated with lifting heavy versus light objects, such as differences in exerted force (Alaerts, Swinnen & Wenderoth, 2010b; Mima, Simpkins, Oluwatimilehin & Hallett, 1999) or expended effort. Indeed, some researchers have suggested that, in adults, perception is heavily shaped by bodily characteristics that increase or reduce the effort associated with producing a given action (Proffitt, 2006). This latter explanation may help to explain why a recent study with infants failed to find differences in mu attenuation as a function of object weight (Marshall, Saby & Meltzoff, 2013). That is, given limitations and variability in infants’ experience with lifting objects of different weights, infants may be unable to recognize differences in expended effort on the basis of object weight, or may be limited in their ability to do so.
In the present paper we investigated whether individual differences in infants’ grip strength would predict mu attenuation during observation of lifting actions with weighted blocks. We hypothesized that infants’ grip strength would predict mu attenuation during observation of lifting actions because grip strength gates both the nature and frequency of experience lifting objects of various weights, which in turn could influence infants’ ability to recognize the differential effort associated with lifting objects of different weights. Because strength profoundly affects one’s ability to lift objects, stronger infants have likely acquired more lifting experience with a broader, and more contrastive, range of object weights than weaker infants (see Wang & Baillargeon, 2008, for evidence that contrastive experience facilitates perceptual discrimination and learning). Thus, we predict that infants’ grip strength will be associated with mu attenuation during observation of lifting actions with weighted blocks. Furthermore, as strength exerts a greater impact on infants’ experience lifting heavy objects versus light objects, we predict that the association between infants’ grip strength and mu attenuation will become stronger as the weight of the block being lifted by the experimenter increases.
Method
Participants
Thirty-four (n = 17 female) 12-month-old infants were recruited from a university-maintained database to participate in the study (M = 12 months, 7 days; range: 11 months, 22 days to 13 months, 1 day). Eleven infants were excluded due to technical problems (n = 7) or not providing sufficient usable EEG data (i.e. at least one artifact-free trial of each type; n = 4). Of the final sample of 23 infants (n = 12 female), the average age was 12 months and 10 days (range: 11 months, 22 days to 13 months, 1 day). Nineteen infants were Caucasian, and four infants were of mixed racial background.
Stimuli
Infants interacted with four plastic blocks (8.9 cm per side) that varied in color (i.e. red, yellow, orange, and green) and weight. For approximately half of the infants, two of the blocks were ‘light’ (70 g) and two were ‘heavy’ (470 g). For the remaining half, two of the blocks were ‘light’ (70 g), one was ‘heavy’ (470 g), and one was ‘super heavy’ (720 g).1 The blocks were paired by contrasting weight (i.e. one light and one heavy, or one light and one super heavy) and color (e.g. red and yellow), and the color–weight pairings were counterbalanced across infants. A bucket (17.1 cm × 14.6 cm × 12.7 cm) and cardboard box (21.6 cm × 12.7 cm × 5.7 cm) were also used during the interaction. A set of 15 (21.6 cm × 27.9 cm) cardboard signs with different abstract patterns served as baseline images.
Procedure
Action task
Infants were seated in their parents’ lap, in front of a table, across from the experimenter. Infants were not familiarized with the blocks or other stimuli before the action task. During the task, infants received intermixed observation trials, action trials and baseline trials. During observation trials, the experimenter randomly selected one of three lifting actions to demonstrate for the infant: hopping a block across the table, lifting a block onto a platform, or lifting a block and dropping it into a bucket. The average duration of the experimenter’s demonstration was 5 seconds, during which approximately two exemplars of the lifting action were performed. Action trials began after the experimenter handed the block (and the bucket or platform, if those actions were demonstrated) to the infants. Action trials ended after infants performed an action with the block or after 30 seconds had elapsed with no block interaction. During action and observation trials, only the block being acted upon was visible to the infant.
For approximately half of the sample (n = 13), half of the action and observation trials were conducted with the 70 g block and half with the 470 g block. For the other half of the sample (n = 10), half of the action and observation trials were conducted with the 70 g block, one-quarter with the 470 g block, and the remaining quarter with the 720 g block.
Baseline trials were administered after action and observation trials and consisted of the experimenter holding an image of an abstract pattern in front of her face for infants to view for approximately 3 seconds.
Breaks were taken as needed throughout the testing session. When breaks failed to re-engage infants, the action task was terminated.
Grip strength assessment
Infants’ grip strength was recorded via a TruStability Silicon Pressure Sensor that was embedded inside a squeezable plastic toy. The embedded pressure sensor was connected to both a switch circuit and a laptop computer running Processing, an open source programming environment (http://processing.org). The purpose of the switch circuit was to activate the playing of the song ‘Old MacDonald’. Processing served to: (1) record infants’ grip strength values (in pounds per square inch, or psi) and (2) gate the activation of the switch circuit. By default, the switch circuit was incomplete (i.e. would not activate the song), but upon pressure, would complete, causing the song to play for 3 seconds. The switch circuit functioned such that, after infants’ initial squeeze, the song would only play for squeezes that met or exceeded 90% of the force of their highest previous squeeze. By implementing this constraint, we hoped to elicit increasingly forceful squeezes on behalf of infants in order to play the song. Only squeezes that succeeded in playing the song were recorded, and the maximum recorded grip strength value was analyzed as infants’ grip strength score.
The grip strength assessment was administered midway through the action task. The experimenter first presented infants with the squeezable toy (containing the embedded pressure sensor), and, using an identical (but inert) toy, modeled forceful squeezes and encouraged infants to do the same. The grip strength assessment continued accordingly for as long as infants remained interested.
EEG recording and analysis
EEG activity was recorded via a 128-channel Geodesic Sensor Net at 250 samples/second (via EGI software; Net Station v4.1; Electrical Geodesics, Inc., Eugene, OR) and filtered online between 0.1 and 100 Hz. EEG activity was recorded with a vertex reference and re-referenced offline to a common average of all leads. Impedances were measured below 40 kΩ at the start of data acquisition.
EEG activity was segmented offline into 1000 ms epochs, extending from 0 ms, indicating the start of the lift, for action and observation trials (i.e. at the point of object contact immediately preceding the block being lifted from the table), or the initial presentation of the baseline image, for baseline trials, to 1000 ms afterward. EEG activity was continuously segmented into 1000 ms epochs until the end of the lift (i.e, when the object was placed back onto the table) or until removal of the baseline image. Epochs from observation and baseline trials in which the infant was not attending to the stimuli, or that were contaminated by infant movement, were identified from video and removed. Artifacts from remaining epochs were removed through NetStation’s artifact detection algorithm which removed epochs that contained 18 or more leads that exceeded 200 μV in raw amplitude, or 18 or more leads that exceeded 100 μV in differential average amplitudes. Overall, this removed 37% of infant action epochs, 20% of infant observation epochs, and 35% of baseline epochs. The average number of artifact-free epochs of each trial type for each infant were: 20.91 action epochs (SE = 3.49), 29.87 observation epochs (SE = 3.27), and 7.52 baseline epochs (SE = .83).
Fast Fourier transforms (FFTs) were performed in Matlab (version 7.11.0.584, R2010b, Natick, MA). Power spectra were calculated based on the average amplitude within each trial type. Mu attenuation was calculated as the natural log of the ratio of power during action or observation trials over power during baseline trials (i.e. [natural log (A/B)], where A is power during action or observation trials, and B is power during baseline trials). A ratio measure was used to account for individual variability in overall EEG power, and the log transformation accounts for the inherent non-normality of ratio data. Accordingly, values of zero indicate no change from baseline activity, negative values indicate mu attenuation relative to baseline, and positive values indicate mu augmentation relative to baseline.
In order to identify the location and frequency of maximal mu attenuation, we focused on oscillatory activity during infants’ production of block lifts over central leads C3 and C4 within the 6 to 9 Hz frequency range. In line with proposed guidelines for investigating the mu rhythm (Cuevas, Cannon, Yoo & Fox, 2014; Marshall & Meltzoff, 2011), the location and frequency of maximal mu attenuation during infants’ production of block lifts was used in subsequent analyses on mu attenuation during observation of block lifts. Consistent with prior work on the infant mu rhythm (Marshall et al., 2011), we defined infants’ mu rhythm band at the group level, rather than at the individual level, in order to statistically confirm the location and frequency of significant attenuation during infants’ action production relative to baseline (an analysis precluded by power limitations when operationalized at the individual level).
Lastly, in order to confirm that our results accurately reflect mu rhythm activity (as opposed to measurement of the occipital alpha rhythm or widespread changes in neural activity), we investigated EEG activity during observation of block lifts at frontal (F3 or F4), parietal (P3 or P4), and occipital (O1 or O2) leads, in the frequency bin and hemisphere that was previously identified (i.e. based on maximal mu attenuation during infants’ production of block lifts).
Ancillary measures
Motor abilities checklist
We assessed infants’ overall level of gross motor development for two reasons: (1) to validate our novel grip strength measure, as we expected a significant positive relation between infants’ motor development and grip strength scores (i.e. muscle strength increases with motor development; Woollacott, 1993) and (2) to ensure that relations between infants’ mu attenuation and grip strength were not underwritten by differences in overall levels of motor development.
Prior to the experiment, parents completed a 24-item Motor Abilities Checklist (MAC; Loucks & Sommerville, 2013), adapted from the Bayley Scales of Motor Development (Bayley, 2006), which lists motor development milestones in order of increasing difficulty. We used the highest consecutive item that parents indicated that their infant could perform as infants’ motor development score (M = 13.50, SD = 6.06; range: 4–24).
Infant weight
As body weight is correlated with grip strength in older children and adults (Wind, Takken, Helders & Engelbert, 2010), we asked parents to report their infants’ weight, taken at their 12-month doctor’s appointment, in order to validate the grip strength measure. Infants’ average weight was 9.85 kg (SE = .25; range: 7.75–12.80 kg).
Infants’ in-task lifting experience
We coded the number of block lifts infants performed during the action task in order to ensure that any relations between infants’ grip strength and mu attenuation were not explained by variability in infants’ in-task lifting experience. Block lifts were operationalized as upward, manual actions that resulted in the infant fully supporting the block’s weight. Infants performed an average of 9.61 (SE = 1.16, range: 0–23) light block lifts, 7.04 (SE = 1.56, range: 0–31) heavy block lifts, and 6.67 (SE = 1.54, range: 1–11) super heavy block lifts, for a grand average of 18.39 (SE = 2.78, range: 1–48) total lifts of all the blocks during the task. Overall, infants performed more one-handed lifts (M = 7.00, SE = 1.09) than bimanual lifts (M = 2.61, SE = .61) with the light blocks, t(22) = 3.29, p = .003, and more bimanual lifts (M = 3.10, SE = 1.11) than one-handed lifts (M = .90, SE = .46) with the super heavy blocks, t(9) = −2.31, p = .05. There was no difference in the number of one-handed (M = 4.83, SE = 1.51) versus bimanual lifts (M = 2.22, SE = .53) performed with the heavy blocks, t (22) = 1.60, p = .13. A second observer independently coded the number of lifts infants performed during the task for a randomly selected subset of infants. Inter-rater reliability (assessed via Pearson’s correlations) was high, r(7) = .98, p < .001.
One possible concern for interpreting these results is that increased grip strength could enable infants to more accurately reproduce the experimenter’s lifting action. Indeed, past work has shown that a greater similarity between produced actions and observed actions leads to greater activation of the shared neural system underlying action perception and production (Reid, Striano & Iacoboni, 2011; Saby, Marshall & Meltzoff, 2012). Accordingly, we coded the number of times, after observing the relevant demonstration, infants lifted the block onto a short platform and/or lifted the block and dropped it into a bucket (depending on which of the two actions was previously demonstrated). Faithful reproduction of the ‘lifting onto a platform action’ was operationalized as lifting a block and placing it completely and securely onto the platform. Faithful reproduction of the ‘lifting and dropping into a bucket’ action was operationalized as lifting a block and dropping it into the bucket, without inverting the bucket. Infants faithfully reproduced the experimenter’s lifting action an average of 3.78 times (SE = .95; range: 0–16) during the task.
Results
Identification of mu rhythm: mu attenuation during infants’ production of block lifts
To identify the location and frequency of maximal mu attenuation, we analyzed EEG activity during infants’ production of block lifts (collapsed over block weight) at sensor locations C3 and C4 from 6 to 9 Hz. As this required conducting eight, one sample t-tests (i.e. two leads and four frequencies), we employed the Bonferroni correction for this analysis and adopted a more stringent significance level of p = .00625. One sample t-tests revealed that significant mu attenuation was only present in the 8 Hz frequency bin at C4, located in the right hemisphere, t(22) = 3.92, p = .001, d = .82 (see Figure 1). Thus, subsequent analyses on mu attenuation during observation of block lifts focused exclusively on EEG activity in the 8 Hz frequency bin at C4.
Figure 1.
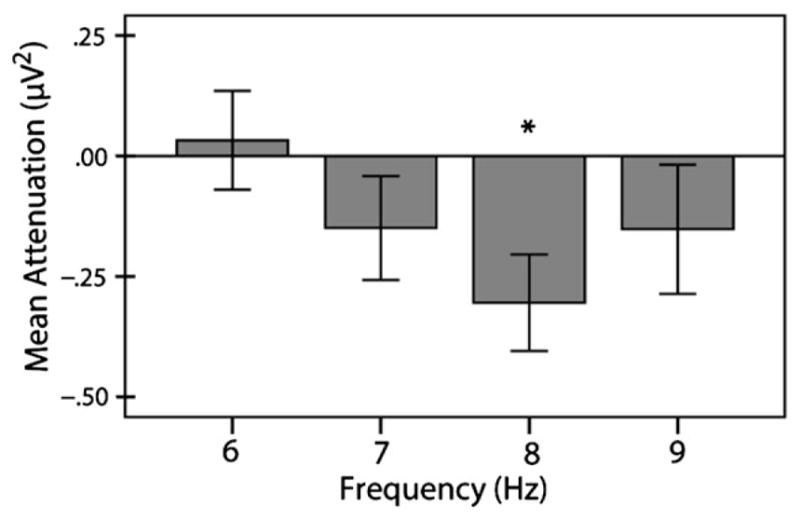
Mu attenuation during infants’ production of block lifts from 6 to 9 Hz at C4. Mu attenuation differed significantly from baseline at 8 Hz only. Error bars represent standard error. * p = .001.
Grip strength: descriptive statistics and relations with ancillary measures
Infants’ maximum grip strength score was, on average, 2.31 psi (SE = 1.86; range: .89–3.73). Overall, infants recorded 3.61 squeezes on the grip strength device (SE = .37; range: 1–6; this number reflects infants’ initial squeeze and as many that met or exceeded 90% of the force of their previous highest squeeze). There were no differences in infants’ maximum grip strength as a function of gender, t(21) = .83, p = .4. Infants’ maximum grip strength was not associated with their frequency of faithful reproductions during the task, r(23) = .13, p = .55, but was marginally associated with the total number of block lifts performed during the task, r(23) = .36, p = .09, such that infants who performed more block lifts had higher maximum grip strength scores. Importantly, infants’ maximum grip strength scores were positively correlated with their motor development scores, r(22) = .54, p = .01, (age-partialled, r(19) = .55, p = .01), such that infants who were more motorically advanced also had higher maximum grip strength scores. In addition, infants’ maximum grip strength scores were related to their body weight, r(22) = .48, p = .02 (age-partialled, r(19) = .48, p = .03), such that heavier infants had higher maximum grip strength scores. Together, these relations provide evidence that our novel paradigm accurately assessed infants’ grip strength.
Mu attenuation during observation of block lifts: relations to grip strength
We hypothesized that mu attenuation during observation of block lifts would vary as a function of infants’ grip strength, such that infants with higher maximum grip strength scores would exhibit greater mu attenuation during observation of block lifts than infants with lower maximum grip strength scores. We conducted a median-split analysis comparing mu attenuation during observation of lifts with all the blocks (i.e. collapsed across block weight) between infants who scored in the lower half on the grip strength assessment (M = 1.52 psi, SE = 1.12, range: .89–2.29) and infants who scored in the upper half (M = 3.04 psi, SE = 1.42, range: 2.39–3.73). This analysis revealed a significant difference in mu attenuation during observation of all block lifts as a function of grip strength group, t(21) = 2.94, p = .01, d = 1.24 (see Figure 2). One sample t-tests against zero confirm that mu attenuation during observation of all block lifts differed from baseline for the high grip strength group, t(11) = 2.35, p = .04, d = .68, but did not for the low grip strength group, t(10) = −1.90, p = .09 (indeed, the low grip strength group showed marginal mu augmentation). These results demonstrate that infants in the high grip strength group exhibited mu attenuation during observation of all block lifts whereas infants in the low grip strength group did not.
Figure 2.
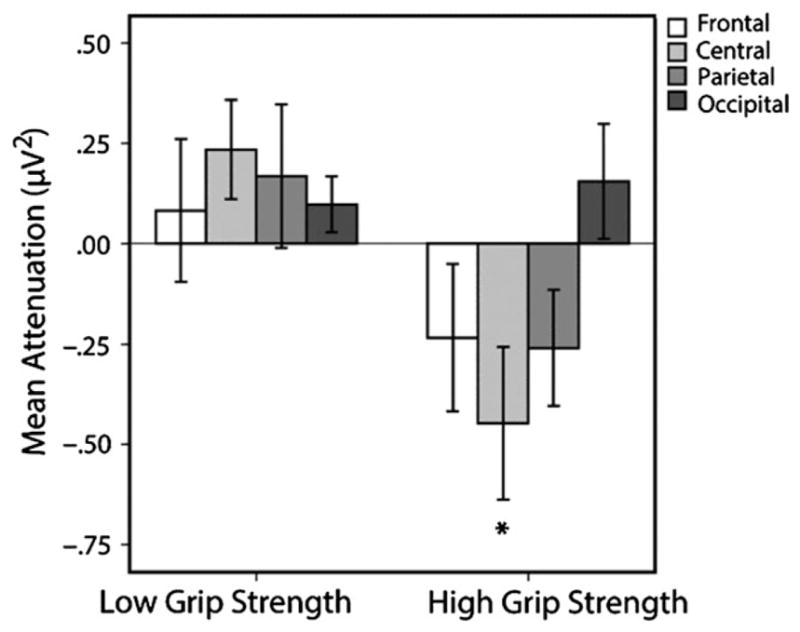
EEG activity in the 8 Hz bin during observation of all block lifts as a function of grip strength group at four scalp locations (F4, C4, P4, and O2). Significant attenuation relative to baseline was only found at the central lead, C4, for the high grip strength group of infants. Error bars represent standard error. * p = .04.
Next, we investigated relations between individual differences in infants’ grip strength and individual differences in mu attenuation during action observation. Because we hypothesized that the relation between mu attenuation and grip strength would become stronger as block weight increased (i.e. because grip strength exerts a greater impact when lifting heavy objects versus light objects), we performed Pearson’s correlations between grip strength and mu attenuation during observation of all block lifts, as well as with mu attenuation during observation of lifts for each block weight independently. In these correlational analyses, we partialled out infants’ age, in-task lifting experience, and motor development scores, in order to examine the unique relation between grip strength and mu attenuation during action observation.
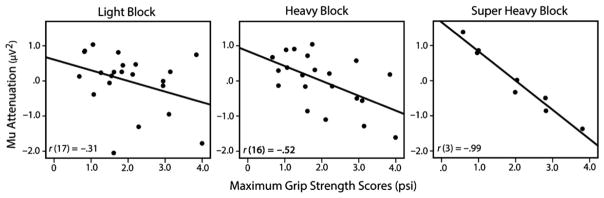
Paralleling the results of the median-split analysis, mu attenuation during observation of all block lifts was predicted by infants’ maximum grip strength scores, r(17) = −.56, p = .01, such that higher maximum grip strength scores were associated with greater mu attenuation during observation of lifts. Furthermore, analyses investigating relations between infants’ grip strength and mu attenuation during observation of lifts for each block weight independently demonstrated a progressively strong relation with increases in block weight: grip strength and mu attenuation during observation of light block lifts, r(17) = −.31, p = .20; grip strength and mu attenuation during observation of heavy block lifts, r(16) = −.52, p = .03; and grip strength and mu attenuation during observation of super heavy block lifts, r(3) = −.99, p = .002 (see Figure 3).2 The results of Steiger’s Z-test (Steiger, 1980), which analyzes the difference between dependent correlations, confirm that there is a marginal difference in the correlation between mu attenuation during observation of light block lifts and grip strength, and the correlation between mu attenuation during observation of super heavy block lifts and grip strength, z = 1.44, p = .08, one-tailed (Lee & Preacher, 2013). The difference between the two correlations is compelling, as it was found with a small sample size (i.e. n = 8, as that is the number of infants who had artifact-free observation trials with the super heavy block), and demonstrating significant differences between dependent correlations requires considerable power (Kenny, 1987). As such, it is likely that with an increased sample size this difference would have reached conventional levels of significance.
Figure 3.
Pearson’s correlations between infants’ maximum grip strength (in psi) and mu attenuation during observation of lifts with the light block, heavy block, and super heavy block. Pearson’s r values reflect the partial correlation (controlling for infants’ age, in-task experience, and motor development scores) between grip strength and mu attenuation during action observation.
Importantly, relations between infants’ grip strength and mu attenuation during observation of block lifts were maintained after controlling for the frequency of infants’ faithful reproductions (in addition to controlling for infants’ age, in-task lifting experience, and motor development scores), which suggests that accurately reproducing the experimenter’s actions did not account for these significant relations: grip strength and mu attenuation during observation of all block lifts, r(16) = −.56, p = .02; grip strength and mu attenuation during observation of light block lifts, r(16) = −.29, p = .25; grip strength and mu attenuation during observation of heavy block lifts, r(15) = −.51, p = .04; and grip strength and mu attenuation during observation of super heavy block lifts, r(2) = −.99, p = .005.
Finally, we directly investigated whether infants’ grip strength was the best predictor of variance in infants’ mu attenuation. Accordingly, we conducted a multiple regression analysis with mu attenuation during observation of all block lifts as the dependent measure, and infants’ age, motor development scores, in-task lifting experience, frequency of faithful reproductions, and grip strength as the predictor variables. The results of the multiple regression indicated that these five predictors explained 42.6% of the variance in mu attenuation, F(5, 16) = 2.37, p = .09. Critically, however, the multiple regression revealed that infants’ grip strength was the only significant predictor of mu attenuation during observation of block lifts (β = −.51, p = .02) while the other four variables were non-significant (all p values > .33).
Specificity of mu rhythm attenuation: examining EEG activity at frontal, parietal, and occipital leads
In order to confirm that the reported mu attenuation was localized over the sensorimotor cortex, we investigated EEG activity during observation of block lifts in the 8 Hz bin at frontal, parietal, and occipital leads located in the right hemisphere (corresponding to 10–20 locations F4, P4, and O2, respectively). Critically, only over the sensorimotor cortex (i.e. C4) does attenuation differ significantly from baseline, and only for the high grip strength infants (see Figure 2; all remaining p values > .10). These findings are similar to those of previous studies on the mu rhythm in both infants (Marshall et al., 2011) and adults (Babiloni, Babiloni, Carducci, Cincotti, Cocozza et al., 2002; Calmels, Hars, Jarry & Stam, 2010; Marshall, Bouquet, Shipley & Young, 2009).
In addition, we more closely investigated EEG activity at occipital lead O2 in order to confirm that the reported mu attenuation was not reflective of the occipital alpha rhythm, a separate neural frequency also located in the 6 to 9 Hz range in infancy (Stroganova, Orekhova & Posikera, 1999). Infants’ grip strength scores, age, in-task lifting experience, frequency of faithful reproductions, and motor development scores were all unrelated to EEG activity at O2 during observation of all block lifts, as well as unrelated to EEG activity at O2 during observation of block lifts for each block weight independently (all p values > .34). These findings demonstrate that the reported attenuation was specific to central leads overlaying the sensorimotor cortex, as attenuation was not found over matched frontal, parietal, or occipital leads, which confirms that our results specifically reflect attenuation of the mu rhythm frequency.
Discussion
The aim of the present study was to investigate the influence of bodily characteristics on the shared neural system underlying action perception and production during action observation in infancy. To do so, we investigated the relation between grip strength and mu attenuation during observation of lifting actions with weighted blocks in 12-month-old infants, an age at which there is significant natural variability in infants’ maximal grip strength. We found that infants with higher grip strength scores exhibited significant mu attenuation during observation of block lifts, whereas infants with lower grip strength scores did not. Furthermore, the relation between grip strength and mu attenuation varied as a function of block weight: grip strength more strongly predicted mu attenuation during observation of lifts with the heavy and super heavy blocks than with the light blocks. These relations were maintained after controlling for infants’ age, in-task lifting experience, and motor development scores, which suggests that this relation was driven by differences in infants’ grip strength per se and not other co-occurring factors.
Critically, our results confirm that differences in neural activity during observation of block lifts were found selectively at central leads, overlaying the sensorimotor cortex, and not over matched frontal, parietal, or occipital leads. These findings, coupled with our use of a well-established method of identifying infants’ mu rhythm band (i.e. based on the location and frequency of maximal mu attenuation during infants’ production of block lifts; Cuevas et al., 2014; Marshall & Meltzoff, 2011), confirm that our results specifically reflect recruitment of the underlying neural assemblies indexed by the mu rhythm, and were not due to widespread changes in neural activity during the task (e.g. changes in visual attention as a function of task complexity; Herrmann, Sensowski & Röttger, 2004). More broadly, our results bear on issues regarding the development of the shared neural system underlying action perception and production. Specifically, scholars are actively debating whether we are equipped at birth with a neural system that links produced actions to observed actions, and whether experience is requisite versus facilitative towards this system’s development (Cook, Bird, Catmur, Press & Heyes, 2014; Gallese, Rochat, Cossu & Sinigaglia, 2009). The present study contributes to this debate in two ways. First, mu attenuation during observation of block lifts was not exhibited by all infants in our sample; indeed, only the high grip strength infants exhibited significant mu attenuation during action observation. This finding, in itself, demonstrates that, irrespective of whether a shared neural system subserving action perception and production is present at birth, there are early emerging differences in activation of this system that are shaped by individual differences. Second, this study highlights the contribution of a previously unexplored factor that influences the development of this system, namely bodily characteristics. While previous work has evidenced the body’s role in perception, broadly construed (Kretch et al., 2014; Proffitt, 2006; Soska et al., 2010), these results demonstrate that bodily characteristics also specifically influence action perception, and do so early in development.
A question that follows from these results is why grip strength was related to mu attenuation during observation of block lifts. One possible explanation is that grip strength and mu attenuation were related because grip strength alters the acquisition of lifting experience –serving to either facilitate or constrain its acquisition. This interpretation would align with prior work showing that activation of the shared neural system underlying action perception and production during action observation is shaped according to bodily experience (Cross et al., 2006; van Elk et al., 2008). Although our results cannot be attributed to in-task differences in lifting experience (as we measured and controlled for this factor in our analysis), it is possible that grip strength influenced infants’ lifetime experience with lifting objects, particularly heavy objects. If this were the case in the present study, it would align with previous work that has found an effect of bodily experience on action perception, and extend it by demonstrating that acquiring experience that specifically corresponds to the actions being observed (in this case, lifting heavy objects) has a stronger influence on activation of the neural system underlying action perception than does generalized action experience (e.g. lifting any object). Moreover, if certain bodily characteristics (e.g. grip strength) prove to be an accurate proxy for aspects of infants’ lifetime experience (e.g. lifting heavy objects), this would present a substantial methodological advance in studying the effects of bodily experience on action perception by providing a quick, in-laboratory measure that captures infants’ everyday action experience (i.e. grip strength assessment).
Another possible explanation for the relation between grip strength and mu attenuation during action observation is that grip strength may have affected infants’ ability to accurately reproduce the observed lifting action, as past work has shown that a greater similarity between produced actions and observed actions leads to greater activation of the shared neural system underlying action perception and production (Reid et al., 2011; Saby et al., 2012). However, if the similarity between the infants’ and the experimenter’s actions were driving the current results, we likely would have found relations between mu attenuation and infants’ faithful reproductions of the experimenter’s actions. As this was not the case, it seems unlikely that the degree of similarity between the infants’ and experimenter’s actions accounts for the current results.
Relatedly, given the inherent kinematic differences among object-directed actions as a function of their weight (even for skilled adult experimenters), as well as differences in the resulting physical outcomes (e.g. louder ‘thumps’ when dropping heavy blocks versus light), it is possible that infants’ grip strength influences their sensitivity to these cues in others’ actions. In this way, grip strength may have been associated with mu attenuation during observation of lifts because high grip strength infants were better at recognizing differences in the experimenter’s actions as a function of block weight, as well as recognizing their associated physical outcomes, than were low grip strength infants.
A final possible explanation for the relation between grip strength and mu attenuation during action observation, and an interpretation that we favor, is that this relation may be driven by differences in infants’ ability to recognize the differential effort associated with lifting objects of various weights. As strength necessarily influences one’s ability to lift objects, it is likely that stronger infants have acquired more contrastive lifting experience (i.e. experience lifting a wider range of objects), which may help these infants recognize that object-directed actions require differing degrees of effort as a function of their weight (among other object properties). This would align with prior work that has demonstrated that experience observing contrastive outcomes allows for comparison between exemplars, which serves to accelerate infants’ learning (Wang & Baillargeon, 2008). Furthermore, weaker infants may allot more cognitive resources towards action production than do stronger infants, particularly when lifting heavy objects, which may come at the expense of attending to and encoding differences in their expended effort as a function of object weight. Thus, increased mu attenuation during action observation, as exhibited by the high grip strength infants in the current study, may reflect their recognition of the effort associated with the observed lifting action, and the progressively close relation between grip strength and mu attenuation with increased block weight may capture their understanding that effort increases with object weight.
Additional support for our interpretation is found in studies that have demonstrated greater activation of the shared neural system underlying action perception and production for individuals with experience and expertise with the witnessed action. Because action experience likely facilitates the ability to recognize the effort associated with a given action, it is possible that the results of prior studies reflect differences in the observers’ ability to recognize expended effort for particular actions (Aglioti, Cesari, Romani & Urgesi, 2008; Calvo-Merino et al., 2005; Cross et al., 2006). This novel interpretation of past results is also consistent with work by Proffitt and colleagues (Proffitt & Linkenauger, 2013) that has demonstrated that perception of the environment is scaled by the effort an individual anticipates expending during action production (e.g. perception of flat expanses is scaled by the anticipated effort to walk from point A to point B). In addition, studies have found that observing actions performed with heavy versus light objects elicits increased activation of the neural system underlying action perception and production (Alaerts et al., 2010a; Alaerts, de Beukelaar, Swinnen & Wenderoth, 2012; Senot et al., 2011), which provides further support that perception of increased effort is associated with greater activation of this system. The present study builds on these findings by suggesting that grip strength either constrains or facilitates the ability to recognize effort, such that weaker individuals may be unable to recognize and appreciate the effort expended by others, whereas stronger individuals can.
Our interpretation – that infants, as a consequence of their own developing strength, are differentially sensitive to effort – may also help to explain why a recent, similar study failed to find group-level differences in mu attenuation during action observation as a function of object weight (Marshall et al., 2013). In that study, mu rhythm activity was recorded while 14-month-old infants observed an experimenter performing actions with objects of different weights. They found no difference in mu attenuation during action observation as a function of object weight, and, moreover, the reported mu attenuation was very subtle. As such, it is likely that infants in their study, as in the current experiment, varied in strength. However, because individual differences in grip strength were not measured and accounted for in their analysis, testing a heterogeneous sample in terms of strength may have served to obscure, rather than illuminate, differences in mu attenuation that would have been found as a function of object weight.
Nevertheless, it is always possible that infants’ grip strength serves as a proxy for another, as-yet uncovered, variable that better explains variability in mu attenuation during action observation. However, future work may provide a critical test of our hypothesis that grip strength leads infants to be differentially sensitive to others’ expended effort. For example, given that individuals seek to minimize expended effort when possible (Proffitt, 2008), and given that infants preferentially select light over heavy objects (Hauf, Paulus & Baillargeon, 2012), we would predict that, after seeing another individual act on objects of varying weight, and in the absence of direct experience, infants’ tendency to select light over heavy objects should vary as a function of grip strength. Specifically, if grip strength gates the ability to recognize effort, stronger infants should be more likely to choose lighter objects over heavier objects, while weaker infants should not differentiate their actions towards the objects. These findings would support the conclusion that stronger infants are better able to recognize the differential effort associated with lifting objects of different weights than are weaker infants.
Another unresolved and contested issue concerns the functional significance of neural activation of the shared system underlying action perception and production. Specifically, some researchers have argued that activation of this system during action observation is the by-product of domain general associative learning and, as such, does not necessarily contribute to action understanding and could even be epiphenomenal (Cook et al., 2014; Heyes, 2010; Hickok, 2009). Alternatively, other researchers have proposed that activation of this system facilitates one’s ability to identify the goals and intentions underlying an observed action (Calvo-Merino, 2013; Iacoboni, Molnar-Szakacs, Gallese, Buccino, Mazziotta et al., 2005; Rizzolatti, Fogassi & Gallese, 2001). Future work can begin to directly test these competing hypotheses. If the latter hypothesis is correct, it is possible that increased mu attenuation during action observation, as exhibited by high grip strength infants in our study, represents a heightened understanding of the goals underlying the experimenter’s lifting actions (e.g. to place the block on top of, versus beside, a platform). Thus, future studies may seek to demonstrate that infants’ ability to identify the experimenter’s goal in this context is driven by their grip strength. For example, stronger infants should outperform weaker infants in visually anticipating the goal of the experimenter’s lifting actions and in predicting her subsequent actions.
To conclude, the present experiment advances prior research by demonstrating that individual differences in bodily characteristics profoundly influence how infants perceive others’ actions. Moreover, the impact of bodily characteristics on action perception emerges early in development. Our findings suggest a potential novel role for the body in perception, by either enabling or constraining infants’ ability to recognize the differential effort associated with various actions. Thus, the present study demonstrates that, beyond the acquisition of experience, the body in itself, its characteristics and capabilities, serves to uniquely shape our perceptions of the nearly ubiquitous actions of other people.
Research highlights.
Variability in 12-month-old infants’ mu attenuation during observation of lifting actions with weighted blocks was associated with their maximal grip strength, such that stronger infants exhibited greater mu attenuation during observation of lifting actions than weaker infants.
Relations between infants’ grip strength and mu attenuation during observation of lifting actions became progressively stronger as the weight of the block being lifted by the experimenter increased.
These results extend the body’s role in perception by demonstrating that activation of the neural system underlying action perception and production is gated not only by bodily experience producing actions, but also by one’s bodily characteristics, and that the influence of these characteristics on action perception emerges early in development.
Acknowledgments
This research was supported in part by grants from NIH (award #: K18OD008069) and from the University of Washington Bridge Funds. We acknowledge Robert Ross, Gerry Ebalaroza-Tunnell, Miranda Sitch, and Monica Dion for their help with data collection and coding, the Early Childhood Cognition Lab for feedback on earlier versions of this manuscript, and most especially the families who participated in this research.
Footnotes
We administered three block weights to half the sample and two block weights to the other half (randomly assigned) in order to (1) ensure that all infants interacted with at least two block weights, as well as to (2) mitigate possible frustration and noncompliance that could be elicited when infants interact with heavy objects.
We conducted another set of Pearson’s correlations between infants’ grip strength and mu attenuation during observation of block lifts, controlling for the number of squeezes on the grip strength device, and significant relations with infants’ grip strength were maintained: mu attenuation during observation of all block lifts, r(16) = −.54, p = .02; mu attenuation during observation of light block lifts, r(16) = −.31, p = .22; mu attenuation during observation of heavy block lifts, r(15) = −.54, p = .03; and mu attenuation during observation of super heavy block lifts, r(2) = −.96, p = .04.
References
- Adolph KE, Cole WG, Komati M, Garciaguirre JS, Badaly D, et al. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychological Science. 2012;23(11):1387–1394. doi: 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglioti SM, Cesari P, Romani M, Urgesi C. Action anticipation and motor resonance in elite basketball players. Nature Neuroscience. 2008;11(9):1109–1116. doi: 10.1038/nn.2182. [DOI] [PubMed] [Google Scholar]
- Alaerts K, de Beukelaar T, Swinnen S, Wenderoth N. Observing how others lift light or heavy objects: time-dependent encoding of grip force in the primary motor cortex. Psychological Research. 2012;76(4):503–513. doi: 10.1007/s00426-011-0380-1. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Senot P, Swinnen S, Craighero L, Wenderoth N, et al. Force requirements of observed object lifting are encoded by the observer’s motor system: a TMS study. European Journal of Neuroscience. 2010a;31(6):1144–1153. doi: 10.1111/j.1460-9568.2010.07124.x. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Swinnen SP, Wenderoth N. Observing how others lift light or heavy objects: which visual cues mediate the encoding of muscular force in the primary motor cortex? Neuropsychologia. 2010b;48(7):2082–2090. doi: 10.1016/j.neuropsychologia.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Cocozza G, et al. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. NeuroImage. 2002;17(2):559–572. doi: 10.1006/nimg.2002.1192. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant and toddler development – third edition: Administration manual. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- Bhalla M, Proffitt D. Visual–motor recalibration in geographical slant perception. Journal of Experimental Psychology: Human Perception and Performance. 1999;25(4):1076–1096. doi: 10.1037//0096-1523.25.4.1076. [DOI] [PubMed] [Google Scholar]
- Braadbaart L, Williams J, Waiter G. Do mirror neuron areas mediate mu rhythm suppression during imitation and action observation? International Journal of Psychophysiology. 2013;89(1):99–105. doi: 10.1016/j.ijpsycho.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink G, Fadiga L, Fogassi L, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13(2):400–404. [PubMed] [Google Scholar]
- Calmels C, Hars M, Jarry G, Stam CJ. Nonlinear EEG synchronization during observation: effects of instructions and expertise. Psychophysiology. 2010;47(5):799–808. doi: 10.1111/j.1469-8986.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B. Neural mechanisms for action observation. In: Johnson K, Shiffrar M, editors. People watching: Social, perceptual, and neurophysiological studies of body perception. Oxford: Oxford University Press; 2013. pp. 283–303. [Google Scholar]
- Calvo-Merino B, Glaser D, Grèzes J, Passingham R, Haggard P. Action observation and acquired motor skills: an fMRI study with expert dancers. Cerebral Cortex. 2005;15(8):1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, et al. Travel broadens the mind. Infancy. 2000;1(2):149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Cannon E, Yoo K, Vanderwert R, Ferrari P, Woodward A, et al. Action experience, more than observation, influences mu rhythm desynchronization. PLoS ONE. 2014;9(3):e92002. doi: 10.1371/journal.pone.0092002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casile A, Giese M. Nonvisual motor training influences biological motion perception. Current Biology. 2006;16(1):69–74. doi: 10.1016/j.cub.2005.10.071. [DOI] [PubMed] [Google Scholar]
- Cole KJ. Lifting a familiar object: visual size analysis, not memory for object weight, scales lift force. Experimental Brain Research. 2008;188(4):551–557. doi: 10.1007/s00221-008-1392-y. [DOI] [PubMed] [Google Scholar]
- Cook R, Bird G, Catmur C, Press C, Heyes C. Mirror neurons: from origin to function. Behavioral and Brain Sciences. 2014;37(02):177–192. doi: 10.1017/S0140525X13000903. [DOI] [PubMed] [Google Scholar]
- Cross E, Hamilton AFdeC, Grafton S. Building a motor simulation de novo: observation of dance by dancers. NeuroImage. 2006;31(3):1257–1267. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Cannon E, Yoo K, Fox N. The infant EEG mu rhythm: methodological considerations and best practices. Developmental Review. 2014;34(1):26–43. doi: 10.1016/j.dr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JR, Beltzner MA. Independence of perceptual and sensorimotor predictions in the size–weight illusion. Nature Neuroscience. 2000;3(7):737–741. doi: 10.1038/76701. [DOI] [PubMed] [Google Scholar]
- Gallese V, Rochat M, Cossu G, Sinigaglia C. Motor cognition and its role in the phylogeny and ontogeny of action understanding. Developmental Psychology. 2009;45(1):103–113. doi: 10.1037/a0014436. [DOI] [PubMed] [Google Scholar]
- Hauf P, Paulus M, Baillargeon R. Infants use compression information to infer objects’ weights: examining cognition, exploration, and prospective action in a preferential-reaching task. Child Development. 2012;83(6):1978–1995. doi: 10.1111/j.1467-8624.2012.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C, Sensowski D, Röttger S. Phase-locking and amplitude modulations of EEG alpha: two measures reflect different cognitive processes in a working memory task. Experimental Psychology. 2004;51(4):311–318. doi: 10.1027/1618-3169.51.4.311. [DOI] [PubMed] [Google Scholar]
- Heyes C. Mesmerising mirror neurons. NeuroImage. 2010;51(2):789–791. doi: 10.1016/j.neuroimage.2010.02.034. [DOI] [PubMed] [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. Journal of Cognitive Neuroscience. 2009;21(7):1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Müsseler J, Aschersleben G, Prinz W. The theory of event coding (TEC): a framework for perception and action planning. Behavioral and Brain Sciences. 2001;24(05):849–878. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta J, et al. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biology. 2005;3(3):e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, et al. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Kenny DA. Testing measures of association. In: Kenny DA, editor. Statistics for the social and behavioral sciences. Boston, MA: Little, Brown; 1987. pp. 270–291. [Google Scholar]
- Keuken M, Hardie A, Dorn B, Dev S, Paulus M, et al. The role of the left inferior frontal gyrus in social perception: an rTMS study. Brain Research. 2011;1383:196–205. doi: 10.1016/j.brainres.2011.01.073. [DOI] [PubMed] [Google Scholar]
- Kretch KS, Franchak JM, Adolph KE. Crawling and walking infants see the world differently. Child Development. 2014;85(4):1503–1518. doi: 10.1111/cdev.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IA, Preacher KJ. Calculation for the test of the difference between two dependent correlations with one variable in common [Computer software] 2013 Sep; Available from http://quantpsy.org.
- Loucks J, Sommerville JA. Developmental changes in the discrimination of dynamic human actions in infancy: development of action discrimination. Developmental Science. 2012;15(1):123–130. doi: 10.1111/j.1467-7687.2011.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks J, Sommerville JA. Attending to what matters: flexibility in adults’ and infants’ action perception. Journal of Experimental Child Psychology. 2013;116(4):856–872. doi: 10.1016/j.jecp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bouquet CA, Shipley TF, Young T. Effects of brief imitative experience on EEG desynchronization during action observation. Neuropsychologia. 2009;47(10):2100–2106. doi: 10.1016/j.neuropsychologia.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Marshall P, Meltzoff AN. Neural mirroring systems: exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1(2):110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P, Saby J, Meltzoff A. Infant brain responses to object weight: exploring goal-directed actions and self-experience. Infancy. 2013;18(6):942–960. doi: 10.1111/infa.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P, Young T, Meltzoff A. Neural correlates of action observation and execution in 14-month-old infants: an event-related EEG desynchronization study. Developmental Science. 2011;14(3):474–480. doi: 10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima T, Simpkins N, Oluwatimilehin T, Hallett M. Force level modulates human cortical oscillatory activities. Neuroscience Letters. 1999;275(2):77–80. doi: 10.1016/s0304-3940(99)00734-x. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S, Johnson B, McNair N. Mu rhythm modulation during observation of an object-directed grasp. Cognitive Brain Research. 2004;19(2):195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Paulus M, Hunnius S, van Elk M, Bekkering H. How learning to shake a rattle affects 8-month-old infants’ perception of the rattle’s sound: electrophysiological evidence for action–effect binding in infancy. Developmental Cognitive Neuroscience. 2012;2(1):90–96. doi: 10.1016/j.dcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J. The functional significance of mu rhythms: translating ‘seeing’ and ‘hearing’ into ‘doing’. Brain Research Reviews. 2005;50(1):57–68. doi: 10.1016/j.brainres-rev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Proffitt D. Embodied perception and the economy of action. Perspectives on Psychological Science. 2006;1(2):110–122. doi: 10.1111/j.1745-6916.2006.00008.x. [DOI] [PubMed] [Google Scholar]
- Proffitt D. An action-specific approach to spatial perception. In: Klatzky RL, MacWhinney B, Behrman M, editors. Embodiment, ego-space, and action. New York: Psychology Press; 2008. pp. 179–202. [Google Scholar]
- Proffitt D, Linkenauger S. Perception viewed as a phenotypic expression. In: Prinz W, Beisert M, Herwig A, editors. Action science: Foundations of an emerging discipline. Cambridge, MA: MIT Press; 2013. pp. 171–197. [Google Scholar]
- Reid V, Striano T, Iacoboni M. Neural correlates of dyadic interaction during infancy. Developmental Cognitive Neuroscience. 2011;1(2):124–130. doi: 10.1016/j.dcn.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2(9):661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Saby J, Marshall P, Meltzoff A. Neural correlates of being imitated: an EEG study in preverbal infants. Social Neuroscience. 2012;7(6):650–661. doi: 10.1080/17470919.2012.691429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senot P, D’Ausilio A, Franca M, Caselli L, Craighero L, et al. Effect of weight-related labels on corticospinal excitability during observation of grasping: a TMS study. Experimental Brain Research. 2011;211(1):161–167. doi: 10.1007/s00221-011-2635-x. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Sommerville J, Hildebrand E, Crane C. Experience matters: the impact of doing versus watching on infants’ subsequent perception of tool-use events. Developmental Psychology. 2008;44(5):1249–1256. doi: 10.1037/a0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville J, Woodward A, Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96(1):B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soska KC, Adolph KE, Johnson SP. Systems in development: motor skill acquisition facilitates three-dimensional object completion. Developmental Psychology. 2010;46(1):129–138. doi: 10.1037/a0014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V, Johnson MH, Karoui IE, Csibra G. Motor system activation reveals infants’ on-line prediction of others’ goals. Psychological Science. 2010;21(3):355–359. doi: 10.1177/0956797610362058. [DOI] [PubMed] [Google Scholar]
- Stroganova T, Orekhova E, Posikera I. EEG alpha rhythm in infants. Clinical Neurophysiology. 1999;110(6):997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Vanderwert R, Fox N, Ferrari P. The mirror mechanism and mu rhythm in social development. Neuroscience Letters. 2013;540:15–20. doi: 10.1016/j.neulet.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, van Schie HT, Hunnius S, Vesper C, Bekkering H. You’ll never crawl alone: neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage. 2008;43(4):808–814. doi: 10.1016/j.neuroimage.2008.07.057. [DOI] [PubMed] [Google Scholar]
- Wang S, Baillargeon R. Can infants be ‘taught’ to attend to a new physical variable in an event category? The case of height in covering events. Cognitive Psychology. 2008;56(4):284–326. doi: 10.1016/j.cogpsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind A, Takken T, Helders P, Engelbert R. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? European Journal of Pediatrics. 2010;169(3):281–287. doi: 10.1007/s00431-009-1010-4. [DOI] [PubMed] [Google Scholar]
- Woollacott M. Early postnatal development of posture control: normal and abnormal aspects. In: Kalveerboer AF, Hopkins B, Geuze R, editors. Motor development in early and later childhood: Longitudinal approaches. Cambridge: Cambridge University Press; 1993. pp. 89–108. [Google Scholar]
- World Health Organization. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height, and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. [Google Scholar]