Vol. 214, No. 7, July 2017. https://doi.org/10.1084/jem.20161070
The authors regret that an error appeared in their original version of Fig. 4. Some of the PE and PS species listed on the x axis of Fig. 4 B were incorrect. The corrected figure and corresponding legend appear below.
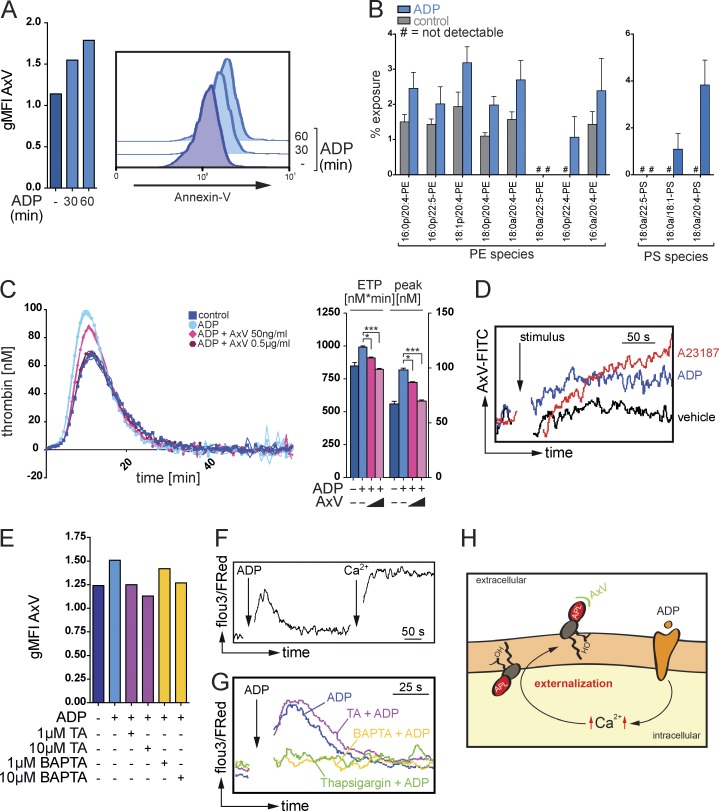
Figure 4.
Ca2+-dependent exposure of aminophospholipids by eosinophils promote thrombin generation. (A) Flow cytometry analysis of the binding of annexin V (AxV) to aminophospholipids on the surface of resting or ADP-stimulated mouse eosinophils. Histograms show representative annexin V stainings, and bar graphs show mean geometric fluorescence intensities (gMFI). (B) LC/MS/MS-based quantification of the exposure of the aminophospholipids PE and PS in mouse eosinophils in response to ADP stimulation. (C, left) Calibrated thrombin generation assays with resting or ADP-stimulated mouse eosinophils in the presence of annexin V. (Right) Bar graphs show endogenous thrombin potential (ETP; nM*min) and peak of thrombin generation (peak; nM). (D) Flow cytometry analysis of annexin V binding on mouse eosinophils over time in the presence of calcium ionophore A23187, ADP, or vehicle. (E) Flow cytometry analysis of annexin V binding on mouse eosinophils in the presence of tannic acid (TA) or intracellular Ca2+-chelator BAPTA/AM. Bar graphs show geometric mean fluorescence intensity. (F) Flow cytometry–based analysis of intracellular Ca2+ signaling, indicated by Fluo3/FuraRed ratio, over time in a Ca2+-free environment. Where indicated (arrow and Ca2+), CaCl2 at a final concentration of 1 mM was added. (G) Flow cytometry–based analysis of intracellular Ca2+ signaling, indicated by Fluo3/FuraRed ratio, over time in a Ca2+-free environment. (H) Postulated mechanism of a sequential generation and Ca2+-dependent externalization of aminophospholipids (APL) at the surface of eosinophils. OH indicates hydroxyl group. Data are representative of at least three independent experiments. Error bars represent SEM. *, P < 0.05; ***, P < 0.001.
The online HTML and PDF versions of this paper have been corrected. The error remains only in the print version.