Janody previews studies from the Knust and Djiane laboratories that identify Big bang as a new growth regulator in Drosophila melanogaster.
Abstract
How tissue growth is regulated during development and cancer is a fundamental question in biology. In this issue, Tsoumpekos et al. (2018. J. Cell Biol. https://doi.org/10.1083/jcb.201705104) and Forest et al. (2018. J. Cell Biol. https://doi.org/10.1083/jcb.201705107) identify Big bang (Bbg) as an important growth regulator of the Drosophila melanogaster wing imaginal disc.
The Drosophila wing imaginal disc is the proliferating larval epithelial tissue, which forms the adult wing appendages of the fly. It is currently one of the best-studied model systems for deciphering growth regulatory mechanisms. The epithelial organization of the wing disc is defined by the cells’ polarity along an apical–basal axis, which establishes distinct membrane domains. Among those, the apical E-cadherin–mediated junctional complex connected to the actin cytoskeleton has been implicated in sensing and transmitting tension into downstream biochemical signaling (Sluysmans et al., 2017). Drosophila is a powerful model to carry out genetic screens, which identified several evolutionarily conserved factors and pathways controlling cell growth, death, and proliferation. Among those, cell polarity pathways and the actomyosin cytoskeleton are now well recognized as essential regulators of tissue growth. They do so by modulating the activity of signal transduction pathways, one of those being the conserved Hippo signaling pathway (Gaspar and Tapon, 2014; Rauskolb et al., 2014; Deng et al., 2015; Richardson and Portela, 2017). These observations indicate that the cell cytoarchitecture has a profound impact on the expression of pro-proliferative and antiapoptotic genes. Despite these major advances, important questions remain, such as how tension on actin filaments is spatially and temporally regulated at the cell membrane to achieve proper tissue growth and how these mechanical cues impact biochemical signaling, leading to changes in gene expression. Addressing these questions has been difficult because the actomyosin cytoskeleton, as well as the plethora of actin-binding proteins involved in actin filament polymerization, depolymerization, and organization, controls multiple cellular functions. In this issue, Tsoumpekos et al. and Forest et al. take a different angle to solve this issue by focusing on the PDZ domain protein Big bang (Bbg), hypothesizing that such scaffolding proteins coordinate cell polarity pathways, actin cytoskeleton regulation, and signaling through their ability to organize multiprotein complexes.
In complementary studies, one team came to study Bbg through a hypothesis-generating genetic screen for wing growth modulators, whereas the second explored Bbg function through a candidate, hypothesis-driven approach. The researchers found that Bbg localized with E-cadherin at the apical cytocortex. Using flies expressing RNA interference constructs knocking down bbg, as well as bbg mutant alleles, the authors demonstrate that bbg sustained wing disc growth, at least partially by promoting cell survival. Bbg has no major effect on the maintenance of cell polarity determinants at the apical membrane but acts as a major organizer of the apical cytocortex, promoting actin filament accumulation, apical cell constriction (Forest et al., 2018; Tsoumpekos et al., 2018), and junctional tension (Tsoumpekos et al., 2018). Mechanistically, Bbg was found by both teams in protein complex with the regulatory light chain of Myosin II Spaghetti squash (Sqh::GFP), and Bbg and Sqh::GFP colocalized at the apical cell cortex, where Bbg stabilized Sqh::GFP (Tsoumpekos et al., 2018) and potentiated its active phosphorylated form (Forest et al., 2018; Tsoumpekos et al., 2018). Like removing Bbg function, Sqh-deficient wing disc cells were apically enlarged and the resulting adult wings were undergrown (Tsoumpekos et al., 2018). In agreement with a role for Sqh in supporting wing growth downstream of Bbg, expressing a constitutive active form of Sqh (ShqE20E21) in bbg-deficient wing cells was sufficient to restore apical cell constriction (Forest et al., 2018), junctional tension, and actin filament accumulation (Tsoumpekos et al., 2018). More importantly, ShqE20E21 also rescued the size of bbg-deficient wings (Forest et al., 2018; Tsoumpekos et al., 2018). Thus, the scaffolding protein Bbg controls wing growth by promoting tension through the regulation of an apical actomyosin network (Fig. 1).
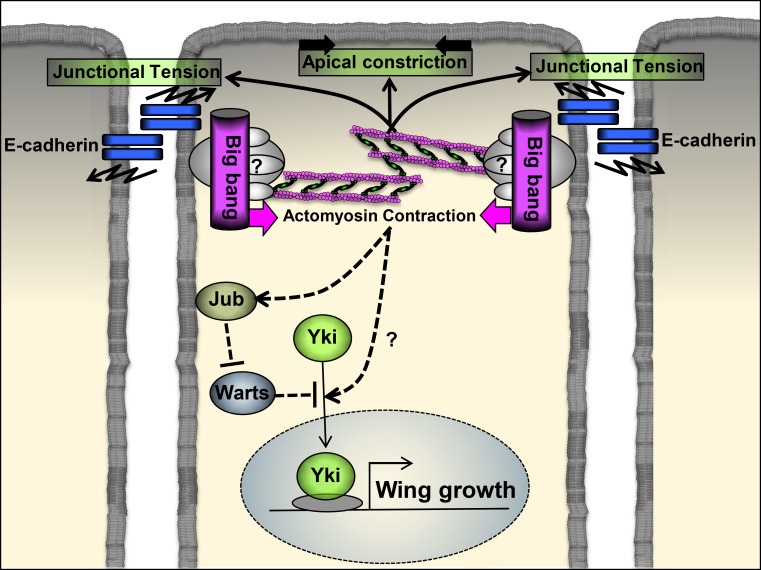
Figure 1.
Cytoskeletal tension promoted by the scaffolding protein Bbg at the apical cortex promotes wing disc growth by potentiating Yki activity. Bbg localizes at the apical cytocortex in proximity to E-cadherin, where it might organize a multiprotein complex, which includes Sqh and other unknown proteins. In addition, Bbg potentiates Sqh phosphorylation, which promotes actomyosin contraction. In turn, tension on actin filaments induces apical contraction, junctional tension, and wing disc growth by triggering Yki activity in part through the local recruitment of Jub, which inhibits Warts activity.
Recent studies have reported that actomyosin activity, promoted by Rok–myosin II or inhibited by the αβ/αβΗ spectrin heterotetramers, potentiates the pro-proliferative and antiapoptotic activity of the cotranscription factor Yorkie (Yki-YAP/TAZ in mammals), normally kept in check by two serine/threonine protein kinases, Hippo (MST1/2 in mammals) and Warts (LATS1/2 in mammals; Rauskolb et al., 2014; Deng et al., 2015). Forest et al. (2018) found that Bbg is required to potentiate the expression of Yki target genes. The authors also observed that Bbg colocalizes with the βH-Spectrin Karst (Kst) and both interact physically, so it is possible that Bbg regulates Hippo pathway activity by counteracting the inhibitory effect of the apical αβΗ spectrin actin network on myosin activity. However, Bbg and Kst may control Hippo signaling activity using independent mechanisms. Forest et al. (2018) propose that cytoskeletal tension promoted by Bbg at the apical cortex potentiates Yki activity in part through the local recruitment of Ajuba (Jub), which inhibits Warts activity (Fig. 1). In contrast, Jub membrane recruitment is not affected by Kst (Deng et al., 2015). This raises the question of whether actomyosin activities generated at different subdomains of the cortical membrane control distinct members of the Hippo signaling pathway. The observation by Forest et al. (2018) that Bbg exists in two pools at the cell cortex, one being dependent of Kst for localization, is consistent with this hypothesis. A pool of Bbg could recruit Jub by promoting actomyosin activity on an actin filament population free of spectrin. In contrast, a fraction of Bbg, recruited by the αβΗ spectrin actin network, could control Hippo pathway activity independently of Jub membrane recruitment by inhibiting the repressive role of spectrin on myosin activity. Identifying additional components recruited by Bbg and unraveling their possible function will further our understanding of the protein network involved in actomyosin activity that fine-tunes tissue growth.
The shared similarities of disc epithelia with human epithelia, as well as the high level of functional conservation of Hippo pathway components and their mode of regulation, have highlighted the relevance of discoveries made with imaginal discs for cancer research (Beira and Paro, 2016). Interestingly, the closest human bbg orthologue, PDZ domain containing 2 (PDZD2), is up-regulated in primary prostate tumors (Chaib et al., 2001). Whether PDZD2 promotes tumor growth and does so by potentiating YAP/TAZ activities via stress on actin filaments would be interesting to test.
We are still a long way from having a complete picture of the diversity and origin of mechanical cues that impinge on growth-regulatory signaling to the nucleus. However, the studies from Tsoumpekos et al. (2018) and Forest et al. (2018) bring an important piece to this puzzle that will undoubtedly inspire further investigation.
Acknowledgments
I apologize to colleagues whose work could not be cited because of length restrictions. I specially thank Eurico Morais-de-Sá and Patricia S. Guerreiro for comments on the manuscript.
Research in the Janody laboratory is supported by funds from Fundação para a Ciência e Tecnologia (FCT; PTDC/BIA-BCM/121455/2010) and from FCT cofinanced by European Regional Development Fund through Programa Operacional Competitividade e Internacionalização (POCI-01-0145-FEDER-016390). Florence Janody is the recipient of Fundação para a Ciência e Tecnologia IF/01031/2012.
The author declares no competing financial interests.
References
- Beira J.V., and Paro R.. 2016. The legacy of Drosophila imaginal discs. Chromosoma. 125:573–592. 10.1007/s00412-016-0595-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaib H., Rubin M.A., Mucci N.R., Li L., Taylor J.M.G., Day M.L., Rhim J.S., and Macoska J.A.. 2001. Activated in prostate cancer: A PDZ domain-containing protein highly expressed in human primary prostate tumors. Cancer Res. 61:2390–2394. [PubMed] [Google Scholar]
- Deng H., Wang W., Yu J., Zheng Y., Qing Y., and Pan D.. 2015. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. eLife. 4:e06567 10.7554/eLife.06567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest E., Logeay R., Géminard C., Kantar D., Frayssinoux F., Heron-Milhavet L., and Djiane A.. 2018. The apical scaffold big bang binds to spectrins and regulates the growth of Drosophila melanogaster wing discs. J. Cell Biol. 10.1083/jcb.201705107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P., and Tapon N.. 2014. Sensing the local environment: Actin architecture and Hippo signalling. Curr. Opin. Cell Biol. 31:74–83. 10.1016/j.ceb.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Rauskolb C., Sun S., Sun G., Pan Y., and Irvine K.D.. 2014. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell. 158:143–156. 10.1016/j.cell.2014.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H.E., and Portela M.. 2017. Tissue growth and tumorigenesis in Drosophila: Cell polarity and the Hippo pathway. Curr. Opin. Cell Biol. 48:1–9. 10.1016/j.ceb.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Sluysmans S., Vasileva E., Spadaro D., Shah J., Rouaud F., and Citi S.. 2017. The role of apical cell-cell junctions and associated cytoskeleton in mechanotransduction. Biol. Cell. 109:139–161. 10.1111/boc.201600075 [DOI] [PubMed] [Google Scholar]
- Tsoumpekos G., Nemetschke L., and Knust E.. 2018. Drosophila Big bang regulates the apical cytocortex and wing growth through junctional tension. J. Cell Biol. 10.1083/jcb.201705104 [DOI] [PMC free article] [PubMed] [Google Scholar]