Abstract
Rationale:
Only a few cases of putative lung adenocarcinoma presenting as carcinoma of unknown primary site (CUP) with epidermal growth factor receptor (EGFR) mutation have been reported, and the efficacy of EGFR-tyrosine kinase inhibitors (TKIs) for these cases is unclear.
Patient concerns and diagnoses:
A 67-year-old man complained of paresis of the right lower extremity, dysarthria, and memory disturbance. Computed tomography and magnetic resonance imaging showed multiple brain tumors with brain edema and swelling of the left supraclavicular, mediastinal, and upper abdominal lymph nodes. Moreover, a metastatic duodenal tumor was detected via upper gastrointestinal endoscopy examination. The biopsy specimen of the lesion was examined and was diagnosed as adenocarcinoma with CK7 and TTF-1 positivity. Finally, the case was diagnosed as EGFR mutation-positive putative lung adenocarcinoma presenting as CUP.
Interventions and outcomes:
Oral erlotinib, an EGFR-TKI, was administered at 150 mg daily. Five weeks later, the brain lesions and several swollen lymph nodes showed marked improvement, and the symptoms of the patient also improved. Three months later, the duodenal lesion was undetected on upper gastrointestinal endoscopy. After an 8-month follow-up, the patient was well with no disease progression.
Lessons:
Putative lung adenocarcinoma presenting as CUP may have EGFR mutation, and EGFR-TKI therapy may be effective for such malignancy.
Keywords: carcinoma of unknown primary site, epidermal growth factor receptor mutation, putative lung adenocarcinoma
1. Introduction
Carcinoma of unknown primary site (CUP) accounts for 3% to 5% of all cancer diagnoses.[1] CUP patients in whom the primary site can be predicted have better outcomes.[2] Therefore, CUP tumors have been categorized according to histopathological structure, metastatic sites, serum tumor markers, and immunohistochemical examinations to determine the primary sites.[3,4] Approximately 60% of CUP tumors are adenocarcinomas.[3] Previous studies reported that adenocarcinomas that are immunohistochemically positive for cytokeratin (CK) 7, thyroid transcription factor (TTF)-1, or Napsin A and negative for CK 20 should be presumed as lung adenocarcinoma, and these tumors should be treated similarly to primary lung adenocarcinoma.[4] Approximately 15% to 40% of primary lung adenocarcinomas have epidermal growth factor receptor (EGFR) mutations,[5] and treatment with EGFR-tyrosine kinase inhibitors (TKIs) prolong the survival of patients with EGFR mutation-positive lung cancer.[5,6] However, case reports of putative lung adenocarcinoma with EGFR mutation are limited,[7–9] and the efficacy of EGFR-TKI for these cases is unclear.
Herein, we report a case of EGFR mutation-positive putative lung adenocarcinoma presenting as CUP showing good response to EGFR-TKI therapy.
2. Case presentation
A 67-year-old man with a 147 pack-year smoking history presented to a hospital with chief complaints of paresis of right lower extremity, dysarthria, and memory disturbance. No particular personal and family medical history was reported, except for his type 2 diabetes mellitus. On physical examination, he had a paresis of right lower extremity and dysarthria.
Brain computed tomography (CT) and magnetic resonance imaging (MRI) revealed multiple brain tumors with brain edema (Fig. 1A). The brain tumors were suspected to be metastatic tumors. The serum carcinoembryonic antigen (CEA) level was increased (29.6 ng/mL). Other tumor markers were within the normal range. Neck, chest, and abdominal CT examination was performed, and swelling of the left supraclavicular, mediastinal, and upper abdominal lymph nodes were detected (Fig. 1B, C). However, the primary site of the tumor could not be determined. He was transferred to our hospital and was initially treated with whole-brain radiation therapy. After, he underwent [18F]-fluorodeoxyglucose (FDG) positron emission tomography, and high FDG uptake was detected at the same lymph nodes detected via CT examination. However, the primary site of the tumor still could not be determined (Fig. 1D). Consequently, he underwent upper gastrointestinal endoscopic examination, and metastatic duodenal tumor was detected (Fig. 2A). Histopathological examination showed that the tumor was an adenocarcinoma via (Fig. 2B). Immunohistochemical staining of the tumor specimen showed CK7 and TTF-1 positivity (Fig. 2C, D). Based on the cytological feature and histological structure of the adenocarcinoma and the results of immunohistochemical staining, the primary site of the adenocarcinoma was presumed to be the lung. The tumor specimen was also examined similar to that for advanced primary lung adenocarcinoma as follows: EGFR mutation, anaplastic lymphoma kinase (ALK) gene rearrangement, c-ros oncogene 1 (ROS1) rearrangement, and programmed death-ligand 1 (PD-L1) expression. EGFR exon 19 deletion and PD-L1 positivity (tumor proportion score [TPS]: 80%) were detected.
Figure 1.
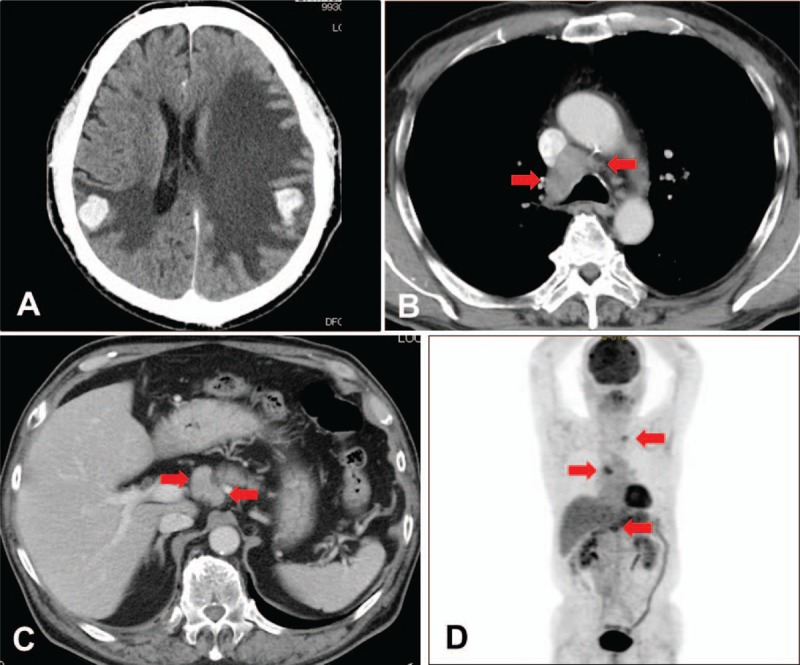
Initial computed tomography (CT) and [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET). (A) CT scan showed multiple nodules and edema in the bilateral cerebral hemispheres. (B) CT scan showed swelling of the mediastinal lymph nodes (arrowhead). (C) CT scan showed upper abdominal lymph node swelling (arrowhead). (D) FDG-PET scan demonstrated high FDG uptake at the same lymph nodes detected via CT scan (arrowhead).
Figure 2.
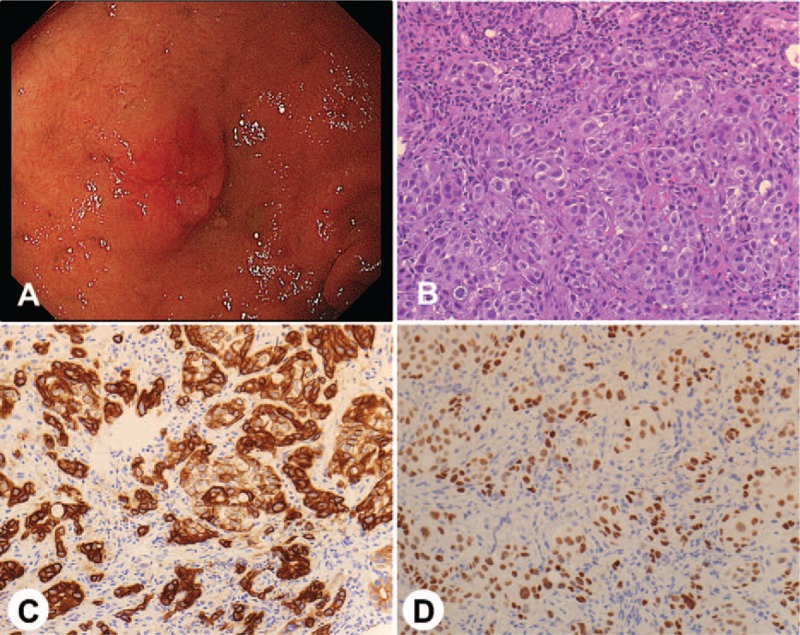
(A) Upper gastrointestinal endoscopy showed a metastatic duodenal tumor. (B) A biopsy specimen showed histopathological findings of adenocarcinoma (hematoxylin and eosin stain, magnification ×200). (C) Immunohistochemical staining of the specimen showed CK7 positivity of the tumor cells (magnification ×200). (D) Immunohistochemical staining of the specimen showed TTF-1 positivity of the tumor cells (magnification ×200).
Oral erlotinib, an EGFR-TKI, was administered daily at 150 mg. Five weeks later, the brain lesions and several swelling lymph nodes showed marked improvement on CT examination (Fig. 3A-C). The patient's paresis of the right lower extremity, dysarthria, and memory disturbance also improved. Three months later, the duodenal lesion was undetected on upper gastrointestinal endoscopic examination (Fig. 3D). The serum CEA level was also decreased (4.0 ng/mL). Regarding erlotinib-related adverse events, only a grade 1 rash occurred. To date, the patient is alive with no complaints and no disease progression and has continued erlotinib for a total of 8 months. Informed consent was signed by the patient.
Figure 3.
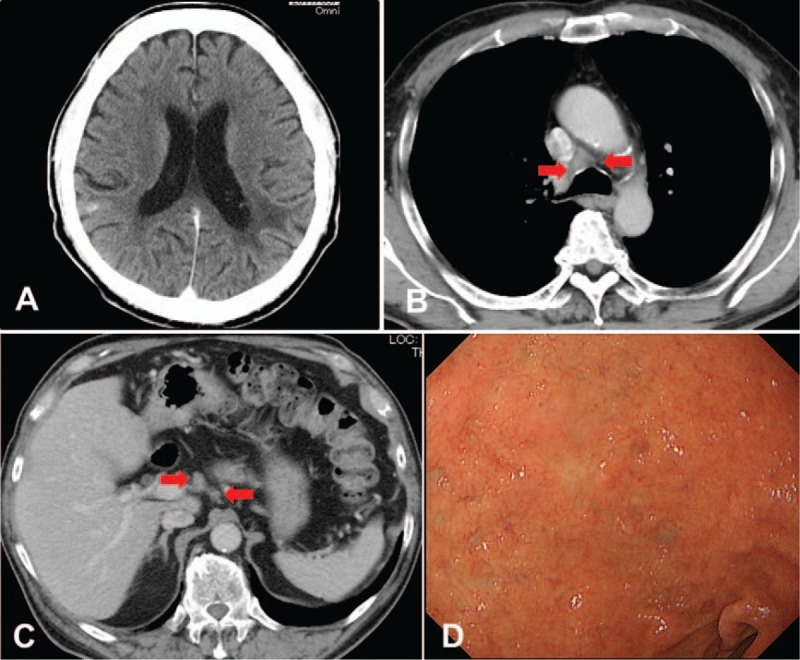
Computed tomography (CT) scan after starting the treatment for the tumor. (A) CT scan showed shrinkage of multiple nodules and improvement of edema in the bilateral cerebral hemispheres. (B) CT scan showed shrinkage of mediastinal lymph nodes (arrowhead). (C) CT scan showed shrinkage of the upper abdominal lymph nodes (arrowhead). (D) Upper gastrointestinal endoscopy demonstrated marked shrinkage of the metastatic duodenal tumor.
3. Discussion
In the present case report, we showed two important clinical observations. First, putative lung adenocarcinoma presenting as CUP may have EGFR mutation. To our knowledge, only three cases of CUP with EGFR mutation has been reported previously,[7–9] and 2 of the 3 cases were putative lung adenocarcinomas as diagnosed via immunohistochemical examinations.[7,8] In the study of CUP with no presumable primary site, EGFR mutation was detected in 6% of the study population.[10] Meanwhile, in the study of putative lung cancers evaluated via a 92-gene molecular cancer classifier assay, EGFR mutation was not detected, but ALK rearrangement was detected in 4 of the 21 cases.[11] The frequency of EGFR mutation in CUP is unknown, but driver oncogenes, such as EGFR mutation and ALK rearrangement, should be at least examined in putative lung adenocarcinomas.
Second, EGFR-TKI therapy may be effective for EGFR mutation-positive putative lung adenocarcinoma. The efficacy of EGFR-TKI was shown in all 3 cases of EGFR mutation-positive CUP previously reported.[7–9] EGFR mutation-positive primary lung cancer should be treated with EGFR-TKI because of its proven efficacy[5]; however, the efficacy of EGFR-TKI for EGFR mutation-positive putative lung cancer is unclear. A prospective study is required to evaluate the efficacy of EGFR-TKI for EGFR mutation-positive CUP.
In clinical practice, routine genetic examination for CUP with no presumable primary site is difficult. Previous studies reported that oncogenic driver mutations, such as EGFR mutation, ALK rearrangement, BRAF mutation, ROS1 rearrangement, RET rearrangement, and MET amplification, have been detected in CUP.[10,12] However, comprehensive genetic examination is required to detect these mutations. The prognosis of CUP with no presumable primary site is poor (6–10 months)[2]; therefore, genetic examination to detect driver oncogenes associated with targeted therapies is reasonable to improve the prognosis of CUP. Currently, the development of gene panels for cancer has progressed.[13–16] Further improvement and cost reduction of genetic examination to detect driver oncogene will ameliorate the prognosis of CUP.
Recently, immune-checkpoint inhibitors have been widely used for primary lung cancer.[17] Moreover, first-line treatment with pembrolizumab, an immune-checkpoint inhibitor, has been shown to improve the survival of patients with primary lung cancer if PD-L1-TPS of the lung cancer is 50% or more.[18] Therefore, PD-L1-TPS examinations is essential to determine the appropriate treatment for the primary lung cancer. However, the efficacy of immune-checkpoint inhibitor therapy for putative lung cancer is unknown. A prospective study is required to establish the usefulness of the PD-L1-TPS examination for putative lung cancer.
In conclusion, we report a case of EGFR mutation-positive putative lung adenocarcinoma presenting as CUP showing good response to EGFR-TKI therapy. Driver oncogenes, such as EGFR mutation and ALK rearrangement, should be examined in putative lung adenocarcinoma similar to that for primary lung adenocarcinoma.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Abbreviations: ALK = anaplastic lymphoma kinase, CEA = carcinoembryonic antigen, CK = cytokeratin, CT = computed tomography, CUP = carcinoma of unknown primary site, EGFR = epidermal growth factor receptor, FDG = fluorodeoxyglucose, MRI = magnetic resonance imaging, PD-L1 = programmed death-ligand 1, ROS1 = c-ros oncogene 1, TKI = tyrosine kinase inhibitor, TPS = tumor proportion score, TTF = thyroid transcription factor.
The authors have no ethical conflicts to disclose.
The authors declare that they have no conflicts of interest.
References
- [1].Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet 2012;379:1428–35. [DOI] [PubMed] [Google Scholar]
- [2].Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin—diagnosis and treatment. Nat Rev Clin Oncol 2011;8:701–10. [DOI] [PubMed] [Google Scholar]
- [3].Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med 2014;371:757–65. [DOI] [PubMed] [Google Scholar]
- [4].Conner JR, Hornick JL. Metastatic carcinoma of unknown primary: diagnostic approach using immunohistochemistry. Adv Anat Pathol 2015;22:149–67. [DOI] [PubMed] [Google Scholar]
- [5].Nan X, Xie C, Yu X, et al. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget 2017;8:75712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Takano T, Fukui T, Ohe Y, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 2008;26:5589–95. [DOI] [PubMed] [Google Scholar]
- [7].Kuwata T, Iwata T, Iwanami T. Napsin A and thyroid transcription factor-1-positive cerebellar tumor with epidermal growth factor receptor mutation. Case Rep Oncol 2011;4:564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yamada T, Ohtsubo K, Ishikawa D, et al. Cancer of unknown primary site with epidermal growth factor receptor mutation for which gefitinib proved effective. Gan To Kagaku Ryoho 2012;39:1291–4. Japanese. [PubMed] [Google Scholar]
- [9].Tan DS, Montoya J, Ng QS, et al. Molecular profiling for druggable genetic abnormalities in carcinoma of unknown primary. J Clin Oncol 2013;31:e237–9. [DOI] [PubMed] [Google Scholar]
- [10].Ross JS, Wang K, Gay L, et al. Comprehensive genomic profiling of carcinoma of unknown primary site new routes to targeted therapies. JAMA Oncol 2015;1:40–9. [DOI] [PubMed] [Google Scholar]
- [11].Hainsworth JD, Anthony Greco F. Lung adenocarcinoma with anaplastic lymphoma kinase (ALK) rearrangement presenting as carcinoma of unknown primary site: recognition and treatment implications. Drugs Real World Outcomes 2016;3:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Löffler H, Pfarr N, Kriegsmann M, et al. Molecular driver alterations and their clinical relevance in cancer of unknown primary site. Oncotarget 2016;7:44322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patel R, Tsan A, Tam R, et al. Mutation scanning using MUT-MAP, a high-throughput, microfluidic chip-based, multi-analyte panel. PLoS ONE 2012;7:e51153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schleifman EB, Tam R, Patel R, et al. Next generation MUT-MAP, a high-sensitivity high-throughput microfluidics chip-based mutation analysis panel. PLoS ONE 2014;9:e90761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tops BB, Normanno N, Kurth H, et al. Development of a semi-conductor sequencing-based panel for genotyping of colon and lung cancer by the Onconetwork consortium. BMC Cancer 2015;15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wing MR, Reeser JW, Smith AM, et al. Analytic validation and real-time clinical application of an amplicon-based targeted gene panel for advanced cancer. Oncotarget 2017;8:75822–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Assi HI, Kamphorst AO, Moukalled NM, et al. Immune checkpoint inhibitors in advanced non–small cell lung cancer. Cancer 2017;124:246–61. [DOI] [PubMed] [Google Scholar]
- [18].Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]


