Abstract
Background:
This trial aimed to evaluate the efficacy and safety of roflumilast for treating Chinese patients with chronic obstructive pulmonary disease (COPD).
Methods:
A total of 120 patients with COPD were recruited and were randomly divided into 2 groups (an intervention group and a placebo group) at a 1:1 ratio. Patients received either roflumilast or placebo 500 μg once daily for a total of 12 months. The primary outcome was lung function, measured by the change from baseline of forced expiratory volume in 1 second (FEV1), FVC = forced vital capacity (FVC), and FEF25–75%. The secondary outcome measurements included the quality of life, measured with the St. George's Respiratory Questionnaire (SGRQ). All outcomes were measured at the end of 12-month treatment and 3-month follow-up after the treatment. In addition, adverse events (AEs) were also recorded during the treatment period.
Results:
FEV1, FVC, FEF25–75%, and SGRQ were significantly better in the intervention group than those in the placebo group at the end of 12-month treatment and 3-month follow up after treatment. Moreover, AEs were much higher with roflumilast than placebo in this study.
Conclusions:
The findings suggest that roflumilast has promising effect to improve lung function in Chinese population with COPD.
Keywords: chronic obstructive pulmonary disease, clinical trial, efficacy, roflumilast
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic pulmonary condition. It often involves the limitation of irreversible airflow and the gradual loss of lung function.[1] It has been reported that the prevalence of COPD was 8.2% in Chinese population aged more than 40 years old.[2] However, this figure may still be underestimated because many people with COPD remain undiagnosed.[3] Additionally, this kind of condition not only brings a significant burden for patients, families, and the social healthcare systems, but also is associated with poor health-related quality of life in patients.[4]
Present therapy for moderate to severe COPD include the combined interventions of smoking cessation, involving long-acting β 2 agonist (LABA)/inhaled corticosteroid (ICS) or LABA/long-acting muscarinic antagonist (LAMA). Although those therapies are effective in decreasing airflow obstruction and enhancing quality of life in patients with COPD, more effective and safe therapies are still looked for preventing and treating its exacerbations and progression.[1,5]
Roflumilast is an oral, potent, and selective inhibitor of phosphodiesterase 4 (PDE4). It has a half-life compatible with once-daily dose. It is reported that roflumilast can inhibit the release of mediators from activated inflammatory cells,[6,7] and can reduce the absolute number of neutrophils and eosinophils in induced sputum.[8] Additionally, clinical trials also reported that roflumilast was associated with significant enhancements of lung function and quality of life in patients with COPD.[9,10] However, ethnic differences may lead to efficacy and safety differences in drug responses.[11] Currently, no study specifically focuses on this issue among the Chinese population. Thus, it is necessary to further explore the efficacy and safety of roflumilast in Chinese population with COPD.
This study aimed to conduct a randomized, double blinded, placebo-controlled trial to evaluate the efficacy and safety of roflumilast for Chinese patients with COPD. We hypothesized that roflumilast for the treatment of COPD in Chinese population would be superior to the efficacy of placebo.
2. Methods/design
2.1. Study design
This study is designed as a two-arm, randomized, double blinded, placebo-controlled trial with participants, investigators, assessor, and analyst blinding. The study was approved by the ethics committee of The People's Hospital of Yan’an, and was also conducted at this hospital from January 2011 to December 2016. A total of 120 patients were included and were randomly divided into 2 groups (intervention group and placebo group) at a 1:1 allocation ratio. The patients received either roflumilast or placebo once daily for a total of 12 months. Outcome evaluation and data analysis were performed at the end of 12-month treatment, and 3-month follow-up after the treatment.
3. Eligibility
3.1. Inclusion criteria
The inclusion criteria were as follows: aged more than 40 years old; confirmed diagnosis of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (postbronchodilator forced expiratory volume in 1 second (FEV1)/FVC ratio ≤ 70%, and a postbronchodilator FEV1 with 30% and 80% predicted);[1] a history of COPD with more than 12 months; clinical stable COPD within 1 month before the study; no medication changes within the 3 months prior the study; and the provision of written informed consent prior to enrollment into the study.
3.2. Exclusion criteria
Patients were excluded if they met the following conditions: pregnancy or breast feeding; asthma or other lung related diseases, such as lung cancer, active tuberculosis; α1-antitrypsin deficiency; treatment with systemic glucocorticosteroids, any short-acting β2-agonist 1 month before the study; and severe mental disorder.
3.3. Randomization and allocation concealment
All eligible included patients were randomly assigned to the intervention group or placebo group in a 1:1 ratio. Randomization schedule was generated using a computerized number generator with SAS package (version 9.1; SAS Institute Inc., Cary, North Carolina) by a professional statistician, who was blinded to the treatment allocation. The patients, investigators, outcome assessors, and data analysts were masked to the treatment allocation.
3.4. Intervention
Patients in the intervention group received roflumilast, 500 μg once daily for a total of 12 months. Subjects in the control group received placebo, the same administration schedule as the roflumilast. In addition, the placebo also has the similar appearance, taste, and color as the roflumilast.
3.5. Outcomes
The primary outcome was lung function, measured by the FEV1, FVC, and FEF25–75% change from the baseline.[12] The secondary outcome measurements included quality of life, measured by the St. George's Respiratory Questionnaire (SGRQ).[13] All the above outcomes were assessed at the end of 12-month treatment and 3-month follow up after the treatment. In addition, adverse events (AEs) were also recorded during the treatment period, and were evaluated at the end of 12-month treatment.
3.6. Statistical analysis
SAS package (version 9.1; SAS Institute Inc., Cary, NC) was used to analyze the outcome data with intention-to-treat analysis. The sample size was calculated based on the primary outcome, that is, the change in the FEV1. Therefore, the required sample size of this study was estimated to be 120 patients (60 per group) in order to detect a minimal difference between groups in the FEV1 of 0.05L with α = 0.5 and β = 0.8. All the outcome data were presented as the mean change from baseline (95% confidence interval). T-test or Mann–Whitney rank sum test was used to analyze the continuous data. Pearson's chi-square test or Fisher's exact test was used to analyze the categorical data. The statistical significance level was set at P < .05.
4. Results
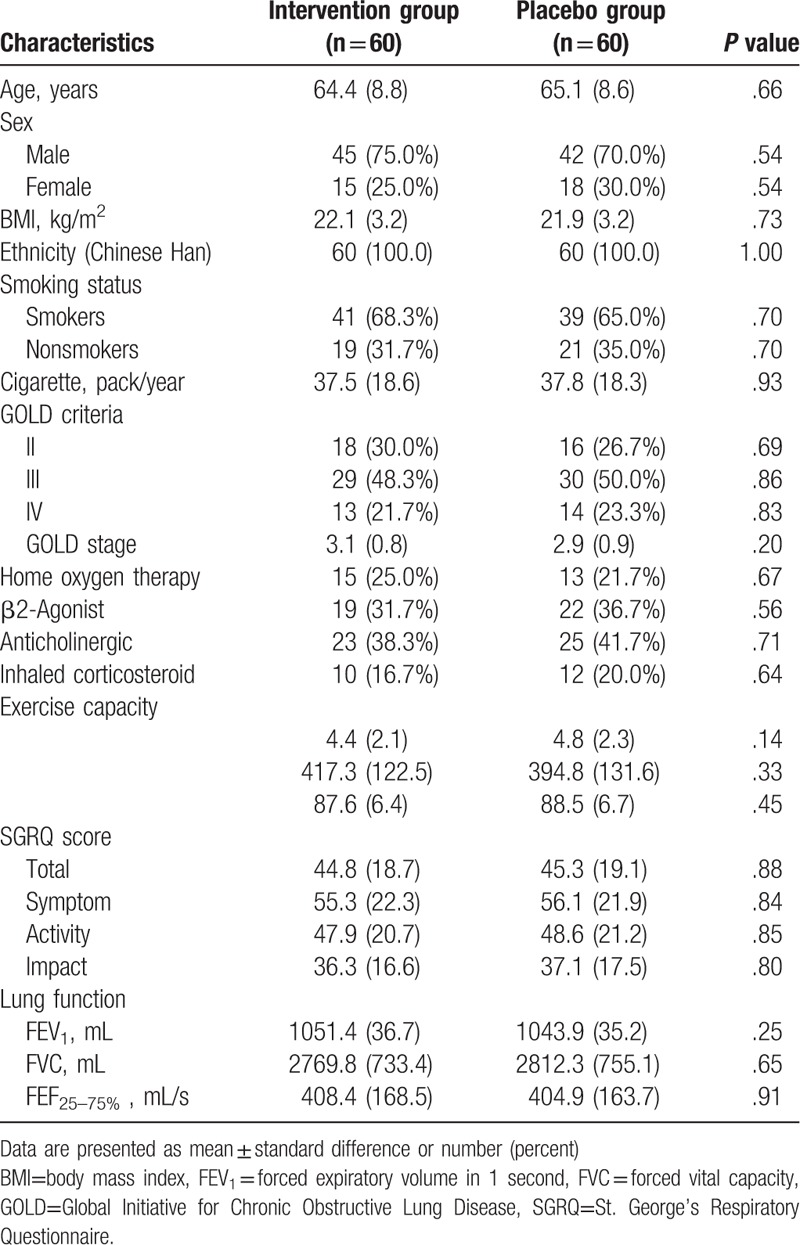
One hundred and eighty three participants were initially recruited the study (Fig. 1). Sixty three patients were excluded. Thus, 120 patients were included in this study, and were equally allocated to the intervention or placebo groups. Five patients withdrew from the intervention group, and 6 participants withdrew from the placebo group because of the AEs, consent withdrawn, and moved to the other cities (Fig. 1). The patient characteristics at baseline are summarized in Table 1. There were not significant differences in all the characteristics and clinical variables at the baseline visit.
Figure 1.
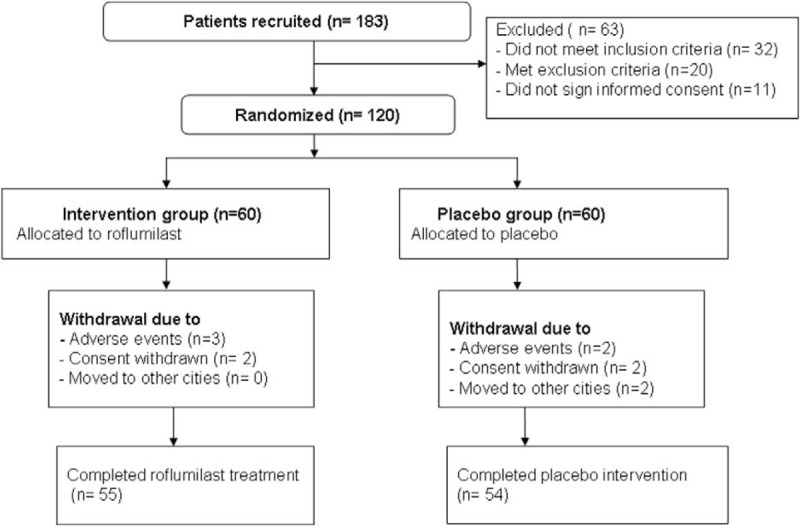
Flowchart of participants throughout the study selection.
Table 1.
Patients characteristics at baseline.
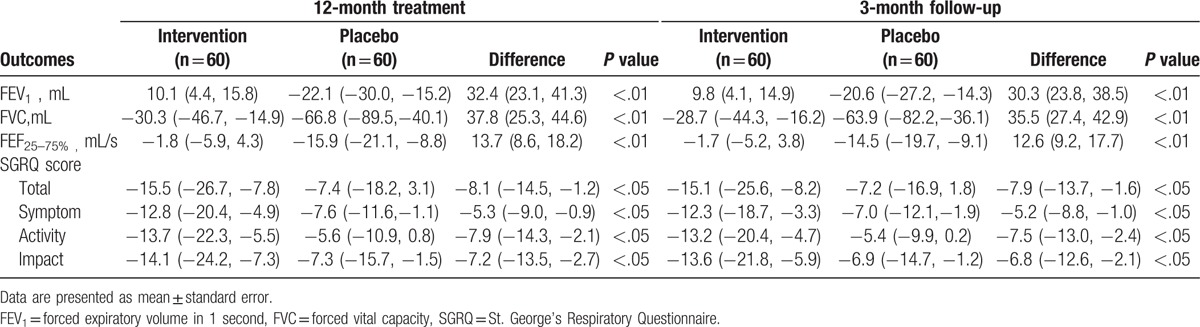
The analysis results of primary and secondary outcome measurements are summarized in Table 2. Roflumilast significantly enhanced the lung function, measured by the FEV1, FVC, and FEF25–75%, when compared with the placebo both at the end of 12-week treatment (P < .01), and 3-month follow-up (P < .01). In addition, roflumilast also exhibit significant improvements in quality of life, measured by SGRQ scale compared with the placebo both at the end of 12-week treatment (P < .05), and 3-month follow-up (P < .05) (Table 2).
Table 2.
Outcome measurements at the end of the 12-month treatment and 3-month follow-up (change from baseline).
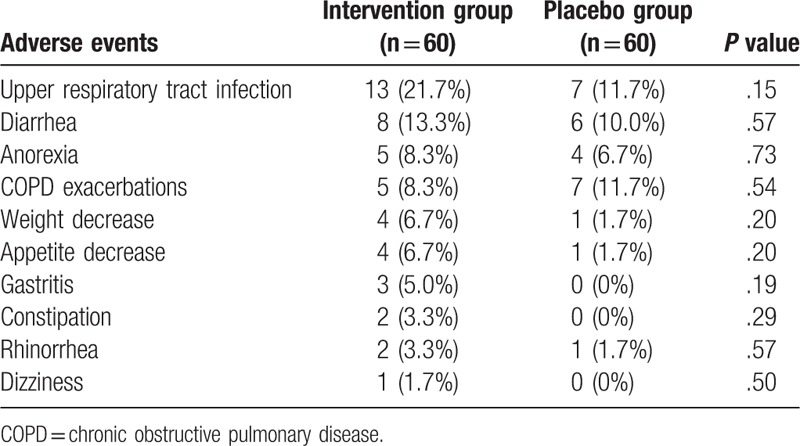
AEs in both groups are showed in Table 3. There were not significant differences in AEs between both groups (Table 3). The most frequency AEs were upper respiratory tract infection (intervention group, 21.7% vs. placebo group, 11.7%, P = 0.15), and diarrhea (intervention group, 13.3% vs. placebo group, 10.0%, P = 0.57). No serious AEs occurred in the either group.
Table 3.
Adverse events between 2 groups.
5. Discussion
Previous studies have evaluated the efficacy of roflumilast for the treatment of COPD. They found that roflumilast plays an important role in improving lung function in Asian population, such as Korea, and European population for treating patients with COPD. Of them, one study evaluated the efficacy and safety of roflumilast in Korean patients with COPD.[14] It was found that roflumilast significantly enhanced lung function with a tolerable safety profile in Korean COPD patients irrespective of the severity of airflow limitation.[14] Other studies explored the efficacy of roflumilast in the Asian population, and also found that roflumilast can improve pulmonary function, and the tolerability profile was similar to the Caucasian population.[15,16] Another study explored the European patients to assess the efficacy and safety of roflumilast and the results demonstrated that roflumilast produced a modest improvement in lung function without changing the exacerbation rate or health status in severe and stable COPD, and fewer exacerbations.[10] However, no study was conducted specifically focused on the Chinese population with COPD.
The results of our study are partly consistent with the previous studies.[14–16] We confirmed our hypothesis that roflumilast has promising efficacy for the treatment of COPD in Chinese population, compared to the placebo. To our best knowledge, this study is the first randomized, double-blinded, placebo-controlled trial to explore the efficacy of roflumilast for treating Chinese population with COPD specifically. Our findings showed the efficacy of roflumilast for Chinese patients with COPD.
In this study, the mean changes of the FEV1, FVC, and FEF25–75% were significantly greater in the intervention group than those in the control group. Furthermore, the total score of SGRQ significantly lower in the intervention group than that in the control group in Chinese patients with COPD. Thus, roflumilast treatment appears to be promising for improving the health-related quality of life of Chinese patients with COPD.
Although the encouraging efficacy of roflumilast for the treatment of COPD, this study still had several limitations. First, this study was only conducted at a single hospital with recruitment of Chinese Han ethnicity. Thus, it may be limited to the generalization to other ethnicities and other hospitals. Second, this study only performed 3-month follow-up evaluation owing to its short duration. Therefore, the studies with longer follow-up assessment of roflumilast on COPD are still needed to be addressed in the future.
6. Conclusion
The results of this study found that roflumilast can either improve lung function, or the health-related quality of life of Chinese patients with COPD. Future studies are still needed to warrant the results of this study.
Footnotes
Abbreviations: AEs = adverse events, COPD = chronic obstructive pulmonary disease, FEV1 = forced expiratory volume in 1 second, GOLD = Global Initiative for Chronic Obstructive Lung Disease, ICS = inhaled corticosteroid, LABA = long-acting β 2 agonist, LAMA = long-acting muscarinic antagonist, PDE4 = phosphodiesterase 4, SGRQ = St. George's Respiratory Questionnaire.
The authors have no conflicts of interest to disclose.
References
- [1].Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: updated 2013. London, UK: Global Initiative for Chronic Obstructive Lung Disease; 2013. http://www.goldcopd.org. Accessed April 2017. [Google Scholar]
- [2].Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med 2007;176:753–60. [DOI] [PubMed] [Google Scholar]
- [4].Menzin J, Boulanger L, Marton J, et al. The economic burden of chronic obstructive pulmonary disease (COPD) in a US Medicare population. Respir Med 2008;102:1248–56. [DOI] [PubMed] [Google Scholar]
- [5].American Thoracic Society; European Respiratory Society Task Force Standards for the Diagnosis and Management of Patients with COPD. New York, NY: American Thoracic Society; 2004. [Google Scholar]
- [6].Field SK. Roflumilast: an oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma. Expert Opin Investig Drugs 2008;17:811–8. [DOI] [PubMed] [Google Scholar]
- [7].McIvor RA. Roflumilast: systemic therapy for COPD. Expert Rev Respir Med 2010;2:539–49. [DOI] [PubMed] [Google Scholar]
- [8].Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax 2007;62:1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rabe KF, Bateman ED, O’Donnell D, et al. Roflumilast-an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005;366:563–71. [DOI] [PubMed] [Google Scholar]
- [10].Calverley PM, Sanchez-Toril F, McIvor A, et al. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:154–61. [DOI] [PubMed] [Google Scholar]
- [11].Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther 2008;84:417–23. [DOI] [PubMed] [Google Scholar]
- [12].American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995;152:1107–36. [DOI] [PubMed] [Google Scholar]
- [13].Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation: the St George's Respiratory Questionnaire. Am Rev Respir Dis 1992;145:1321–7. [DOI] [PubMed] [Google Scholar]
- [14].Lee JS, Hong YK, Park TS, et al. Efficacy and safety of roflumilast in Korean patients with COPD. Yonsei Med J 2016;57:928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee SD, Hui DS, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology 2011;16:1249–57. [DOI] [PubMed] [Google Scholar]
- [16].Zheng J, Yang J, Zhou X, et al. Roflumilast for the treatment of COPD in an Asian population: a randomized, double-blind, parallel-group study. Chest 2014;145:44–52. [DOI] [PubMed] [Google Scholar]


