Supplemental Digital Content is available in the text
Keywords: arthroplasty, asymptomatic bacteriuria, infection, systematic review
Abstract
Background:
A high prevalence of asymptomatic bacteriuria exists in patients prior to arthroplasty, and urinary tract infection is considered to be a source of postoperative superficial wound and prosthetic joint infections. There is no consensus whether to screen for and treat asymptomatic bacteriuria before arthroplasty.
Objective:
To summarize the association between asymptomatic bacteriuria and complications after arthroplasty and to evaluate the clinical benefits of treating asymptomatic bacteriuria prior to arthroplasty.
Method:
We systematically searched PubMed, Embase, and the Cochrane Library to retrieve potentially eligible articles. By screening the titles and abstracts of retrieved records and then reading the full texts of the remaining papers, we finally included 8 English-language articles in this systematic review.
Results:
Asymptomatic bacteriuria prior to arthroplasty is significantly associated with an increased occurrence of postoperative prosthetic joint and superficial wound infections. However, there is little evidence for direct or hematogenous seeding of urinary infections, and treating asymptomatic bacteriuria before arthroplasty did not decrease the incidence of postoperative infectious complications.
Conclusion:
Asymptomatic bacteriuria is not a contraindication for arthroplasty, and the practice of routine preoperative screening for and treatment of asymptomatic bacteriuria should not be continued.
1. Introduction
Asymptomatic bacteriuria (ASB), otherwise known as hidden bacteriuria, is diagnosed when patients suffer from true bacteriuria without any symptoms or signs of urinary tract infection (UTI).[1–4] It is reported that ASB exists in approximately 20% of healthy women over 80 years of age, and the prevalence increases with increasing age.[5]Escherichia coli is the most common type of pathogenic bacteria identified in ASB.[6] After a series of case reports was published in the 1970s, orthopedists first noticed a relationship between UTI and prosthetic joint infection (PJI).[7–10] In a case reported by Hall[10], a female patient with rheumatoid arthritis underwent total knee arthroplasty (TKA), and 5.5 years later, E coli was isolated from a symptomatic UTI. After antimicrobial treatment for 3 weeks, inflammation, warmth, and pain occurred in her right knee, and the bacteria isolated from the joint cavity and the urinary tract infection had the same antibiotic sensitivity.
With the substantially increasing demand for arthroplasty in recent decades, the number of postoperative infections and the related economic burden are also elevated. For infirm or immunocompromised patients, the signs and symptoms of UTI are not apparent. Therefore, several reviews suggested administrating postoperative oral antibiotics to patients with ASB for 8 to 10 days,[11] administering specific perioperative antibiotics,[12] or administering cefuroxime if preoperative pyuria existed.[13]
Although controversy over the benefits of prophylactic treatment of ASB continues,[14] more than two-thirds of orthopedists in the United Kingdom treat ASB before joint replacement.[15] However, several questions remain. For patients undergoing arthroplasty, if there are no symptoms or signs of UTI, is it necessary to routinely screen for ASB? Once ASB is identified, should antibacterial treatment be initiated? Some researchers suggest that patients with ASB have a higher incidence of postoperative superficial wound or prosthetic joint infections,[6,16,17] but does a causal relationship exist? In this study, we systematically review the related literature and provide references to help surgeons make informed treatment decisions.
2. Methods
2.1. Source of literature
ZQY and LLH independently searched PubMed, Embase, and the Cochrane Library by using the keywords “asymptomatic bacteriuria” and (“arthroplasty” OR “joint replacement”) to retrieve records without language limitations. The last retrieval was performed on September 30, 2017. Any disagreements were settled by consensus or were arbitrated by a third reviewer (SW).
2.2. Inclusion criteria and literature selection
We identified eligible studies according to the following criteria: those evaluating preoperative ASB before joint replacement and clinical research. Repeat and unoriginal research was excluded. By evaluating the titles and abstracts, we screened all retrieved citations. Following this, we downloaded and read the full text of the remaining articles and identified the eligible studies. Additional literature was identified by reviewing the reference lists of relevant articles.
2.3. Data extraction and analysis
Useful data including authors’ names, published year, number and demographic characteristic of participants, treatment regimen, duration of follow-up, and complications were extracted and recorded in an Excel spreadsheet. As all data we analyzed were drawn from published literature, no informed consent or ethical approval was needed.
3. Results
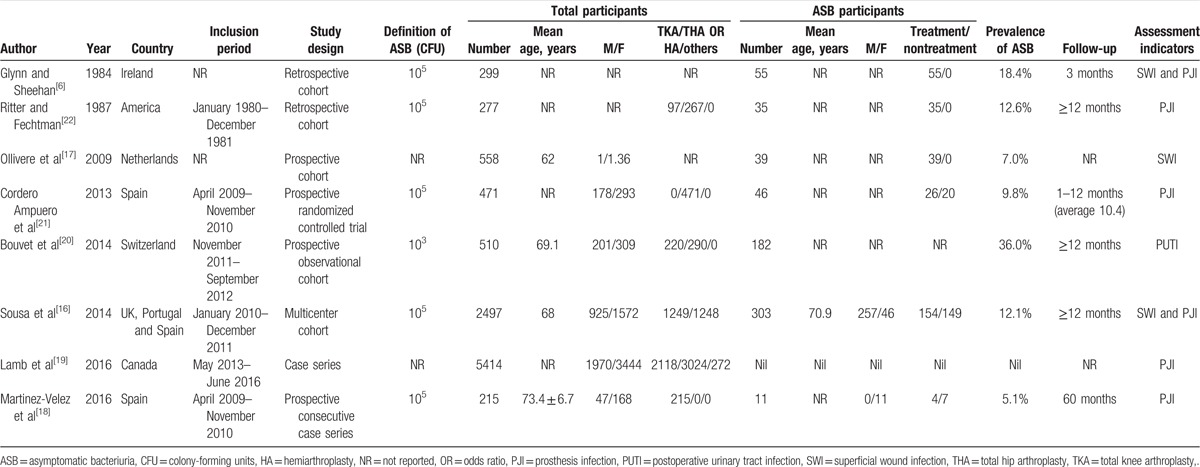
By searching 3 electronic databases and screening the reference lists of pertinent articles, a total of 28 nonduplicated articles were identified. Following this, 2 articles that did not meet the inclusion criteria were excluded after screening the titles and abstracts. After downloading and reading the full texts of the remaining articles, we selected 8 articles [6,16–22] that were in line with the research purpose of this article. All were published in English. The study selection process and the reasons for exclusion are shown in Figure 1 (see table, Supplemental Content, for studies excluded in the first-round screening). Among them, there were 5 cohort studies,[6,16,17,20,22] 2 case series,[18,19] and only 1 randomized controlled trial.[21] Only 2 studies[16,19] included more than 1000 participants. These 8 studies included a total of 10241 patients who underwent joint replacement, of which 671 patients with preoperative ASB were noted in 7 studies.[6,16–18,20–22] Various incidences of ASB were reported across these papers, ranging from 5.1% to 35.7%.[18,20] Basic information and demographic characteristics of the patients in the included studies are shown in Table 1.
Figure 1.
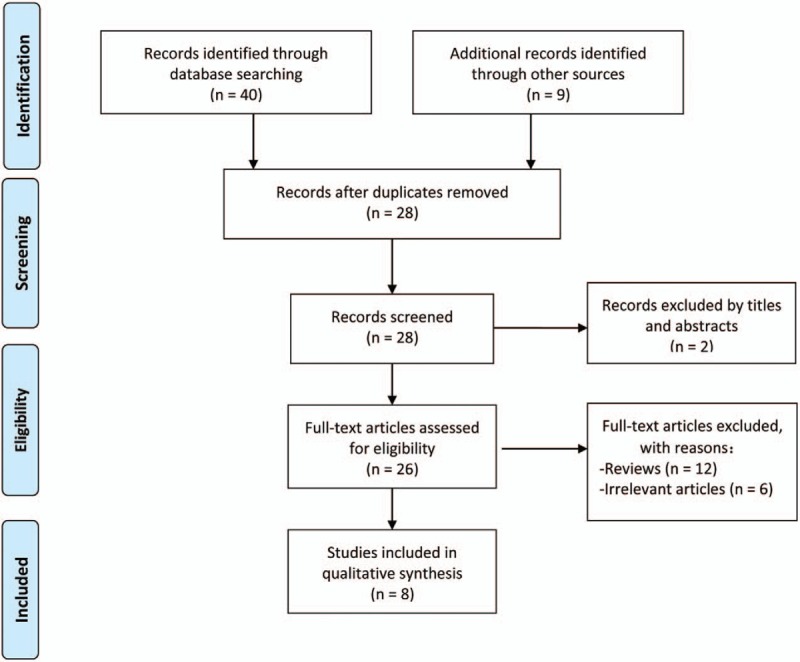
Flowchart summarizing the study selection process.
Table 1.
Characteristics of included studies assessing asymptomatic bacteriuria before arthroplasty.
3.1. Preoperative ASB and postoperative PJI
Prosthetic joint infection is one of the most disastrous complications after artificial joint replacement, and its treatment course can be very difficult. PJI can not only result in repeated surgery but also increases the cost burden and wastes medical resources.[23–25] Hematogenous seeding of bacteria from UTI is often viewed as a risk factor for PJI.[26,27] With the progress of operative procedures, the incidence of PJI has become quite low, which makes research about the relationship between preoperative ASB and PJI difficult.[28–30]
Cordero-Ampuero et al[21] enrolled 471 patients undergoing total hip arthroplasty (THA) or hemiarthroplasty without perioperative indwelling urethral catheterization or urinary tract symptoms and administered a routine urinalysis and a urine culture if urinalysis was abnormal. A total of 46 patients with ASB were identified and randomly divided into treatment and nontreatment groups. Patients in the treatment group were administered appropriate antibiotics for 7 days. During an average follow-up of 10.4 months, 13 cases of PJI occurred, which included 7 patients with normal preoperative urinalysis, 5 patients with abnormal preoperative urinalysis but normal urine culture, and 1 patient with ASB. For the 1 patient with both preoperative ASB and postoperative PJI, the appropriate antibiotic treatment was administered, and the isolation of the postoperative culture was not in accordance with that of the preoperative urine culture.
Another prospective study[18] from the same center recruited 215 candidates for TKA with no perioperative urethral catheterization or urinary tract symptoms. Eventually, 11 patients with preoperative ASB were identified and divided into 2 groups. The 4 patients in group A received antibiotic treatment for 7 days based on urine culture results, and the 7 patients in group B did not undergo antibiotic treatment other than the traditional prophylaxis regimen. Postoperative follow-up lasted for at least 48 months, and only 1 PJI occurred within 3 months after operation in group A. The results of the postoperative bacterial cultures were also different from the preoperative urine culture results.
In 2014, Sousa and his co-workers[16] published a multicenter cohort study including 2497 patients who underwent arthroplasty surgeries. Urine culture revealed 303 patients with preoperative ASB. Advanced age, female sex, a body mass index (BMI) of >30 kg/m2, and a high American Society of Anesthesiologists (ASA) score were found to be risk factors for ASB. Eventually, 154 patients with ASB underwent specific oral antibiotic treatment for 8 days. A total of 43 cases of postoperative PJI occurred, of which 13 cases had preoperative ASB. Multivariate analysis revealed that for patients with ASB, the risk of PJI after arthroplasty was significantly higher than for those without ASB, with odds ratio (OR) being 3.95% and 95% confidence interval (CI) ranging from 1.52 to 10.26). Postoperative UTI was also significantly related to a high incidence of PJI (OR 6.64, 95% CI: 1.24–35.64). However, for patients with either preoperative ASB or postoperative UTI, the pathogens cultured from urine were dissimilar to those isolated from PJI. Meanwhile, treating preoperative ASB did not reduce the incidence of postoperative PJI (OR 0.82, 95% CI: 0.27–2.51). This study is the largest scale one regarding this topic; however, several limitations including nonrandom allocation and inclusion of patients with perioperative urinary catheters merit consideration.
Ritter et al[22] enrolled 277 patients who underwent arthroplasty, and a total of 35 cases of preoperative ASB were identified. During the follow-up period, varying from 1 to 16 years, 3 cases of PJI were identified, and none of them were related to preoperative ASB.
Lamb et al[19] announced that beginning on May 1, 2015, preoperative routine urine cultures were discontinued at their bone and joint center. A total of 3523 patients (3069 underwent preoperative urine culture, of which 352 cases were positive) were admitted during the preceding 2 years, and 1891 patients (of which 10 underwent urine culture and none received corresponding antibacterial treatment) were admitted during the 1 year following the announcement, and these groups of patients were analyzed. There were 1 and 3 cases of PJI occurring in each group, respectively, and none corresponded to the results of preoperative urine culture.
Although Sousa et al[16] found a higher prevalence of postoperative PJI in patients with preoperative ASB, none of the pathogens isolated from the infected endoprostheses in these clinical studies discussed above were a result of direct or hematogenous seeding from the urinary tract, which refutes their causative relationship.
3.2. Preoperative ASB and postoperative SWI
A study[6] published in 1984 retrospectively analyzed 299 patients who underwent arthroplasty. For 57 patients with preoperative bacteriuria (55 cases with ASB), an appropriate 10-day antibiotic regimen was administered according to susceptibility testing. The patients were followed-up for 3 months, and 2 cases of SWI and no PJI occurred in patients with preoperative UTI of E coli, and both bacterial cultures of infected swabs showed Staphylococcus aureus. Although the authors suggested perioperative oral antibiotic treatment for ASB, the results of the study did not support their recommendations.
Ollivere et al[17] included 558 patients who underwent elective joint replacement, identified preoperative ASB in 39 patients, and then treated them with appropriate antibiotics. A higher incidence of delayed incision healing and wound infection was found in the ASB group compared with the non-ASB group (P < .02). However, as the results of postoperative urine culture were not reported, it was unclear whether the same bacteria were isolated from the wound infection. This study could not confirm a causal link between preoperative ASB and postoperative wound complications. More rigorous studies are needed in order to verify this finding.
3.3. Preoperative ASB and postoperative UTI
Bouvet et al[20] analyzed 510 patients who underwent arthroplasty, including 182 with preoperative ASB, and found that the prevalence of UTI after treatment did not decrease although the antibiotic used could cover 65% of the preoperative ASB isolations. The urine culture results preoperatively and 3 days postoperatively were consistent in only 49% of patients. Sousa et al[16] showed that the incidence of postoperative UTI in the ASB group was not higher than that of the non-ASB group (OR 1.74, 95% CI: 0.65 to 4.64). Another study[6] revealed that E coli accounted for 78% of preoperative UTIs but only 39% of postoperative UTIs.
4. Discussion
Arthroplasty is the standard operation used to treat degenerative osteoarthritis, rheumatoid arthritis, osteonecrosis, and other joint disorders of the extremities. It can effectively correct malformation, ameliorate function, and decrease joint pain in addition to improving patients’ quality of life. Preoperative urinary tract infection is believed to be a potential cause of bacterial contamination after arthroplasty surgeries. However, the real effect of asymptomatic bacteriuria prior to joint replacement surgeries remains controversial.
In this systematic review, we included a total of 8 studies, of which only 1[21] was a randomized controlled trial, but it was small in scale. The heterogeneous prevalence reported in included studies may be attributed to multiple factors: the definition of ASB, the sex and age distribution of participants, as well as the care used in taking samples. True bacteriuria was defined as an isolation of ≥ 105 colony-forming units (CFU)/mL in 5 included studies,[6,16,18,21,22] while 1[20] used a nonstandard criterion of ≥ 103 CFU/mL. Only 2 studies[18,21] excluded patients with perioperative indwelling catheters, which acted as a risk factor for postoperative infection.
Different guidelines had inconsistent viewpoints on the management of preoperative ASB. The French Infectious Diseases Society objected to screening for asymptomatic bacteriuria before orthopedic surgery procedures,[31] while the British Orthopaedic Association supported routine preoperative urine screening but did not state whether ASB needed management.[32] The Scottish Intercollegiate Guidelines Network (SIGN), the National Institute for Health and Care Excellence (NICE), and the Infectious Diseases Society of America proposed that, with the exception of pregnant women, patients with ASB do not need corresponding antibiotic treatment; nevertheless, these guidelines were not specifically drafted for arthroplasty patients.[33,34] Other guidelines indicated that conventional urinary screening was no longer obligatory before joint replacement unless the patient had symptoms of a UTI[35] or a history of frequent urinary infections.[36]
One study randomly divided women with ASB into the treatment group and the nontreatment group. For the treatment group, the incidence of symptomatic UTI was not improved, and the side-effects of antimicrobial treatment, such as antibiotic resistance and Clostridium difficile diarrhea, significantly increased.[37,38] Some surgeons hold the opinion that after symptoms of UTI occur, sufficient time still exists to commence targeted treatment to prevent the formation of sepsis and prosthetic joint infection caused by hematogenous seeding of urinary tract infection.[39] Although urine culture is not expensive, given the large number of arthroplasties performed worldwide annually, routine screening and treatment of preoperative ASB will result in a considerable economic burden.[40] According to a survey in the United States in 1989, about $1.5 million was required in order to prevent 1 wound infection caused by preoperative ASB after joint replacement.[41] Considering hospitals’ increasingly limited resources, the low value of antimicrobial treatment for ASB is questionable.
Explanations for the finite value of treating asymptomatic bacteriuria are not well established. The first plausible reason is that patients with ASB are at high risk of recurrent urinary tract infections. A similar urinary tract infection rate was observed before and after appropriate antibiotic treatment for ASB in arthroplasty candidates, and more than half of them showed a different result in the urine culture.[6,20] However, analysis of the relationship between recurrent UTI after treatment and PJI was not available due to the limited data.[21] The second reason is that ASB may be a surrogate for a neglected marker. As most PJI in the ASB group occurred in the early postoperative period (i.e., 6 weeks),[16] different skin flora between patients with and without ASB and ASB and associated contamination during surgery might contribute to the elevated infection rate. Meanwhile, Ollivere et al[17] also revealed an increased rate of superficial wound infection (SWI) in patients with ASB and noticed different proportions of anaerobic infections in wound swab cultures between the ASB and non-ASB groups. However, they neglected to analyze other preoperative risk factors and only used a univariate analysis method, which limited the reliability of their conclusions; meanwhile, they did not present whether the isolation from urine was same as that of the postoperative wound swab. Future studies should focus on the relationships among preoperative asymptomatic bacteriuria, recurrent UTI after treatment, microbial flora of the skin, and postoperative infectious complications.
The limitations of this systematic review merit consideration. First of all, studies concerning ASB before joint replacement are scarce, and most of them are small scale; therefore, the power to detect a small difference in the infection rate is restricted. Secondly, there was heterogeneity throughout the literature regarding the definition of ASB, inclusion criteria for participants, demographic characteristics, and antibiotic regimen. Last but not least, only 1[21] randomized controlled study was retrieved, and therefore selection bias was inevitable.
5. Conclusion
Asymptomatic bacteriuria is a common finding in candidates for arthroplasty. Based on the currently available evidence, regardless of the fact that there is a high prevalence of postoperative prosthetic joint and SWIs in patients with preoperative asymptomatic bacteriuria, the relationship is not causal. ASB does not act as a source of hematogenous dissemination or direct spread of the pathogen that causes infection after joint replacement. For patients receiving arthroplasty, perioperative treatment of ASB does not provide obvious benefits; conversely, it will lead to augmentation of antibiotic resistance, economic burden, and allergies. ASB is not a contraindication for arthroplasty, and the practice of routine preoperative screening for and treatment of ASB should not be continued. Meanwhile, it must be recognized that more rigorous studies about the mechanism for the elevated postoperative infection rate in the ASB group are still imperative.
Supplementary Material
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, ASB = asymptomatic bacteriuria, BMI = body mass index, CFU = colony-forming units, CI = confidence interval, HA = hemiarthroplasty, NICE = National Institute for Health and Care Excellence, OR = odds ratio, PJI = prosthetic joint infection, SIGN = Scottish Intercollegiate Guidelines Network, SWI = superficial wound infection, THA = total hip arthroplasty, TKA = total knee arthroplasty, UTI = urinary tract infection.
QZ and LL contribute equally to this work.
This study was supported by the Beijing Municipal Natural Science Foundation (7174346) and National Natural Science Foundation of China (81372013, 81672236).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Aizen E, Shifrin B, Shugaev I, et al. Clinical and microbiological outcomes of asymptomatic bacteriuria in elderly stroke patients. Israel Med Assoc J 2017;3:147–51. [PubMed] [Google Scholar]
- [2].Ramos JA, Lemos EV, Ruano-Ravina A. Duration of surgical antibiotic prophylaxis in patients with asymptomatic bacteriuria. Lancet Infect Dis 2017;4:370. [DOI] [PubMed] [Google Scholar]
- [3].Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America Guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clinical Infectious Diseases 2005;5:643–54. [DOI] [PubMed] [Google Scholar]
- [4].Gleckman R, Esposito A, Crowley M, et al. Reliability of a single urine culture in establishing diagnosis of asymptomatic bacteriuria in adult males. J Clin Microbiol 1979;5:596–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nicolle LE. Asymptomatic bacteriuria: when to screen and when to treat. Infect Dis Clin North Am 2003;2:367–94. [DOI] [PubMed] [Google Scholar]
- [6].Glynn MK, Sheehan JM. The significance of asymptomatic bacteriuria in patients undergoing hip/knee arthroplasty. Clin Orthop Relat Res 1984;185:151–4. [PubMed] [Google Scholar]
- [7].D’Ambrosia RD, Shoji H, Heater R. Secondarily infected total joint replacements by hematogenous spread. J Bone Joint Surg Am 1976;4:450–3. [PubMed] [Google Scholar]
- [8].Burton DS, Schurman DJ. Hematogenous infection in bilateral total hip arthroplasty. Case report. J Bone Joint Surg Am 1975;7:1004–5. [PubMed] [Google Scholar]
- [9].Cruess RL, Bickel WS, VonKessler KL. Infections in total hips secondary to a primary source elsewhere. Clin Orthop Relat Res 1975;106:99–101. [DOI] [PubMed] [Google Scholar]
- [10].Hall AJ. Late infection about a total knee prosthesis. Report of a case secondary to urinary tract infection. J Bone Joint Surg Br 1974;1:144–7. [PubMed] [Google Scholar]
- [11].David TS, Vrahas MS. Perioperative lower urinary tract infections and deep sepsis in patients undergoing total joint arthroplasty. J Am Acad Orthop Surg 2000;1:66–74. [DOI] [PubMed] [Google Scholar]
- [12].Rajamanickam A, Noor S, Usmani A. Should an asymptomatic patient with an abnormal urinalysis (bacteriuria or pyuria) be treated with antibiotics prior to major joint replacement surgery? Cleve Clin J Med 2007;74:S17–8. [DOI] [PubMed] [Google Scholar]
- [13].Otermin I, Rivero M, Hidalgo A. Is it necessary to delay or to put off surgery in the case of possible asymptomatic bacteriuria and orthopaedic surgery with implants? Enferm Infecc Microbiol Clin 2009;4:252–3. [DOI] [PubMed] [Google Scholar]
- [14].Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis 2014;7:980–3. [DOI] [PubMed] [Google Scholar]
- [15].Finnigan TKP, Bhutta MA, Shepard GJ. Asymptomatic bacteriuria prior to arthroplasty: how do you treat yours? Orthop Proc 2012;94suppl XXIX:58–158. [Google Scholar]
- [16].Sousa R, Munoz-Mahamud E, Quayle J, et al. Is asymptomatic bacteriuria a risk factor for prosthetic joint infection? Clin Infect Dis 2014;1:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ollivere BJ, Ellahee N, Logan K, et al. Asymptomatic urinary tract colonisation predisposes to superficial wound infection in elective orthopaedic surgery. Int Orthop 2009;3:847–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Martinez-Velez D, Gonzalez-Fernandez E, Esteban J, et al. Prevalence of asymptomatic bacteriuria in knee arthroplasty patients and subsequent risk of prosthesis infection. Eur J Orthop Surg Traumatol 2016;2:209–14. [DOI] [PubMed] [Google Scholar]
- [19].Lamb MJ, Baillie L, Pajak D, et al. Elimination of screening urine cultures prior to elective joint arthroplasty. Clin Infect Dis 2017;64:806–9. [DOI] [PubMed] [Google Scholar]
- [20].Bouvet C, Lubbeke A, Bandi C, et al. Is there any benefit in pre-operative urinary analysis before elective total joint replacement? Bone Joint J 2014;3:390–4. [DOI] [PubMed] [Google Scholar]
- [21].Cordero-Ampuero J, Gonzalez-Fernandez E, Martinez-Velez D, et al. Are antibiotics necessary in hip arthroplasty with asymptomatic bacteriuria? Seeding risk with/without treatment. Clin Orthop Relat Res 2013;12:3822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ritter MA, Fechtman RW. Urinary tract sequelae: possible influence on joint infections following total joint replacement. Orthopedics 1987;3:467–9. [DOI] [PubMed] [Google Scholar]
- [23].Flury D, Behrend H, Sendi P, et al. Brucella melitensis prosthetic joint infection. J Bone Jt Infect 2017;3:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marang-van DMP, Bragan TE, Liew S, et al. Variation in prosthetic joint infection and treatment strategies during 4.5 years of follow-up after primary joint arthroplasty using administrative data of 41397 patients across Australian, European and United States Hospitals. BMC Musculoskelet Disord 2017;1:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wouthuyzen-Bakker M, Nijman JM, Kampinga GA, et al. Efficacy of antibiotic suppressive therapy in patients with a prosthetic joint infection. J Bone Jt Infect 2017;2:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dennison T, Alentorn-Geli E, Assenmacher AT, et al. Management of acute or late hematogenous infection after shoulder arthroplasty with irrigation, debridement, and component retention. J Shoulder Elbow Surg 2017;1:73–8. [DOI] [PubMed] [Google Scholar]
- [27].Triantafyllopoulos GK, Soranoglou V, Memtsoudis SG, et al. Implant retention after acute and hematogenous periprosthetic hip and knee infections: whom, when and how? World J Orthop 2016;9:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kurtz SM, Lau E, Watson H, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012;8:61–5. [DOI] [PubMed] [Google Scholar]
- [29].Kurtz SM, Lau E, Schmier J, et al. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty 2008;7:984–91. [DOI] [PubMed] [Google Scholar]
- [30].Maderazo EG, Judson S, Pasternak H. Late infections of total joint prostheses. A review and recommendations for prevention. Clin Orthop Relat Res 1988;229:131–42. [PubMed] [Google Scholar]
- [31].Marmor S, Kerroumi Y. Patient-specific risk factors for infection in arthroplasty procedure. Orthop Traumatol Surg Res 2016;102:S113–9. [DOI] [PubMed] [Google Scholar]
- [32].British Orthopaedic Association Primary Total Hip Replacement: A Guide to Good Practice. London: British Orthopaedic Association; 2006. [Google Scholar]
- [33].Scottish Intercollegiate Guidelines Network (SIGN). SIGN guideline 88. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/recommendations.html. Accessed September 30, 2017. [Google Scholar]
- [34].Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40:643–54. [DOI] [PubMed] [Google Scholar]
- [35].Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection. Bone Joint J 2013;11:1450–2. [DOI] [PubMed] [Google Scholar]
- [36].No authors listed. American Academy of Orthopaedic Surgeons. OrthoInfo. Available at: http://orthoinfo.aaos.org/topic.cfm?topic=a00389. Accessed 17 December, 2013. [Google Scholar]
- [37].Rotjanapan P, Dosa D, Thomas KS. Potentially inappropriate treatment of urinary tract infections in two Rhode Island nursing homes. Arch Intern Med 2011;5:438–43. [DOI] [PubMed] [Google Scholar]
- [38].Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987;1:27–33. [DOI] [PubMed] [Google Scholar]
- [39].Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008;7:1710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lourenco T, Pickard R, Vale L, et al. Minimally invasive treatments for benign prostatic enlargement: systematic review of randomised controlled trials. BMJ 2008;337:a1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lawrence VA, Gafni A, Gross M. The unproven utility of the preoperative urinalysis: economic evaluation. J Clin Epidemiol 1989;12:1185–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


